Seven studies were included, reporting 5938 children (mean age, 11.3 ± 2.8 years). Incidental findings were present in 16.4% of healthy children, intracranial cysts being the most frequent (10.2%). Nonspecific white matter hyperintensities were reported in 1.9%, Chiari I malformation was found in 0.8%, and intracranial neoplasms were reported in 0.2%. In total, the prevalence of incidental findings needing follow-up was 2.6%. The prevalence of incidental findings is much more frequent in children than previously reported in adults, but clinically significant incidental findings were present in <1 in 38 children.
Abstract
BACKGROUND:
The detection of incidental findings on children's brain MR imaging poses various practical issues because the life-long implications of such findings may be profound.
PURPOSE:
Our aim was to assess the prevalence and characteristics of incidental brain MR imaging findings in children.
DATA SOURCES:
Electronic databases (PubMed, EMBASE, and Cochrane) were searched for articles published between 1985 to July 2018, with the following search terms: “incidental,” “findings,” “brain,” “MR imaging.”
STUDY SELECTION:
Inclusion criteria were the following: 1) patients younger than 21 years of age, 2) healthy children without any clinical condition, 3) MR images obtained with at least a 1.5T magnet, 4) original articles, and 5) a methodologic quality score of ≥10.
DATA ANALYSIS:
Two observers independently extracted data and assessed data quality and validity. The number and type of incidental findings were pooled. Heterogeneity was assessed using the Cochran Q statistic and the I2 statistic.
DATA SYNTHESIS:
Seven studies were included, reporting 5938 children (mean age, 11.3 ± 2.8 years). Incidental findings were present in 16.4% (99% CI, 9.8–26.2; Q = 117.5, I2= 94.9%) of healthy children, intracranial cysts being the most frequent (10.2%, 99% CI, 3.1–28.5; Q = 306.4, I2 = 98.0%). Nonspecific white matter hyperintensities were reported in 1.9% (99% CI, 0.2–16.8; Q = 73.6, I2 = 94.6%), Chiari 1 malformation was found in 0.8% (99% CI, 0.5–1.3; Q = 7.6, I2 = 60.5%), and intracranial neoplasms were reported in 0.2% (99% CI, 0.1–0.6; Q = 3.4, I2 = 12.3%). In total, the prevalence of incidental findings needing follow-up was 2.6% (99% CI, 0.5–11.7; Q = 131.2, I2 = 95.4%). Incidental findings needing specific treatment were brain tumors (0.2%) and cavernomas (0.2%).
LIMITATIONS:
Limitations were no age stratification or ethnicity data and variation in the design of included studies.
CONCLUSIONS:
The prevalence of incidental findings is much more frequent in children than previously reported in adults, but clinically meaningfull incidental findings were present in <1 in 38 children.
MR imaging of the brain is increasingly used in both pediatric research and clinical routine, with constantly improving image quality due to advances in hardware and sequence development. Performing MR imaging at a higher resolution and/or field strength using more sensitive sequences may lead to the detection of subtle brain abnormalities that would not have been previously detected. Furthermore, with the steadily increasing number of brain MR imaging scans obtained each year,1 these technical advances will result in more patients and physicians being confronted with and needing to manage incidental brain findings.2 Incidental findings (IFs) are previously undetected abnormalities of potential clinical relevance that are unexpectedly discovered and, by definition, unrelated to the purpose of the examination. The detection of IFs poses various practical and ethical issues, particularly when the subjects/patients are children, in whom the life-long implications of such findings may be profound. Detection is potentially detrimental because the treatment can have harmful as well as beneficial consequences. Estimating the probability of discovering incidental brain findings is of importance to help clinicians inform patients of these risks and to guide researchers to adequately inform either healthy volunteers in imaging research or individuals being considered for screening by brain MR imaging.
The clinical relevance and natural course of these unexpected asymptomatic findings have been studied in adults but remain largely unexplored in the pediatric population. Previous studies have investigated pediatric IFs in healthy research volunteers and in populations of children who underwent MR imaging examinations for various reasons.3–8 Recently, Jansen et al,9 in a monocentric study of 3966 children, reported that at least 1 IF was present in 25.6% of the children (95% CI, 24.2–27.0), though the prevalence of findings requiring clinical follow-up was only 0.43% (95% CI, 0.26–0.70). A systematic review and meta-analysis of the published literature has been recommended10 to provide more precise estimates of the range of IFs on brain MR imaging and explore the influence of study design, patient characteristics, and imaging parameters on the detection of incidental brain findings. In the present study, our aim was to assess the prevalence and characteristics of incidental brain MR imaging findings in children through a systematic review and meta-analysis of the current literature.
