Abstract
Extracellular vesicles, including exosomes and microvesicles, play a fundamental role in the activity of the nervous system, participating in signal transmission between neurons and providing the interaction of central nervous system with all body systems. In many neurodegenerative diseases, neurons pack toxic substances into vesicles and release them into the extracellular space, which leads to the spread of misfolded neurotoxic proteins. The contents of neuron-derived extracellular vesicles may indicate pathological changes in the central nervous system, and the analysis of extracellular vesicle molecular content contributes to the development of non-invasive methods for the diagnosis of many central nervous system diseases. Extracellular vesicles of neuronal origin can be isolated from various biological fluids due to their ability to cross the blood-brain barrier. Today, the diagnostic potential of almost all toxic proteins involved in nervous system disease pathogenesis, specifically α-synuclein, tau protein, superoxide dismutase 1, FUS, leucine-rich repeat kinase 2, as well as some synaptic proteins, has been well evidenced. Special attention is paid to extracellular RNAs mostly associated with extracellular vesicles, which are important in the onset and development of many neurodegenerative diseases. Depending on parental cell type, extracellular vesicles may have different therapeutic properties, including neuroprotective, regenerative, and anti-inflammatory. Due to nano size, biosafety, ability to cross the blood-brain barrier, possibility of targeted delivery and the lack of an immune response, extracellular vesicles are a promising vehicle for the delivery of therapeutic substances for the treatment of neurodegenerative diseases and drug delivery to the brain. This review describes modern approaches of diagnosis and treatment of central nervous system diseases using extracellular vesicles.
Keywords: biomarkers, cell-mediated therapy, central nervous system diseases, diagnosis, exosomes, extracellular RNAs, extracellular vesicles, microRNAs, microvesicles, neurodegenerative diseases
Introduction
Extracellular vesicles (EVs) are membrane particles of cellular origin involved in the regulation of many physiological and pathological processes in the body (Chulpanova et al., 2018a; Hessvik and Llorente, 2018; Galieva et al., 2019). EVs are produced by almost all the cells of body and provide intercellular communication, and transport of biologically active molecules to target cells (Hartmann and Burg, 1989; Merchant et al., 2017). EVs play an important role in the functioning of nervous system, providing a mechanism of communication not only between nerve and glial cells, but also to allow the interconnection of the central nervous system (CNS) with all body systems (Chivet et al., 2012). In CNS pathologies EVs play a dual role. On the one hand, they help maintain cellular homeostasis, cleaning the nervous system of protein aggregates and other pathogenic agents. On the other hand, they can also transfer toxic substances to healthy nerve cells, mediating pathogenic progress. The pathogenic role of EVs is shown in neurodegeneration, neuroinflammation, cancers and disorders that affect the CNS, for example, lysosomal storage disorders (Caruso Bavisotto et al., 2019).
In various pathological processes, EVs undergo significant changes in composition, quantity and size. Knowledge of these changes may make it possible to identify new biomarkers of various diseases for sensitive and specific diagnosis (Wong and Chen, 2019). Today, special attention is paid to the diagnostic potential of pathogenic proteins, synaptic proteins, and RNAs inside EVs.
Several studies have investigated the therapeutic potential of native exosomes isolated from dendritic cells (Pitt et al., 2016; Sousa et al., 2017), macrophages (Choo et al., 2018), hematopoietic stem cells (Radosinska and Bartekova, 2017), endothelial cells (Xiao et al., 2017) and mesenchymal stem cells (MSCs) (Lopez-Verrilli et al., 2016; Mathew et al., 2019). MSC-derived exosomes contain cytokines, trophic growth factors, signal lipids, messenger RNAs and regulatory microRNAs (miRNAs), which makes them an attractive therapeutic agent for use in cell-free regenerative medicine (Phinney and Pittenger, 2017).
When developing new strategies for CNS disease treatment, special attention is paid to EVs due to their ability to cross the blood-brain barrier (BBB) (Matsumoto et al., 2017a). The mechanisms for EVs passing through the BBB remain controversial and require further investigation. Malignant neoplasms, inflammatory processes and other pathological conditions of the CNS can lead to dysfunction of the BBB and the flow of substances across it, in part through disruption of the tight junctions (Garcia-Romero et al., 2017). It is assumed that normal transport through the BBB can occur through vesicular transcytosis, which is divided into receptor-mediated transcytosis and adsorptive-mediated transcytosis. The receptor-mediated transcytosis process occurs via binding of the EV with specific cellular receptors. Whereas, adsorptive-mediated transcytosis is mediated by adsorption of cationic particles to the anionic components of the plasma membrane. In the case of adsorptive-mediated transcytosis, there is less affinity and higher throughput. Whilst EVs can cross the BBB through the mechanism of adsorptive-mediated transcytosis (Matsumoto et al., 2017b), the process of receptor-mediated transcytosis is more commonly exploited for delivery of therapeutic drugs to the brain, when specificity can be increased and controlled through the modification of surface ligands (Preston et al., 2014), for example the rabies virus glycoprotein is often used to target EV binding to the acetylcholine receptor of nerve cells (Alvarez-Erviti et al., 2011; Huey et al., 2017; Phoolcharoen et al., 2017).
The articles used in this extracellular vesicles of central nervous system review were retrieved by replicating the search terms Kawikova and Askenase (2015) and Rufino-Ramos et al (2017). An electronic search of the NCBI PubMed database for literature from 1981 to 2019 was performed. The results were further screened by title and abstract to present EVs in CNS. This included publications prior to 2019, with the following search criteria: extracellular vesicles (EVs), exosomes, microvesicles (MVs), neurodegenerative diseases, microRNAs, extracellular RNAs, central nervous system (CNS) diseases.
Cells of Nervous System – Potential Source and Targets of Extracellular Vesicles
Nervous tissue is composed of neurons and neuroglia. Neurons are highly polarized cells, most of them consist of a body and two functionally and morphologically different processes: dendrites and axon (Giordano-Santini et al., 2016), that provide information flow through the nervous system (Takano et al., 2015). Neuroglia are involved in the metabolism and maintenance of brain homeostasis, neuron survival, development and modulation of synaptic transmission, distribution of nerve impulses, determination of CNS structure and many other physiological processes. The role of neuroglia in the many pathologies of the nervous system, including some mental illness, epilepsy and neurodegenerative diseases, is also defined. Neuroglia include astrocytes, oligodendrocytes, Schwann cells, NG2-glia and microglia (Giordano-Santini et al., 2016). Astrocytes interact with neurons, blood vessels and many structures of the nervous system and are involved in synaptic transmission. The complex interaction of astrocytes-neurons-blood vessel is generally known as a neurovascular unit of the BBB. Oligodendrocytes in the CNS and Schwann cells in the peripheral nervous system produce myelin, which provides transmission speed along axons. In addition, these cells provide trophic support and affect the structure of axons (von Bernhardi et al., 2016; Mukhamedshina et al., 2019). At the periphery, Schwann cells provide the regeneration of axons and neuromuscular junctions. NG2-glia cells are precursors of oligodendrocytes and astrocytes, they provide remyelination in case of some neurodegenerative diseases and can also modulate neuron properties and activity. Microglia are CNS immune cells, which provide neuron protection against various pathogenic factors (von Bernhardi et al., 2016; Akhmetzyanova et al., 2018).
Classification of Extracellular Vesicles
There are three main types of EVs that differ in the mechanism of release into the intercellular space, in size and cargo: exosomes, microvesicles (MVs) and apoptotic bodies (Hessvik and Llorente, 2018). Exosomes are the smallest EVs, at 30 to 100 nm in diameter, they are formed inside endosomal organelles called multivesicular bodies (MVBs). MVBs were initially regarded as prelysosomal structures participating in protein degradation. However, new studies showed that MVBs are involved in the intra- and intercellular turnover of molecules (Kawikova and Askenase, 2015). The release of exosomes occurs in several stages: formation of intraluminal vesicles within MVBs, transport of MVBs to the plasma membrane and fusion of MVBs with the plasma membrane (Hessvik and Llorente, 2018). MVBs can also fuse with lysosomes to degrade the content (Raposo and Stoorvogel, 2013). MVs are released directly from the plasma membrane and are 100 to 1000 nm in diameter (Raposo and Stoorvogel, 2013). MV formation occurs as a result of the aminophospholipid translocase-mediated dynamic redistribution of phospholipids and following constriction of the actin cytoskeleton due to the actin-myosin interaction (Akers et al., 2013). Apoptotic bodies are 50 nm to 5 μm in diameter (Rufino-Ramos et al., 2017) and are released only in advanced stages of apoptosis by caspase-mediated cleavage (Todorova et al., 2017).
Uptake of EV cargo can occur through a variety of mechanisms, including micropynocytosis, phagocytosis, caveolin-mediated, lipid-raft mediated and clathrin-dependent endocytosis. Proteins and glycoproteins presented on the surface of both EVs and target cells influence the uptake mechanism (Rufino-Ramos et al., 2017). In this review, we will consider exosomes and MVs, and their potential in the diagnosis and treatment of CNS diseases.
