Abstract
Extracellular matrix (ECM) influences cell differentiation through its structural and biochemical properties. In nervous system, neuronal behavior is influenced by these ECMs structures which are present in a meshwork, fibrous, or tubular forms encompassing specific molecular compositions. In addition to contact guidance, ECM composition and structures also exert its effect on neuronal differentiation. This short report reviewed the native ECM structure and composition in central nervous system and peripheral nervous system, and their impact on neural regeneration and neuronal differentiation. Using topographies, stem cells have been differentiated to neurons. Further, focussing on engineered biomimicking topographies, we highlighted the role of anisotropic topographies in stem cell differentiation to neurons and its recent temporal application for efficient neuronal differentiation.
Keywords: biomimetic platforms, biophysical cues, contact guidance, extracellular matrix, neuronal development, neural regeneration, neural stem cell niche, neuronal differentiation, neuronal maturation, stem cell, topography
Introduction
Nervous system extracellular matrix (ECM) plays a major role in neural development and regeneration processes such as neocortex folding, stem cell niche maintenance, peripheral nerve regeneration, neural cell migration, axonal growth, and many more (Moore et al., 2002; Colognato et al., 2005; Gonzalez-Perez et al., 2013; Gattazzo et al., 2014; Herrera-Perez et al., 2015; Long et al., 2018). ECM mediates these processes via cell-ECM interactions which provides the cells with a wealth of signals such as growth factors, bioactive molecules, and biophysical cues in a spatiotemporal manner (Mecham, 2001; Yue, 2014). The physical properties of ECM that are of prime importance for its function include elasticity, matrix pore size, and the ECM structure or topography. Biophysical cues play a major role in neural development and regeneration processes such as neurogenesis, nerve repair, neuronal cell migration, and axonal growth (Moore and Sheetz, 2011; Franze, 2013; Franze et al., 2013; Wieringa et al., 2018). Of these different biophysical properties, matrix elasticity and topographies are well studied and have been shown to drastically affect neural cell behavior. ECM structure and topography influences many different neural cell processes including neuron migration, neuronal stem cell fate regulation, and nerve regeneration (Hoffman-Kim et al., 2010; Ankam et al., 2013b; Marcus et al., 2017; Simitzi et al., 2017).
Neural differentiation processes have wide clinical and industrial applications. Stem cell-derived neurons have been widely used for the development of cell-based therapies for the treatment of neurological diseases, such as Parkinson’s disease, Huntington’s disease and multiple sclerosis, and neurological damage such as spinal cord injury or stroke (Lindvall et al., 2004; Martino and Pluchino, 2006; Tomita et al., 2013; Trounson and McDonald, 2015; Shi et al., 2016). In addition, these cells have also been used for fundamental neurological studies, drug-screening platforms, neurodevelopmental and neurodegenerative disease modelling (Avior et al., 2016; Shi et al., 2016; Dutta et al., 2017). Conventional methods of neural differentiation rely primarily on the use of biochemical factors to regulate the differentiation process. However, the use of biochemical factors alone has proven to result in long differentiation periods and provide minimal control over lineage-commitment (Schuldiner et al., 2001; Chambers et al., 2009; Hu and Zhang, 2009). Hence, appropriate integration of ECM biophysical cues in stem cell related regenerative therapies is much desired. In this review, we focused on understanding the composition of native ECM in central nervous system (CNS) and peripheral nervous system (PNS) and the resulting native ECM architecture and topography. We briefly discuss the role of native ECM composition and topographical cues in neural regeneration and neuronal progenitor differentiation. In addition, we also provide an overview of the parameters that affect the topography efficiency in synthetic platforms used to enhance neuronal differentiation of stem cells, including pattern isotropy, feature dimensions, and temporal and spatial applications. Lastly, we concluded by emphasising the need for further understanding of the native structures and topography dynamics for better development of biomimetic platforms to optimize functional neuronal differentiation. For the present review, we searched the literature using keywords such as “Surface topography”, “Neuronal differentiation and surface topography”, “Nervous system extracellular matrix”, “Central nervous system”, “Peripheral nervous system”, “Stem cells and surface topography”, “Embryonic stem cells and neuronal differentiation”, “Neural stem cells”, “Induced pluripotent stem cells and neuronal differentiation”, “Nervous system stem cell niche”, “Mechanotransduction”, and “Topography dynamics” on PubMed and Google scholar. In addition, we also used modifications of the above main keywords to thoroughly search the literature. The major inclusion criteria preferred the literature comprising native ECM or surface topography.
Composition and Topographies of the Nervous System Extracellular Matrix
Cells secrete and deposit distinct molecules in their parenchyma resulting in the formation of native ECM structures (Frantz et al., 2010; Lau et al., 2013). Throughout human body, the ECM in different tissues are present in various structural and molecular compositions (Frantz et al., 2010). The nervous system ECM has specialized structures encompassing molecular compositions secreted or deposited by surrounding neural cells in a spatiotemporal manner (Barros et al., 2011; Lau et al., 2013). The deposited ECM proteins form functional 3-dimension (3D) architectures to support cellular functions. In addition to cell membrane surface structures (Blumenthal et al., 2014), ECM architectures and topographies also have the potential to affect neural cell processes such as cell migration, differentiation, cellular function, and morphology (Yim et al., 2007; Hoffman-Kim et al., 2010; Barros et al., 2011; Ankam et al., 2013b; Marcus et al., 2017; Sathe et al., 2017; Tan et al., 2018). Mutations of ECM molecules in nervous system have provided hints towards the role of molecular composition in forming native ECM surface topographies. For example, mutations in laminin γ1 causes the surface fragmentation of the embryonic plial basement membrane directly affecting radial glia cell attachment and dysplasia of cortical plate neurons (Halfter et al., 2002). Similarly, in perlecan mutants (one of the members of heparan sulphate proteoglycans), basement membrane shows partial disruption leading to inadequate neuronal composition of the cerebral cortex (Haubst et al., 2006). CNS and PNS cells exhibit different functional characteristics. ECM topography and molecular composition also differs in CNS and PNS which might influence these cell characteristics. Hence, in this section, we discuss the various molecular composition of CNS and PNS ECM, and the resulting ECM architectures and topographies present in these systems.
Native neural ECM structure and composition
During late embryonic and early post-natal stages, brain ECM is present in a “juvenile” state providing appropriate cues for blood-brain barrier development, neural stem cell (NSC) differentiation, proliferation, and migration (Zimmermann and Dours-Zimmermann, 2008; Barros et al., 2011; Lau et al., 2013; Barnes et al., 2017). It is mainly composed of versicans, neurocan, laminins, tenascin-C, hyaluronan, and cartilage link proteins (HAPLN1), which are deposited by NSCs, glia cells, and neurons in their microenvironment in a spatiotemporal manner (Colognato and Yurchenco, 2000; Rauch, 2004; Zimmermann and Dours-Zimmermann, 2008; Barros et al., 2011; Lau et al., 2013; Mouw et al., 2014; Barnes et al., 2017). In this state of ECM, hyaluronan acts as a backbone around which versicans, neurocan, laminins, tenascin-C, and HAPLN1 aggregate and interact with each other (Colognato and Yurchenco, 2000; Mouw et al., 2014).
After 2–3 weeks of post-natal stage in mouse embryonic CNS, juvenile ECM remodels into a more mature form through changes in gene expression patterns as well as proteolysis by matrix metalloproteinases (matrix metalloproteinase-2 and matrix metalloproteinase-9) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) enzymes (ADAMTS1 and ADAMTS4). Subsequently, the juvenile ECM will be replaced by other variant ECM molecules of the same family (Bandtlow and Zimmermann, 2000; Rauch, 2004; Zimmermann and Dours-Zimmermann, 2008). For example, versican V1 is replaced by another splice variant versican V2; tenascin-C is replaced by tenascin-R and tenascin-N; and HAPLN1 is replaced by brain link proteins (Rauch, 2004; Zimmermann and Dours-Zimmermann, 2008). This remodelling process is termed as the maturation process, where neural migration is restricted and stable neuronal connections are formed, leading to improvement in neural cell conductance and neuroprotective functions (Zimmermann and Dours-Zimmermann, 2008). Scanning electron microscopy images of decellularized adult rat brain shows microlobular structure arranged in a non-specific orientation (Baiguera et al., 2014). Similar to rat brain, native porcine brain also has similar micro-architecture (DeQuach et al., 2011). However, dimensions and spacings between these structures are not well elucidated. This matured ECM can be majorly categorized into three different types of structure, namely basement membrane, perineuronal nets, and neural interstitial matrix (Figure 1A). Basement membrane plays a major role in formation and maintenance of blood-brain barrier; acting as a boundary between endothelial cells and CNS parenchymal tissue. It is composed of various ECM proteins such as collagen IV, laminin-nidogen complexes (Laminin 111, Laminin 211, Laminin 411, Laminin 511, Nidogen-1, and Nidogen-2), fibronectin, dystroglycan, agrin, and perlecan (Lau et al., 2013; Thomsen et al., 2017). Using transmission electron microscopy, basement membrane in mice cerebral cortex is measured to be 40–60 μm in thickness and present as a continuous layer. In addition, high resolution transmission electron microscopy cross-sectional images of this basement membrane show convex-shape nanostructures (Dong et al., 2002). However, these convex shapes are yet to be revealed if they are present as individual domes, or if the convex shapes are the cross-sectional view of continuous structures such as folds, ridges or fibers. Fractone is also another type of basement membrane possessing similar composition as vascular basement membrane. However, the location and structure of this matrix form is different from vascular basement membrane. Fractones are primarly located in ventricles and spinal canal, and present as an isolated branch shape structure ranging between 1–6 µm in size (Mercier et al., 2003; Kerever et al., 2007; Mercier, 2016).
