Keywords: active ingredients, Alpinia oxyphylla, apoptosis, ethanol crude extract, fraction, hydrogen peroxide, nerve regeneration, neuroprotective agent, neuroprotective effects, PC12 cells, traditional herb
Abstract
Alpinia oxyphylla, a traditional herb, is widely used for its neuroprotective, antioxidant and memory-improving effects. However, the neuroprotective mechanisms of action of its active ingredients are unclear. In this study, we investigated the neuroprotective effects of various organic extracts of Alpinia oxyphylla on PC12 cells exposed to hydrogen peroxide-induced oxidative injury in vitro. Alpinia oxyphylla was extracted three times with 95% ethanol (representing extracts 1–3). The third 95% ethanol extract was dried and resuspended in water, and then extracted successively with petroleum ether, ethyl acetate and n-butanol (representing extracts 4–6). The cell counting kit-8 assay and microscopy were used to evaluate cell viability and observe the morphology of PC12 cells. The protective effect of the three ethanol extracts (at tested concentrations of 50, 100 and 200 µg/mL) against cytotoxicity to PC12 cells increased in a concentration-dependent manner. The ethyl acetate, petroleum ether and n-butanol extracts (each tested at 100, 150 and 200 μg/mL) had neuroprotective effects as well. The optimum effective concentration ranged from 50–200 μg/mL, and the protective effect of the ethyl acetate extract was comparatively robust. These results demonstrate that organic extracts of Alpinia oxyphylla protect PC12 cells against apoptosis induced by hydrogen peroxide. Our findings should help identify the bioactive neuroprotective components in Alpinia oxyphylla.
Chinese Library Classification No. R452; R363; R364
Introduction
Alpinia (A.) oxyphylla is commonly used in traditional Chinese medicine to treat dyspepsia, diarrhea (Zhang et al., 2013; Wang et al., 2015), abdominal pain (Song et al., 2014; Zhang et al., 2015a), poor memory (Shi et al., 2015; He et al., 2019), inflammatory conditions (He et al., 2010; Zhang et al., 2018; Qi et al., 2019) and cancer (Lin et al., 2013). Recently, the medicinal properties of A. oxyphylla and its pharmaceutical products have received considerable attention (Bian et al., 2013; Zhang et al., 2015b, 2018). The protective effects of A. oxyphylla extract in chronic kidney disease have been explored using metabolomics (Li et al., 2016). Various biomarkers, such as agmatine, CAMP and 7-methylguanine, are restored to control levels after treatment with A. oxyphylla extract, suggesting that it has protective effects. Wang et al. (2015) showed that the 95% ethanol extract and 90% ethanol-eluted fractions of A. oxyphylla have antidiarrheal activity. Some studies focusing on the effectiveness of A. oxyphylla extract ignored identifying the bioactive components. A. oxyphylla contains numerous potentially bioactive compounds, including flavonoids (Zhang et al., 2015b; Sun et al., 2016), tepenes (Lv et al., 2011; Xie et al., 2014; Hou et al., 2015; Zhao et al., 2015), alkaloids (Zhou et al., 2013) and diphenylheptanes (Bian et al., 2013).
Peripheral nerve injuries, such as those caused by accidental trauma, birth injury, ischemia or iatrogenic injury, often result in temporary or life-long neurological dysfunctions, which can be devastating and severely impact the patient’s quality of life (Cao et al., 2019; Han et al., 2019; Zhang et al., 2019). It is necessary to promote neural cell proliferation to restore the injured nerves in adults. A few studies have shown that protocatechuic acid modulates the MAPK (ERK1/2, JNK and p38)/PA (uPA, tPA)/MMP (MMP2, MMP9) regeneration and migration signaling pathways in Schwann cells (Ju et al., 2015a). Furthermore, protocatechuic acid promotes cell proliferation and survival via the insulin-like growth factor-I signaling pathway (Ju et al., 2015b). Li et al. (2016) reported that a novel lead compound, oxyphylla A, is a neuroprotective agent for Parkinson’s disease.
In the present study, we investigated the neuroprotective effects of various organic extracts of A. oxyphylla on hydrogen peroxide (H2O2)-induced apoptosis in cultured PC12 cells. Our aim is to lay the foundation for the purification and identification of the bioactive components in A. oxyphylla for use in future clinical application.
Materials and Methods
Materials
Ethanol, petroleum ether, ethyl acetate and n-butanol were of analytical grade (Guangdong Guanghua Sci-Tech Co., Ltd., Guangzhou, China). Undifferentiated rat PC12 cells were from Procell Life Science & Technology Co., Ltd., Wuhan, China.
Sample extraction and fractionation
The air-dried fruits of A. oxyphylla (10.0 kg) were extracted three times for 1.5 hours each by refluxing in 95% ethanol (1:10, w/v) to obtain 95% ethanol extracts 1–3 (95% EE-1–3). A portion of 95% EE-3 was concentrated by vacuum evaporation and dried by water bath evaporation, and then resuspended and dissolved in ultrapure water by ultrasonication for 30 minutes, resulting in a brown-yellow suspension. The suspension was extracted with petroleum ether several times until the upper layer of the extract was colorless to obtain the petroleum ether fraction (PF). The residual extract was extracted with ethyl acetate until the upper layer of the extract was colorless to obtain the ethyl acetate fraction (EF). The final raffinate was extracted with n-butanol until the upper layer of the extract was colorless to obtain the n-butanol fraction (BF). All six extracts were concentrated by rotary evaporation and dried in a vacuum at 45°C.