Exosome and Microvesicle Cargo
Depending on the type of parental cell, exosomes and MVs may have different content including bioactive molecules, membrane receptors, proteins, lipids, and genetic material that can be transported to target cells (Merchant et al., 2017; Rufino-Ramos et al., 2017; Todorova et al., 2017). Exosomes and MVs contain both constitutive proteins, found in cells of different origins, and unique proteins, the presence of which depends on the cell type and the microenvironment conditions. Unique proteins may serve as potential biomarkers for the diagnosis of various diseases (Haraszti et al., 2016). The proteomic profile of MVs and their parental cells has significant homology, in contrast to exosome proteomic profile, for which significant differences from the parental cell have been reported (Haraszti et al., 2016). Furthermore, using EVs isolated from U87 glioblastoma cells, Huh7 hepatocellular carcinoma cells and human bone marrow-derived MSCs, Haraszti et al. (2016) demonstrated exosomes contain extracellular matrix proteins, heparin-binding proteins, receptors, immune response and cell adhesion proteins, while MVs are enriched in proteasomes, endoplasmic reticulum proteins and mitochondria. Most constitutive proteins of exosomes and MVs are involved in the biogenesis process, for example, tetraspanins, Rab proteins and the endosomal sorting complex required for transport, which is the main engine of exosome biogenesis (Kalluri and LeBleu, 2016; Rufino-Ramos et al., 2017).
Exosomes and MVs also differ in lipid content. Glycolipids and free fatty acids predominate in exosomes, and the lipid composition of MVs is rich in ceramides and sphingomyelins (Haraszti et al., 2016). The lipid composition of exosomes and MVs, in contrast to their parental cells, is distinguished by a high content of phosphatidylserine, which is a determinant for vesicle entry into target cells (Record et al., 2018). The entry capacity is also determined by surface receptors and ligands of exosomes and MVs (Rufino-Ramos et al., 2017). Exosomes and MVs can contain a wide range of genetic material: chromosomal DNA, mitochondrial DNA, single-stranded and double-stranded RNAs, encoding messenger RNAs and non-coding RNAs (long noncoding RNAs, miRNAs, and circular RNAs) (Kalluri and LeBleu, 2016; Xu et al., 2016; Kim et al., 2017). Exosome and MV nucleic acids are potential biomarkers for the diagnosis of many diseases (Kinoshita et al., 2017; Szabo and Momen-Heravi, 2017).
Exosomes and Microvesicles in Normal Physiology and Central Nervous System Diseases
It is known that exosomes and MVs mediate the interaction of nervous system cells between themselves, as well as the communication of peripheral organs with the CNS (Batiz et al., 2015; Kumar et al., 2018). Neurons, astrocytes, oligodendrocytes and microglial cells release EVs and exchange signal molecules through them (Bakhti et al., 2011; Goetzl et al., 2016a; Sun et al., 2017; Vinuesa et al., 2018). It is assumed that neural exosome cargo modulates local synaptic transmission. For example, it was found that neural exosomes released after activation of glutamatergic synapses merge only with neurons, providing interneuronal communication. Thus, exosome and MV cargo can affect the interneuronal communication and synapse activity (Chivet et al., 2014; Lu and Xu, 2016).
In case of CNS diseases, such as neurodegenerative, neuroinflammatory diseases and brain tumors, exosomes and MVs, on the one hand, can remove toxic proteins and aggregates from the affected cells, and on the other, distribute pathogenic agents to healthy cells (Rufino-Ramos et al., 2017; Sardar Sinha et al., 2018).
Exosomes carrying a prion protein (PrPC) on the surface play a protective role in beta-amyloid (Aβ)-mediated neurodegeneration. The prion protein on the surface of neuronal cells acts as an Aβ receptor which activates neurotoxic signaling. However, as part of exosome cargo, PrPC binds to the neurotoxic Aβ oligomer and contributes to its fibrillation and detoxification (Falker et al., 2016).
Exosomes and MVs also contribute to angiogenesis, coagulopathy, and metastasis, in particular in CNS cancers (Kumar et al., 2018). As long as exosomes and MVs are able to overcome BBB, they can reach distant tissues and aggravate nervous system disease pathogenesis (Selmaj et al., 2017). Exosomes and MVs were shown to contribute to the transport of superoxide dismutase 1 and RNA-binding protein FUS in amyotrophic lateral sclerosis (Sproviero et al., 2018), as well as TAR DNA-binding protein 43 (TDP-43) in frontotemporal lobar degeneration and amyotrophic lateral sclerosis (Feneberg et al., 2014; Iguchi et al., 2016). It was found that TDP-43 exhibits higher toxicity as exosome cargo than in free form (Feiler et al., 2015). Exosomes and MVs also contribute to the spread of huntingtin expansion in Huntington’s disease (Jeon et al., 2016), α-synuclein in Parkinson’s disease (PD) (Ngolab et al., 2017), tau protein in Alzheimer’s disease (AD) and some other neurodegenerative diseases (Shi et al., 2016; Wang et al., 2017). The identification of these proteins in EVs isolated from patient’s body fluids may help diagnose CNS diseases (Koniusz et al., 2016).
MVs derived from microglia after traumatic brain injury are reported to activate microglia and neuroinflammation. It has been shown that in the blood of mice after traumatic brain injury, the number of microglia-derived MVs increases by about 2 times, which can cause pro-inflammatory reactions in non-activated microglia cells. Stimulation with lipopolysaccharides increases the release of MVs from microglia in vitro and the content of pro-inflammatory mediators in MVs. MVs from activated microglia in vitro or CD11b-isolated microglia/macrophage from the traumatic brain injury brain ex vivo are sufficient to initiate neuroinflammation following their injection into the cortex of naïve animals. These data confirm that MVs act as independent activators of microglia and promote the spread of inflammation (Kumar et al., 2017).
The Use of Exosomes and Microvesicles in the Diagnosis of Central Nervous System Diseases
The effectiveness of CNS disease treatment largely depends on early diagnosis, improvements in which can be achieved by the development of new molecular methods, including EV analysis methods (Hirshman et al., 2016). An important feature for diagnosis is the increase in the various inflammatory and signaling molecules, including RNA and pathogenic proteins, which are selectively packaged into exosomes (Harischandra et al., 2018) (Figure 1A). An EV-based diagnostic approach is particularly relevant for diseases of the CNS, for which direct access to the affected tissues for subsequent molecular analysis is difficult. However, as exosomes can pass the BBB they can be obtained as surrogate markers present within accessible biological fluids (Manek et al., 2018). For diagnostic use, EVs can be isolated from many body fluids, such as plasma, urine, cerebrospinal fluid (CSF), saliva, amniotic fluid and bile (Ko et al., 2016; Manek et al., 2018). However, to date the labor-intensive and variable sample preparation techniques have limited the widespread use of EVs as a diagnostic approach.
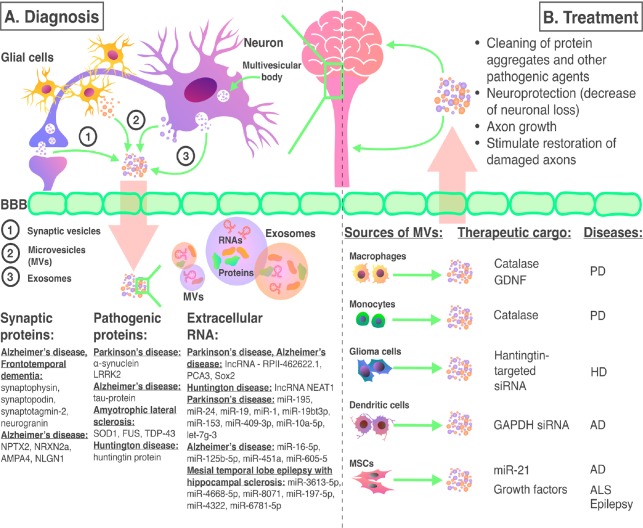
Figure 1.
The use of exosomes and microvesicles in the diagnosis and treatment of CNS diseases.
(A) In CNS pathologies exosomes and microvesicles derived from neurons and glial cells carry various disease biomarkers. Due to their ability to overcome the BBB and get into body fluids non-invasive diagnostic methods which allow identifying many CNS diseases are under investigation. (B) Exosomes and microvesicles derived from various cell lines can be a promising tool for CNS disease treatment. AD: Alzheimer’s disease; ALS: amyotrophic lateral sclerosis; AMPA4: alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate alpha 4; BBB: blood-brain barrier; CNS: central nervous system; GDNF: glial cell line-derived neurotrophic factor; HD: Huntington’s disease; lncRNA: long non-coding RNA; miR: microRNA; MV: microvesicles; NEAT1: nuclear paraspeckle assembly transcript 1; NLGN1: neuroligin 1; NPTX2: neuronal pentraxin 2; NRXN2a: neurexin 2a; PCA3: prostate cancer antigene 3; PD: Parkinson’s disease; RPII: lncRNA-RP11-462622.1; siRNA: small interfering RNA; SOD1: superoxide dismutase 1; TDP-43: TAR DNA-binding protein 43.
