Abstract
Background
The long-term safety was assessed in patients with psoriatic arthritis who were treated with ixekizumab in three clinical trials (SPIRIT-P1/-P2/-P3).
Methods
Integrated safety data from three trials (controlled and uncontrolled), including two pivotal phase 3, randomized, double-blind clinical trials: SPIRIT-P1 and SPIRIT-P2, were assessed. Safety data were integrated from the all ixekizumab exposure safety population (defined as all patients receiving ≥ 1 dose of ixekizumab). We report exposure-adjusted incidence rates (IRs) per 100 patient-years (PY) at 1-year intervals up to 3 years for adverse events.
Results
Total exposure to IXE reached 1822.2 PY (1118 patients). The IRs/100 PY for the following treatment discontinuations were as follows: adverse events (5.3); serious infections (1.3); injection-site reactions (12.7); infections (34.2); and deaths (0.3). The IRs for treatment-emergent adverse events decreased or remained stable over time, the most common being upper respiratory tract infection, nasopharyngitis, and injection-site reactions. The IRs for serious adverse events and serious infections remained stable over time, whereas for injection-site reactions and general infections, IRs decreased with longer ixekizumab exposure. Opportunistic infections were limited to oral and esophageal candida and localized herpes zoster. No suicide or self-injury-related behaviors were reported. The IRs/100 PY for safety topics of special interest included inflammatory bowel disease (adjudicated; 0.1), depression (1.6), malignancies (0.7), and major adverse cardiovascular events (0.6).
Conclusions
The findings of this integrated safety analysis in patients with psoriatic arthritis are consistent with the known safety profile of ixekizumab. No unexpected safety signals were observed with ixekizumab treatment in patients with psoriatic arthritis.
Trial registration
SPIRIT-P1 (NCT01695239; Registered August 08, 2012), SPIRIT-P2 (NCT02349295; September 23, 2014), and SPIRIT-P3 (NCT02584855; August 04, 2015).
Keywords: Ixekizumab, Safety, Psoriatic arthritis, Inflammatory bowel disease, Major adverse cardiovascular events
Background
Psoriatic arthritis (PsA) is a chronic, inflammatory disease, which is characterized by peripheral arthritis, axial disease, enthesitis, dactylitis, and skin and nail manifestations [1]. Ixekizumab (IXE) is a high-affinity monoclonal antibody that selectively targets interleukin 17A (IL-17A) [2]. The United States Food and Drug Administration has approved IXE for the treatment of psoriasis, psoriatic arthritis, and axial spondyloarthritis [3]. Due to the chronic nature of this disease, long-term safety data on IXE are critical.
In clinical the SPIRIT-P1 trial, IXE was superior to placebo (PBO) in improving several measures including disease activity, radiographic disease progression, physical function, and patient-reported quality of life in biologic-naïve patients with active PsA [4]. In clinical trial SPIRIT-P2, IXE improved the signs and symptoms of patients with active PsA (inadequate responders to tumor necrosis factor [TNF] inhibitor) along with a safety profile consistent with previous studies involving both PsA and psoriasis [5, 6].
A previously published integrated analysis paper by Mease et al., from three clinical trials showed no unexpected safety signals with IXE treatment up to week 96 [7]. We report the results of integrated analysis that evaluated long-term safety and tolerability of up to 3 years of exposure to IXE using data from three clinical trials for 1822.2 patient-years (PY) in patients with active PsA.
Methods
Patients and study design
The present report includes integrated safety analysis data derived from SPIRIT-P1 [4], SPIRIT-P2 [5], and SPIRIT-P3 (Fig. 1). The analysis used data from the All-IXE Exposure-Safety Population, defined as all patients with PsA receiving ≥ 1 dose of IXE. This database includes data from all study periods of SPIRIT-P1 and SPIRIT-P2, along with the open-label period of SPIRIT-P3. The results presented here are from a database lock in March 2018 of these three clinical trials.
Fig. 1.