Cell culture
PC12 cells were cultured in RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco), 100 U/mL penicillin (Gibco) and 100 μg/mL streptomycin (Gibco) in a water-saturated atmosphere of 5% CO2 in 96-well plates at 37°C for 24 hours. Five wells each were treated with vehicle alone or different concentrations of the six A. oxyphylla extracts. The cultures were incubated for 24 hours. After culture for 48 hours, cell viability was analyzed with the cell counting kit-8 assay (Biosharp, Hefei, China), and morphology was observed on an inverted microscope (MZ16FA; Leica, Wetzlar, Hesse-Darmstadt, Germany).
Analysis of cell viability
The cell counting kit-8 assay was used to evaluate cell viability. PC12 cells were seeded into 96-well plates at a density of 4 × 104 cells/well (100 μL/well) for 24 hours, and then pretreated with vehicle alone or different concentrations of the various A. oxyphylla extracts for 24 hours. The supernatants were discarded, the wells were washed twice with phosphate-buffered saline, and 1 mL serum-free 1640 medium and 100 μL cell counting kit-8 solution were added, followed by incubation for 3 hours at 37°C. The optical density was determined at 450 nm. Five parallel experiments were done. The cell viability of the tested compounds was calculated using the following equation:
Cell viability (%) = (cell viability of drug group – cell viability of model group)/(cell viability of control group – cell viability of model group) × 100.
Cell viability was assessed with the cell counting kit-8 assay to identify the optimal H2O2 concentration (70 μM in this study). The cells were exposed to 70 μM H2O2 for 2 hours before supernatant removal to observe the protective effects of the different concentrations of the various A. oxyphylla extracts.
Statistical analysis
Each experiment was performed at least three times, and the results were expressed as the mean ± SD. The values followed a Gaussian distribution. Differences between means were compared by one-way analysis of variance followed by Dunnett’s post hoc test using SPSS 22.0 software (IBM, Armonk, NY, USA).
Results
Effects of the different extracts of A. oxyphylla on cell viability
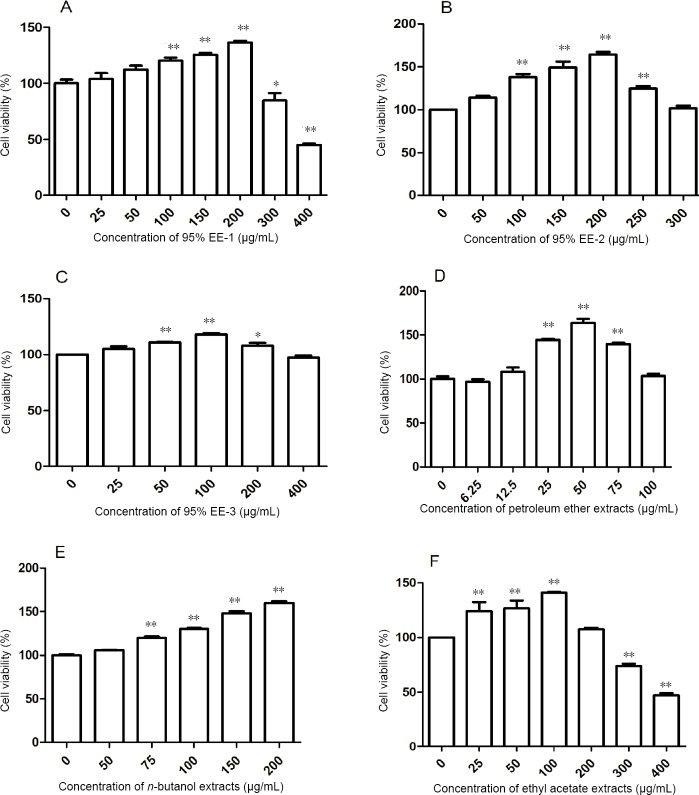
PC12 cells were treated with different concentrations of the various A. oxyphylla extracts for 24 hours. Each extract was tested using a different concentration range, based on pilot studies. The EF, 95% EE-1 and EE-3 extracts were tested at 0–400 μg/mL; 95% EE-2 was tested at 0–300 μg/mL; PF was tested at 0–100 μg/mL; BF was tested at 0–200 μg/mL.
As shown in Figure 1A, B and E, at concentrations of 100–200 μg/mL, 95% EE-1, 95% EE-2 and BF increased cell viability, without affecting cellular morphology. The 200 μg/mL concentration of these extracts increased cell viability to 136.4%, 164.3% and 159.9% of that in the control group, respectively. Figure 1C shows that 95% EE-3 slightly increased cell viability at concentrations of 50–200 μg/mL, with the 100 μg/mL concentration increasing cell viability to 118.0% of that in the control group. At concentrations of 25–75 μg/mL, PF markedly increased cell viability, with the 50 μg/mL concentration increasing viability to 163.8% of that in the control (Figure 1D). As shown in Figure 1F, at concentrations of 25–100 μg/mL, EF increased cell viability, with the 100 μg/mL concentration increasing it to 141.1% of that in the control.
Figure 1.
Effects of different concentrations of the organic extracts of Alpinia oxyphylla on the viability of PC12 cells.