As noted, EV cargo may differ between the normal physiological condition and in different pathologies. A study of CSF in patients with AD showed that exosome trafficking is different in patients with AD compared to a healthy control group (Riancho et al., 2017). In addition, it has been reported that following traumatic brain injury, the size of secreted exosomes and MVs differs. EVs obtained from control CSF samples ranged between 99–104 nm in size, whereas after traumatic injury the size decreased to 74–98 nm. The total amount of EVs also increased and the proportion of some proteins present within the EVs was also altered (Manek et al., 2018).
The presence of proteins within EVs that have a known involvement in neurodegenerative disease pathogenesis have also been proposed as useful biomarkers when obtained from body fluids such as plasma and CSF. For example, α-synuclein (Shi et al., 2014; Stuendl et al., 2016) and Leucine-rich repeat kinase 2 (Fraser et al., 2013) in PD, tau protein in asthma and PD spontaneous manifestation (Shi et al., 2016). In chronic traumatic encephalopathy, which occurs as a result of repeated blows to the head and is more common in athletes, elevated levels of tau protein in plasma exosomes have been observed (Stern et al., 2016). In patients with AD and frontotemporal dementia, synaptic dysfunction occurs in the early stages of the disease as a result of a decrease in the level of functional synaptic proteins. Analysis of plasma neuron-derived exosomes showed that the level of functional synaptic proteins synaptophysin, synaptopodin, synaptotagmin-2 and neurogranin was significantly less when compared to healthy controls. Moreover, the levels of these proteins in neuron-derived exosomes correlated with the cognitive function of the patients indicating their prognostic potential (Goetzl et al., 2016b). Similar results were also obtained when the levels of presynaptic proteins neuronal pentraxin 2, neurexin 2a and postsynaptic proteins GluA4-containing glutamate (alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate alpha 4) receptor and neuroligin 1 were measured in the same patient samples (Goetzl et al., 2018).
The Extracellular RNAs in Extracellular Vesicles as Biomarkers to Diagnose Central Nervous System Diseases
Extracellular RNAs are most frequently mentioned as a potential biomarker of diseases (Figure 1A). Today, extracellular RNAs contained in neuronal cell-derived exosomes and MVs are used as biomarkers to diagnose CNS tumors, neurological and neurodegenerative diseases (Rao et al., 2013). Circular RNAs (Yang et al., 2018), miRNAs (Su et al., 2016), piwi-interacting RNAs (Clark and Lau, 2014) and long non-coding RNAs (Fernandes et al., 2019) are essential for maintaining cellular homeostasis. Where RNA regulation is disrupted as part of various CNS pathological conditions, changes in RNA expression levels may indicate various diseases (Maniati et al., 2019). For example, circulating U2 fragments of small nuclear RNAs have been proposed as a biomarker for primary CNS lymphoma, whilst the expression levels of long non-coding RNAs RP11-462G22.1, prostate cancer antigen 3 (PCA3) and Sox2OT are associated with PD and AD (Gui et al., 2015), and nuclear paraspeckle assembly transcript 1 with Huntington’s disease (Lu and Xu, 2016).
A number of studies have reported both the diagnostic and prognostic potential of miRNAs in a number of neurodegenerative diseases. The functions of miR-195, miR-24 and miR-19b effect neuronal apoptosis, regeneration and neurodegenerative processes. In patients with PD, decreased miR-19b level and increased miR-195 and miR-24 levels were found in EVs isolated from plasma potentially indicating tissue-wide changes in these miRNAs and alteration of the neuronal processes controlled by them (Cao et al., 2017). Gui et al. (2015) reported decreased abundance of CSF exosomal miR-1 and miR-19b-3p and increased abundance of miR-153, miR-409-3p, miR-10a-5p and let-7g-3p in patients with PD compared to healthy controls.
Investigation of miRNA profiles of exosomes isolated from the CSF of patients with AD, showed that miR-16-5p, miR-125b-5p, miR-451a and miR-605-5p expression in patients with early disease onset differs from the control group. The same has been also observed in a late-onset cohort, with the exception of miR-16-5p for which there was no difference when compared to the control group, the authors suggest miR-16-5p expression may be age-related hence no difference is detected in age-matched healthy controls (McKeever et al., 2018). Similar studies of patients with dementia revealed changes in the expression level of miR-137, miR-155 and miR-223 in exosomes isolated from the serum of patients with dementia. Moreover, a correlation between the level of exosomal miR-223 and serum interleukin (IL)-1β, IL-6, tumor necrosis factor-α and C-reactive protein has been observed (Wei et al., 2018).
Exosomal miRNAs have also been investigated in the diagnosis of mesial temporal lobe epilepsy with hippocampal sclerosis. Exosomal miR-3613-5p, miR-4668-5p, miR-8071, miR-197-5p, miR-4322 and miR-6781-5p were all found to have diagnostic potential, and miR-8071 exhibited the best correlation with seizure severity (Yan et al., 2017).
The miRNA profile of brain-derived EVs also changes after traumatic brain injury. It has been reported that the expression of miR-21, miR-146, miR-7a, and miR-7b increases, and the expression of miR-212 decreases after damage (Harrison et al., 2016).
The Use of Exosomes and Microvesicles in Central Nervous System Disease Treatment
CNS damage can be caused by various factors, including vascular disease, injures, infectious and hereditary diseases. For example, in case of ischemia, inadequate tissue oxygenation leads to prolonged hypoxia, depletion of energy reserves in neurons and glial cells. This causes an energy-dependent membrane ion-pump function decreases, membrane potential loss and, as a result, cell damage and cell death (Pratt and McPherson, 1997). As a consequence of this, ischemic stroke may occur, resulting in damage to the BBB damage and disruption of normal neuron function (Jiang et al., 2018). Head injuries often cause neurological disorders (Wright, 2017), a CNS inflammatory response (Russo and McGavern, 2016), and have been associated with accelerated neurodegeneration and in some cases increased risk of the later development of AD and PD (McKee and Daneshvar, 2015). Some infectious agents, causing encephalitis (Venkatesan and Murphy, 2018), neuroborreliosis, neurosyphilis (Halperin, 2018), streptococcal meningoencephalitis (Gres et al., 2019), can also lead to damage of the BBB and lead to acute inflammation, changes in the brain immune cells and neuron damage. Some lysosomal storage diseases also lead to neurodegeneration, causing accumulation of pathogenic compounds in nerve cells, such as cholesterol and sphingolipids in Niemann-Pick disease (Strauss et al., 2010), GM2 ganglioside in Tay-Sachs disease (Solovyeva et al., 2018) and Sandhoff disease (Hooper et al., 2017).
The main limitation in the treatment of nervous system diseases is the selective permeability of the BBB. Currently, to solve this problem invasive methods including neurosurgery (Timbie et al., 2015) and BBB osmotic opening (Bhattacharjee et al., 2001) are used to overcome the BBB in applied medicine. New experimental methods of drug delivery through the BBB using nanoparticles (Zhang et al., 2018) and MR-guided focused ultrasound (Mainprize et al., 2019) offer an alternative to such invasive approaches. However, further development of effective systems to deliver drugs to the brain at a level of efficacy continues to be relevant.
In addition to being endogenous biomarkers of disease, EVs are also promising vectors for therapeutic agent delivery into the nervous system, as they are protected from degradation, retain their original state, and most importantly, are able to overcome the BBB (Kourembanas, 2015) (Figure 1B). The ability of EVs to pass the BBB is assumed to be related to their expression of claudin-5 protein, potentially enabling EVs to “unzip” the tight contacts of the BBB using a similar mechanism to that used by claudin-5 expressing leukocytes (Paul et al., 2016).
The use of exosomes and MVs also represents a promising alternative to cell based therapy, circumventing side effects, such as cell oncogenic transformation or undesirable cellular differentiation (Chulpanova et al., 2018b). Moreover, EVs have several advantages over cell therapy. Unlike cells, EVs are small in size which prevents the risk for emboli and provides a better distribution of therapeutic agents. EVs are also able to overcome biological barriers and reach the CNS (Phinney and Pittenger, 2017). In addition, therapeutic molecules inside EVs are protected by the natural lipid bilayer, which ensures stability, biocompatibility, low immunogenicity, ability to overcome body biological barriers (for example, BBB), as well as targeted drug delivery ability dependent upon the proteins embedded in the bilayer (Rufino-Ramos et al., 2017).
Despite promising prospects for the use of EVs, there are a number of limitations that need to be considered when constructing treatment strategies. The endogenous cargo of patient produced EVs has been reported to contribute to the progression of certain diseases by spreading pathogenic agents into healthy cells (Bakhshandeh et al., 2017). Therefore, the effects of therapeutic drugs loaded into EVs could be counteracted by the EV endogenous cargo.