Figure 1.
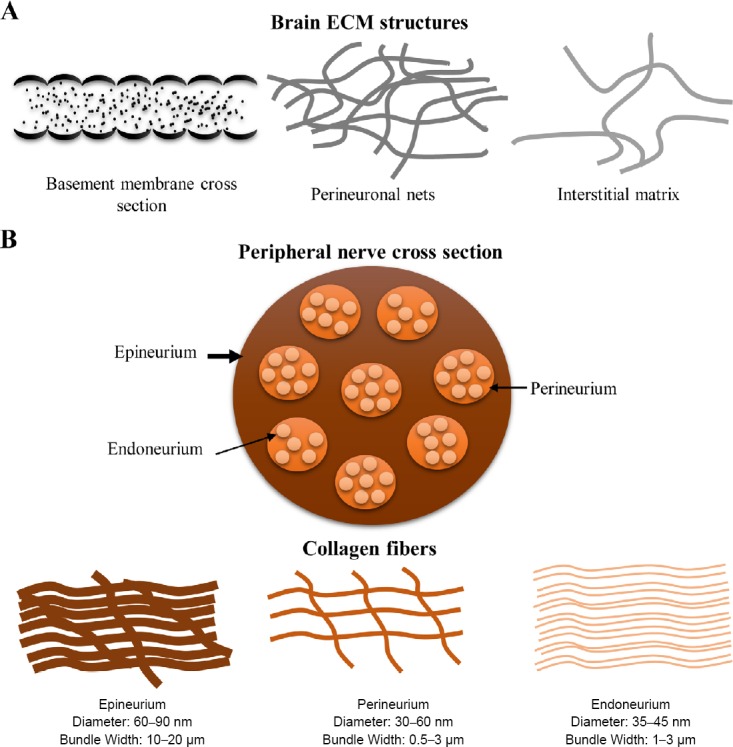
Nervous system components architecture.
(A) Schematic diagram of brain extracellular matrix (ECM) components and their architecture. (B) Schematic diagram of peripheral nerve cross-section and the collagen fibers.
In contrast to basement membrane, perineuronal nets are predominantly composed of proteoglycans, tenascin-R, hyaluronan, and link proteins in the brain parenchyma (Barros et al., 2011; Lau et al., 2013). The extracellular proteoglycans interacts with the linear hyaluronic acid molecules deposited on the plasma membrane that is further stabilized by tenascin R and link proteins, forming a dense 3D meshwork surrounding neurons (Kwok et al., 2011; Arranz et al., 2014). Perineuronal net appear to be in micro-fibrous form and the fibrous structure does not have a particular orientation (Giamanco et al., 2010; Kwok et al., 2011; Arranz et al., 2014; de Winter et al., 2016). In contrast to perineuronal net meshwork structure, neural interstitial matrix is dispersed in the parenchyma, majorly comprising a dense networks of proteoglycans, hyaluronan, tenascins, and link proteins, as well as relatively small amounts of collagen XV, elastin, laminin 511, and fibronectin forming fibrous structures (Seppänen et al., 2007; Barros et al., 2011; Lau et al., 2013; Happel and Frischknecht, 2016; Omar et al., 2017). Even though this matrix is present in the brain in a ubiquitous manner and it may impart essential 3D biochemical and biophysical cues. In comparison to other forms, this matrix type is relatively less studied in terms of both structural and functional properties (Lau et al., 2013).
Similar to CNS, PNS also consists of basement membrane composed of collagens (Collagen types I, III, IV, and XV), laminins (Laminin 211, Laminin 411, and Laminin 511), nidogen-1, heparin sulfate proteoglycans, and fibronectin in a tubular form, which surrounds Schwann cells along the axon in a continuous manner (Chernousov and Carey, 2000; Barros et al., 2011; Catala and Kubis, 2013; Gonzalez-Perez et al., 2013; Chen et al., 2015). Outside of these individual units, fascicle interior between axon-Schwann cells units is majorly composed of collagen type I and III fibrils oriented in a longitudinal direction (Shellswell et al., 1979; Montes et al., 1984). This interior layer of the nerve is known as endoneurium (Figure 1B). In rat sciatic nerves, the individual endoneurium collagen fibrils have an average diameter of 41.31 ± 4.2 nm forming collagen bundles of 1–3 µm thickness (Junqueira et al., 1979; Ushiki and Ide, 1990). This layer is further protected by another layer composed of perineurial cells, basement membrane, and collagen type I and III fibrils (average 30–60 nm collagen fibril diameter, about 0.5–3 µm thick collagen bundles in rat sciatic nerve), forming a perineurium layer in a tubular shape structures with collagen meshwork (Junqueira et al., 1979; Ushiki and Ide, 1990). In humans, the basement membrane of sural nerve perineurium has been shown to be 405.86 ± 4.2 nm thick (Johnson et al., 1981; Hill and Williams, 2004). In addition, multiple bundles of perineurium layers are surrounded by epineurium consisting longitudinally oriented collagen type I fibrils. These epineurium collagen fibrils have an average 77.33 ± 11 nm diameter in rat sciatic nerve and collectively, these epineurium collagen bundles are 10–20 µm thick (Junqueira et al., 1979; Ushiki and Ide, 1990; Catala and Kubis, 2013; Gonzalez-Perez et al., 2013; Sridharan et al., 2015). Junqueira et al. (1979) also observed that the collagen fibril diameter in human femoral nerve shared similar dimensions of endoneurium and epineurium in rat sciatic nerve. However, the collagen bundle thickness in human peripheral nerves is yet to be determined. Molecular composition, structure, dimensions, and orientation of PNS ECM in different layers are very well studied; however, the effect of fiber dimensionality of the basement membrane on the directly contacting neurites and Schwann cells is not well known. Taken together, there are many important questions still need to be addressed regarding the structure and dimensions of the topographies in both CNS and PNS ECM at different ECM development or remodelling stages. Indeed, the functions of these ECM topographies in the in vivo system remains an important question to explore such as their role in radial glia cell attachment, neuron orientation, neuron migration, and neuronal differentiation. Before discussing the potential of topographies for neural regeneration, we will briefly discuss the role of native ECM structures and composition in PNS regeneration and CNS neuronal differentiation, respectively.
Native ECM impact on neural regeneration and neuronal stem cell differentiation
Weiss coined the term “selective contact guidance” by postulating a relationship between surface structures and directed movement of cell through cell attachment (Weiss, 1947). Peripheral nerve regeneration provides an ideal platform to understand the relationship between surface structure and contact guidance. During PNS regeneration, PNS basement membrane provides the structural support to the surrounding cells. Schwann cells attach to the longitudinally aligned basement membrane structures that guide the neurites from proximal to distal stump, resulting in reattachment of proximal neurons to their appropriate distal targets (Cattin and Lloyd, 2016). Using repetitive freeze-thaw cycles (Ide et al., 1983), the decellularized nerve segments enabled regenerating neurites attached and migrated along the fiber orientation, when these decellularized nerve segments were grafted to sciatic nerve. With the advent of optimized decellularization processes, various studies also observed similar regeneration efficacy when the decellularized ECM grafts were implanted at injured peripheral nerves (Adarsh, 1988; Sondell et al., 1998; Hudson et al., 2004). However, the regenerative capability in peripheral nerve is drastically affected by the injury gap length, which is the distance between proximal and distal stump, and the regeneration rate. If the gap length is increased beyond a critical gap length (~15 mm in rat, ~40 mm in human (Kaplan et al., 2015)), then this can lead to “chronically denervated” conditions where Schwann cells and basal lamina in distal stump deteriorate due to the long-term absence of proximal neurite contact (Höke, 2006).