(A–F) Effects of 95% ethanol extracts 1–3 (95% EE-1, 95% EE-2, 95% EE-3), petroleum ether extract, ethyl acetate extract and n-butanol extract on cultured PC12 cell viability. Cells (4 × 104 cells/mL) were treated with different extracts for 24 hours at 37°C after normal culture for 24 hours. *P < 0.05, **P < 0.01, vs. control group (0 μg/mL). Data are expressed as the mean ± SD (n = 5; one-way analysis of variance followed by Dunnett’s post hoc test). EE: Ethanol extract.
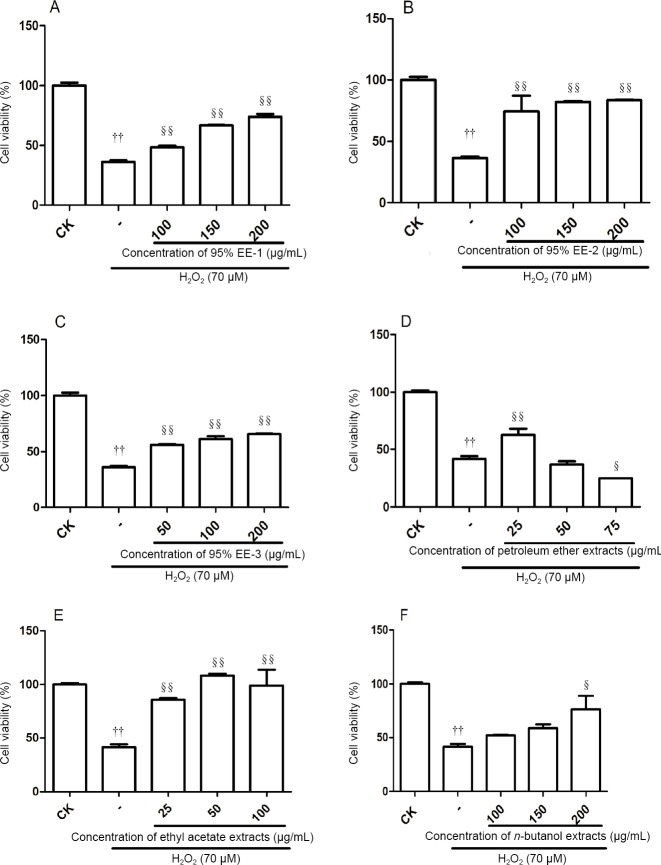
Effects of different extracts of A. oxyphylla on the viability of H2O2-exposed PC12 cells
The pilot study revealed that H2O2 at 50–90 μM induced cell death in a dose-dependent manner (Figure 2). To evaluate the cytoprotective effects of the A. oxyphylla extracts, PC12 cells were pretreated with the extracts and a moderate concentration (70 μM) of H2O2. Cell viability was assessed with the cell counting kit-8 assay. As shown in Figure 3A–C, incubation with 70 μM H2O2 for 2 hours resulted in a cell viability rate of 36.2% compared with the control. However, viability increased to 48.4%, 66.8% and 73.9% in cells pretreated with 95% EE-1 (100, 150 and 200 μg/mL, respectively) for 24 hours. Cell viability rate increased to 74.4%, 82.0% and 83.4% in cells pretreated with 95% EE-2 (100, 150 and 200 μg/mL, respectively) for 24 hours. Cell viability increased to 56.1%, 61.3% and 65.6% in cells pretreated with 95% EE-3 (50, 100 and 200 μg/mL, respectively) for 24 hours. As shown in Figure 3D and E, incubation with 70 μM H2O2 for 2 hours resulted in a cell viability rate of 41.6% compared with the control. Viability increased to 62.4% in cells pretreated with 25 μg/mL PF for 24 hours, but was only 24.9% in cells pretreated with 75 μg/mL PF (Figure 3D). Cell viability rate increased dramatically to 85.4%, 108.5% and 99.2% in cells pretreated with EF (25, 50 and 100 μg/mL, respectively) for 24 hours (Figure 3E). Moreover, the cell viability rate increased slightly to 52.1%, 58.5% and 76.3% in cells pretreated with BF (100, 150 and 200 μg/mL, respectively) for 24 hours (Figure 3F). Together, these results suggest that extracts of A. oxyphylla are neuroprotective against H2O2-induced oxidative stress. The neuroprotective effect of the ethyl acetate extract was the most robust.
Figure 2.
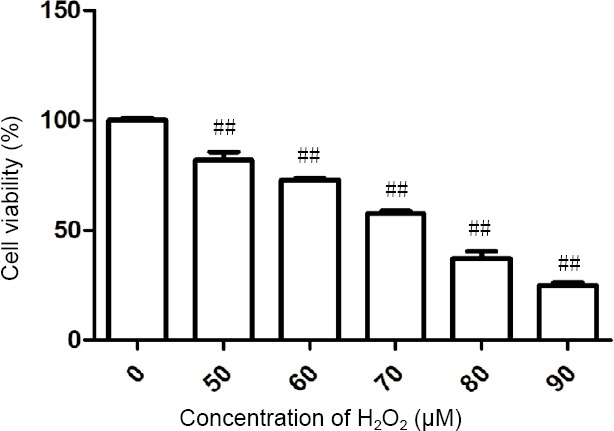
Effects of H2O2 on PC12 cell viability.
Cell counting kit-8 assay shows that H2O2 decreased cell viability in a concentration-dependent manner. ##P < 0.01, vs. control group (0 μM H2O2). Data are expressed as the mean ± SD (n = 5; one-way analysis of variance followed by Dunnett’s post hoc test). Cell viability (%) = (cell viability of drug group – cell viability of model group)/(cell viability of control group – cell viability of model group) × 100.