Native MSC-derived exosomes are used for the regeneration of nervous tissue. For example, the exosomes were shown to stimulate the restoration of damaged axons. It is believed that this effect is due to growth factors such as vascular endothelial growth factor, hepatocyte growth factor, epidermal growth factor, brain-derived neurotrophic factor and neurotrophin-3, which are necessary for neuron growth and recovery (Lopez-Verrilli et al., 2016). Similar results were shown for native exosomes from Schwann cells (Lopez-Verrilli et al., 2013). As well as modulation of microglia to reduce the activation of their inflammatory response (Jaimes et al., 2017). Table 1 summarizes the use of EVs in the treatment of CNS diseases.
Table 1.
Examples of the use of extracellular vesicles for the treatment of central nervous system diseases
| Disease | Extracellular vesicle type | Therapeutic effect | Injection | Reference |
|---|---|---|---|---|
| Parkinson’s disease | Exosomes from monocytes and macrophages loaded with catalase | Neuroprotection | Intranasal Intravenous | Haney et al. (2013, 2015), Kojima et al. (2018) |
| Exosomes from mesenchymal stem cell (MSC) 3D culture | Neuroprotection, protection of dopaminergic neurons | In vitro | Jarmalaviciute et al. (2015) | |
| Exosomes from macrophages transfected with glial cell line-derived neurotrophic factor encoding plasmid | Neuroprotection, protection of dopaminergic neurons | In vitro | Zhao et al. (2014) | |
| Alzheimer’s disease | Exosomes with increased miR-21 expression isolated from hypoxia-preconditioned MSCs | Memory and learning ability improvement, beta-amyloid-peptide accumulation reduction | Intravenous | Cui et al. (2018) |
| Exosomes from dendritic cells with glyceraldehyde 3-phosphate dehydrogenase small interfering RNA and RVG peptide | glyceraldehyde 3-phosphate dehydrogenase gene knockdown, reduction of neurodegeneration and apoptotosis | Intravenous | Alvarez-Erviti et al. (2011) | |
| Huntington’s disease | Exosomes from human U87 glioblastoma cell with huntingtin-targeted small interfering RNA | Huntingtin gene knockdown | Intracranial | Didiot et al. (2016) |
| Epilepsy | Native exosomes from bone marrow MSCs | Neuronal loss and inflammation reduction, neurogenesis normalization, preservation of cognitive functions and memory | Intranasal | Long et al. (2017) |
| Multiple sclerosis | Native exosomes and microvesicles from human periodontal ligament stem cells | Regeneration and immunomodulation | Intravenous | Rajan et al. (2016) |
| Plasma exosomes after environmental enrichment | Myelination increase | In vitro | Pusic et al. (2016) | |
| Amyotrophic lateral sclerosis | Native exosomes from MSCs | Superoxide dismutase 1 aggregation relief | In vitro | Lee et al. (2016) |
Parkinson’s disease
PD is the second most common neurodegenerative disease (Tomiyama et al., 2015). PD is characterized by the presence of Lewy bodies formed due to α-synuclein aggregation and the death of dopamine neurons (Fan et al., 2017). Dopamine deficiency leads to motor impairment, particularly tremor, rigidity and bradykinesia (Tysnes and Storstein, 2017). It is known that oxidative stress aggravates neurodegeneration in PD patients. Intranasal administration of exosomes loaded with catalase into PD model mice resulted in a significant neuroprotective effect. Exosomes isolated from monocytes and macrophages were used in the work, these vesicles avoid capture by the immune cells, are able to overcome the BBB and effectively bind with brain cells. Various methods were used for catalase loading, the most effective was the use of saponin, sonication and extrusion (Haney et al., 2015; Kojima et al., 2018). Exosomes, isolated from human exfoliated deciduous teeth-derived stem cells maintained in 3D culture, possess neuroprotective potential and prevent apoptosis in dopaminergic neurons by approximately 80%. It is noteworthy that exosomes obtained from cells cultured under standard 2D conditions do not show such an effect (Jarmalaviciute et al., 2015).
Alzheimer’s disease
AD is a degenerative disease of the CNS, one of the most common causes of dementia, characterized by the formation of two major protein aggregates: senile (amyloid) plaques and neurofibrillary tangles, which are involved in processes leading to progressive neurodegeneration and death (Thei et al., 2018). Senile plaques are formed by the deposition of Aβ peptide fibrils in the human brain is the main component of paired helical filaments, which form compact filamentous structures called neurofibrillary tangles. In vitro experiments with hippocampal cells have shown a relationship between amyloid fibrils and signaling pathways which cause excessive phosphorylation of tau protein, which leads to destabilization of microtubules and axonal transport blocking (Dhiman et al., 2019). It was also shown that abnormal phosphorylation of tau protein involves two protein kinases: cyclin-dependent kinase 5 and glycogen synthase kinase 3β. In vitro studies of brain cells and neuroblastoma cells showed that cyclin-dependent kinase 5 is involved in the processes of cortex maturation and neuron migration, and also plays an important role in normal brain development. Deregulation of this protein kinase leads to excessive tau protein phosphorylation, thereby causing a sequence of molecular events leading to neuron degeneration (Liu et al., 2016). A number of studies showed that oxidative stress is a major factor in normal signaling pathway altering in neurons, which leads to their biochemical, structural abnormalities and degeneration. The main genes involved in the development of AD encode proteins such as amyloid precursor protein, presenilins 1 and 2, alpha-2-macroglobulin and apoliprotein E (Qu et al., 2019). However, so far these components of pathophysiology have not yet led to EV studies.
miRNAs play an important role in the regulation of various inflammatory responses. It is known that miR-21 controls the balance between pro-inflammatory, immunoregulatory and anti-inflammatory reactions. Dysregulation of miR-21 causes a chronic inflammation (Stuendl et al., 2016). Hypoxia-preconditioned MSCs have increased miR-21 expression. Injection of exosomes isolated from hypoxia-preconditioned MSCs to AD model animals (amyloid precursor protein/presenilin 1) led to improvement in their memory and ability to learn, and also reduced the accumulation of Aβ-peptide. The results confirmed the ability of native MSC-derived exosomes without surface molecule modification to penetrate into the CNS (Cui et al., 2018). In AD, glyceraldehyde 3-phosphate dehydrogenase glycolytic enzyme is involved in neurodegenerative processes and in apoptotic cell death (El Kadmiri et al., 2014). The administration of glyceraldehyde 3-phosphate dehydrogenase small interfering RNA into mice using exosomes isolated from autologous dendritic cells modified with neuron-specific RVG peptide for targeting resulted in specific gene knockdown in neurons, microglia, and oligodendrocytes in the brain. Exosomes were loaded with exogenous small interfering RNA by electroplating (Alvarez-Erviti et al., 2011).
Huntington’s disease
Huntington’s disease is an autosomal dominant neurodegenerative disease which leads to impaired motor and cognitive functions. Neurodegeneration is caused by the accumulation of mutant huntingtin protein, which negatively affects many cell processes. Mutant protein occurs due to CAG repeats, the CAG repeat length is positively correlated with the disease severity and negatively with age of onset (Pagan et al., 2017). It is known that mutations in the huntingtin protein are the main cause of Huntington’s disease. Hydrophobically modified small interfering RNAs (hsiRNAs) aimed at huntingtin messenger RNA were used to eliminate the toxic protein. During joint incubation, hsiRNAs were loaded into exosomes isolated from the human U87 glioblastoma cell. It was shown that the use of such exosomes improved hsiRNA spread in the brain of model animals, due to which huntingtin gene silencing was achieved. The authors recognize that use of glioblastoma cell-derived exosomes can provoke tumor formation, due to which it is necessary to optimize methods for obtaining exosomes from other cell types in order to introduce this approach into clinical practice (Didiot et al., 2016).
Epilepsy
Epilepsy is a widespread chronic neurological disorder characterized by recurrent convulsive seizures. A variety of brain damaging insults, including injuries, CNS infections and tumors can lead to epilepsy (Vezzani et al., 2016). The seizures cause an increase in extracellular glutamate level which contributes to cell damage and changes in neuronal signaling (Barker-Haliski and White, 2015). Epilepsy can result in mental disorders and mental retardation (Guerreiro, 2016).
Chronic hippocampus dysfunction is another consequence of epilepsy. In order to prevent this type of dysfunction, exosomes from human bone marrow-derived MSCs, which have strong anti-inflammatory and neuroprotective properties was tested. Intranasal administration of exosomes to model mice led to a decrease in neuronal loss, reduced inflammation, normal neurogenesis maintenance, unimpaired cognitive functions and memory preservation (Long et al., 2017).