MATERIALS AND METHODS
Before conducting this review, we developed a detailed protocol, including objectives and plans for collecting and analyzing data. The manuscript was prepared in accordance with the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA) guidelines.11,12 This study was designed, conducted, and analyzed, and the article was written independent of industry or any financial support.
Search Strategy and Selection Criteria
The medical literature on incidental brain MR imaging findings, published between1985 to July 2018, was reviewed using PubMed, EMBASE, and the Cochrane data bases. Candidate studies were searched using the following keywords/MeSH terms and Boolean logic operators: “incidental,” “findings,” “brain,” “MR imaging.” No age keyword was used in the data base search. The search was supplemented by hand searching the reference list of each selected article and each review article.
Inclusion criteria for study selection were the following: 1) pediatric patients younger than 21 years of age (according to the guidelines of the American Academy of Pediatrics13), 2) healthy children without any clinical condition (ie, research controls or research cohort), 3) MR images obtained with at least a 1.5T magnet, 4) original articles (ie, not a review), 5) published in English or French, and 6) a methodologic quality score of ≥10, as defined below. Studies were excluded if patients' ages were not specified.
Data Extraction
Data from the included studies were extracted by 2 pediatric neuroradiologists (V.D.-R. and C.-J.R.) using a standardized critical appraisal and data-extraction form, and any disagreements were resolved by consensus. The data-extraction form was subdivided into 4 sections: 1) study characteristics, 2) patients characteristics, 3) imaging methods, and 4) imaging findings.
Study Characteristics
We extracted the following data: single institution/multicenter, prospective/retrospective data collection, consecutive/sporadic cases, midyear of study (defined as median calendar year of the MR imaging acquisition period), and country.
Patient Characteristics
Children's baseline extracted data included sex, number, mean age of the eligible children, and inclusion criteria (research cohort versus research controls).
Imaging Methods
We recorded the MR imaging magnet field used (1.5T or 3T), available MR imaging sequences, and occupation of the imaging reviewer (radiologist or not). We distinguished between standard and high-resolution MR imaging protocols. High-resolution brain MR imaging protocols were defined as those performed on a 3T MR imaging unit with an effective voxel resolution of at best 2 × 2 × 2 mm3 and as standard protocols otherwise.
Imaging Findings
We recorded the presence and number of IFs per patient, the number of IFs needing follow-up or treatment, the number of IFs according to sex, and detailed IFs. Six categories were created to further analyze common IFs: 1) normal variation (except those included in the other categories, such as pineal cysts), 2) cysts, 3) vascular abnormalities (developmental venous anomaly, cavernoma, capillary telangiectasia), 4) developmental disorders (Chiari 1 malformation, corpus callosum anomaly), 5) white matter hyperintensities, and 6) neoplasms.
Intracranial cysts were recorded as a whole group and according to subtypes (pineal cyst, choroid plexus cyst, arachnoid cyst, and so forth). White matter hyperintensities were gathered in 1 group, regardless of their localization. Findings outside the cranial vault (ie, nasopharynx and sinus conditions) were not considered.
Quality of Reporting in Included Studies
We assessed the quality of reporting of all included studies based on the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement checklist,14 used to build a quality score of 0–22.
Risk of Bias Assessment
After the selection of studies, quality and risk of biases when pooling in meta-analysis were critically appraised on the basis of the scheme suggested by the Cochrane Collaboration tool.15
Statistical Analysis
In all analyses, inconsistency of findings across studies was assessed using the Cochran Q statistic and the I2 statistic.15 We used a fixed-effects weighted model to calculate the pooled estimates, except with P (heterogeneity) < .10 or I2 > 30%, in which case a random-effects weighted model was used. We assessed publication bias using visual inspection of scatterplots according to study size or precision (ie, funnel plot.)16 Due to marked heterogeneity, uncontrolled nature of the data, and multiplicity of testing, a 2-sided P value of < .01 was prespecified to indicate a convincing statistical difference. All P values were 2-tailed. Analyses were performed using Comprehensive Meta-Analysis 2.0 for Windows (Biostat, Englewood, New Jersey).