Figure 3.
Effects of the three sequential 95% ethanol extracts (EE-1–3), petroleum ether extract, ethyl acetate extract and n-butanol extract on H2O2-induced PC12 cell damage.
(A) EE-1; (B) EE-2; (C) EE-3. (D–F) Effects of petroleum ether, ethyl acetate and n-butanol extracts, respectively. PC12 cells (4 × 104 cells/mL) were treated with 70 μM H2O2 in the absence or presence of the extracts. Viability is calculated as the percentage of living cells in treated cultures compared with control cultures (CK). Data are expressed as the mean ± SD (n = 5; one-way analysis of variance followed by Dunnett’s post hoc test). ††P < 0.05, vs. CK; §P < 0.05, §§P < 0.01, vs. cells exposed to H2O2 alone. Cell viability (%) = (cell viability of drug group – cell viability of model group)/(cell viability of control group – cell viability of model group) × 100. EE: Ethanol extract.
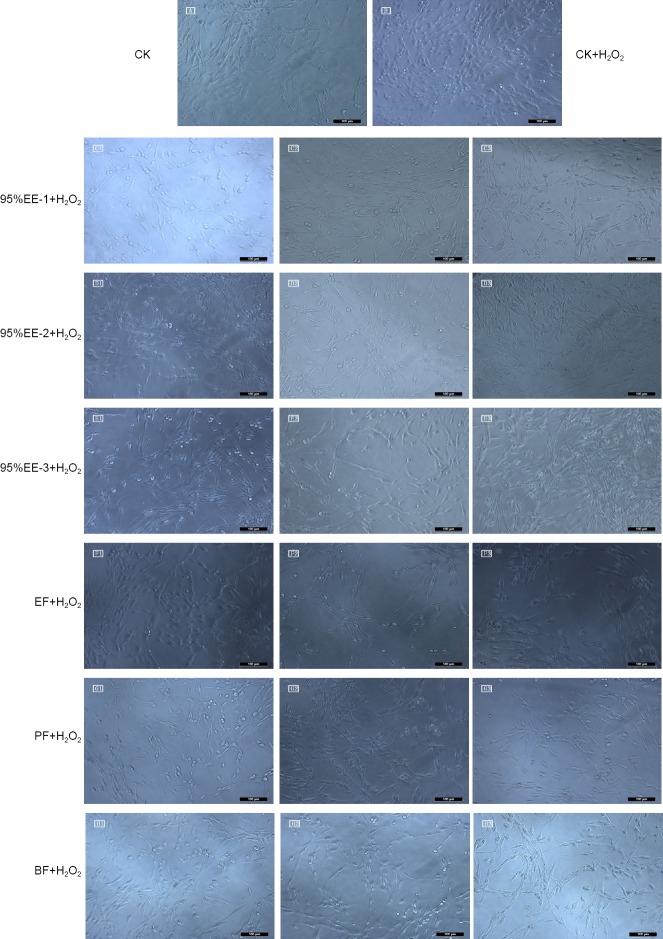
Under the optical microscope, PC12 cells were small and translucent immediately after passage in suspension. The cells were plump and formed a network after 48 hours (Figure 4A). After exposure to H2O2 for 2 hours, the cells in the model group were severely damaged (Figure 4B). The cells in the various extract treatment groups exhibited varying degrees of morphological changes (Figures 4C1, 2, 3–H1, 2, 3). The six extracts of A. oxyphylla effectively inhibited H2O2-induced cytotoxicity at the different concentrations. The cytoprotective effects of 95% EE-1, 95% EE-2 and 95% EE-3 increased with increasing concentration from 100–200 μg/mL. As shown in Figure 4C3, D3 and E3, morphology was good and axons grew well. The EF, PF and BF extracts had optimal effects on morphology at 25, 50 and 200 μg/mL, respectively. Thus, the EF extract had the best effect.
Figure 4.
Morphology of PC12 cells pretreated with different concentrations of the various organic extracts of Alpinia oxyphylla and exposed to H2O2.
Control group (CK) (A); model group (CK + H2O2) (B); 95% EE-1 + H2O2 (C1, 100 μg/mL; C2, 150 μg/mL; C3, 200 μg/mL); 95% EE-2 + H2O2 (D1, 100 μg/mL; D2, 150 μg/mL; D3, 200 μg/mL); 95% EE-3 + H2O2 (E1, 50 μg/mL; E2, 100 μg/mL; E3, 200 μg/mL); ethyl acetate fraction (EF) + H2O2 (F1, 25 μg/mL; F2, 50 μg/mL; F3, 75 μg/mL); petroleum ether fraction (PF) + H2O2 (G1, 25 μg/mL; G2, 50 μg/mL; G3, 100 μg/mL); n-butanol fraction (BF) + H2O2 (H1, 100 μg/mL; H2, 150 μg/mL; H3, 200 μg/mL). The cells were not stained. Original magnification: 20×. Scale bars: 100 μm.
Discussion
H2O2 induces apoptosis in many different cell types, including PC12 cells, by initiating mitochondrial dysfunction (Jang et al, 2001; Huang et al, 2015; Chen et al., 2019). H2O2, an inducer of neuronal injury, is extensively used to explore the neuroprotective potential of new pharmacotherapies (Porres-Martínez et al., 2016; Liu et al., 2018; Chu et al., 2019). Exploration of natural compounds that support neurite outgrowth against the toxicity of H2O2 is critical for treating neurodegenerative diseases.