Multiple sclerosis
Multiple sclerosis is a chronic inflammatory CNS disease leading to demyelination and neurodegeneration (Correale et al., 2017). MS etiology remains unclear, but it is assumed that MS is an autoimmune disease. Progressive MS leads to the loss of axons and trophic support (Nicholas and Rashid, 2013). It was shown that native exosomes and MVs from human periodontal ligament stem cells exhibit regenerative and immunomodulating properties in the treatment of multiple sclerosis. After their administration to a mouse model of MS, a decrease in proinflammatory cytokines IL-17, interferon γ, IL-1β, IL-6, tumor necrosis factor-α, induction of anti-inflammatory IL-10 and expression attenuation of signal transducer and activator of transcription 1, p53, caspase-3 and Bax, which are associated with cell apoptosis were observed (Rajan et al., 2016).
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis is characterized by the degeneration of the upper and lower motor neurons, which leads to muscle weakness, convulsions, paralysis, and death (Hardiman et al., 2017). The causes of amyotrophic lateral sclerosis remain unknown, but the genes associated with the disease have been identified (Chia et al., 2018). Neuronal cytoplasmic inclusions of TDP-43, FUS, C9orf72, TDP-43 are characterized of amyotrophic lateral sclerosis (Saberi et al., 2015). The use of native exosomes from adipose tissue-derived stem cells on mouse neuronal cell culture amyotrophic lateral sclerosis model showed superoxide dismutase 1 aggregation relief, it is assumed that the effect is achieved due to the restoration of mitochondrial functions (Lee et al., 2016).
Conclusion
Exosomes and MVs are membrane nanoparticles of endosomal and membrane origin, which provide intercellular communication through the transport of biological molecules. It was found that exosomes and MVs are released by many CNS cells and play an important role in its functioning, as well as contributing to the spread of pathogenic agents in various diseases. The identification of novel vesicle biomarkers demonstrates the potential for the application of exosomes and MVs for the early diagnosis of the nervous system diseases. Furthermore, the ability to overcome the BBB, protect therapeutic agents from degradation, lack of immunoreactivity, biosafety and the possibility of targeted delivery make exosomes and MVs promising tools for use in clinical practice for cell-free therapy of CNS diseases.
Acknowledgments
Study was partially accomplished in Center of the National Technology Initiative at the M.M. Shemyakin–Yu.A. Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: The work was performed according to the Russian Government Program of Competitive Growth of Kazan Federal University. AAR was supported by state assignment 20.5175.2017/6.7 of the Ministry of Education and Science of Russian Federation and the President of the Russian Federation grant НШ-3076.2018.4.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Jonathan M. Borkum, University of Maine, USA.
Funding: The work was financially supported by the Russian Government Program of Competitive Growth of Kazan Federal University. AAR was supported by state assignment 20.5175.2017/6.7 of the Ministry of Education and Science of Russian Federation and the President of the Russian Federation grant НШ-3076.2018.4.
P-Reviewer: Borkum JM; C-Editors: Zhao M, Yu J; T-Editor: Jia Y
References
- 1.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akhmetzyanova ER, Mukhamedshina YO, Zhuravleva MN, Galieva LR, Kostennikov AA, Garanina EE, Rizvanov AA. Transplantation of microglia in the area of spinal cord injury in an acute period increases tissue sparing, but not functional recovery. Front Cell Neurosci. 2018;12:507. doi: 10.3389/fncel.2018.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 4.Bakhshandeh B, Kamaleddin MA, Aalishah K. A comprehensive review on exosomes and microvesicles as epigenetic factors. Curr Stem Cell Res Ther. 2017;12:31–36. doi: 10.2174/1574888x11666160709211528. [DOI] [PubMed] [Google Scholar]
- 5.Bakhti M, Winter C, Simons M. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J Biol Chem. 2011;286:787–796. doi: 10.1074/jbc.M110.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker-Haliski M, White HS. Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb Perspect Med. 2015;5:a022863. doi: 10.1101/cshperspect.a022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batiz LF, Castro MA, Burgos PV, Velasquez ZD, Munoz RI, Lafourcade CA, Troncoso-Escudero P, Wyneken U. Exosomes as novel regulators of adult neurogenic niches. Front Cell Neurosci. 2015;9:501. doi: 10.3389/fncel.2015.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharjee AK, Nagashima T, Kondoh T, Tamaki N. Quantification of early blood-brain barrier disruption by in situ brain perfusion technique. Brain Res Brain Res Protoc. 2001;8:126–131. doi: 10.1016/s1385-299x(01)00094-0. [DOI] [PubMed] [Google Scholar]
- 9.Cao XY, Lu JM, Zhao ZQ, Li MC, Lu T, An XS, Xue LJ. MicroRNA biomarkers of Parkinson’s disease in serum exosome-like microvesicles. Neurosci Lett. 2017;644:94–99. doi: 10.1016/j.neulet.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 10.Caruso Bavisotto C, Scalia F, Marino Gammazza A, Carlisi D, Bucchieri F, Conway de Macario E, Macario AJL, Cappello F, Campanella C. Extracellular vesicle-mediated cell(-)cell communication in the nervous system: focus on neurological diseases. Int J Mol Sci. 2019;20:E434. doi: 10.3390/ijms20020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chia R, Chiò A, Traynor BJ. Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications. Lancet Neurol. 2018;17:94–102. doi: 10.1016/S1474-4422(17)30401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chivet M, Hemming F, Pernet-Gallay K, Fraboulet S, Sadoul R. Emerging role of neuronal exosomes in the central nervous system. Front Physiol. 2012;3:145. doi: 10.3389/fphys.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, Sadoul R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3:24722. doi: 10.3402/jev.v3.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choo YW, Kang M, Kim HY, Han J, Kang S, Lee JR, Jeong GJ, Kwon SP, Song SY, Go S, Jung M, Hong J, Kim BS. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano. 2018;12:8977–8993. doi: 10.1021/acsnano.8b02446. [DOI] [PubMed] [Google Scholar]
- 15.Chulpanova DS, Kitaeva KV, James V, Rizvanov AA, Solovyeva VV. Therapeutic prospects of extracellular vesicles in cancer treatment. Front Immunol. 2018a;9:1534. doi: 10.3389/fimmu.2018.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chulpanova DS, Kitaeva KV, Tazetdinova LG, James V, Rizvanov AA, Solovyeva VV. Application of mesenchymal stem cells for therapeutic agent delivery in anti-tumor treatment. Front Pharmacol. 2018b;9:259. doi: 10.3389/fphar.2018.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark JP, Lau NC. Piwi Proteins and piRNAs step onto the systems biology stage. Adv Exp Med Biol. 2014;825:159–197. doi: 10.1007/978-1-4939-1221-6_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Correale J, Gaitan MI, Ysrraelit MC, Fiol MP. Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain. 2017;140:527–546. doi: 10.1093/brain/aww258. [DOI] [PubMed] [Google Scholar]
- 19.Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, Fang J, Xu YW, Dong YR, Liu JR, Guo HD. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018;32:654–668. doi: 10.1096/fj.201700600R. [DOI] [PubMed] [Google Scholar]
- 20.Dhiman K, Blennow K, Zetterberg H, Martins RN, Gupta VB. Cerebrospinal fluid biomarkers for understanding multiple aspects of Alzheimer’s disease pathogenesis. Cell Mol Life Sci. 2019;76:1833–1863. doi: 10.1007/s00018-019-03040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Didiot MC, Hall LM, Coles AH, Haraszti RA, Godinho BM, Chase K, Sapp E, Ly S, Alterman JF, Hassler MR, Echeverria D, Raj L, Morrissey DV, DiFiglia M, Aronin N, Khvorova A. Exosome-mediated delivery of hydrophobically modified siRNA for huntingtin mRNA silencing. Mol Ther. 2016;24:1836–1847. doi: 10.1038/mt.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Kadmiri N, Slassi I, El Moutawakil B, Nadifi S, Tadevosyan A, Hachem A, Soukri A. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer’s disease. Pathol Biol (Paris) 2014;62:333–336. doi: 10.1016/j.patbio.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Falker C, Hartmann A, Guett I, Dohler F, Altmeppen H, Betzel C, Schubert R, Thurm D, Wegwitz F, Joshi P, Verderio C, Krasemann S, Glatzel M. Exosomal cellular prion protein drives fibrillization of amyloid beta and counteracts amyloid beta-mediated neurotoxicity. J Neurochem. 2016;137:88–100. doi: 10.1111/jnc.13514. [DOI] [PubMed] [Google Scholar]