RESULTS
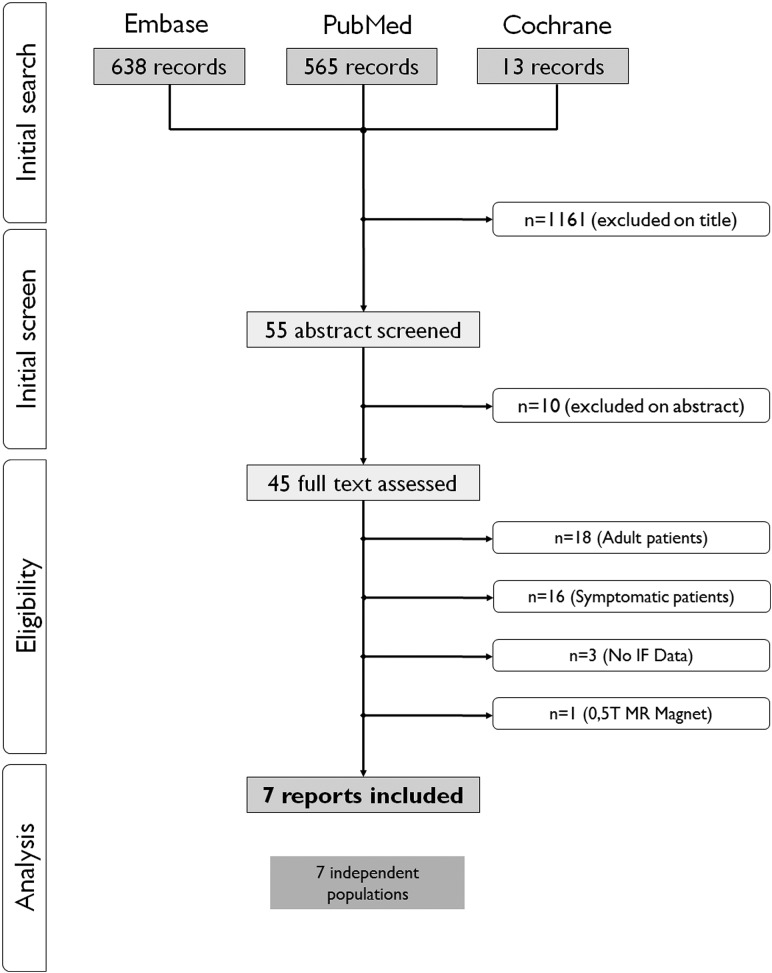
The initial search identified 1216 publications, 55 of which were evaluated in full text (see the flow chart in Fig 1). Seven studies, with 5938 children (mean age, 11.3 ± 2.8 years) were included.3–9 One study published as a short report9 was fully evaluated (methodologic data) thanks to the related methodologic report.17
Fig 1.
Flow chart of included studies.
Study and Patient Characteristics
Table 1 and the On-line-Table describe the characteristics of the included studies.
Table 1:
Characteristics of included studies
| Study | Country | Total Sample Size | Mean Age (Range) (yr) | % Male | MR Imaging Magnet | <2 mm3 Resolution | T2 or FLAIR WI | 3D |
|---|---|---|---|---|---|---|---|---|
| Kim et al, 20023 | US | 225 | NA (1–18) | 44 | 1.5 | No | Yes | No |
| Kumra et al, 20064 | US | 60 | NA (10–21) | NA | 1.5 | No | Yes | Yes |
| Seki et al, 20105 | Japan | 110 | NA (5–8) | 54 | 1.5 | No | Yes | Yes |
| Gur et al, 20136 | US | 1400 | 14.8 (8–21) | 48 | 3 | Yes | No | Yes |
| Kaiser et al, 20157 | US | 114 | 8.3 (0.2–18) | 41 | 3 | Yes | Yes | Yes |
| Monterrey et al, 20178 | US | 65 | 10.1 (NA) | 71 | 3 | Yes | No | Yes |
| Jansen et al, 20179 | Netherlands | 3966 | 10.1 (8.6–11.9) | 49 | 3 | Yes | Yes | Yes |
Note:—NA indicates not applicable; WI, weighted imaging.