In comparison to peripheral nerves, CNS shows different responses upon injuries and damages. For example, in spinal cord injury, adult spinal cord tissues do not possess similar regenerative ability due to formation of lesion and glial scar boundary. This lesion mostly consists of pericytes, perivascular-derived fibroblasts, macrophages, and ependymal cells along with inhibitory ECM molecules which restricts the neuronal regeneration (Busch and Silver, 2007; Adams and Gallo, 2018). While the surrounding glial boundary broadly comprised of reactive astrocytes, NG2 glia and microglia also participate in isolating the lesioned environment from their surroundings to prevent secondary injury, and they also secrete inhibitory ECM molecules (Busch and Silver, 2007; Adams and Gallo, 2018).
Even though CNS does not show similar regenerative capacity as PNS, two major NSCs populations have been identified in the subventricular zone of the lateral ventricles and subgranular zone of the dentate gyrus in the hippocampus, and they are playing major roles in tissue homeostasis. The subventricular zone stem cells differentiate into neuroblasts that migrate to the olfactory bulb forming olfactory bulb interneurons and corpus callosum oligodendrocytes. Meanwhile, the NSCs in subgranular zone region differentiate into immature neurons before differentiating into dentate granule neurons in granule cell layer (Kazanis and ffrench-Constant, 2011; Gattazzo et al., 2014; Bond et al., 2015; Faissner and Reinhard, 2015; Gonçalves et al., 2016; de Miranda et al., 2017; Tian et al., 2018). These NSCs are closely associated with fractone and vascular basement membrane structures in subventricular zone or subgranular zone, to maintain their stemness through interacting with their surrounding microenvironment (Kazanis and ffrench-Constant, 2011; Gattazzo et al., 2014; Bond et al., 2015).
ECM composition also plays a critical role in mediating the differentiation of neuronal progenitor cells. During early developmental stages, mutations of basement membrane components laminin β2 and γ3 affect radial glial attachment and alignment disrupting asymmetric division of these progenitors (Radner et al., 2012). Similarly, prelecan knockout mice also exhibit the defects in neurogenesis process of neural progenitor populations (Girós et al., 2007; Barros et al., 2011). In addition, the degradation of ECM component chondroitin sulfate proteoglycans, which are enriched in NSC growth environment during development and in adult NSC niche, results in reduced radial glia proliferation and reduction in neurogenesis (Sirko et al., 2007). Although ECM composition has been well-known to be one of the important microenvironmental signal for efficient neuronal differentiation, how ECM structures affect neuronal differentiation still remains unclear. Currently, it is technically challenging to understand the role of these structures in the in vivo system. Hence, in vitro studies provide clues to delineate the role of these topographies in neuronal differentiation.
The Use of Nanotopographies to Enhance Neural Tissue Engineering Platforms
Since contact guidance plays a major role in many neural processes, there is interest in developing technologies that mimic the biophysical cues of the native neural ECM to help enhance neuronal processes on synthetic platforms. Of particular interest is neuronal differentiation, which has many important applications including the development of cell-based therapies for the treatment of neurological diseases, neuronal regeneration, and in vitro platforms for fundamental neurological studies, drug-screen platforms, disease modelling and precision medicine platforms (Avior et al., 2016; Shi et al., 2016; Dutta et al., 2017). Conventional methods of neural differentiation rely primarily on the use of biochemical factors as a means of process regulation, however this method alone has proven to be expensive, result in long differentiation periods, and provide minimal control over lineage-commitment (Schuldiner et al., 2001; Chambers et al., 2009; Hu and Zhang, 2009). Incorporation of biophysical cues, such as topography, in synthetic substrates has been shown to significantly enhance neuronal differentiation. These platforms increase differentiation rate, reduce cost, and promote lineage specific differentiation improving neuronal yield (Schuldiner et al., 2001; Chambers et al., 2009; Hu and Zhang, 2009; Ankam et al., 2013a, 2015; Anh Tuan et al., 2016; Nguyen et al., 2018; Tan et al., 2018). There are many factors that affect the efficiency of topography. Parameters such as pattern isotropy, feature dimensions, temporal application, and sequential application should all be considered. Furthermore, these parameters should also be considered with respect to the cell type and the stage of differentiation they are undergoing, as this will affect which topographies are optimal. Commonly used stem cell types include: embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), NSCs, and mesenchymal stem cells (MSCs). This section will focus on the key parameters that need to be considered when designing a topographically enhanced system, provide a brief overview of the efforts to optimize these parameters, and identify parameters that still need further study and optimization.
Optimization of pattern alignment and dimensions
Inspired by contact guidance phenomenon and fiber structures in the native ECM, topographical studies majorly focussed on the anisotropic topographies. Indeed, these patterns have been shown to enhance neuronal differentiation induction, reducing differentiation times, and decreasing the dependence on soluble biochemical cues required to induce differentiation. Enhancements such as upregulation of neuronal markers such as class III β-tubulin (TuJ1) or microtubule associated protein 2, earlier expression of neuronal markers, increased percentage of cell population expression neuronal markers (neuronal yield), neurite outgrowth, elongation and branching, and earlier electrophysiological activity have all been observed. Further, this enhancement by anisotropic patterns is not only limited to a certain cell type or differentiation pathway, but also in both pluripotent and multipotent cells undergoing either direct differentiation or trans-differentiation. The effects of anisotropic patterns stem largely from their ability to provide contact guidance, which can influence cell morphology and gene expression. The anisotropic patterns cause cells to elongate, altering focal adhesions and promoting cytoskeletal reorganization (Teo et al., 2010; Ankam et al., 2013b, 2015; Teo et al., 2013; Wong et al., 2014; Nguyen et al., 2016; Marcus et al., 2017; Yip et al., 2018). Isotropic patterns have been shown to promote the induction of glial differentiation (Moe et al., 2012; Ankam et al., 2013b; Qi et al., 2013). The reason this occurs is not well understood, but it is suggested that it may be due to isotropic patterns’ inability to induce cell morphologies that encourage focal adhesion formation and cytoskeletal changes required for inducing neuronal lineage (Ankam et al., 2013b, 2015). The mechanosensing of the extracellular topography involves a complex interplay between distinct mechanoreceptors, cell structure, and intracellular pathways. A variety of mechanotransduction pathways have been implicated in topography-induced improvements in neuronal differentiation. Examples include, focal adhesion kinase and focal adhesion signaling pathway, cytoskeletal contractility (Yim et al., 2010; Teo et al., 2013; Ankam et al., 2015), the Rho family GTPase (Keung et al., 2011) pathway, Hippo/yes-associated protein (Sun et al., 2014) pathway, Src and PI3K (Sawada et al., 2006) pathways, mitogen-activated protein kinase and the extracellular-signal-regulated kinase 1/2 (Chen et al., 2013) pathways. In addition, other factors such as nuclei shape or nuclei mechanical properties and epigenetic modifications have also been shown to correlate with differentiation (Huang et al., 2015; Ankam et al., 2018). For an in-depth review of these mechanisms, it is recommended that readers reference Stukel and Willits (2015), and Yim and Sheetz (2012). In this review, anisotropic patterns used to enhance neuronal differentiation, with the most commonly studied gratings and fibers, will be discussed. A summary can be found in Table 1.
Table 1.