The H2O2 concentration that induces 50% PC12 cell lethality is around 70 μM, when incubated for 2 hours. Other researchers have found it to be around 150 μM for 24 hours of exposure and 750 μM for 6 hours of exposure (Tusi et al., 2014; Cheong et al., 2016). The reasons for the discrepancies may include differences in experimental conditions, including reagent quality.
In recent years, an increasing number of studies have focused on natural substances isolated from Chinese herbal medicines, particularly as synthetic chemicals can have serious adverse effects (Wang et al., 2014; Hu and Sun, 2017; Liu et al., 2017; Ai et ai., 2019; Dai et al., 2019). Huang et al. (2015) found that forsythiaside provides protective effects against H2O2-induced death of neurons. Divate et al. (2017) demonstrated the neuroprotective effects of Xylaria nigripes mycelia extracts on H2O2-induced cytotoxicity in PC12 cells.
In this study, we found that A. oxyphylla extracts had no negative effect on the proliferation of PC12 cells, even positively impacting proliferation within a certain range. Among the extracts, EF was particularly effective, closely followed by EE-2. Consistent with previous reports, A. oxyphylla extracts possessed significant neuroprotective activity. Wong et al. (2004) reported that the ethanol extract of A. oxyphylla fructus improves spatial learning by affecting the serum levels of cytokines. Studies suggest that neuroprotection is achieved via multiple mechanisms, including decreased Bax/Bcl-2 ratio (Peng et al., 2012; Ip et al., 2016; Phatak et al., 2016; Rivero-Segura et ai., 2017; Lima et al., 2018), restored mitochondrial membrane potential (Chtourou et al., 2015; Chiang et al., 2016; Chen et al., 2018; Tian et al., 2018; Wang et al., 2019), and downregulated caspase-3 (Chen et al., 2016; Zhang et al., 2016; Zhou et al., 2016; Ding et al., 2017; Rivero-Segura et al., 2017; Lima et al., 2018). Furthermore, studies show that Xylaria nigripes mycelia extracts inhibit the release of lactate dehydrogenase and decrease DNA damage (Divate et al., 2017). Huang et al. (2015) found that forsythiaside decreased reactive oxygen species levels and lipid peroxidation. Thus, the neuroprotective effect of natural substances have some common mechanisms as well as some unique ones. Therefore, the neuroprotection afforded by A. oxyphylla extracts against H2O2-induced apoptosis in PC12 cells may involve unique mechanisms. A shortcoming of this study is that the underlying neuroprotective mechanisms were not investigated. In future studies, we will focus on identifying the bioactive components in the EF extract and on elucidating the cell and molecular pathways involved in neuroprotection.
In summary, we investigated the neuroprotective effects of six organic extracts of A. oxyphylla on apoptosis in PC12 cells induced by H2O2. The EF extract had the best neuroprotective effect. In a following study, we will aim to isolate the bioactive components in this extract and clarify their mechanisms of action. Despite the shortcomings of this study, our findings provide substantial insight into the neuroprotective action of A. oxyphylla and its therapeutic potential for the treatment of neurodegenerative disorders.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was financially supported by the National Natural Science Foundation of China, No. 81574038 (to ZZW); the Natural Science Foundation of Guangdong Province of China, No. 2017A030313842 (to LHD); the Science and Technology Foundation of Guangdong Province of China, No. 2017A050506007 (to YHL); the Technology Research Foundation of Basic Research Project of Shenzhen City of China, No. JCYJ20170412161254416 (to ZZW). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was financially supported by the National Natural Science Foundation of China, No. 81574038 (to ZZW); the Natural Science Foundation of Guangdong Province of China, No. 2017A030313842 (to LHD); the Science and Technology Foundation of Guangdong Province of China, No. 2017A050506007 (to YHL); the Technology Research Foundation of Basic Research Project of Shenzhen City of China, No. JCYJ20170412161254416 (to ZZW).