The mean value of the methodologic quality scores was 15.3 ± 2.1/22. No study scored positively on all items, and all studies were monocentric. The case series were from the United States (n = 5), the Netherlands (n = 1), and Japan (n = 1). Table 2 details patient characteristics.
Table 2:
| No. Available (Studies) | Rate (%) (No.) or Mean Value ± SD | 99% CI | P (Het) | I²/Q Value | |
|---|---|---|---|---|---|
| Patients | 5938 (7) | ||||
| Sex (female) | 5878 (6) | 50.2 (2990) | 45.0–55.4 | .001 | 76.0/20.9 |
| Age (mean) (SD) (yr) | 5543 (4) | 11.3 ± 2.8 | |||
| Incidental findings | |||||
| No IF | 5938 (7) | 79.9 (4648) | 66.1–89.0 | <.001 | 96.7/183.3 |
| ≥1 IF | 5938 (7) | 16.4 (1189) | 9.8–26.2 | <.001 | 94.9/117.6 |
| ≥1 IF, high resolution | 5543 (4) | 18.9 (1128) | 9.4–34.6 | <.001 | 97.1/104.6 |
| ≥1 IF, standard resolution | 395 (3) | 13.2 (61) | 5.8–27.2 | .016 | 75.7/8.2 |
| ≥1 IF, boys | 816 (3) | 17.6 (133) | 14.1–21.7 | <.001 | 97.5/81.0 |
| ≥1 IF, girls | 874 (3) | 16.3 (100) | 9.2–19.9 | .002 | 83.4/12.0 |
| IF to follow | 5938 (7) | 2.6 (67) | 0.5–11.7 | <.001 | 95.4/131.2 |
Note:—Het indicates heterogeneity.
Values are expressed as absolute number of patients (studies) or a percentage, unless otherwise specified.
Imaging Methods
Imaging was performed using either a 1.5T (n = three; 395 children) or 3T magnet (n = four; 5543 children). All the imaging protocols included a T1-weighted sequence (6/7 studies using 3D, 1/7 using 2D or 3D images [53%/47%]), and 4 studies also reported 2D T2-weighted or FLAIR sequences. Four studies, including 5545 patients, were defined as high-resolution MRI.6–9 Images were reviewed by neuroradiologists, except for 1 study in which the reviewer was a pediatric neurologist.5
Incidental Findings
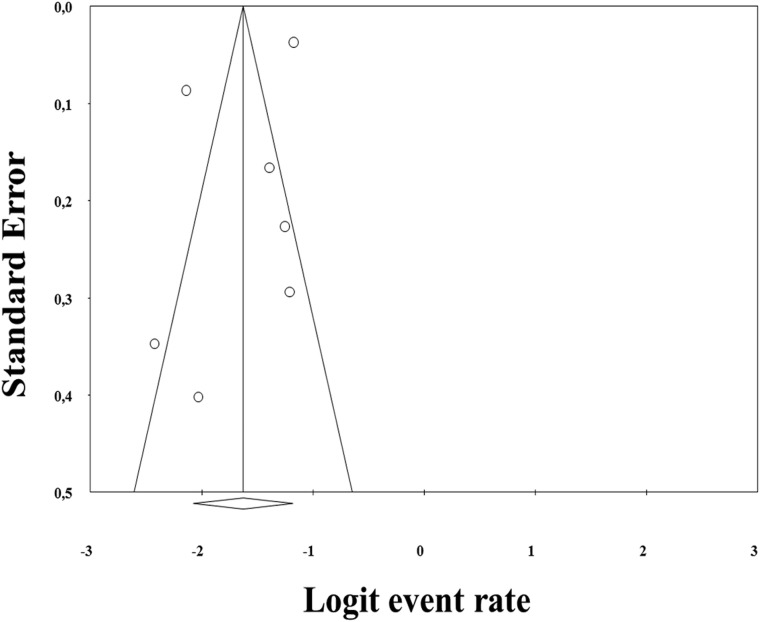
A total of 1189 children (16.4%, 99% CI, 9.8–26.2) had at least 1 IF (Table 2), corresponding to a number needed to scan ∼6 (99% CI, 3.8–10.2) to identify 1 IF. Publication bias is presented in the funnel plot analysis (Fig 2). A referral or a follow-up was needed in 67/5938 children (2.6%; 99% CI, 0.5–11.7; the number needed to scan ∼38). There was no significant difference in IF prevalence between boys and girls (relative risk, 1.86; 99% CI, 0.70–4.94; P = .10).