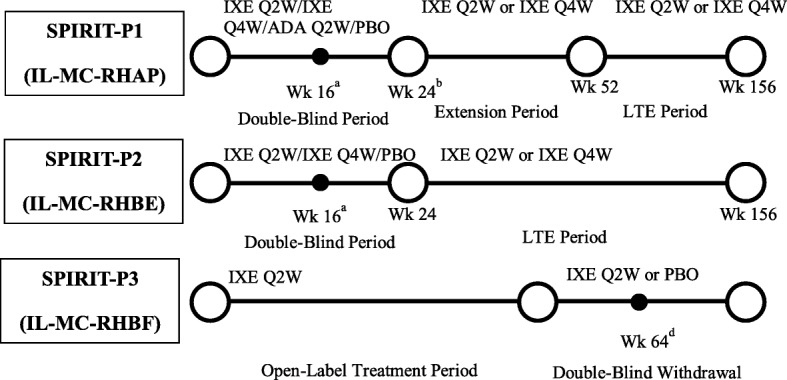
Study design. All patients treated with IXE had a loading dose of 160 mg at week 0. ADA dose was 40 mg Q2W unless stated otherwise. aPatients determined to be inadequate responders by blinded criteria given adjustments to their background/existing therapy. Inadequate responders in the non-IXE groups randomized to IXE Q2W or IXE Q4W with washout for ADA inadequate responders. bResponders in ADA or PBO groups re-randomized to either IXE Q2W or IXE Q4W. cPatients randomized to IXE Q2W or PBO if they met the randomized withdrawal (RW) criteria (i.e., those who met Coates criteria for MDA for ≥ 3 consecutive months across ≥ 4 consecutive visits) at week 36 or later up to week 64. dPatients who had not met RW criteria at week 64 were given IXE Q2W; patients who relapsed (no longer met MDA criteria) during the double-blind withdrawal period were switched to, or continued, IXE Q2W. ADA Q2W: 40 mg of adalimumab every 2 weeks; IXE Q2W: 80 mg of ixekizumab every 2 weeks; IXE Q4W: 80 mg of ixekizumab every 4 weeks; LTE: long-term extension; MDA: minimal disease activity; PBO: placebo; Wk: week
Clinical trials SPIRIT-P1 and SPIRIT-P2 are phase 3 randomized, double-blind, PBO-controlled, parallel-group trials involving patients with active PsA [4, 5]. Patients were randomized to subcutaneous injections of PBO, adalimumab 40 mg (ADA), IXE 80 mg once every 2 weeks (IXE Q2W), or IXE 80 mg once every 4 weeks (IXE Q4W). Both IXE regimens included a 160-mg starting dose. Patients who received PBO and ADA were re-randomized to either IXE Q2W or IXE Q4W for the open-label extension period (weeks 24–156); patients who initially received IXE remained on their original dose. Both trials have similar study designs, except SPIRIT-P1 patients are biologic naive whereas SPIRIT-P2 patients are conventional (c) disease-modifying antirheumatic drugs (DMARDs) and biologic (b) DMARDs experienced. SPIRIT-P1 included assessments of radiographic progression and used ADA as an active control. The primary efficacy and safety analyses of both trials are published [4, 5]. SPIRIT-P3 is a phase 3 study with an open-label period (weeks 0–36) followed by a randomized double-blind withdrawal period from week 36 to week 104, examining the effect of single-arm IXE Q2W in patients with active PsA who are cDMARD-inadequate responders and bDMARD naive.
All studies included in this analysis were compliant with ethical guidelines including the Declaration of Helsinki and other relevant laws and regulations. The study protocols were approved by each site’s ethical review committee/institutional review board, and all patients provided written informed consent.
Safety evaluations
Adverse events (AEs) were classified based upon the Medical Dictionary for Regulatory Activities (MedDRA) versions 19.0 and 19.1. A treatment-emergent AE (TEAE) was defined as an event that first occurred or worsened in severity from baseline until, or prior to, the last visit within the treatment period, and which did not necessarily have a causal relation with the study drug.
Prespecified safety topics of special interest included serious infections (SIs), injection-site reactions (ISRs), allergic reaction/hypersensitivity, opportunistic infections (including candidiasis), major adverse cardiovascular events (MACEs), malignancies (excluding non-melanoma skin cancer [NMSC]), tuberculosis (TB), depression, and suicidality. Each adjudicator reviewed suspected inflammatory bowel disease (IBD) cases and reported their findings as definite, probable, or possible using the EPIMAD registry methodology for diagnosis of IBD cases [8]. Only patients with definite or probable Crohn’s disease (CD) or ulcerative colitis (UC) were classified as having IBD. MACEs were adjudicated by a Clinical Events Committee (CEC).
TB screening was performed at week 52 and annually in all patients per the protocol in patients with no history of TB. In SPIRIT-P1, patients were screened for latent TB infection and were required to be negative or to complete 4 weeks of treatment before enrolment. Patients who tested positive were discontinued. In SPIRIT-P2 or SPIRIT-P3, patients continued if active TB were excluded and if they received a full course of treatment for latent TB with no evidence of hepatotoxicity.
Statistical methods
Overall exposure of IXE was summarized in total PY. This was calculated as follows: PY = sum of duration of exposure in days (for all patients in treatment group)/365.25. TEAEs were summarized by frequencies and exposure-adjusted incidence rates (IRs). IRs per 100 PY were calculated by dividing the total number of patients experiencing the TEAE for the events of interest by the sum of all patients’ time (in 100 years) of exposure during the treatment period. The entire exposure time during the treatment period was used. Frequencies and exposure-adjusted IRs of AEs over time by 1-year time intervals through 156 weeks (3 years) were summarized. The patients who had multiple events across the yearly intervals were counted once in each yearly interval.
Results
A total of 1118 patients who received IXE from 3 studies were included and accounted for 1822.2 PY of exposure (median exposure was 645 days ranging from 8 to 1219 days). The number of patients exposed to study drug over a period of 3 years is shown in Fig. 2. For the pooled population with PsA, the mean age was 49.5 years and 53.8% were female. The mean (SD) duration of the PsA symptoms was 9.71 (8.7) (Table 1).
Fig. 2.
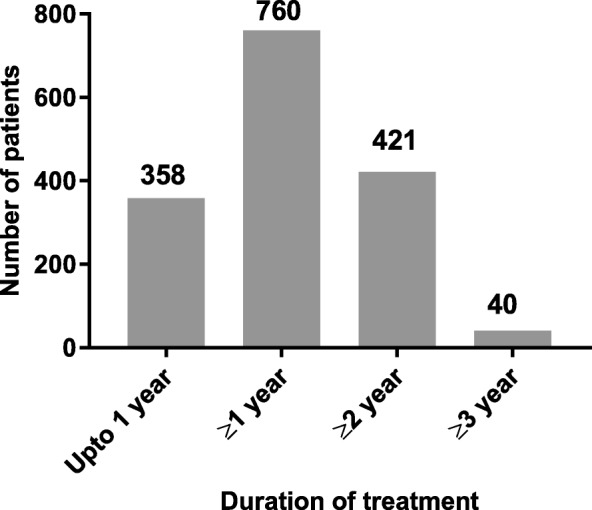
Number of patients by treatment duration. The number of patients exposed to ixekizumab over a period of 3 years. Total N = 1118; total exposure = 1822.2 patient-years
Table 1.