Summary of anisotropic topographies influence on neuronal differentiation
| Type(s) of topography | Material | Starting cell type | Mechanistic study (Y/N) | Major findings | Study |
|---|---|---|---|---|---|
| Nano-/micro-patterning | |||||
| Equally space nanogratings (H = 150 nm and 560 nm, W = 500 and 1000 nm). Hexagonally arranged nanopillars (H = 150 or 560 nm, D = 500 nm) |
Poly(dimethylsiloxan) incubate with 1% Geltrex | iPSC | Y (YAP expression) | Gratings with heights of 560 nm showed the best performance, reducing cell proliferation, enhancing cytoplasmic localization of YAP and promoting neuronal differentiation (compared to the flat control). YAP localizations are critical to induce neural differentiation. | Song et al. (2016) |
| Nanogratings (H = 250 nm, W = 250 nm, 1 μm, 10 μm, S = 500 nm, 2 μm, 20 μm) |
Poly(dimethylsiloxan) coated with bovine fibronectin | MSC | Y (focal adhesion kinase) | Gratings with 250 nm line widths upregulated neurogenic and myogenic differentiation markers. Focal adhesions on nanogratings were smaller and more elongated than those seem on micro gratings or control. | Teo et al. (2013) |
| Nanogratings (H = 300 nm, W = 350 nm, 2 μm, and 5 μm) | Poly(dimethylsiloxan) coated with 1:80 diluted Matrigel | iPSC | N | Neuronal marker expression was inversely proportional to width. | Pan et al. (2013) |
| Nanogratings (H = 350 nm, W = 350 nm, 1 μm, 10 μm; S = 700 nm, 2 μm, 20 μm) | Poly(dimethylsiloxan) coated with bovine collagen I | MSC | N | The effect of nanogratings alone was greater than the effect of retinoic acid on flat substrates, regarding upregulation of neuronal markers. | Yim et al. (2007) |
| Nanogratings (H = 500 nm, S = 250 nm) | Polyurethane acrylate on glass coverslip | ESC | N | Nanoscale gratings alone can induce the differentiation of ESC into a neuronal lineage without the use of differentiation-inducing agents. | Lee et al. (2010) |
| Nanogratings (H = 625 nm, W = S = 1.5 μm) Nanopores (S = 28 nm, pore size = 10 nm) Hierarchical (combination of nanopores and nanogratings) | Polystyrene-poly(methyl methacrylate) random copolymer and polystyrene-poly(methyl methacrylate) block copolymer | NSC | Y (β1 integrin-mediated binding, intracellular Rho-associated protein kinase pathway | Cells have a mechanical memory of the conditions under which neuronal differentiation was induced. Enhanced neuronal differentiation persisted even after the removal of the hierarchical pattern. | Yang et al. (2014) |
| Microgratings (H = W = S = 2 μm) | Poly(dimethylsiloxan) coated with Matrigel | ESC and iPSC | N | The effect of topography is additive. An initial exposure to 2 μm increase neural differentiation rate and an additional culture period can improve neural differentiation. | Chan et al. (2012) |
| Microgratings (H = W = S = 2 μm) Micropillars (H = 2 μm, P = 12 μm and D = 2 μm) | Poly(dimethylsiloxan) coated with poly-L-ornithine, fibronectin and laminin | iPSC | N | Gratings are beneficial for early stage of differentiation (lineage commitment). Pillars are beneficial for later stages (maturation). Sequential application resulted in significantly increased overall differentiation rate. | Tan et al. (2018) |
| Nanogratings and microgratings: i. H = W = S = 250 nm ii. H = 120 nm, S = 1 μm, W = 2 μm iii. H = 80 nm, S = 2 μm, W = 1 μm iv. H = W = S = 2 μm Nanopillars: v. H = 1 μm, P = 6.5 μm, H = 1 μm Nanowells: vi. H = 2 μm, P = 12 μm, H = 2 μm Hierarchical: vii. 250 nm gratings with 250 nm space perpendicular to 2 μm gratings. |
Poly(dimethylsiloxan) coated with poly-L-ornithine and laminin | ESC | N | High throughput topography screening. Anisotropic patterns promote neuronal differentiation and isotropic patterns promote glial differentiation. | Ankam et al. (2013) |
| Nanogratings and Microgratings (H = 0.35 μm, 0.8 μm, 2 μm and 4 μm, W = 2 μm, S = 2 μm) | Poly(dimethylsiloxan) coated with poly-L-ornithine and laminin | NPC | Y (cytoskeletal bending) | Cells can sense the depth of micro-gratings. Neurite elongation, alignment and neuronal differentiation increased with grating depth. Filopodial adhesion in growth cones favour elongation but the neurite cytoskeleton resists it. | Chua et al. (2014) |
| Electrospun fibers | |||||
| Aligned and random nanofibers, D = 250 nm | Electrospun polycaprolactone | ESC | N | Aligned nanofibers enhanced differentiation into neural lineage and directed neurite outgrowth | Xie et al. (2008) |
| Aligned and randomly oriented nanofibers, D = 260 nm, 480 nm and 930 nm | Electrospun polycaprolactone coated with poly-L-ornithine and laminin | NSC | Y (Wnt Signaling) | Highest yield of neuronal progenitors on 480 nm aligned fibers, due to selectivity against oligodendrocytes and increase in canonical Wnt signaling. | Lim et al. (2010) |
| Aligned nanofibers, D = 270 nm | Electrospun polycaprolactone and gelatin | MSC | N | Aligned fibers up-regulated neural markers at both the protein and mRNA level, compared to the control. | Jiang et al. (2011) |
| Aligned and randomly oriented nanofibers, D = 400 nm and 800 nm | Tussah silk fibroin | ESC-derived NPCs | N | Aligned fibers significantly promoted neuronal differentiation and neurite outgrowth. Cells on 400 nm fibers had higher viability, differentiation and neurite outgrowth. | Wang et al. (2011) |
| Randomly oriented nanofibers, D = 283 nm, 749 nm, 153 nm, 1452 nm. | Laminin coated electrospun polyether sulfone | NSC | N | Fiber diameter was found to be inversely proportional to proliferation and cell spreading, and directly proportional to degree of cell aggregation. | Christopherson et al. (2009) |
Stem cell behavior on gratings and electrospun fibers. D: Diameter; ESC: embryonic stem cell; H: height; iPSC: induced pluripotent stem cell; MSC: mesenchymal stem cell; NPC: neural precursor cell; NSC: neural stem cell; P: pitch; S: spacing; W: width; YAP: yes-associated protein.
Gratings
Gratings, also commonly referred to as ridges or grooves, are patterns of parallel line channels that can be rectangular, rounded or v-shaped. Methods commonly used for fabrication of micro- and nanoscale grating topographies include, soft lithography, photolithography, thermal lithography, plasma lithography, electron beam lithography, nano-imprinting, and electric field-aided casting (Teo et al., 2010; Jeon et al., 2014; Marcus et al., 2017). It is recommended that the reader reference Teo et al., Jeon et al. and, Norman and Desai for reviews of topography fabrication techniques (Norman and Desai, 2006; Teo et al., 2010; Jeon et al., 2014). Key parameters that can determine how gratings will affect cell behaviors include the width, depth, and the aspect ratio of the depth to width. In general, features wider than soma diameter (12 ± 3 μm) or the average filopodia extension length do not encourage efficient interaction with cells resulting in differentiation efficiency that is comparable to that of un-patterned substrates (Recknor et al., 2006; Béduer et al., 2012; Chan et al., 2013). In addition, known native ECM topographies are also primarily less than 10 μm in diameter. Therefore, grating widths in the range of 250 nm–10 μm that have been shown to enhance neuronal differentiation of a variety of cell types are normally considered (Yim et al., 2007; Lee et al., 2010; Béduer et al., 2012; Moe et al., 2012; Ankam et al., 2013b; Chan et al., 2013; Qi et al., 2013; Teo et al., 2014; Tan et al., 2015). Using 250 nm, 350 nm, and 2 µm wide gratings, direct neuronal differentiation was induced in ESCs and iPSCs without or with minimal use of biochemical factors. In these systems, earlier upregulation of neuronal lineage markers, and increased preference for the neuronal lineage were observed (Figure 2) (Lee et al., 2010; Ankam et al., 2013b; Chan et al., 2013; Pan et al., 2013; Tan et al., 2015). Furthermore, it was also found that alignment, extension, and neurite outgrowth of individual cells and colonies decreased with grating width (Chan et al., 2013; Song et al., 2016). For NSCs or NPCs, grating widths in the range of 250 nm–10 µm have been shown to increase early upregulation of neuronal markers such as TUJ1 and increase preference for the neuronal lineage compared to planar substrates (Moe et al., 2012). In this range, an inverse relationship between grating width and neuronal marker upregulation has been observed; however, no similar relationships could be drawn between the preference for neuronal lineage commitment (Qi et al., 2013; Yang et al., 2013). For MSCs only nano-widths (250 nm and 350 nm) were shown to have a significant impact on the directed neuronal differentiation. Interestingly, the effect was even stronger than that of retinoic acid alone on an un-patterned surface (Yim et al., 2007, 2010; Teo et al., 2013; Ankam et al., 2018). It has also been proposed that an upper limit on grating width of 1.9 µm may also exist for MSC. In a combination of transmission electron microscopy observation and computational modeling, Zeng et al. (2018) proposes a model showing that for grating widths above 1.9 µm, the basal cell membrane tend to bend into the grooves, which correlates with reducing elongation and alignment of MSC on grating surfaces.
Figure 2.