Fig 2.
Funnel plot of incidental findings in brain MR imaging of healthy children. Each dot represents a study; the y-axis represents the size of the study (ie, number of subjects) and the x-axis shows the result of the study (ie, prevalence of incidental findings). Aysmmetric funnel plot suggests no relationship between IF prevalence and study size.
IF prevalence appeared significantly higher (relative risk, 1.32; 99% CI, 1.04–1.67; P = .02) in studies with high-resolution MR imaging protocols (relative risk, 18.9%; 99% CI, 9.4–34.6) than in studies with standard-resolution MR imaging protocols (relative risk, 13.2%; 99% CI, 5.8–27.2).
Cysts were the most frequent IF (Table 3), found in 10.2% (1096) of children, with pineal cyst being the most frequently reported (2.3%, 704/5878), followed by arachnoid cyst (2.2%, 89/4256). The proportion of enlarged perivascular spaces was high (7.4%) but was reported in only 2 small cohorts (24/175 children). White matter hyperintensities were found in 1.9% (27/4428) (most being focal [19/27], the others being less clearly defined [8/27]); venous developmental anomaly, in 1.6% (66/4256); and Chiari I malformation, in 0.8% (32/4263). Corpus callosum anomalies were reported in 0.7% (7/4143), 5 with partial corpus callosum agenesis.
Table 3:
| No. Available (Studies) | Rate % (No.) | 99% CI | P (Heterogeneity) | I²/Q Value | |
|---|---|---|---|---|---|
| Normal variations | |||||
| Mega cisterna magna | 4428 (5) | 2.5 (116) | 0.8–7.4 | <.001 | 78.0/22.7 |
| Cavum septum pellucidum | 5653 (5) | 2.2 (105) | 1.1–4.6 | .007 | 71.4/14.0 |
| Empty sella | 3966 (1) | 0.2 (7) | 0.1–0.5 | 1 | 0/0 |
| Cysts | 5938 (7) | 10.2 (1096) | 3.1–28.5 | <.001 | 98.0/306.4 |
| Pineal | 5878 (6) | 2.3 (704) | 0.4–12.4 | <.001 | 97.1/175.4 |
| Arachnoid | 4256 (3) | 2.2 (89) | 1.7–2.9 | .10 | 52.8/6.4 |
| Choroid plexus | 4031 (2) | 0.4 (8) | 0.1–6.4 | .04 | 75.6/4.1 |
| Porencephalic | 4143 (3) | 0.2 (4) | 0.1–0.6 | .05 | 69.7/5.8 |
| Ventricular dilation | 5656 (4) | 1.1 (46) | 0.1–12.4 | <.001 | 95.5/65.9 |
| Enlarged perivascular spaces | 175 (2) | 7.4 (24) | 0.1–93.9 | <.001 | 93.6/15.5 |
| Vascular anomalies | |||||
| Developmental venous anomaly | 4256 (3) | 1.6 (66) | 1.1–2.1 | .72 | 0/0.66 |
| Cavernoma | 3966 (1) | 0.2 (7) | 0.1–0.5 | 1 | 0/0 |
| Capillary telangiectasia | 3966 (1) | 0.1 (2) | 0–0.3 | 1 | 0/0 |
| Developmental disorders | |||||
| Gray matter heterotopia | 4031 (2) | 0.5 (19) | 0.3–0.9 | .75 | 0/0.1 |
| Gray matter dysplasia | 3966 (1) | 0.1 (2) | 0–0.3 | 1 | 0/0 |
| Corpus callosum anomaly | 4143 (3) | 0.7 (7) | 0.1–15.1 | <.001 | 90.3/20.5 |
| Chiari I malformation | 4263 (4) | 0.8 (32) | 0.5–1.3 | .06 | 60.5/7.6 |
| White matter hyperintensities | 4428 (5) | 1.9 (27) | 0.2–16.8 | <.001 | 94.6/73.6 |
| Neoplasm | 4368 (4) | 0.2 (9) | 0.1–0.6 | .33 | 12.3/3.4 |
IFs that required therapeutic management were rare, with asymptomatic tumors reported in 9 children (0.2%, 9/4368). Eight were low-grade tumors (4 low-grade gliomas, 1 neuroepithelial dysembryoplastic tumor, 1 craniopharyngioma, 2 nonspecified lesions), and only 1 patient had a high-grade tumor, an ependymoma.9 Cavernomas were present in 7 children (0.2%, 7/3966).