- 24.Fan CH, Lin CY, Liu HL, Yeh CK. Ultrasound targeted CNS gene delivery for Parkinson’s disease treatment. J Control Release. 2017;261:246–262. doi: 10.1016/j.jconrel.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Feiler MS, Strobel B, Freischmidt A, Helferich AM, Kappel J, Brewer BM, Li D, Thal DR, Walther P, Ludolph AC, Danzer KM, Weishaupt JH. TDP-43 is intercellularly transmitted across axon terminals. J Cell Biol. 2015;211:897–911. doi: 10.1083/jcb.201504057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feneberg E, Steinacker P, Lehnert S, Schneider A, Walther P, Thal DR, Linsenmeier M, Ludolph AC, Otto M. Limited role of free TDP-43 as a diagnostic tool in neurodegenerative diseases. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:351–356. doi: 10.3109/21678421.2014.905606. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes JCR, Acuna SM, Aoki JI, Floeter-Winter LM, Muxel SM. Long non-coding RNAs in the regulation of gene expression: physiology and disease. Noncoding RNA. 2019;5:E17. doi: 10.3390/ncrna5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraser KB, Moehle MS, Daher JP, Webber PJ, Williams JY, Stewart CA, Yacoubian TA, Cowell RM, Dokland T, Ye T, Chen D, Siegal GP, Galemmo RA, Tsika E, Moore DJ, Standaert DG, Kojima K, Mobley JA, West AB. LRRK2 secretion in exosomes is regulated by 14-3-3. Hum Mol Genet. 2013;22:4988–5000. doi: 10.1093/hmg/ddt346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galieva LR, James V, Mukhamedshina YO, Rizvanov AA. Therapeutic potential of extracellular vesicles for the treatment of nerve disorders. Front Neurosci. 2019;13:163. doi: 10.3389/fnins.2019.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Romero N, Carrion-Navarro J, Esteban-Rubio S, Lazaro-Ibanez E, Peris-Celda M, Alonso MM, Guzman-De-Villoria J, Fernandez-Carballal C, de Mendivil AO, Garcia-Duque S, Escobedo-Lucea C, Prat-Acin R, Belda-Iniesta C, Ayuso-Sacido A. DNA sequences within glioma-derived extracellular vesicles can cross the intact blood-brain barrier and be detected in peripheral blood of patients. Oncotarget. 2017;8:1416–1428. doi: 10.18632/oncotarget.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giordano-Santini R, Linton C, Hilliard MA. Cell-cell fusion in the nervous system: Alternative mechanisms of development, injury, and repair. Semin Cell Dev Biol. 2016;60:146–154. doi: 10.1016/j.semcdb.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Goetzl EJ, Abner EL, Jicha GA, Kapogiannis D, Schwartz JB. Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer’s disease. FASEB J. 2018;32:888–893. doi: 10.1096/fj.201700731R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goetzl EJ, Mustapic M, Kapogiannis D, Eitan E, Lobach IV, Goetzl L, Schwartz JB, Miller BL. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 2016a;30:3853–3859. doi: 10.1096/fj.201600756R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goetzl EJ, Kapogiannis D, Schwartz JB, Lobach IV, Goetzl L, Abner EL, Jicha GA, Karydas AM, Boxer A, Miller BL. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 2016b;30:4141–4148. doi: 10.1096/fj.201600816R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gres V, Kolter J, Erny D, Henneke P. The role of CNS macrophages in streptococcal meningoencephalitis. J Leukoc Biol. 2019 doi: 10.1002/JLB.4MR1118-419R. doi: 101002/JLB4MR1118-419R. [DOI] [PubMed] [Google Scholar]
- 36.Guerreiro CA. Epilepsy: Is there hope? Indian J Med Res. 2016;144:657–660. doi: 10.4103/ijmr.IJMR_1051_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gui Y, Liu H, Zhang L, Lv W, Hu X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget. 2015;6:37043–37053. doi: 10.18632/oncotarget.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halperin JJ. Neuroborreliosis and neurosyphilis. Continuum (Minneap Minn) 2018;24:1439–1458. doi: 10.1212/CON.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 39.Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haney MJ, Zhao Y, Harrison EB, Mahajan V, Ahmed S, He Z, Suresh P, Hingtgen SD, Klyachko NL, Mosley RL, Gendelman HE, Kabanov AV, Batrakova EV. Specific transfection of inflamed brain by macrophages: a new therapeutic strategy for neurodegenerative diseases. PLoS One. 2013;8:e61852. doi: 10.1371/journal.pone.0061852. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Haraszti RA, Didiot MC, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, Gao F, Narain NR, DiFiglia M, Kiebish MA, Aronin N, Khvorova A. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. 2016;5:32570. doi: 10.3402/jev.v5.32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, Shaw PJ, Simmons Z, van den Berg LH. Amyotrophic lateral sclerosis. Nat Rev Dis Primers. 2017;3:17071. doi: 10.1038/nrdp.2017.71. [DOI] [PubMed] [Google Scholar]
- 43.Harischandra DS, Ghaisas S, Rokad D, Zamanian M, Jin H, Anantharam V, Kimber M, Kanthasamy A, Kanthasamy AG. Environmental neurotoxicant manganese regulates exosome-mediated extracellular miRNAs in cell culture model of Parkinson’s disease: Relevance to alpha-synuclein misfolding in metal neurotoxicity. Neurotoxicology. 2018;64:267–277. doi: 10.1016/j.neuro.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrison EB, Hochfelder CG, Lamberty BG, Meays BM, Morsey BM, Kelso ML, Fox HS, Yelamanchili SV. Traumatic brain injury increases levels of miR-21 in extracellular vesicles: implications for neuroinflammation. FEBS Open Bio. 2016;6:835–846. doi: 10.1002/2211-5463.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hartmann AA, Burg G. Fulminant acne in Klinefelter syndrome treated with testosterone. A side effect of anti-tallness therapy. Monatsschr Kinderheilkd. 1989;137:466–467. [PubMed] [Google Scholar]
- 46.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirshman BR, Kras RT, Akers JC, Carter BS, Chen CC. Extracellular vesicles in molecular diagnostics: an overview with a focus on CNS diseases. Adv Clin Chem. 2016;76:37–53. doi: 10.1016/bs.acc.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Hooper AWM, Alamilla JF, Venier RE, Gillespie DC, Igdoura SA. Neuronal pentraxin 1 depletion delays neurodegeneration and extends life in Sandhoff disease mice. Hum Mol Genet. 2017;26:661–673. doi: 10.1093/hmg/ddw422. [DOI] [PubMed] [Google Scholar]
- 49.Huey R, Hawthorne S, McCarron P. The potential use of rabies virus glycoprotein-derived peptides to facilitate drug delivery into the central nervous system: a mini review. J Drug Target. 2017;25:379–385. doi: 10.1080/1061186X.2016.1223676. [DOI] [PubMed] [Google Scholar]
- 50.Iguchi Y, Eid L, Parent M, Soucy G, Bareil C, Riku Y, Kawai K, Takagi S, Yoshida M, Katsuno M, Sobue G, Julien JP. Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain. 2016;139:3187–3201. doi: 10.1093/brain/aww237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaimes Y, Naaldijk Y, Wenk K, Leovsky C, Emmrich F. Mesenchymal stem cell-derived microvesicles modulate lipopolysaccharides-induced inflammatory responses to microglia cells. Stem Cells. 2017;35:812–823. doi: 10.1002/stem.2541. [DOI] [PubMed] [Google Scholar]
- 52.Jarmalaviciute A, Tunaitis V, Pivoraite U, Venalis A, Pivoriunas A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy. 2015;17:932–939. doi: 10.1016/j.jcyt.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 53.Jeon I, Cicchetti F, Cisbani G, Lee S, Li E, Bae J, Lee N, Li L, Im W, Kim M, Kim HS, Oh SH, Kim TA, Ko JJ, Aube B, Oueslati A, Kim YJ, Song J. Human-to-mouse prion-like propagation of mutant huntingtin protein. Acta Neuropathol. 2016;132:577–592. doi: 10.1007/s00401-016-1582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163-164:144–171. doi: 10.1016/j.pneurobio.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalluri R, LeBleu VS. Discovery of double-stranded genomic dna in circulating exosomes. Cold Spring Harb Symp Quant Biol. 2016;81:275–280. doi: 10.1101/sqb.2016.81.030932. [DOI] [PubMed] [Google Scholar]
- 56.Kawikova I, Askenase PW. Diagnostic and therapeutic potentials of exosomes in CNS diseases. Brain Res. 2015;1617:63–71. doi: 10.1016/j.brainres.2014.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip Rev RNA. 2017;8:e1413. doi: 10.1002/wrna.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kinoshita T, Yip KW, Spence T, Liu FF. MicroRNAs in extracellular vesicles: potential cancer biomarkers. J Hum Genet. 2017;62:67–74. doi: 10.1038/jhg.2016.87. [DOI] [PubMed] [Google Scholar]