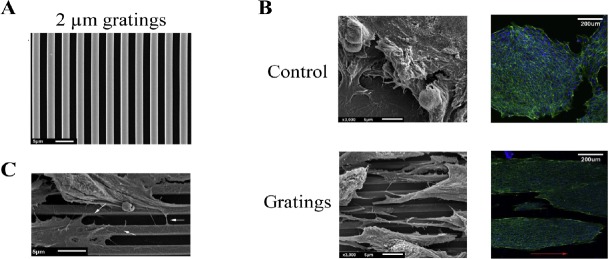
Human pluripotent stem cell (hPSC) colonies and hPSC elongate along the grating axis of 2 μm gratings.
(A) 2 μm gratings are reproduced with high fidelity, as demonstrated by scanning electron microscope. PSC cultured on the unpatterned control. (B) hPSC cultured on the unpatterned control exhibited a spread morphology in contrast to the elongated morphology observed on 2 μm gratings. Cells were stained for phalloidin (green) and 4′,6-diamidino-2-phenylindole (blue). Scale bars: 5 μm (left) and 200 μm (right). (C) The filopodia of hPSC on 2 μm gratings extend along, across and between gratings (white arrows). Adapted from Chan et al. (2013).
In general, grating depths in the range of 250 nm–4 µm are most commonly studied (Yim et al., 2007; Lee et al., 2010; Béduer et al., 2012; Moe et al., 2012; Ankam et al., 2013b; Chan et al., 2013; Qi et al., 2013). Not many studies have investigated the effect of grating depth on neuronal differentiation. However, a correlation between grating depth and preferential neuronal differentiation of NPCs has been observed. Using NPC on 2 μm wide gratings and media favouring astrocyte differentiation, Chua et al. (2014) showed that if the 2 μm wide gratings were fabricated with depths of 2–4 µm, a higher percentage of cells expressed neuronal markers with a lower percentage of cells expressed astrocyte markers. This trend of increased neuron differentiation with increased depth, was maintained up until a saturation point of 2–4 μm in depth. Beyond this point, perpendicular neurite growth was hindered due to the high energy costs of neurites bending in deep grooves. A similar trend was shown using iPSCs, wherein increasing grating depth from 150 nm to 560 nm (using grating widths of 500 nm and 1 µm), significantly increased the fraction of cells committed to the neuronal lineage (Song et al., 2016). It was also shown, that grating depth relative to width, also referred to as aspect ratio, can also impact cell behavior. Chan et al. (2013) found that ESC on micro gratings with 1:1 aspect ratios has the greatest elongation and alignment. A similar result using MSC was observed by Wong et al. (2014), wherein aspect ratio was shown to be better at predicting cell elongation and alignment than either depth or width alone. Thus, depth, width, and aspect ratio play a significant role in the topographical modification of cell behavior.
Fibers
Fibrous scaffolds closely resemble the native nervous system ECM fibrils, as they are made of mesh of micro- and/or nanoscale fibers. In these meshes, fibers can either be aligned in parallel or randomly oriented as present in native CNS and PNS ECM structures. There are many methods of fibrous scaffold fabrication, however, the most commonly used for tissue engineering purposes are electrospinning, phase separation and self-assembly (Jhala and Vasita, 2015; Marcus et al., 2017). It is recommended that the reader reference Sultana (2015) for a review on fibrous scaffold fabrication and materials for tissue engineering purposes. Diameter and orientation of fibers are key dimensions that can determine how they will affect cell behavior. Almost all published studies use fibers with diameters in the range of 200 nm–2 μm similar to collagen fiber bundle thickness observed in PNS endoneurium and perineurium structures (Christopherson et al., 2009; Xie et al., 2009; Lim et al., 2010; Jiang et al., 2012). Similar to gratings, there is no consensus on the optimal fiber diameter. However, there seems to be a consensus on the optimal orientation. Early reports indicated that fiber orientation did not impact neuronal differentiation efficiency (Yang et al., 2005), but subsequent studies overwhelmingly showed the opposite. Fiber alignment was shown to significantly improved neuronal differentiation in a variety of cell types including ESCs, iPSCs (Xie et al., 2009; Mohtaram et al., 2015; Abbasi et al., 2016), NSCs (Christopherson et al., 2009; Lim et al., 2010; Bakhru et al., 2011; Lins et al., 2017), and MSCs (Cho et al., 2010; Jiang et al., 2012). Embryoid bodies on aligned fibers extended along the fiber axis and had more extensive neurite outgrowth compared to random and planar substrates. On randomly oriented fibers, both ESCs and NSCs tended to extend in multiple directions and became glial-like in morphology. For neuronal differentiation of ESCs, biochemical factors were still required. However, when used in combination with aligned fibers, embryoid bodies showed higher upregulation of neuronal markers, and the neuronal lineage was promoted over the glial (astrocyte) lineage, which was suppressed (Xie et al., 2009; Abbasi et al., 2016). Similar results were observed when using NSCs, wherein on aligned fibers, cells had a more elongated morphology compared to random or planar substrates (Yang et al., 2005; Christopherson et al., 2009; Lim et al., 2010; Mahairaki et al., 2010; Bakhru et al., 2011; Wang et al., 2012). Upregulation of neuronal markers and increased neuronal yield were observed in NSCs grown on fibers (Christopherson et al., 2009; Lim et al., 2010; Mahairaki et al., 2010; Bakhru et al., 2011; Wang et al., 2012; Lins et al., 2017). Similar to what was observed with ESCs, in addition to a bias in neuronal lineage commitment, a negative selection against the glial lineage (oligodendrocytes) was also observed in NSCs (Lim et al., 2010). The effect of alignment was even more significant for MSCs. Only MSCs grown on aligned substrates showed improvement in neuronal differentiation, specifically neuronal marker upregulation and a preference to the neuronal lineage over the glial lineage. MSCs grown on random fibers performed equally as well as planar substrates (Cho et al., 2010; Jiang et al., 2012).
The effect of fiber diameter is less clear and optimal fiber diameters widely vary depending on the study design. Early studies using NSCs reported that nanoscale fibers were much more effective that microfibers (300 nm vs. 1 µm) for increasing the rate at which cells took on the elongated neuronal morphology, in other words, the rate of differentiation (Yang et al., 2005). Two later studies have shown that while nanofibers outperform microfibers, larger nanofibers are more effective than smaller nanofibers (749 nm vs. 283 nm and 480 nm vs. 260 nm) at increasing the rate at which cells began expressing neuronal markers and overall neuronal yield when using NSC (Christopherson et al., 2009; Lim et al., 2010). However, using ESC derived neural progenitors, it was reported that 400 nm fibers performed better than 800 nm fibers, so the optimal fiber diameter may be in the mid-range of the nanoscale (Wang et al., 2012). Thus, fiber diameter plays a significant role in the topographical modification of cell behavior. It is of interest for future studies using different cell types than those mentioned here, such as iPSCs or MSCs, to incorporate this parameter into their analysis.
Optimization of topography application sequence and combination
Previous studies, like those outlined above, tended to focus on early induction and lineage commitment. However, to develop optimal systems resulting in fully functional neurons, enhancement of all differentiation phases is likely required. Interestingly, it has been shown that sequentially applied topographies can further improve neuronal differentiation as cells can “remember” past topographies (Chan et al., 2013; Yang et al., 2014b). Additionally, topographies that were once thought to be of no value to neuronal differentiation, such as isotropic patterns, have recently been shown to improve differentiation, when applied during later phases, such as maturation. Thus, there is potential to create systems where the benefits of certain topographies applied at different stages can be “added” together to improve the overall differentiation process.
It has previously been shown that cells store information from past environments and that this information can continue to influence cell fate in the future (Chan et al., 2013; Yang et al., 2014a; Muhammad et al., 2015). Using ESC and iPSC, Chan et al. (2013) demonstrated this memory effect for pluripotent cells undergoing neuronal differentiation. They showed that ESC and iPSC have a mechanical memory of their past environments and the topographical signals that they were exposed to during different stages of the maintenance and neuronal differentiation. PSCs were exposed to topography during maintenance, and/or during neuronal differentiation. Remarkably, when PSCs were exposed to grating pattern in the maintenance phase, and then PSCs were moved to planar substrate, enhanced neuronal differentiation was observed. Furthermore, they showed that the effect of topography can indeed be additive. By incorporating a topographical priming step, they were able to decrease the differentiation period and reduce the amount of biochemical cues required (Chan et al., 2013). Similarly, Yang et al. (2014b) demonstrated hierarchical pattern-induced cells continued to exhibit enhanced neuronal differentiation even after the topography was removed. In addition, in vivo regional priming of primary NPC was also shown to play a major role in cell response to in vitro application of topography (Sathe et al., 2017). Notably, differentiation efficiency and morphological changes depended more on the cell source than on the topography being used. For example, although hippocampal cells were slightly more elongated than cortical cells aligned along the gratings, only hippocampal neurons showed significant elongation on pillars (Sathe et al., 2017).