DISCUSSION
In this systematic review and meta-analysis, we report a prevalence of 16.4% of IFs on brain MR imaging within a population of 5938 healthy children. Most of these IFs were benign and did not require routine or urgent referral, treatment, or follow-up.
Relatively few studies have examined incidental findings in healthy children, and prior studies have been limited by both the effective resolution of the imaging sequence used and small sample sizes. By synthesizing all the published data on incidental brain findings in children, we have increased the precision of existing estimates of their prevalence by showing that IFs were encountered in about 1 in every 6 children, whereas referral or follow-up was needed in about 1 in every 38 children. Given the increasingly common use of high-resolution brain MR imaging in pediatric populations, it is important to establish both a baseline rate for IFs and a framework for their evaluation.
A meta-analysis of 16 adult studies, including nearly 20,000 scans, found that the rate of IFs was related to image resolution.18 In the case of sequences considered high-resolution by present standards, we found an IF prevalence that was significantly higher than with standard sequences. In such a situation (ie, with an imaging resolution <2 × 2 × 2 mm3 on a 3T MR imaging unit), the prevalence of pediatric brain IFs is likely to be in the region of 1 in 5 children. However, the high-resolution studies were the most recent ones (2013–2017 versus 2002–2010 for low-resolution studies), which may suggest that the difference could be also due to the MR imaging scan generation, and not only to the magnet strength.
The rate of IFs was much higher in our study (16.4%) than in the adult meta-analysis18 (2.7%), but direct comparison remains difficult. First, the nature of IFs strongly varies. In adults, Vernooij et al19 demonstrated that asymptomatic brain infarcts were present in 7.2%, and cerebral aneurysms, in 1.8%, whereas they were not observed in pediatric studies. Second, the definition of incidental findings varies between adults and children. Hence, focal white matter abnormalities are considered “age-related modifications” in adults, but not in children. If we excluded these findings in our study, the IF rate changes from 16.4% to 9.6%. Third, imaging protocols strongly vary between adult and pediatric studies (resolution, duration, 3D) and may account for rate differences.
We did not encounter differences in IFs or subtype of IF according to sex, whereas a previous study3 reported significant differences in IF prevalence between male and female subjects.
Pineal cysts were the most frequent IF, encountered in 2.3% of children. The prevalence of these cysts in clinical cohorts varies, from 1.8% when cysts are defined as having a diameter of >10 mm20 up to 57%,21 most likely due to differences in the definition of the cyst versus normal gland. The clinical meaning of these cysts may be challenging.22 Jussila et al20 recently reported that even above 10 mm in diameter, pineal cysts most often remain unchanged or display minimal growth. Consequently, size is probably less relevant than mass effect seen on the aqueduct. In the absence of such mass effect, pineal cysts may be overlooked and considered as a nonevolutive normal variant.
Our review found that Chiari I malformation was reported in 0.8% of these asymptomatic children. As in the case of pineal cysts, the clinical relevance of the tonsillar position remains problematic22 because no definite morphologic criterion is predictive of symptomatology23 and tonsillar position may be corrected with age.24 Chiari I malformation, considered asymptomatic after careful specialized clinical evaluation, should not be systematically followed.
White matter FLAIR hyperintensities were observed in 1.9% of children, a finding that is far less frequent than in adults, in whom they are encountered in 8%–28% according to age.25 Therefore, these anomalies imply the need for cautious evaluation by pediatric neuroradiologists. Eidlitz-Markus et al26 reported that focal hyperintensities are more frequent (up to 10%) in children with migraine.