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Patel B, Wysong S, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Ai S, Tang W, Guo RL, Li JQ, Yang W, He ZG. Research progress on Chinese herbal medicine fermentation and profile of active substances derived. Zhongguo Zhong Yao Za Zhi. 2019;44:1110–1118. doi: 10.19540/j.cnki.cjcmm.20181227.002. [DOI] [PubMed] [Google Scholar]
- 2.Bian QY, Wang SY, Xu L J, Chan CO, Mok DK, Chen SB. Two new antio-xidant diarylheptanoids from the fruits of Alpinia oxyphylla. J Asian Nat Prod Res. 2013;15:1094–1099. doi: 10.1080/10286020.2013.816297. [DOI] [PubMed] [Google Scholar]
- 3.Cao LZ, Feng NB, Wang J, Chen JF. Mesenchymal stem cells: present status and prospect for its application in peripheral nerve injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23:5385–5391. [Google Scholar]
- 4.Chen L, Zhang B, Shan S, Zhao X. Neuroprotective effects of vitexin against isoflurane-induced neurotoxicity by targeting the TRPV1 and NR2B signaling pathways. Mol Med Rep. 2016;14:5607–5613. doi: 10.3892/mmr.2016.5948. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Wu X, Shen T, Wang X, Wang S, Wang J, Ren D. Protective effects of ethyl gallate on H2O2-induced mitochondrial dysfunction in PC12 cells. Metab Brain Dis. 2019;34:545–555. doi: 10.1007/s11011-019-0382-z. [DOI] [PubMed] [Google Scholar]
- 6.Cheong CU, Yeh CS, Hsieh YW, Lee YR, Lin MY, Chen CY, Lee CH. Protective effects of costunolide against hydrogen peroxide-induced injury in PC12 cells. Molecules. 2016;21:898. doi: 10.3390/molecules21070898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Chen J, Sun X, Shi X, Wang L, Huang L, Zhou W. Evaluation of the neuroprotective effect of EGCG: a potential mechanism of mitochondrial dysfunction and mitochondrial dynamics after subarachnoid hemorrhage. Food Funct. 2018;9:6349–6359. doi: 10.1039/c8fo01497c. [DOI] [PubMed] [Google Scholar]
- 8.Chiang MC, Cheng YC, Chen SJ, Yen CH, Huang RN. Metformin activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced mitochondrial dysfunction. Exp Cell Res. 2016;347:322–331. doi: 10.1016/j.yexcr.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Chtourou Y, Slima AB, Gdoura R, Fetoui H. Naringenin mitigates iron-induced anxiety-like behavioral impairment, mitochondrial dysfunctions, ectonucleotidases and acetylcholinesterase alteration activities in rat hippocampus. Neurochem Res. 2015;40:1563–1575. doi: 10.1007/s11064-015-1627-9. [DOI] [PubMed] [Google Scholar]
- 10.Chu Q, Yu L, Zheng Z, Chen M, Hua Z, Hang M, Li Y, Li X, Liu Y, Yang Y, Zheng X. Apios americana Medik flowers extract protects PC12, cells against H2O2 induced neurotoxicity via regulating autophagy? Food Chem Toxicol. 2019;124:231–238. doi: 10.1016/j.fct.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Dai ZR, Ning J, Sun GB, Wang P, Zhang F, Ma HY, Zou LW, Hou J, Wu JJ, Ge GB, Sun XB, Yang L. Cytochrome P450 3A enzymes are key contributors for hepatic metabolism of bufotalin, a natural constitute in Chinese Medicine Chansu. Front Pharmacol. 2019;10:52. doi: 10.3389/fphar.2019.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding H, Fu YJ, Zheng LR, Chen J, Shi XQ. Neuroprotection mechanism of lidocaine in rabbits with early brain injury resulted from subarachnoid hemorrhage. Sichuan Da Xue Xue Bao Yi Xue Ban. 2017;48:230–233. [PubMed] [Google Scholar]
- 13.Divate RD, Wang PM, Wang CC, Chou ST, Chang CT, Chung YC. Protective effect of medicinal fungus Xylaria nigripes mycelia extracts against hydrogen peroxide-induced apoptosis in PC12 cells. Int J Immunopathol Pharmacol. 2017;30:105–112. doi: 10.1177/0394632017695280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han GH, Peng J, Liu P, Ding X, Wei S, Lu S, Wang Y. Therapeutic strategies for peripheral nerve injury: decellularized nerve conduits and Schwann cell transplantation. Neural Regen Res. 2019;14:1343–1351. doi: 10.4103/1673-5374.253511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He B, Xu F, Yan T, Xiao F, Wu B, Wang Y, Bi K, Jia Y. Tectochrysin from Alpinia Oxyphylla Miq, alleviates Aβ1-42 induced learning and memory impairments in mice. Eur J Pharmacol. 2019;842:365–372. doi: 10.1016/j.ejphar.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 16.He ZH, Ge W, Yue GG, Lau CBS, He MF, But PPH. Anti-angiogenic effects of the fruit of Alpinia oxyphylla. J Ethnopharmacol. 2010;132:443–449. doi: 10.1016/j.jep.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 17.Hou L, Ding G, Guo B, Huang W, Zhang X, Sun Z, Shi X. New sesquiterpenoids and a diterpenoid from Alpinia oxyphylla. Molecules. 2015;20:1551–1559. doi: 10.3390/molecules20011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu RF, Sun XB. Design of new traditional Chinese medicine herbal formulae for treatment of type 2 diabetes mellitus based on network pharmacology. Chin J Nat Med. 2017;15:436–441. doi: 10.1016/S1875-5364(17)30065-1. [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Lin Y, Su H, Ye D. Forsythiaside protects against hydrogen peroxide-induced oxidative stress and apoptosis in PC12 Cell. Neurochem Res. 2015;40:27–35. doi: 10.1007/s11064-014-1461-5. [DOI] [PubMed] [Google Scholar]