Interestingly, optimal topography was found to differ in different phases of differentiation. While gratings enhance the initial stages of neuronal differentiation of iPSCs into dopaminergic neurons, pillars were better at enhancing maturation. Maturation of cells on pillars resulted in increased neurite branching, and enhanced maturation with more cells being capable of repetitive firing, as compared to cells that matured on gratings or planar substrates (Figure 3) (Tan et al., 2018). This is rather surprising as pillars were previously thought to enhance glial morphologies and have no effects on neuronal differentiation (Moe et al., 2012; Ankam et al., 2013b; Qi et al., 2013). Using a combination of gratings for the early stages of differentiation and pillars for maturation, it is possible to increase both the yield of mature dopaminergic neurons and differentiation rate (repetitive firing at 4 weeks compared to 55–70 days) (Tan et al., 2018). Thus, there is great potential to increase control and enhancement of differentiation into functional neurons by optimizing the temporal and sequential parameters of topography.
Figure 3.
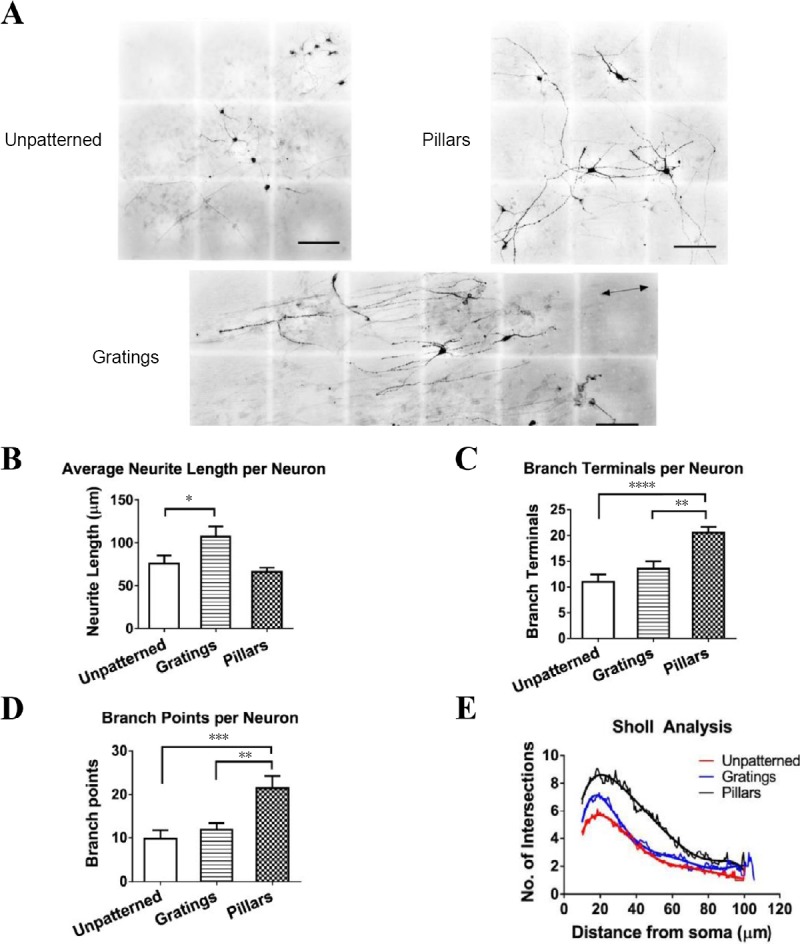
Morphology of tyrosine hydroxylase-positive (TH) neurons on patterned and unpatterned substrates after 21 days of differentiation.
(A) Representative images of human induced pluripotent stem cell (iPSC)-derived TH neurons on unpatterned, gratings and pillared PDMS substrates. Images were digitally stitched from overlapping fields of view to capture the entire length of neurites. Cells on gratings were aligned on the gratings axis (arrows). Scale bars: 100 μm. (B) Average neurite length per neuron. TH neurons were more elongated when differentiated on gratings than pillars and unpatterned control. (C) Number of terminals per neuron. (D) Number of branch points per neuron. (E) Sholl analysis. TH neurons have significantly more branching and increased neuronal complexity when differentiated on pillars than gratings and unpatterned control. All data are represented as the mean ± SEM of three independent experiments with over 30 TH-positive neurons analyzed on each pattern. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Adapted from Tan et al. (2018).
Conclusions and Future Perspectives
In conclusion, studies on the biophysical cues of ECM in the native microenvironment can provide a more in-depth understanding of cell processes and develop better biomimetic platforms. In both PNS and CNS, the spatiotemporally dynamic nature of ECM composition and structure helps to regulate many neural processes including cell migration, differentiation, and regeneration. However, a clear relationship between composition and functional architecture still needs to be determined along with better understanding of surface topography functions. Knowledge on the topography related mechanobiological mechanisms will help to provide better understanding of biological processes, and to enhance the development of biomimetic substrates for neuronal differentiation. Through the use of biomimetic in vitro systems, the parameters of alignment, feature dimensions, temporal application and/or sequential application have been identified to be important for controlling the neuronal differentiation. Most works to optimize these systems have been done for the early stages of differentiation and have focused on the parameters of pattern alignment and dimensions. In general, anisotropic patterns tend to increase both the rate of differentiation and neuronal yield during early differentiation. Feature sizes resembling native structure dimensions have been identified as optimal. There are few studies that focus on later stages of differentiation. However, it has been shown that isotropic patterns were shown to be optimal for enhancing maturation indicating that patterns can be optimized temporally. The effect of topography was also shown to be additive, as sequential application of topography showed that cells have mechanical memory of past environments, which is a promising parameter for future optimization of biomimetic systems. Future work should also consider optimizing these parameters for not only neuronal differentiation of stem cells in to functional neuron cells but also the assembly and organization of neural organoids. While this review focused on how microenvironment signals, such as the biochemical composition and the ECM architecture, affect neuronal differentiation; subsequent development of biomimetic platforms should also investigate topography and other biophysical cues, such as substrate stiffness, in conjunction to produce systems closely resembling native microenvironment. Collectively, the knowledge gained from studying the functionality of native structures in a spatiotemporal manner for mature neuronal differentiation in conjunction with temporal behavior of topographies on stem cells will bring a step closer to fabricate systems capable of forming functional matured neurons.
Additional file: Open peer review reports 1 (99.4KB, pdf) and 2 (98.7KB, pdf) .
Acknowledgments
The authors would like to thank Dr. Rizwan Muhammad and Grace Pohan at University of Waterloo for editing this work and their invaluable feedback.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: The work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery (NSERC 2016040; to DJ, SM and EKFY) and University of Waterloo start up fund (to DJ, SM and EKFY) for their generous funding. In addition, NSERC Undergraduate Student Research Awards (USRA; to SM and EKFY) and Collaborative Research and Training Experience (CREATE, 401207296; to SM and EKFY) for their generous partial funding.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Olga Kopach, University College London, UK; Amadi Ogonda Ihunwo, University of the Witwatersrand, South Africa.
Funding: The work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery (NSERC 2016040; to DJ, SM and EKFY) and University of Waterloo start up fund (to DJ, SM and EKFY) for their generous funding. In addition, NSERC Undergraduate Student Research Awards (USRA; to SM and EKFY) and Collaborative Research and Training Experience (CREATE, 401207296; to SM and EKFY) for their generous partial funding.