Corpus callosum anomalies were very rare (0.7%), with no complete agenesis reported and only 0.5% with partial agenesis. Nevertheless, the review by Sotiriadis and Makrydimas27 showed that up to 75% of patients with isolated corpus callosum agenesis have normal development; caution is therefore needed when assessing these patients because this anomaly appears to be so rare in asymptomatic children.
Meaningful IFs (ie, implying the need for therapeutic management) were exceptionally reported, with neoplasms in 0.2% (99% CI, 0.1–0.6) of children and only 1 high-grade tumor. If this prevalence is lower than in adults18,28,29 mainly because meningiomas are much rarer in children, it was higher than expected, according to estimates from cancer registries, which have shown a prevalence of 35 in 100 000 (0.04%).30 Therefore, reviewing images from research pediatric protocols in healthy volunteers, readers should be aware of the possibility of discovering a brain tumor in about 1 in every 500 children.
Our study has several limitations. First, we do not have patient-level data allowing IFs to be stratified by age, which could be of interest because some of the IFs may appear or vanish with age (such as tonsillar ptosis). We may also have missed data included in published studies that did not detail the subjects' ages. Second, data according to ethnicity were not available, and this may be a potential confounding factor in the present meta-analysis. Third, the influence of variations in study design may have impacted the precision of IF rates and explain the substantial heterogeneity of studies assessed by Cochran statistics (I2 > 75%) for most IFs, in particular for IF s that could be considered normal variants (cysts, enlarged perivascular spaces). However, the prevalence of meaningful IFs, such as tumors, was more homogeneous (I2 = 12.3). Fourth, in our systematic review, while the comprehensive search was designed to include as many pertinent studies as possible, some publications may have been missed. Finally, baseline characteristics were often limited to the entire series, without details by subgroups. Detailed data about IFs needing follow-up or specific treatment were not reported in the included studies, precluding any detailed subgroup or meta-regression analyses, and none of our results could be corrected for confounders.
Conclusions
Incidental findings in healthy children's brain MR imaging are frequent (16.4%), but rarely require referral, follow-up, or, even rarer, any treatment (0.4%), and parents and children should be informed accordingly before imaging examination for research purposes. Researchers must develop specific procedures, in a cost-effective fashion, including management of brain MR imaging examinations, detection of IF, disclosure, and reporting to parents and appropriate follow-up by specialized clinicians.
ABBREVIATION:
- IF
incidental finding
References
- 1. Trost MJ, Robison N, Coffey D, et al. Changing trends in brain imaging technique for pediatric patients with ventriculoperitoneal shunts. Pediatr Neurosurg 2018;53:116–20 10.1159/000485923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shoemaker JM, Cole C, Petree LE, et al. Evolution of universal review and disclosure of MRI reports to research participants. Brain Behav 2016;6:e00428 10.1002/brb3.428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim BS, Illes J, Kaplan RT, et al. Incidental findings on pediatric MR images of the brain. AJNR Am J Neuroradiol 2002;23:1674–77 [PMC free article] [PubMed] [Google Scholar]
- 4. Kumra S, Ashtari M, Anderson B, et al. Ethical and practical considerations in the management of incidental findings in pediatric MRI studies. J Am Acad Child Adolesc Psychiatry 2006;45:1000–06 10.1097/01.chi.0000222786.49477.a8 [DOI] [PubMed] [Google Scholar]
- 5. Seki A, Uchiyama H, Fukushi T, et al. Incidental findings of brain magnetic resonance imaging study in a pediatric cohort in Japan and recommendation for a model management protocol. J Epidemiol 2010;20:S498–504 10.2188/jea.JE20090196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gur RE, Kaltman D, Melhem ER, et al. Incidental findings in youths volunteering for brain MRI research. AJNR Am J Neuroradiol 2013;34:2021–25 10.3174/ajnr.A3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaiser D, Leach J, Vannest J, et al. Unanticipated findings in pediatric neuroimaging research: prevalence of abnormalities and process for reporting and clinical follow-up. Brain Imaging Behav 2015;9:32–42 10.1007/s11682-014-9327-7 [DOI] [PubMed] [Google Scholar]