Demographic and baseline characteristics (All PsA ixekizumab-exposure safety population)
| Characteristics | All-IXE treatment periods(N = 1118) |
|---|---|
| Age, years, mean (SD) | 49.5 (11.9) |
| Sex, n (%) | |
| Male | 517 (46.2) |
| Female | 601 (53.8) |
| Race, n (%) | |
| White | 1056 (94.5) |
| Asian | 39 (3.5) |
| American Indian or Alaska Native | 9 (0.8) |
| Multiple | 8 (0.7) |
| Black or African American | 4 (0.4) |
| Native Hawaiian or other Pacific Islander | 1 (0.1) |
| Weight, kg, mean (SD) | 86.31 (20.4) |
| BMI, kg/m2, mean (SD) | 29.95 (6.9) |
| Previous PsA systemic therapya, n (%) | |
| No prior treatment | 218 (19.5) |
| Non-biologic only | 562 (50.3) |
| Biologic only | 71 (6.4) |
| Biologic and non-biologic | 267 (23.9) |
| Duration of PsA symptoms in years, mean (SD) | 9.71 (8.7) |
All-IXE treatment period defined as all patients who received ≥ 1 dose of IXE
aSystemic therapy includes biologic (such as anti-TNF inhibitors) and non-biologic (such as cDMARDs, NSAIDs, and corticosteroids) medications that were used prior to the study entry
bDMARDs biologic disease-modifying antirheumatic drugs, BMI body mass index, cDMARDs conventional disease-modifying antirheumatic drugs, IXE ixekizumab, N population size, n number in each group, NSAIDs non-steroidal anti-inflammatory drugs, PsA psoriatic arthritis, SD standard deviation
The n (IRs/100 PY) for TEAEs at years 1, 2, and 3 were 844 (89.3/100 PY), 465 (72.5/100 PY), and 170 (72.4/100 PY), respectively. The most common TEAEs (n [IRs/100 PY]) were upper respiratory tract infection (161 [8.8/100 PY]), nasopharyngitis (150 [8.2/100 PY]), and ISR (142 [7.8/100 PY]) (Table 2).
Table 2.
Summary of most commonly reported adverse events (incidence rates per 100 PY)
| Event type | Double-blind period (weeks 0–24) |
All-IXE treatment periods (N = 1822.2) IR (95% CI) |
||
|---|---|---|---|---|
| Placebo N = 224 n (IR) |
IXE80Q4W (N = 229) n (IR) |
IXE80Q2W (N = 225) n (IR) |
||
| Patients with ≥ 1 TEAE | 127 (148.2) | 153 (155.6) | 156 (163.4) | 50.0 (46.9, 53.4) |
| Milda | 60 (70.0) | 91 (92.6) | 81 (84.8) | 20.4 (18.4, 22.5) |
| Moderatea | 63 (73.5) | 54 (54.9) | 61 (63.9) | 24.3 (22.1, 26.6) |
| Severea | 4 (4.7) | 8 (8.1) | 14 (14.7) | 5.4 (4.5, 6.6) |
| Patients discontinuing from study drug due to AEs | 8 (9.3) | 7 (7.1) | 12 (12.6) | 5.3 (4.3, 6.4) |
| Patients with ≥ 1 SAEs | 6 (7.0) | 9 (9.2) | 11 (11.5) | 6.4 (5.3, 7.6) |
| Deaths | 0 (0) | 0 (0) | 0 (0) | 0.3 (0.1, 0.7) |
| Patients with ≥ 1 most frequent TEAEs (preferred term) | ||||
| Upper respiratory tract infection | 16 (18.7) | 16 (16.3) | 15 (15.7) | 8.8 (7.6, 10.3) |
| Nasopharyngitis | 9 (10.5) | 15 (15.3) | 7 (7.3) | 8.2 (7.0, 9.7) |
| Injection-site reaction | 1 (1.2) | 22 (22.4) | 32 (33.5) | 7.8 (6.6, 9.2) |
| Bronchitis | 7 (8.2) | 4 (4.1) | 7 (7.3) | 4.4 (3.6, 5.5) |
| Sinusitis | 5 (5.8) | 9 (9.2) | 6 (6.3) | 3.7 (2.9, 4.7) |
| Urinary tract infection | 5 (5.8) | 8 (8.1) | 4 (4.2) | 3.2 (2.5, 4.1) |
| Injection-site erythema | 0 (0.0) | 9 (9.2) | 17 (17.8) | 2.9 (2.2, 3.7) |
| Patients with ≥ 1 AESIs | ||||
| Cytopenias | 2 (2.3) | 2 (2.0) | 4 (4.2) | 2.5 (1.9, 3.4) |
| Hepatic | 10 (11.7) | 7 (7.1) | 11 (11.5) | 4.9 (4.0, 6.0) |