P-Reviewers: Kopach O, Ihunwo AO; C-Editors: Zhao M, Yu J; T-Editor: Jia Y
References
- 1.Abbasi N, Hashemi SM, Salehi M, Jahani H, Mowla SJ, Soleimani M, Hosseinkhani H. Influence of oriented nanofibrous PCL scaffolds on quantitative gene expression during neural differentiation of mouse embryonic stem cells. J Biomed Mater Res A. 2016;104:155–164. doi: 10.1002/jbm.a.35551. [DOI] [PubMed] [Google Scholar]
- 2.Adams KL, Gallo V. The diversity and disparity of the glial scar. Nat Neurosci. 2018;21:9–15. doi: 10.1038/s41593-017-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adarsh KG. Evaluation of acellular and cellular nerve grafts in repair of rat peripheral nerve. J Neurosurg. 1988;68:117–123. doi: 10.3171/jns.1988.68.1.0117. [DOI] [PubMed] [Google Scholar]
- 4.Anh Tuan N, Sharvari RS, Evelyn KFY. From nano to micro: topographical scale and its impact on cell adhesion, morphology and contact guidance. J Phys Condens Matter. 2016;28:183001. doi: 10.1088/0953-8984/28/18/183001. [DOI] [PubMed] [Google Scholar]
- 5.Ankam S, Lim CK, Yim EK. Actomyosin contractility plays a role in MAP2 expression during nanotopography-directed neuronal differentiation of human embryonic stem cells. Biomaterials. 2015;47:20–28. doi: 10.1016/j.biomaterials.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Ankam S, Teo BK, Kukumberg M, Yim EK. High throughput screening to investigate the interaction of stem cells with their extracellular microenvironment. Organogenesis. 2013a;9:128–142. doi: 10.4161/org.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ankam S, Teo BK, Pohan G, Ho SW, Lim CK, Yim EK. Temporal changes in nucleus morphology, lamin A/C and histone methylation during nanotopography-induced neuronal differentiation of stem cells. Front Bioeng Biotechnol. 2018;6:69. doi: 10.3389/fbioe.2018.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ankam S, Suryana M, Chan LY, Moe AA, Teo BK, Law JB, Sheetz MP, Low HY, Yim EK. Substrate topography and size determine the fate of human embryonic stem cells to neuronal or glial lineage. Acta Biomater. 2013b;9:4535–4545. doi: 10.1016/j.actbio.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Arranz AM, Perkins KL, Irie F, Lewis DP, Hrabe J, Xiao F, Itano N, Kimata K, Hrabetova S, Yamaguchi Y. Hyaluronan deficiency due to has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space. J Neurosci. 2014;34:6164. doi: 10.1523/JNEUROSCI.3458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avior Y, Sagi I, Benvenisty N. Pluripotent stem cells in disease modelling and drug discovery. Nat Rev Mol Cell Biol. 2016;17:170. doi: 10.1038/nrm.2015.27. [DOI] [PubMed] [Google Scholar]
- 11.Baiguera S, Del Gaudio C, Lucatelli E, Kuevda E, Boieri M, Mazzanti B, Bianco A, Macchiarini P. Electrospun gelatin scaffolds incorporating rat decellularized brain extracellular matrix for neural tissue engineering. Biomaterials. 2014;35:1205–1214. doi: 10.1016/j.biomaterials.2013.10.060. [DOI] [PubMed] [Google Scholar]
- 12.Bakhru S, Nain AS, Highley C, Wang J, Campbell P, Amon C, Zappe S. Direct and cell signaling-based, geometry-induced neuronal differentiation of neural stem cells. Integr Biol (Camb) 2011;3:1207–1214. doi: 10.1039/c1ib00098e. [DOI] [PubMed] [Google Scholar]
- 13.Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- 14.Barnes JM, Przybyla L, Weaver VM. Tissue mechanics regulate brain development, homeostasis and disease. J Cell Sci. 2017;130:71. doi: 10.1242/jcs.191742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barros CS, Franco SJ, Müller U. Extracellular matrix: functions in the nervous system. Cold Spring Harb Perspect Biol. 2011;3:a005108. doi: 10.1101/cshperspect.a005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Béduer A, Vieu C, Arnauduc F, Sol JC, Loubinoux I, Vaysse L. Engineering of adult human neural stem cells differentiation through surface micropatterning. Biomaterials. 2012;33:504–514. doi: 10.1016/j.biomaterials.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 17.Blumenthal NR, Hermanson O, Heimrich B, Shastri VP. Stochastic nanoroughness modulates neuron–astrocyte interactions and function via mechanosensing cation channels. Proc Natl Acad Sci U S A. 2014;111:16124. doi: 10.1073/pnas.1412740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bond Allison M, Ming GL, Song H. Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell. 2015;17:385–395. doi: 10.1016/j.stem.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Catala M, Kubis N. Chapter 3 - Gross anatomy and development of the peripheral nervous system. In: Said G, Krarup C, editors. Handbook of Clinical Neurology. Amsterdam: Elsevier; 2013. pp. 29–41. [DOI] [PubMed] [Google Scholar]
- 21.Cattin AL, Lloyd AC. The multicellular complexity of peripheral nerve regeneration. Curr Opin Neurobiol. 2016;39:38–46. doi: 10.1016/j.conb.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan LY, Birch WR, Yim EK, Choo AB. Temporal application of topography to increase the rate of neural differentiation from human pluripotent stem cells. Biomaterials. 2013;34:382–392. doi: 10.1016/j.biomaterials.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 24.Chen P, Cescon M, Bonaldo P. The role of collagens in peripheral nerve myelination and function. Mol Neurobiol. 2015;52:216–225. doi: 10.1007/s12035-014-8862-y. [DOI] [PubMed] [Google Scholar]
- 25.Chen WH, Cheng SJ, Tzen JT, Cheng CM, Lin YW. Probing relevant molecules in modulating the neurite outgrowth of hippocampal neurons on substrates of different stiffness. PLoS One. 2013;8:e83394. doi: 10.1371/journal.pone.0083394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chernousov MA, Carey DJ. Schwann cell extracellular matrix molecules and their receptors. Histol Histopathol. 2000;15:593–601. doi: 10.14670/HH-15.593. [DOI] [PubMed] [Google Scholar]
- 27.Cho YI, Choi JS, Jeong SY, Yoo HS. Nerve growth factor (NGF)-conjugated electrospun nanostructures with topographical cues for neuronal differentiation of mesenchymal stem cells. Acta Biomater. 2010;6:4725–4733. doi: 10.1016/j.actbio.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Christopherson GT, Song H, Mao HQ. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials. 2009;30:556–564. doi: 10.1016/j.biomaterials.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Chua JS, Chng CP, Moe AA, Tann JY, Goh EL, Chiam KH, Yim EK. Extending neurites sense the depth of the underlying topography during neuronal differentiation and contact guidance. Biomaterials. 2014;35:7750–7761. doi: 10.1016/j.biomaterials.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Colognato H, Yurchenco PD. Form and function: The laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 31.Colognato H, ffrench-Constant C, Feltri ML. Human diseases reveal novel roles for neural laminins. Trends Neurosci. 2005;28:480–486. doi: 10.1016/j.tins.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 32.de Miranda AS, Zhang CJ, Katsumoto A, Teixeira AL. Hippocampal adult neurogenesis: Does the immune system matter? J Neurol Sci. 2017;372:482–495. doi: 10.1016/j.jns.2016.10.052. [DOI] [PubMed] [Google Scholar]
- 33.de Winter F, Kwok JCF, Fawcett JW, Vo TT, Carulli D, Verhaagen J. The chemorepulsive protein semaphorin 3A and perineuronal net-mediated plasticity. Neural Plast. 2016;2016:14. doi: 10.1155/2016/3679545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeQuach JA, Yuan SH, Goldstein LS, Christman KL. Decellularized porcine brain matrix for cell culture and tissue engineering scaffolds. Tissue Eng Part A. 2011;17:2583–2592. doi: 10.1089/ten.tea.2010.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong L, Chen Y, Lewis M, Hsieh JC, Reing J, Chaillet JR, Howell CY, Melhem M, Inoue S, Kuszak JR, DeGeest K, Chung AE. Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1/Nidogen-1. Lab Invest. 2002;82:1617. doi: 10.1097/01.lab.0000042240.52093.0f. [DOI] [PubMed] [Google Scholar]
- 36.Dutta D, Heo I, Clevers H. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med. 2017;23:393–410. doi: 10.1016/j.molmed.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Faissner A, Reinhard J. The extracellular matrix compartment of neural stem and glial progenitor cells. Glia. 2015;63:1330–1349. doi: 10.1002/glia.22839. [DOI] [PubMed] [Google Scholar]
- 38.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franze K. The mechanical control of nervous system development. Development. 2013;140:3069. doi: 10.1242/dev.079145. [DOI] [PubMed] [Google Scholar]
- 40.Franze K, Janmey PA, Guck J. Mechanics in neuronal development and repair. Annu Rev Biomed Eng. 2013;15:227–251. doi: 10.1146/annurev-bioeng-071811-150045. [DOI] [PubMed] [Google Scholar]