- 20.Ip FC, Zhao YM, Chan KW, Cheng EY, Tong EP, Chandrashekar O, Fu GM, Zhao ZZ, Ip NY. Neuroprotective effect of a novel Chinese herbal decoction on cultured neurons and cerebral ischemic rats. BMC Complement Altern Med. 2016;16:437. doi: 10.1186/s12906-016-1417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang J H, Surh Y J. Protective effects of resveratrol on hydrogen peroxide-induced apoptosis in rat pheochromocytoma (PC12) cells. Mutat Res. 2001;496:181–190. doi: 10.1016/s1383-5718(01)00233-9. [DOI] [PubMed] [Google Scholar]
- 22.Ju DT, Liao HE, Shibu MA, Ho TJ, Padma VV, Tsai FJ, Chung LC, Day CH, Lin CC, Huang CY. Nerve regeneration potential of protocatechuic acid in rsc96 Schwann cells by induction of cellular proliferation and migration through IGFR-PI3K-Akt signaling. Chin J Physiol. 2015a;58:412–419. doi: 10.4077/CJP.2015.BAD340. [DOI] [PubMed] [Google Scholar]
- 23.Ju DT, Kuo WW, Ho TJ, Paul CR, Kuo CH, Viswanadha VP, Lin CC, Chen YS, Chang YM, Huang CY. Protocatechuic acid from Alpinia oxyphylla induces Schwann cell migration via ERK1/2, JNK and p38 activation. Am J Chin Med. 2015b;43:653–665. doi: 10.1142/S0192415X15500408. [DOI] [PubMed] [Google Scholar]
- 24.Li G, Zhang Z, Quan Q, Jiang R, Szeto SS, Yuan S, Wong WT, Lam HH, Lee SM, Chu IK. Discovery, synthesis, and functional characterization of a novel neuroprotective natural product from the fruit of Alpinia oxyphylla for use in Parkinson’s disease through LC/MS-based multivariate data analysis-guided fractionation. J Proteome Res. 2016;15:2595–2606. doi: 10.1021/acs.jproteome.6b00152. [DOI] [PubMed] [Google Scholar]
- 25.Li YH, Tan YF, Cai HD, Zhang JQ. Metabonomic study of the fruits of Alpinia oxyphylla as an effective treatment for chronic renal injury in rats. J Pharm Biomed Anal. 2016;124:236–245. doi: 10.1016/j.jpba.2016.02.035. [DOI] [PubMed] [Google Scholar]
- 26.Lima LKF, Pereira SKS, Junior RDSS, Santos FPDS, Nascimento AS, Feitosa CM, Figuerêdo JS, Cavalcante ADN, Araújo ECDC, Rai M. A brief review on the neuroprotective mechanisms of vitexin. Biomed Res Int. 2018;2018:4785089. doi: 10.1155/2018/4785089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin RJ, Yen CM, Chou TH, Chiang FY, Wang GH, Tseng YP, Wang L, Huang TW, Wang HC, Chan LP, Ding HY, Liang CH. Antioxidant, anti-adipocyte differentiation, antitumor activity and anthelmintic activities against anisakis simplex and hymenolepis nana of yakuchinone A from Alpinia oxyphylla. BMC Complement Altern Med. 2013;13:237. doi: 10.1186/1472-6882-13-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Zhang L, Liu D, Li B, Zhang M. Neuroprotective effects of extracts from the radix curcuma aromatica on H2O2-induced damage in PC12 cells. Comb Chem High Throughput Screen. 2018;21:571–582. doi: 10.2174/1386207321666181005121457. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q, Zhu L, Cheng C, Hu YY, Feng Q. Natural active compounds from plant food and Chinese herbal medicine for nonalcoholic fatty liver disease. Curr Pharm Des. 2017;23:5136–5162. doi: 10.2174/1381612823666170918120643. [DOI] [PubMed] [Google Scholar]
- 30.Lv XQ, Luo JG, Wang XB, Wang JS, Luo J, Kong LYi. Four new sesquiterpenoids from the fruits of Alpinia oxyphylla. Chem Inform. 2011;59:402–406. doi: 10.1248/cpb.59.402. [DOI] [PubMed] [Google Scholar]
- 31.Peng Y, Jiang B, Wu H, Dai R, Tan L. Effects of genistein on neuronal apoptosis, and expression of Bcl-2 and Bax proteins in the hippocampus of ovariectomized rats. Neural Regen Res. 2012;7:2874–2881. doi: 10.3969/j.issn.1673-5374.2012.36.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phatak NR, Stankowska DL, Krishnamoorthy RR. Bcl-2, Bcl-xL, and p-AKT are involved in neuroprotective effects of transcription factor Brn3b in an ocular hypertension rat model of glaucoma. Mol Vis. 2016;22:1048–1061. [PMC free article] [PubMed] [Google Scholar]
- 33.Porres-Martínez M, González-Burgos E, Carretero ME, Gómez-Serranillos MP. In vitro neuroprotective potential of the monoterpenes α-pinene and 1, 8-cineole against H2O2-induced oxidative stress in PC12 cells. Z Naturforsch C, 2016;71:191–199. doi: 10.1515/znc-2014-4135. [DOI] [PubMed] [Google Scholar]
- 34.Qi Y, Cheng X, Jing H, Yan T, Xiao F, Wu B, Bi K, Jia Y. Effect of Alpinia oxyphylla-Schisandra chinensis herb pair on inflammation and apoptosis in Alzheimer’s disease mice model. J Ethnopharmacol. 2019;237:28–38. doi: 10.1016/j.jep.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 35.Rivero-Segura NA, Flores-Soto E, García de la Cadena S, Coronado-Mares I, Gomez-Verjan JC, Ferreira DG, Cabrera-Reyes EA, Lopes LV, Massieu L, Cerbón M. Prolactin-induced neuroprotection against glutamate excitotoxicity is mediated by the reduction of [Ca2+]i overload and NF-κB activation. PLoS One. 2017;12:e0176910. doi: 10.1371/journal.pone.0176910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi SH, Zhao X, Liu AJ, B Liu, Li H, Wu B, Bi KS, Jia Y. Protective effect of n-butanol extract from Alpinia oxyphylla on learning and memory impairments. Physiol Behav. 2015;139:13–20. doi: 10.1016/j.physbeh.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Song W, Li Y, Wang J, Li Z, Zhang J. Characterization of nucleobases and nucleosides in the fruit of Alpinia oxyphylla collected from different cultivation regions. Drug Test Anal. 2014;6:239–245. doi: 10.1002/dta.1462. [DOI] [PubMed] [Google Scholar]