| Infection | 62 (72.3) | 77 (78.3) | 72 (75.4) | 34.2 (31.6, 37.0) |
| Serious infections | 0 (0) | 1 (1.0) | 5 (5.2) | 1.3 (0.8, 1.9) |
| Candida infections | 1 (1.2) | 4 (4.1) | 8 (8.4) | 2.1 (1.6, 2.9) |
| Esophageal candidiasis | 0 (0) | 0 (0) | 1 (1.0) | 0.1 (0.0, 0.4) |
| Active tuberculosis | 0 (0) | 0 (0) | 0 (0) | 0 (0.0, 0.0) |
| Latent tuberculosis | 0 (0) | 0 (0) | 0 (0) | 0.7 (0.4, 1.2) |
| Injection-site reactions | 10 (11.7) | 40 (40.7) | 57 (59.7) | 12.7 (11.2, 14.5) |
| Allergic reactions/hypersensitivities | 4 (4.7) | 10 (10.2) | 14 (14.7) | 4.8 (3.9, 6.0) |
| Confirmed cerebro-cardiovascular events | 2 (2.3) | 0 (0) | 0 (0) | 1.2 (0.8, 1.8) |
| Confirmed MACE events | 0 (0) | 0 (0) | 0 (0) | 0.6 (0.3, 1.1) |
| Malignancies | 0 (0) | 2 (2.0) | 0 (0) | 0.7 (0.4, 1.2) |
| Depression | 3 (3.5) | 4 (4.1) | 4 (4.2) | 1.6 (1.2, 2.4) |
| Adjudicated inflammatory bowel disease (narrow and broad terms) | 0 (0) | 0 (0) | 0 (0) | 0.1 (0.0, 0.4)b |
| Adjudicated Crohn’s disease | 0 (0) | 0 (0) | 0 (0) | 0.1 (0.0, 0.4) |
| Adjudicated ulcerative colitis | 0 (0) | 0 (0) | 0 (0) | 0.1 (0.0, 0.4) |
All-IXE treatment period defined as all patients who received ≥ 1 dose of IXE
aPatients with multiple occurrences of the same event are counted under the highest severity
AEs are listed according to the preferred term in MedDRA, and AEs occurred in ≥ 3.0% of the patients in the combined (total) ixekizumab group
bThe data presented is for All-IXE treatment period
AEs adverse events, AESIs adverse events of special interest, CI confidence interval, IR incidence rate, IXE ixekizumab, MACE major adverse cardiac events, MedDRA Medical Dictionary for Regulatory Activities, N population size, n number in group, PsA psoriatic arthritis, PY patient-years, Q2W every 2 weeks, Q4W every 4 weeks, SAE serious adverse event, TEAE treatment-emergent adverse event
Likewise, the IRs for serious AEs (SAEs) remained stable with longer IXE treatment (Fig. 3). SAEs (n [IRs/100 PY]) occurring in ≥ 3 patients were cholelithiasis and pneumonia (5 [0.3/100 PY] each), bronchitis, and fall (4 [0.2/100 PY] each), coronary artery disease, meniscus injury, and osteoarthritis (3 [0.2/100 PY] each). Six deaths (0.3/100 PY) were reported (cerebrovascular accident, metastatic renal cell carcinoma, cardiorespiratory arrest, myocardial infarction, drowning, and pneumonia). None of these deaths were determined related to IXE treatment. TEAEs leading to IXE discontinuation (n [IRs/100 PY]) included latent TB (19 [1.0/100 PY]), ISR (3 [0.2/100 PY]), and pneumonia, myalgia, and cerebrovascular accident in which the exposure-adjusted IRs were 2 [0.1/100 PY] for each TEAE.
Fig. 3.
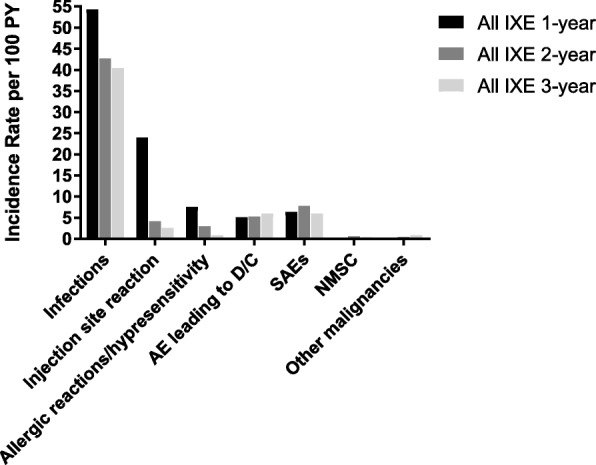
Treatment-emergent adverse events per 100 patient-years by years of treatment. AE: adverse event; D/C: discontinuation; IXE: ixekizumab; NMSC: non-melanoma skin cancer; PY: patient-years; SAE: serious adverse event
Adverse events of special interest
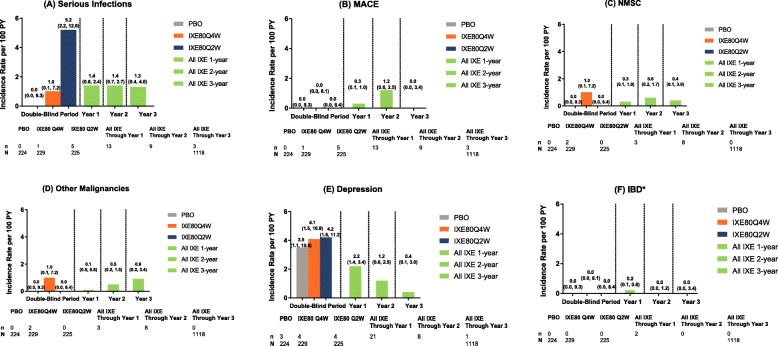
The IRs at 1-year intervals up to year 3 including double-blind treatment are shown in Fig. 4 for (a) serious infections, (b) MACE (CEC-adjudicated), (c) NMSC, (d) other malignancies (excluding NMSC), (e) depression, and (f) IBD related.