- 8. Monterrey JC, Philips J, Cleveland S, et al. Incidental brain MRI findings in an autism twin study. Autism Res 2017;10:113–20 10.1002/aur.1720 [DOI] [PubMed] [Google Scholar]
- 9. Jansen PR, Dremmen M, van den Berg A, et al. Incidental findings on brain imaging in the general pediatric population. N Engl J Med 2017;377:1593–95 10.1056/NEJMc1710724 [DOI] [PubMed] [Google Scholar]
- 10. Wolf SM, Lawrenz FP, Nelson CA, et al. Managing incidental findings in human subjects research: analysis and recommendations. J Law Med Ethics 2008;36:219–48 10.1111/j.1748-720X.2008.00281.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting—Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hardin AP, Hackell JM, COMMITTEE ON PRACTICE AND AMBULATORY MEDICINE. Age limit of pediatrics. Pediatrics 2017;140:e20172151 10.1542/peds.2017-2151 [DOI] [PubMed] [Google Scholar]
- 14. Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg 2014;12:1500–24 10.1016/j.ijsu.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 15. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54:1046–55 10.1016/s0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- 17. White T, Muetzel RL, El Marroun H, et al. Paediatric population neuroimaging and the Generation R Study: the second wave. Eur J Epidemiol 2018;33:99–125 10.1007/s10654-017-0319-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morris Z, Whiteley WN, Longstreth WT, et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2009;339:b3016 10.1136/bmj.b3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med 2007;357:1821–28 10.1056/NEJMoa070972 [DOI] [PubMed] [Google Scholar]
- 20. Jussila M-P, Olsén P, Salokorpi N, et al. Follow-up of pineal cysts in children: is it necessary? Neuroradiology 2017;59:1265–73 10.1007/s00234-017-1926-8 [DOI] [PubMed] [Google Scholar]
- 21. Whitehead MT, Oh CC, Choudhri AF. Incidental pineal cysts in children who undergo 3-T MRI. Pediatr Radiol 2013;43:1577–83 10.1007/s00247-013-2742-x [DOI] [PubMed] [Google Scholar]
- 22. Maher CO, Piatt JH Jr, Section on Neurologic Surgery, American Academy of Pediatrics. Incidental findings on brain and spine imaging in children. Pediatrics 2015;135:e1084–96 10.1542/peds.2015-0071 [DOI] [PubMed] [Google Scholar]
- 23. Khalsa SSS, Geh N, Martin BA, et al. Morphometric and volumetric comparison of 102 children with symptomatic and asymptomatic Chiari malformation Type I. J Neurosurg Pediatr 2018;21:65–71 10.3171/2017.8.PEDS17345 [DOI] [PubMed] [Google Scholar]
- 24. Whitson WJ, Lane JR, Bauer DF, et al. A prospective natural history study of nonoperatively managed Chiari I malformation: does follow-up MRI surveillance alter surgical decision making? J Neurosurg Pediatr 2015;16:159–66 10.3171/2014.12.PEDS14301 [DOI] [PubMed] [Google Scholar]
- 25. Vermeer SE, Longstreth WT, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007;6:611–19 10.1016/S1474-4422(07)70170-9 [DOI] [PubMed] [Google Scholar]
- 26. Eidlitz-Markus T, Zeharia A, Haimi-Cohen Y, et al. MRI white matter lesions in pediatric migraine. Cephalalgia 2013;33:906–13 10.1177/0333102413480955 [DOI] [PubMed] [Google Scholar]
- 27. Sotiriadis A, Makrydimas G. Neurodevelopment after prenatal diagnosis of isolated agenesis of the corpus callosum: an integrative review. Am J Obstet Gynecol 2012;206:337.e1–5 10.1016/j.ajog.2011.12.024 [DOI] [PubMed] [Google Scholar]
- 28. Bos D, Poels MM, Adams HH, et al. Prevalence, clinical management, and natural course of incidental findings on brain MR images: the population-based Rotterdam Scan Study. Radiology 2016;281:507–15 10.1148/radiol.2016160218 [DOI] [PubMed] [Google Scholar]
- 29. Gibson LM, Paul L, Chappell FM, et al. Potentially serious incidental findings on brain and body magnetic resonance imaging of apparently asymptomatic adults: systematic review and meta-analysis. BMJ 2018;363:k4577 10.1136/bmj.k4577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Porter KR, McCarthy BJ, Freels S, et al. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol 2010;12:520–27 10.1093/neuonc/nop066 [DOI] [PMC free article] [PubMed] [Google Scholar]