- 59.Ko J, Carpenter E, Issadore D. Detection and isolation of circulating exosomes and microvesicles for cancer monitoring and diagnostics using micro-/nano-based devices. Analyst. 2016;141:450–460. doi: 10.1039/c5an01610j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kojima R, Bojar D, Rizzi G, Hamri GC, El-Baba MD, Saxena P, Auslander S, Tan KR, Fussenegger M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat Commun. 2018;9:1305. doi: 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koniusz S, Andrzejewska A, Muraca M, Srivastava AK, Janowski M, Lukomska B. Extracellular vesicles in physiology, pathology, and therapy of the immune and central nervous system, with focus on extracellular vesicles derived from mesenchymal stem cells as therapeutic tools. Front Cell Neurosci. 2016;10:109. doi: 10.3389/fncel.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 63.Kumar A, Stoica BA, Loane DJ, Yang M, Abulwerdi G, Khan N, Kumar A, Thom SR, Faden AI. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation. 2017;14:47. doi: 10.1186/s12974-017-0819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar D, Manek R, Raghavan V, Wang KK. Protein characterization of extracellular microvesicles/exosomes released from cytotoxin-challenged rat cerebrocortical mixed culture and mouse N2a cells. Mol Neurobiol. 2018;55:2112–2124. doi: 10.1007/s12035-017-0474-x. [DOI] [PubMed] [Google Scholar]
- 65.Lee M, Ban JJ, Kim KY, Jeon GS, Im W, Sung JJ, Kim M. Adipose-derived stem cell exosomes alleviate pathology of amyotrophic lateral sclerosis in vitro. Biochem Biophys Res Commun. 2016;479:434–439. doi: 10.1016/j.bbrc.2016.09.069. [DOI] [PubMed] [Google Scholar]
- 66.Liu SL, Wang C, Jiang T, Tan L, Xing A, Yu JT. The role of Cdk5 in Alzheimer’s disease. Mol Neurobiol. 2016;53:4328–4342. doi: 10.1007/s12035-015-9369-x. [DOI] [PubMed] [Google Scholar]
- 67.Long Q, Upadhya D, Hattiangady B, Kim DK, An SY, Shuai B, Prockop DJ, Shetty AK. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci U S A. 2017;114:E3536–3545. doi: 10.1073/pnas.1703920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61:1795–1806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- 69.Lopez-Verrilli MA, Caviedes A, Cabrera A, Sandoval S, Wyneken U, Khoury M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016;320:129–139. doi: 10.1016/j.neuroscience.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 70.Lu D, Xu AD. Mini review: Circular RNAs as potential clinical biomarkers for disorders in the central nervous system. Front Genet. 2016;7:53. doi: 10.3389/fgene.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, Heyn C, Alkins R, Trudeau M, Sahgal A, Perry J, Hynynen K. Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep. 2019;9:321. doi: 10.1038/s41598-018-36340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Manek R, Moghieb A, Yang Z, Kumar D, Kobessiy F, Sarkis GA, Raghavan V, Wang KKW. Protein biomarkers and neuroproteomics characterization of microvesicles/exosomes from human cerebrospinal fluid following traumatic brain injury. Mol Neurobiol. 2018;55:6112–6128. doi: 10.1007/s12035-017-0821-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maniati MS, Maniati M, Yousefi T, Ahmadi-Ahangar A, Tehrani SS. New insights into the role of microRNAs and long noncoding RNAs in most common neurodegenerative diseases. J Cell Biochem. 2019;120:8908–8918. doi: 10.1002/jcb.28361. [DOI] [PubMed] [Google Scholar]
- 74.Mathew B, Ravindran S, Liu X, Torres L, Chennakesavalu M, Huang CC, Feng L, Zelka R, Lopez J, Sharma M, Roth S. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials. 2019;197:146–160. doi: 10.1016/j.biomaterials.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsumoto J, Stewart T, Banks WA, Zhang J. The transport mechanism of extracellular vesicles at the blood-brain barrier. Curr Pharm Des. 2017a;23:6206–6214. doi: 10.2174/1381612823666170913164738. [DOI] [PubMed] [Google Scholar]
- 76.Matsumoto J, Stewart T, Sheng L, Li N, Bullock K, Song N, Shi M, Banks WA, Zhang J. Transmission of alpha-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol Commun. 2017b;5:71. doi: 10.1186/s40478-017-0470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McKee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015;127:45–66. doi: 10.1016/B978-0-444-52892-6.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McKeever PM, Schneider R, Taghdiri F, Weichert A, Multani N, Brown RA, Boxer AL, Karydas A, Miller B, Robertson J, Tartaglia MC. MicroRNA expression levels are altered in the cerebrospinal fluid of patients with young-onset Alzheimer’s disease. Mol Neurobiol. 2018;55:8826–8841. doi: 10.1007/s12035-018-1032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merchant ML, Rood IM, Deegens JKJ, Klein JB. Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery. Nat Rev Nephrol. 2017;13:731–749. doi: 10.1038/nrneph.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mukhamedshina YO, Gracheva OA, Mukhutdinova DM, Chelyshev YA, Rizvanov AA. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural Regen Res. 2019;14:227–237. doi: 10.4103/1673-5374.244778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ngolab J, Trinh I, Rockenstein E, Mante M, Florio J, Trejo M, Masliah D, Adame A, Masliah E, Rissman RA. Brain-derived exosomes from dementia with Lewy bodies propagate alpha-synuclein pathology. Acta Neuropathol Commun. 2017;5:46. doi: 10.1186/s40478-017-0445-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Nicholas R, Rashid W. Multiple sclerosis. Am Fam Physician. 2013;87:712–714. [PubMed] [Google Scholar]
- 83.Pagan F, Torres-Yaghi Y, Altshuler M. The diagnosis and natural history of Huntington disease. Handb Clin Neurol. 2017;144:63–67. doi: 10.1016/B978-0-12-801893-4.00005-5. [DOI] [PubMed] [Google Scholar]
- 84.Paul D, Baena V, Ge S, Jiang X, Jellison ER, Kiprono T, Agalliu D, Pachter JS. Appearance of claudin-5(+) leukocytes in the central nervous system during neuroinflammation: a novel role for endothelial-derived extracellular vesicles. J Neuroinflammation. 2016;13:292. doi: 10.1186/s12974-016-0755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 86.Phoolcharoen W, Prehaud C, van Dolleweerd CJ, Both L, da Costa A, Lafon M, Ma JK. Enhanced transport of plant-produced rabies single-chain antibody-RVG peptide fusion protein across an in cellulo blood-brain barrier device. Plant Biotechnol J. 2017;15:1331–1339. doi: 10.1111/pbi.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pitt JM, Andre F, Amigorena S, Soria JC, Eggermont A, Kroemer G, Zitvogel L. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest. 2016;126:1224–1232. doi: 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pratt BM, McPherson JM. TGF-beta in the central nervous system: potential roles in ischemic injury and neurodegenerative diseases. Cytokine Growth Factor Rev. 1997;8:267–292. doi: 10.1016/s1359-6101(97)00018-x. [DOI] [PubMed] [Google Scholar]
- 89.Preston JE, Joan Abbott N, Begley DJ. Transcytosis of macromolecules at the blood-brain barrier. Adv Pharmacol. 2014;71:147–163. doi: 10.1016/bs.apha.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 90.Pusic KM, Pusic AD, Kraig RP. Environmental enrichment stimulates immune cell secretion of exosomes that promote CNS myelination and may regulate inflammation. Cell Mol Neurobiol. 2016;36:313–325. doi: 10.1007/s10571-015-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qu Y, Liu Y, Noor AF, Tran J, Li R. Characteristics and advantages of adeno-associated virus vector-mediated gene therapy for neurodegenerative diseases. Neural Regen Res. 2019;14:931–938. doi: 10.4103/1673-5374.250570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Radosinska J, Bartekova M. Therapeutic potential of hematopoietic stem cell-derived exosomes in cardiovascular disease. Adv Exp Med Biol. 2017;998:221–235. doi: 10.1007/978-981-10-4397-0_15. [DOI] [PubMed] [Google Scholar]
- 93.Rajan TS, Giacoppo S, Diomede F, Ballerini P, Paolantonio M, Marchisio M, Piattelli A, Bramanti P, Mazzon E, Trubiani O. The secretome of periodontal ligament stem cells from MS patients protects against EAE. Sci Rep. 2016;6:38743. doi: 10.1038/srep38743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rao P, Benito E, Fischer A. MicroRNAs as biomarkers for CNS disease. Front Mol Neurosci. 2013;6:39. doi: 10.3389/fnmol.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Record M, Silvente-Poirot S, Poirot M, Wakelam MJO. Extracellular vesicles: lipids as key components of their biogenesis and functions. J Lipid Res. 2018;59:1316–1324. doi: 10.1194/jlr.E086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Riancho J, Vazquez-Higuera JL, Pozueta A, Lage C, Kazimierczak M, Bravo M, Calero M, Gonalezalez A, Rodriguez E, Lleo A, Sanchez-Juan P. MicroRNA profile in patients with Alzheimer’s disease: analysis of mir-9-5p and mir-598 in raw and exosome enriched cerebrospinal fluid samples. J Alzheimers Dis. 2017;57:483–491. doi: 10.3233/JAD-161179. [DOI] [PubMed] [Google Scholar]