Fig. 4.
Exposure-adjusted incidence rate of TEAEs at 1-year intervals to year 3. The data points on the graph are the IR (95% CI)/100 PY at successive 1-year interval to year 3 for all ixekizumab-treated dataset (SPIRIT-P1, SPIRIT-P2, SPIRIT-P3) for a serious infections, b MACE (CEC-adjudicated), c NMSC, d other malignancies (excluding NMSC), e depression, and f IBD related. The CIs for the IRs are from likelihood ratio test of treatment effect from the Poisson regression model. The AEs were coded using MedDRA Version 19.1. *95% CI was not evaluated for IBD. AE: adverse event; CEC: Clinical Events Committee; CI: confidence interval; IBD: inflammatory bowel disease; IR: incidence rate; IXE: ixekizumab; MACE: major adverse cardiovascular events; MedDRA: Medical Dictionary for Regulatory Activities; Ns: number of patients entered in each time interval; n: number in group; PBO: placebo; PY: patient-years; Q2W: every 2 weeks; Q4W: every 4 weeks; TEAEs: treatment-emergent adverse events
Infections
The IRs of infection-related TEAEs decreased with increasing duration of IXE exposure (Fig. 3). The most common infections (n [IRs/100 PY]) were upper respiratory tract infection (161 [8.8/100 PY]), nasopharyngitis (150 [8.2/100 PY]), and bronchitis (81 [4.4/100 PY]). The overall incidence of SI (n [IRs/100 PY]) was 23 patients (1.3/100PY). SIs (n [IRs/100 PY]) occurring in > 1 patient were pneumonia (5 [0.3/100 PY]), bronchitis (4 [0.2/100 PY]), and latent TB (hospitalization for testing to exclude active TB), lower respiratory tract infection, and esophageal candidiasis (2 [0.1/100 PY each]). The IRs for candida infections were 39 (2.1/100PY). No treatment-emergent candida infection resulted in IXE discontinuation.
There were 15 patients (0.8/100 PY) with localized herpes zoster. Twenty-one patients (1.2/100 PY) discontinued IXE because of infections: 6 patients (0.3/100 PY) due to latent TB, 2 patients (0.1/100 PY) due to pneumonia, and 1 patient (0.1/100 PY) each due to septic arthritis, bronchitis, cellulitis, dermatitis, folliculitis, hepatitis B, nasopharyngitis, otitis media, staphylococcal infection, subcutaneous abscess, tonsillitis, tooth abscess, and urinary tract infection. Grade 3 neutropenia (< 1000 cells/mm3 and ≥ 500 cells/mm3) occurred in 6 patients (0.3/100PY). The majority of cases of neutropenia were either Grade 2 (< 1500 cells/mm3 and ≥ 1000 cells/mm3) in 59 patients (3.2/100PY) or Grade 1 (<2000 cells/mm3 and ≥ 1500 cells/mm3) in 137 patients (7.5/100PY). No patients had infections temporally associated with neutropenia of Grade 3. The reported events were common types of non-opportunistic infections such as nasopharyngitis and otitis externa and influenza (1 patient each); none was a serious adverse event.
Injection-site reactions
The incidence of ISRs decreased substantially from the first year and remained stable over time (Fig. 3). The most common preferred terms of ISRs (n [IRs/100 PY]) were unspecified ISR (142 [7.8/100 PY]), injection-site erythema (52 [2.9/100 PY]), and injection-site pain (18 [1.0/100 PY]). There were 3.5 ISRs per 100 active injections. In most cases, ISRs did not result in treatment discontinuation, 6 patients (0.3/100 PY). There were no serious ISRs.
MACE
The incidence of MACE did not increase with longer IXE exposure (Fig. 3). Eleven patients (0.6/100 PY) had CEC-confirmed MACE (2 vascular deaths, 5 nonfatal myocardial infarctions, and 4 nonfatal strokes). Approximately 72% of the patients had one or more cardiovascular risk factors including hypertension, dyslipidemia, diabetes, and pre-existing cardiovascular disease.
Malignancy
With longer IXE exposure, there was no increase in the malignancy rate (Fig. 3). Thirteen patients (0.7/100 PY) developed malignancy. Of these, 8 patients had NMSC and 6 patients had breast cancer (n = 1), prostate cancer (n = 1), invasive ductal breast carcinoma (n = 1), malignant melanoma in situ (n = 1), metastatic renal cell carcinoma (n = 1), and papillary thyroid cancer (n = 1). These events were considered SAEs and led to discontinuation of study drug.
Hypersensitivity events
The IRs of hypersensitivity events decreased with increasing durations of IXE exposure (Fig. 3). There was one case of SAE of angioedema (non-anaphylactic reactions) and no case of anaphylaxis. Eight patients discontinued due to hypersensitivity including drug eruption, angioedema, dermatitis infected, injection-related reaction, rash, rash pruritic, and solar urticaria.
Inflammatory bowel disease
Two patients (IR = 0.1/100 PY; 1 CD, 1 UC) had adjudicated IBD, and these two patients did not have reported IBD history. Both of these events occurred at 6 months to 1 year of treatment with IXE Q2W group. Three patients (IR = 0.2/100 PY; 1 CD, 2 UC) had un-adjudicated IBD.