- 38.Sun Z, Kong X, Zuo L, Kang J, Hou L, Zhang X. Rapid extraction and determination of 25 bioactive constituents in Alpinia oxyphylla using microwave extraction with ultra high performance liquid chromatography with tandem mass spectrometry. J Sep Sci. 2016;39:603–610. doi: 10.1002/jssc.201501056. [DOI] [PubMed] [Google Scholar]
- 39.Tian T, Zeng J, Zhao G, Zhao W, Gao S, Liu L. Neuroprotective effects of orientin on oxygen-glucose deprivation/reperfusion-induced cell injury in primary culture of rat cortical neurons. Exp Biol Med (Maywood) 2018;243:78–86. doi: 10.1177/1535370217737983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tusi S K, Khodagholi F. Salvia macilenta exhibits antiglycating activity and protects PC12 cells against H2O2-induced apoptosis. Cytotechnology. 2014;66:169–179. doi: 10.1007/s10616-013-9550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Zhao Y, Zhang JQ, Huang XX, Wang YF, Xu XT, Zheng B, Zhou X, Tian HJ, Liu L, Mei QB. Antidiarrheal effect of Alpinia oxyphylla Miq. (Zingiberaceae) in experimental mice and its possible mechanism of action. J Ethnopharmacol. 2015;168:182–190. doi: 10.1016/j.jep.2015.03.066. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Liu Y, Li Y, Liu B, Wu P, Xu S, Shi H. Protective effects of astaxanthin on subarachnoid hemorrhage-induced early brain injury: Reduction of cerebral vasospasm and improvement of neuron survival and mitochondrial function. Acta Histochem. 2019;121:56–63. doi: 10.1016/j.acthis.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Wang YF, Que HF, Wang YJ, Cui XJ. Chinese herbal medicines for treating skin and soft-tissue infections. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD010619.pub2. doi: 101002/14651858CD010619pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong KK, Wan CC, Shaw PC. P4-427 Ethanol extract of Alpinia oxyphylla fructus, shows inhibition of tau protein phosphorylation in cell culture. Neurobiol Aging. 2004;25:1387–1394. [Google Scholar]
- 45.Xie BB, Hou L, Guo BL, Huang WH, Yu JG. The compounds from n-butanol fraction of Alpinia oxyphylla. Yao Xue Xue Bao. 2014;49:1569–1573. [PubMed] [Google Scholar]
- 46.Zhang J, Wang S, Li Y, Xu P, Chen F, Tan Y, Duan J. Anti-diarrheal constituents of Alpinia oxyphylla. Fitoterapia. 2013;89:149–156. doi: 10.1016/j.fitote.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Zhang LQ, Xu CG, Li ZY, Yao F, Zha XW, Qi L, Jing YH. Low-frequency pulsed electromagnetic field promotes neurologic function recovery after delayed repair of perioheral nerve injury in rats. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23:1711–1716. [Google Scholar]
- 48.Zhang Q, Cui C, Chen CQ, Hu XL, Liu YH, Fan YH, Meng WH, Zhao QC. Anti-proliferative and pro-apoptotic activities of Alpinia oxyphylla on HepG2 cells through ROS-mediated signaling pathway. J Ethnopharmacol. 2015a;169:99–108. doi: 10.1016/j.jep.2015.03.073. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Q, Zheng Y, Hu X, Hu X, Lv W, Lv D, Chen J, Wu M, Song Q, Shentu J. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Alpinia oxyphylla Miquel: a review. J Ethnopharmacol. 2018;224:149–168. doi: 10.1016/j.jep.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Xue X, Xian L, Guo Z, Ito Y, Sun W. Potential neuroprotection of protodioscin against cerebral ischemia-reperfusion injury in rats through intervening inflammation and apoptosis. Steroids. 2016;113:52–63. doi: 10.1016/j.steroids.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z, Li G, Szeto SS, Chong CM, Quan Q, Huang C, Cui W, Guo BJ, Wang YQ, Han YF, Siu KWM, Lee SMY, Chu IK. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson’s disease. Free Radical Biol Med. 2015b;84:331–343. doi: 10.1016/j.freeradbiomed.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 52.Zhao H, Ji Z H, Liu C, Yu XY. Neuroprotective mechanisms of 9-hydroxy epinootkatol against glutamate-induced neuronal apoptosis in primary neuron culture. J Mol Neurosci. 2015;56:808–814. doi: 10.1007/s12031-015-0511-z. [DOI] [PubMed] [Google Scholar]
- 53.Zhou H, Yang Y, Zhang J, Peng T, Zhao L, Xu L, Ding Z. Alkaloids from an endophytic streptomyces sp. YIM66017. Nat Prod Commun. 2013;8:1393–1396. [PubMed] [Google Scholar]
- 54.Zhou JM, Gu SS, Mei WH, Zhou J, Wang ZZ, Xiao W. Ginkgolides and bilobalide protect BV2 microglia cells against OGD/reoxygenation injury by inhibiting TLR2/4 signaling pathways. Cell Stress Chaperones. 2016;21:1037–1053. doi: 10.1007/s12192-016-0728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]