- 98.Rufino-Ramos D, Albuquerque PR, Carmona V, Perfeito R, Nobre RJ, Pereira de Almeida L. Extracellular vesicles: Novel promising delivery systems for therapy of brain diseases. J Control Release. 2017;262:247–258. doi: 10.1016/j.jconrel.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 99.Russo MV, McGavern DB. Inflammatory neuroprotection following traumatic brain injury. Science. 2016;353:783–785. doi: 10.1126/science.aaf6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saberi S, Stauffer JE, Schulte DJ, Ravits J. Neuropathology of amyotrophic lateral sclerosis and its variants. Neurol Clin. 2015;33:855–876. doi: 10.1016/j.ncl.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sardar Sinha M, Ansell-Schultz A, Civitelli L, Hildesjo C, Larsson M, Lannfelt L, Ingelsson M, Hallbeck M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018;136:41–56. doi: 10.1007/s00401-018-1868-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Selmaj I, Mycko MP, Raine CS, Selmaj KW. The role of exosomes in CNS inflammation and their involvement in multiple sclerosis. J Neuroimmunol. 2017;306:1–10. doi: 10.1016/j.jneuroim.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 103.Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, Li Y, Aro P, Dator R, He C, Hipp MJ, Zabetian CP, Peskind ER, Hu SC, Quinn JF, Galasko DR, Banks WA, Zhang J. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014;128:639–650. doi: 10.1007/s00401-014-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi M, Kovac A, Korff A, Cook TJ, Ginghina C, Bullock KM, Yang L, Stewart T, Zheng D, Aro P, Atik A, Kerr KF, Zabetian CP, Peskind ER, Hu SC, Quinn JF, Galasko DR, Montine TJ, Banks WA, Zhang J. CNS tau efflux via exosomes is likely increased in Parkinson’s disease but not in Alzheimer’s disease. Alzheimers Dement. 2016;12:1125–1131. doi: 10.1016/j.jalz.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Solovyeva VV, Shaimardanova AA, Chulpanova DS, Kitaeva KV, Chakrabarti L, Rizvanov AA. New approaches to tay-sachs disease therapy. Front Physiol. 2018;9:1663. doi: 10.3389/fphys.2018.01663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sousa C, Pereira I, Santos AC, Carbone C, Kovacevic AB, Silva AM, Souto EB. Targeting dendritic cells for the treatment of autoimmune disorders. Colloids Surf B Biointerfaces. 2017;158:237–248. doi: 10.1016/j.colsurfb.2017.06.050. [DOI] [PubMed] [Google Scholar]
- 107.Sproviero D, La Salvia S, Giannini M, Crippa V, Gagliardi S, Bernuzzi S, Diamanti L, Ceroni M, Pansarasa O, Poletti A, Cereda C. Pathological proteins are transported by extracellular vesicles of sporadic amyotrophic lateral sclerosis patients. Front Neurosci. 2018;12:487. doi: 10.3389/fnins.2018.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stern RA, Tripodis Y, Baugh CM, Fritts NG, Martin BM, Chaisson C, Cantu RC, Joyce JA, Shah S, Ikezu T, Zhang J, Gercel-Taylor C, Taylor DD. Preliminary study of plasma exosomal tau as a potential biomarker for chronic traumatic encephalopathy. J Alzheimers Dis. 2016;51:1099–1109. doi: 10.3233/JAD-151028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Strauss K, Goebel C, Runz H, Mobius W, Weiss S, Feussner I, Simons M, Schneider A. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J Biol Chem. 2010;285:26279–26288. doi: 10.1074/jbc.M110.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stuendl A, Kunadt M, Kruse N, Bartels C, Moebius W, Danzer KM, Mollenhauer B, Schneider A. Induction of alpha-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain. 2016;139:481–494. doi: 10.1093/brain/awv346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Su W, Aloi MS, Garden GA. MicroRNAs mediating CNS inflammation: small regulators with powerful potential. Brain Behav Immun. 2016;52:1–8. doi: 10.1016/j.bbi.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun B, Dalvi P, Abadjian L, Tang N, Pulliam L. Blood neuron-derived exosomes as biomarkers of cognitive impairment in HIV. AIDS. 2017;31:F9–F17. doi: 10.1097/QAD.0000000000001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14:455–466. doi: 10.1038/nrgastro.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Takano T, Xu C, Funahashi Y, Namba T, Kaibuchi K. Neuronal polarization. Development. 2015;142:2088–2093. doi: 10.1242/dev.114454. [DOI] [PubMed] [Google Scholar]
- 115.Thei L, Imm J, Kaisis E, Dallas ML, Kerrigan TL. Microglia in Alzheimer’s disease: a role for ion channels. Front Neurosci. 2018;12:676. doi: 10.3389/fnins.2018.00676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Timbie KF, Mead BP, Price RJ. Drug and gene delivery across the blood-brain barrier with focused ultrasound. J Control Release. 2015;219:61–75. doi: 10.1016/j.jconrel.2015.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F. Extracellular vesicles in angiogenesis. Circ Res. 2017;120:1658–1673. doi: 10.1161/CIRCRESAHA.117.309681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tomiyama H, Lesage S, Tan EK, Jeon BS. Familial Parkinson’s disease/parkinsonism. Biomed Res Int. 2015;2015:736915. doi: 10.1155/2015/736915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 2017;124:901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 120.Venkatesan A, Murphy OC. Viral encephalitis. Neurol Clin. 2018;36:705–724. doi: 10.1016/j.ncl.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 121.Vezzani A, Fujinami RS, White HS, Preux PM, Blumcke I, Sander JW, Loscher W. Infections, inflammation and epilepsy. Acta Neuropathol. 2016;131:211–234. doi: 10.1007/s00401-015-1481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vinuesa A, Bentivegna M, Calfa G, Filipello F, Pomilio C, Bonaventura MM, Lux-Lantos V, Matzkin ME, Gregosa A, Presa J, Matteoli M, Beauquis J, Saravia F. Early exposure to a high-fat diet impacts on hippocampal plasticity: implication of microglia-derived exosome-like extracellular vesicles. Mol Neurobiol. 2018 doi: 10.1007/s12035-018-1435-8. doi: 101007/s12035-018-1435-8. [DOI] [PubMed] [Google Scholar]
- 123.von Bernhardi R, Eugenin-von Bernhardi J, Flores B, Eugenin Leon J. Glial cells and integrity of the nervous system. Adv Exp Med Biol. 2016;949:1–24. doi: 10.1007/978-3-319-40764-7_1. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y, Balaji V, Kaniyappan S, Kruger L, Irsen S, Tepper K, Chandupatla R, Maetzler W, Schneider A, Mandelkow E, Mandelkow EM. The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener. 2017;12:5. doi: 10.1186/s13024-016-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wei H, Xu Y, Xu W, Zhou Q, Chen Q, Yang M, Feng F, Liu Y, Zhu X, Yu M, Li Y. Serum exosomal miR-223 serves as a potential diagnostic and prognostic biomarker for dementia. Neuroscience. 2018;379:167–176. doi: 10.1016/j.neuroscience.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 126.Wong CH, Chen YC. Clinical significance of exosomes as potential biomarkers in cancer. World J Clin Cases. 2019;7:171–190. doi: 10.12998/wjcc.v7.i2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wright JN. CNS injuries in abusive head trauma. AJR Am J Roentgenol. 2017;208:991–1001. doi: 10.2214/AJR.16.17602. [DOI] [PubMed] [Google Scholar]
- 128.Xiao B, Chai Y, Lv S, Ye M, Wu M, Xie L, Fan Y, Zhu X, Gao Z. Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int J Mol Med. 2017;40:1201–1209. doi: 10.3892/ijmm.2017.3106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. 2016;126:1152–1162. doi: 10.1172/JCI81129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yan S, Zhang H, Xie W, Meng F, Zhang K, Jiang Y, Zhang X, Zhang J. Altered microRNA profiles in plasma exosomes from mesial temporal lobe epilepsy with hippocampal sclerosis. Oncotarget. 2017;8:4136–4146. doi: 10.18632/oncotarget.13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang Q, Wu J, Zhao J, Xu T, Zhao Z, Song X, Han P. Circular RNA expression profiles during the differentiation of mouse neural stem cells. BMC Syst Biol. 2018;12:128. doi: 10.1186/s12918-018-0651-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang H, Wang T, Qiu W, Han Y, Sun Q, Zeng J, Yan F, Zheng H, Li Z, Gao M. Monitoring the opening and recovery of the blood-brain barrier with noninvasive molecular imaging by biodegradable ultrasmall Cu2-xSe nanoparticles. Nano Lett. 2018;18:4985–4992. doi: 10.1021/acs.nanolett.8b01818. [DOI] [PubMed] [Google Scholar]
- 133.Zhao Y, Haney MJ, Gupta R, Bohnsack JP, He Z, Kabanov AV, Batrakova EV. GDNF-transfected macrophages produce potent neuroprotective effects in Parkinson’s disease mouse model. PLoS One. 2014;9:e106867. doi: 10.1371/journal.pone.0106867. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]