Other adverse events of special interest
There was no evidence of an increase in depression-related events over time (Fig. 3). The incidence of depression-related events were 1.6/100 PY. One patient (0.1/100PY) had a SAE of depression. Another patient discontinued due to a depression event; this patient was on IXE treatment and had a prior history of depression. The event was not considered related to the study drug. No suicide or self-injury-related behaviors were reported. One patient met laboratory criteria for potential drug-induced liver injury: a 59-year-old male who had received first dose of IXE during the blinded treatment period and was diagnosed with cholelithiasis approximately 2 years after starting the study. The patient underwent surgery and recovered; the event was considered not related to IXE.
Discussion
Here, we report data from the IXE PsA program that includes 3 studies and 1822.2 PY of exposure. The overall TEAEs decreased or remained stable with longer IXE exposure. Consistent with previous reports, ISRs and upper respiratory tract infections are reported as the most frequent TEAEs [7]; the IR for these events decreased with an increase in the duration of IXE exposure. This is consistent with the pattern observed in the psoriasis clinical trials and with the findings associated with the use of secukinumab in PsA [9, 10]. This is similar to the reports for biologic agents neutralizing TNF [11].
The overall incidence of SIs was low which is consistent with this class of biologics [12–14]. Due to the impact on the immune-mediated natural defense, anti-TNF-α, a pro-inflammatory cytokine has been associated with an increased risk of infection, particularly reactivation of latent TB and fungal infections [15]. Results from the British Society for Rheumatology Biologics Register have reported non-significant increase in the rate of SIs between TNF-treated and control [16]. The German and Swedish Biologics Registries have reported a small but significant increase in the risk of SIs [17]. Similarly, for the Italian GISEA registry, the overall incidence of SIs was 31.8/1000 PY in a long-term treatment with anti-TNF therapy [18].
Patients with latent TB were allowed into the clinical trials if treatment was completed per the standard guidelines or were ongoing at the time of study inception; 32 (1.8/100PY) had treatment-emergent latent TB infection. There were no cases of TB reactivation or active TB in the PsA clinical program [7]. Several analyses, primarily from the European registries for biologics, have reported the association between TNF-α inhibitor administration and risk of TB infections; this is particularly true for anti-TNF monoclonal antibodies such as infliximab and ADA when compared with etanercept [19–21].
Consistent with the known mechanism of action of IXE and the role of IL-17 signaling in mucocutaneous defense, candida infections were the most common opportunistic infections [22]. The IRs/100 PY of candida infections and esophageal candidiasis in the present data from PsA.
were 2.1 and 0.1, respectively; most were mild or moderate in nature and there was no discontinuation due to candida infections. Consistent with reports from the psoriasis program and with studies on other IL-17 inhibitors, there were no deep organ or blood stream fungal infections [9, 12].
The role of IL-17 in the pathogenesis of IBD has not been clearly delineated, and patients with PsA have an increased risk for IBD compared with the background population [23, 24]. The IR for IBD for IXE remained consistent with background rates with 2 patients (0.1/100 PY) adjudicated with IBD; one each for CD and UC, respectively, both cases were new onset. Reports from other IL-17 inhibitors such as secukinumab have reported 3 cases of UC, 3 cases of CD and 2 cases of IBD unclassified (EAIRs 0.08, 0.08, and 0.05); 7 of these represented new onset cases [25].
Patients with PsA have an increased risk of MACE, and subjects at entry into the IXE PsA program had a prevalence of known cardiovascular risk factors of obesity (body mass index > 30) of 479 (42.8%), diabetes 78 (7.0%), dyslipidemia 30 (2.7%), and hypertension 434 (38.8%). The IR of CEC-confirmed MACE was 0.6/100 PY, with no trend for an increase with increasing IXE exposure. These findings are consistent with that reported in a pooled safety analysis of IXE from 3 clinical trials (0.7/100PY) [7].
Though severe psoriasis has been associated with increased risk of self-harm and suicide attempts relative to the general population (incidence rate ratios = 1.69), the literature in patients with PsA has been limited [26]. In compliance with the ICH guidelines, only patients with significant uncontrolled neuropsychiatric disorders were excluded, thus patients with a wide spectrum of stable neuropsychiatric disorders including depression were allowed into the ixekizumab PsA clinical trials. One serious depression-related event was reported; there was no suicide ideation, behavior, or completed suicide in the IXE PsA program. These findings are consistent with reports from other IL-17 inhibitors [27].
There were six deaths reported, with the causes being cerebrovascular accident, metastatic renal cell carcinoma, cardiopulmonary arrest, myocardial infarction, drowning, and pneumonia. Upon medical review, no deaths were attributed to IXE by the sponsor. These findings are consistent with previous reports in the larger psoriasis IXE-treated population [9].
Although this study covers up to 1822.2 PYs of exposure with IXE in patients with PsA, the duration of the program and the small number of AEs limit the conclusions that can be drawn for rare events or events. Due to limitations in the clinical trial setting including limited follow-up time with IXE exposure, ongoing long-term studies and post-marketing data will provide additional data to delineate the safety profile of IXE in this treatment population.
Conclusions
The data presented in this report indicate a consistent safety profile for IXE over a period of 3 years. Additionally, the safety profile reported in the PsA treatment population remains consistent with the larger IXE psoriasis clinical trial program [9].
Acknowledgements
The authors thank the SPIRIT-P1, SPIRIT-P2, and SPIRIT-P3 study team for their contribution to the study. Also, the authors thank Lahari N.A., an employee of Eli Lilly and Company, for medical writing support and assistance with preparation and submission of this paper.
Funding
This study was funded by Eli Lilly and Company.
The work described in this manuscript was designed by the funder, Eli Lilly and Company, with input from the academic authors. The data were analyzed and interpreted by Eli Lilly and Company in collaboration with the academic authors. All authors had final responsibility for the decision to submit for publication.
Availability of data and materials
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment for up to 2 years per proposal. For details on submitting a request, see the instructions provided at www.clinicalstudydatarequest.com.
Abbreviations
- ADA
Adalimumab 40 mg
- AEs
Adverse events
- bDMARDs
Biologic disease-modifying antirheumatic drugs
- cDMARDs
Conventional disease-modifying antirheumatic drugs
- CEC
Clinical Events Committee
- CD
Crohn’s disease
- DMARDs
Disease-modifying antirheumatic drugs
- IBD
Inflammatory bowel disease
- ICH
International Council for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use
- IL-17A
Interleukin 17A
- IRs
Incidence rates
- ISRs
Injection-site reactions
- IXE
Ixekizumab
- MACE
Major adverse cardiovascular event
- MedDRA
Medical Dictionary for Regulatory Activities
- NMSC
Non-melanoma skin cancer
- PBO
Placebo
- PsA
Psoriatic arthritis
- PY
Patient-years
- Q2W
Every 2 weeks
- Q4W
Every 4 weeks
- SAEs
Serious adverse events
- SD
Standard deviation
- SIs
Serious infections
- TB
Tuberculosis
- TEAE
Treatment-emergent adverse event
- TNF
Tumor necrosis factor
- TNF-α
Tumor necrosis factor alpha
- UC
Ulcerative colitis
Authors’ contributions
All the authors contributed to the preparation of this manuscript and approved the final version for submission. All authors take responsibility for the accuracy and completeness of data and data analyses. All the authors were involved in data interpretation. BC was also involved in conception of study design. JDC was also involved in the data analysis. WX was the study statistician and was additionally involved in conception of study design, design of the work, and data analysis. MCG was also involved in the conception of study design, and design of the work. The sponsor provided the study drugs, planned and performed the statistical analyses, and provided editorial and writing assistance.
Authors’ information
Not applicable.
Ethics approval and consent to participate
SPIRIT-P1, SPIRIT-P2, and SPIRIT-P3 were conducted in accordance with Good Clinical Practice, the principles of the Declaration of Helsinki, and local laws and regulations. SPIRIT-P1 was approved by the Western Institutional Review Board (approval #1-778053-1) and SPIRIT-P2 was approved by the Bellberry Human Research Ethics Committee (Application #2015-01-049-AA). SPIRIT-P3 Institutional Review Board tracking number # 20151638. For all the three studies, approval was also obtained from each additional site. All patients in both studies gave written informed consent.
Consent for publication
Not applicable.
Competing interests
B. Combe received grant/research support from Pfizer, MSD, and Roche-Chugai; is a consultant for Pfizer, UCB, Bristol-Myers Squibb, Janssen, Eli Lilly and Company, MSD, Roche-Chugai, AbbVie, Sanofi, and Gilead; and is on Speakers bureau for Pfizer, Bristol-Myers Squibb, Gilead, Eli Lilly and Company, Roche-Chugai, and MSD.
P. Rahman has received consulting fees or other remuneration and is on Speakers bureau for AbbVie, Eli Lilly and Company, Janssen, Pfizer, Novartis, and UCB and received grant/research support from Janssen.
H. Kameda has received consulting fees or other remuneration and is on Speakers bureau for AbbVie, Asahi Kasei Pharma, Astellas, Bristol-Myers Squibb, Eisai, Eli Lilly and Company, Janssen, Mitsubishi Tanabe Pharma, Novartis, Chugai, and Pfizer.
J.D. Cañete has received consulting fees or other remuneration from Janssen, Novartis, Mylan, Eli Lilly and Company, Pfizer, and UCB and is on Speakers bureau for Janssen, and Novartis.
G. Gallo, N. Agada, and W. Xu are employees and shareholders of Eli Lilly and Company.
M.C. Genovese received grant/research support and is a consultant for Eli Lilly and Company, and Novartis.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gladman DD. Clinical features and diagnostic considerations in psoriatic arthritis. Rheum Dis Clin N Am. 2015;41:569–579. doi: 10.1016/j.rdc.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Lu J, Allan BW, Tang Y, Tetreault J, Chow CK, et al. Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17a. J Inflamm Res. 2016;9:39–50. doi: 10.2147/JIR.S100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taltz (ixekizumab) prescribing information. Indianapolis: Eli Lilly and Company; 2019. https://pi.lilly.com/us/taltz-uspi.pdf.
- 4.Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, et al. Ixekizumab, an interleukin-17a specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76:79–87. doi: 10.1136/annrheumdis-2016-209709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nash P, Kirkham B, Okada M, Rahman P, Combe B, Burmester GR, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet. 2017;389:2317–2327. doi: 10.1016/S0140-6736(17)31429-0. [DOI] [PubMed] [Google Scholar]
- 6.Strober B, Leonardi C, Papp KA, Mrowietz U, Ohtsuki M, Bissonnette R, et al. Short- and long-term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: etanercept comparisons and integrated data. J Am Acad Dermatol. 2017;76:432–440. doi: 10.1016/j.jaad.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Mease P, Roussou E, Burmester GR, Goupille P, Gottlieb A, Moriarty SR, et al. Safety of ixekizumab in patients with psoriatic arthritis: results from a pooled analysis of three clinical trials. Arthritis Care Res (Hoboken) 2019;71:367–378. doi: 10.1002/acr.23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gower-Rousseau C, Salomez JL, Dupas JL, Marti R, Nuttens MC, Votte A, et al. Incidence of inflammatory bowel disease in northern France (1988-1990) Gut. 1994;35:1433–1438. doi: 10.1136/gut.35.10.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langley RG, Kimball AB, Nak H, Xu W, Pangallo B, Osuntokun OO, et al. Long-term safety profile of ixekizumab in patients with moderate-to-severe plaque psoriasis: an integrated analysis from 11 clinical trials. J Eur Acad Dermatol Venereol. 2019;33:333–339. doi: 10.1111/jdv.15242. [DOI] [PubMed] [Google Scholar]
- 10.Patel NU, Vera NC, Shealy ER, Wetzel M, Feldman SR. A review of the use of secukinumab for psoriatic arthritis. Rheumatol Ther. 2017;4:233–246. doi: 10.1007/s40744-017-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mease PJ. Tumour necrosis factor (TNF) in psoriatic arthritis: pathophysiology and treatment with TNF inhibitors. Ann Rheum Dis. 2002;61:298–304. doi: 10.1136/ard.61.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Kerkhof PC, Griffiths CE, Reich K, Leonardi CL, Blauvelt A, Tsai TF, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83–98. doi: 10.1016/j.jaad.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Papp KA, Griffiths CE, Gordon K, Lebwohl M, Szapary PO, Wasfi Y, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844–854. doi: 10.1111/bjd.12214. [DOI] [PubMed] [Google Scholar]
- 14.Minozzi S, Bonovas S, Lytras T, Pecoraro V, Gonzalez-Lorenzo M, Bastiampillai AJ, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf. 2016;15:11–34. doi: 10.1080/14740338.2016.1240783. [DOI] [PubMed] [Google Scholar]
- 15.Murdaca G, Spano F, Contatore M, Guastalla A, Penza E, Magnani O, et al. Infection risk associated with anti-TNF-alpha agents: a review. Expert Opin Drug Saf. 2015;14:571–582. doi: 10.1517/14740338.2015.1009036. [DOI] [PubMed] [Google Scholar]
- 16.Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DP, et al. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54:2368–2376. doi: 10.1002/art.21978. [DOI] [PubMed] [Google Scholar]
- 17.Listing J, Strangfeld A, Kary S, Rau R, von Hinueber U, Stoyanova-Scholz M, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005;52:3403–3412. doi: 10.1002/art.21386. [DOI] [PubMed] [Google Scholar]
- 18.Atzeni F, Sarzi-Puttini P, Botsios C, Carletto A, Cipriani P, Favalli EG, et al. Long-term anti-TNF therapy and the risk of serious infections in a cohort of patients with rheumatoid arthritis: comparison of adalimumab, etanercept and infliximab in the GISEA registry. Autoimmun Rev. 2012;12:225–229. doi: 10.1016/j.autrev.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, Ustianowski A, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR) Ann Rheum Dis. 2010;69:522–528. doi: 10.1136/ard.2009.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallis RS, Broder M, Wong J, Beenhouwer D. Granulomatous infections due to tumor necrosis factor blockade: correction. Clin Infect Dis. 2004;39:1254–1255. doi: 10.1086/424455. [DOI] [PubMed] [Google Scholar]
- 21.Salmon-Ceron D, Tubach F, Lortholary O, Chosidow O, Bretagne S, Nicolas N, et al. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann Rheum Dis. 2011;70:616–623. doi: 10.1136/ard.2010.137422. [DOI] [PubMed] [Google Scholar]
- 22.Ling Y, Puel A. IL-17 and infections. Actas Dermosifiliogr. 2014;105(Suppl 1):34–40. doi: 10.1016/S0001-7310(14)70016-X. [DOI] [PubMed] [Google Scholar]
- 23.Reich K, Leonardi C, Langley RG, Warren RB, Bachelez H, Romiti R, et al. Inflammatory bowel disease among patients with psoriasis treated with ixekizumab: a presentation of adjudicated data from an integrated database of 7 randomized controlled and uncontrolled trials. J Am Acad Dermatol. 2017;76:441–448. doi: 10.1016/j.jaad.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Catana CS, Berindan Neagoe I, Cozma V, Magdas C, Tabaran F, Dumitrascu DL. Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2015;21:5823–5830. doi: 10.3748/wjg.v21.i19.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schreiber S, Colombel JF, Feagan BG, Reich K, Deodhar AA, McInnes IB, et al. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann Rheum Dis. 2019;78:473–479. doi: 10.1136/annrheumdis-2018-214273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egeberg A, Hansen PR, Gislason GH, Skov L, Mallbris L. Risk of self-harm and nonfatal suicide attempts, and completed suicide in patients with psoriasis: a population-based cohort study. Br J Dermatol. 2016;175:493–500. doi: 10.1111/bjd.14633. [DOI] [PubMed] [Google Scholar]
- 27.Strober BE, Langley RGB, Menter A, Magid M, Porter B, Fox T, et al. No elevated risk for depression, anxiety or suicidality with secukinumab in a pooled analysis of data from 10 clinical studies in moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178:e105–e1e7. doi: 10.1111/bjd.16051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment for up to 2 years per proposal. For details on submitting a request, see the instructions provided at www.clinicalstudydatarequest.com.