- 41.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840:2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giamanco KA, Morawski M, Matthews RT. Perineuronal net formation and structure in aggrecan knockout mice. Neuroscience. 2010;170:1314–1327. doi: 10.1016/j.neuroscience.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 43.Girós A, Morante J, Gil-Sanz C, Fairén A, Costell M. Perlecan controls neurogenesis in the developing telencephalon. BMC Dev Biol. 2007;7:29. doi: 10.1186/1471-213X-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonçalves JT, Schafer ST, Gage FH. Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell. 2016;167:897–914. doi: 10.1016/j.cell.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Perez F, Udina E, Navarro X. Chapter ten - Extracellular matrix components in peripheral nerve regeneration. In: Geuna S, Perroteau I, Tos P, Battiston B, editors. International Review of Neurobiology. Academic Press; 2013. pp. 257–275. [DOI] [PubMed] [Google Scholar]
- 46.Halfter W, Dong S, Yip YP, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci. 2002;22:6029. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Happel MFK, Frischknecht R. Neuronal plasticity in the juvenile and adult brain regulated by the extracellular matrix. In: Travascio F, editor. Composition and function of the extracellular matrix in the human body. New York: ExLi4EvA; 2016. [Google Scholar]
- 48.Haubst N, Georges-Labouesse E, De Arcangelis A, Mayer U, Götz M. Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development. 2006;133:3245. doi: 10.1242/dev.02486. [DOI] [PubMed] [Google Scholar]
- 49.Herrera-Perez M, Voytik-Harbin SL, Rickus JL. Extracellular matrix properties regulate the migratory response of glioblastoma stem cells in three-dimensional culture. Tissue Eng Part A. 2015;21:2572–2582. doi: 10.1089/ten.tea.2014.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill RE, Williams PE. Perineurial cell basement membrane thickening and myelinated nerve fibre loss in diabetic and nondiabetic peripheral nerve. J Neurol Sci. 2004;217:157–163. doi: 10.1016/j.jns.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Hoffman-Kim D, Mitchel JA, Bellamkonda RV. Topography, cell response, and nerve regeneration. Annu Rev Biomed Eng. 2010;12:203–231. doi: 10.1146/annurev-bioeng-070909-105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Höke A. Mechanisms of disease: what factors limit the success of peripheral nerve regeneration in humans? Nat Clin Pract Neurol. 2006;2:448. doi: 10.1038/ncpneuro0262. [DOI] [PubMed] [Google Scholar]
- 53.Hu BY, Zhang SC. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat Protoc. 2009;4:1295. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang B, Li G, Jiang XH. Fate determination in mesenchymal stem cells: a perspective from histone-modifying enzymes. Stem Cell Res Ther. 2015;6:35. doi: 10.1186/s13287-015-0018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10:1641–1651. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 56.Ide C, Tohyama K, Yokota R, Nitatori T, Onodera S. Schwann cell basal lamina and nerve regeneration. Brain Res. 1983;288:61–75. doi: 10.1016/0006-8993(83)90081-1. [DOI] [PubMed] [Google Scholar]
- 57.Jeon H, Simon CG, Jr, Kim G. A mini-review: Cell response to microscale, nanoscale, and hierarchical patterning of surface structure. J Biomed Mater Res B Appl Biomater. 2014;102:1580–1594. doi: 10.1002/jbm.b.33158. [DOI] [PubMed] [Google Scholar]
- 58.Jhala D, Vasita R. A review on extracellular matrix mimicking strategies for an artificial stem cell niche. Polym Rev. 2015;55:561–595. [Google Scholar]
- 59.Jiang X, Cao HQ, Shi LY, Ng SY, Stanton LW, Chew SY. Nanofiber topography and sustained biochemical signaling enhance human mesenchymal stem cell neural commitment. Acta Biomater. 2012;8:1290–1302. doi: 10.1016/j.actbio.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 60.Johnson PC, Brendel K, Meezan E. Human diabetic perineurial cell basement membrane thickening. Lab Invest. 1981;44:265–270. [PubMed] [Google Scholar]
- 61.Junqueira LC, Montes GS, Krisztán RM. The collagen of the vertebrate peripheral nervous system. Cell Tissue Res. 1979;202:453–460. doi: 10.1007/BF00220437. [DOI] [PubMed] [Google Scholar]
- 62.Kaplan HM, Mishra P, Kohn J. The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. J Mater Sci Mater Med. 2015;26:226. doi: 10.1007/s10856-015-5558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kazanis I, ffrench-Constant C. Extracellular matrix and the neural stem cell niche. Dev Neurobiol. 2011;71:1006–1017. doi: 10.1002/dneu.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- 65.Keung AJ, de Juan-Pardo EM, Schaffer DV, Kumar S. Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells. 2011;29:1886–1897. doi: 10.1002/stem.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011;71:1073–1089. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- 67.Lau LW, Cua R, Keough MB, Haylock-Jacobs S, Yong VW. Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat Rev Neurosci. 2013;14:722. doi: 10.1038/nrn3550. [DOI] [PubMed] [Google Scholar]
- 68.Lee MR, Kwon KW, Jung H, Kim HN, Suh KY, Kim K, Kim KS. Direct differentiation of human embryonic stem cells into selective neurons on nanoscale ridge/groove pattern arrays. Biomaterials. 2010;31:4360–4366. doi: 10.1016/j.biomaterials.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 69.Lim SH, Liu XY, Song H, Yarema KJ, Mao HQ. The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials. 2010;31:9031–9039. doi: 10.1016/j.biomaterials.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders–how to make it work. Nat Med. 2004;10:S42. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 71.Lins LC, Wianny F, Livi S, Dehay C, Duchet-Rumeau J, Gérard JF. Effect of polyvinylidene fluoride electrospun fiber orientation on neural stem cell differentiation. J Biomed Mater Res B Appl Biomater. 2017;105:2376–2393. doi: 10.1002/jbm.b.33778. [DOI] [PubMed] [Google Scholar]
- 72.Long KR, Newland B, Florio M, Kalebic N, Langen B, Kolterer A, Wimberger P, Huttner WB. Extracellular matrix components hapln1, lumican, and collagen I cause hyaluronic acid-dependent folding of the developing human neocortex. Neuron. 2018;99:702–719e6. doi: 10.1016/j.neuron.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 73.Mahairaki V, Lim SH, Christopherson GT, Xu L, Nasonkin I, Yu C, Mao HQ, Koliatsos VE. Nanofiber matrices promote the neuronal differentiation of human embryonic stem cell-derived neural precursors in vitro. Tissue Eng Part A. 2010;17:855–863. doi: 10.1089/ten.tea.2010.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marcus M, Baranes K, Park M, Choi IS, Kang K, Shefi O. Interactions of neurons with physical environments. Adv Healthc Mater. 2017;6:1700267. doi: 10.1002/adhm.201700267. [DOI] [PubMed] [Google Scholar]
- 75.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 76.Mecham RP. Overview of extracellular matrix. Curr Protoc Cell Biol Chapter 10:Unit 101. 2001 doi: 10.1002/0471143030.cb1001s00. [DOI] [PubMed] [Google Scholar]
- 77.Mercier F. Fractones: extracellular matrix niche controlling stem cell fate and growth factor activity in the brain in health and disease. Cell Mol Life Sci. 2016;73:4661–4674. doi: 10.1007/s00018-016-2314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mercier F, Kitasako JT, Hatton GI. Fractones and other basal laminae in the hypothalamus. J Comp Neurol. 2003;455:324–340. doi: 10.1002/cne.10496. [DOI] [PubMed] [Google Scholar]
- 79.Moe AA, Suryana M, Marcy G, Lim SK, Ankam S, Goh JZ, Jin J, Teo BK, Law JB, Low HY, Goh EL, Sheetz MP, Yim EK. Microarray with micro- and nano-topographies enables identification of the optimal topography for directing the differentiation of primary murine neural progenitor cells. Small. 2012;8:3050–3061. doi: 10.1002/smll.201200490. [DOI] [PubMed] [Google Scholar]
- 80.Mohtaram NK, Ko J, King C, Sun L, Muller N, Jun MB, Willerth SM. Electrospun biomaterial scaffolds with varied topographies for neuronal differentiation of human-induced pluripotent stem cells. J Biomed Mater Res A. 2015;103:2591–2601. doi: 10.1002/jbm.a.35392. [DOI] [PubMed] [Google Scholar]
- 81.Montes GS, Bezerra MSF, Junqueira LCU. Collagen distribution in tissues. In: Ruggeri A, Motta PM, editors. Ultrastructure of the Connective Tissue Matrix. Boston, USA: Springer US; 1984. pp. 65–88. [Google Scholar]
- 82.Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, Hoshi T, Campbell KP. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- 83.Moore SW, Sheetz MP. Biophysics of substrate interaction: Influence on neural motility, differentiation, and repair. Dev Neurobiol. 2011;71:1090–1101. doi: 10.1002/dneu.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat Rev Mol Cell Biol. 2014;15:771. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muhammad R, Peh GS, Adnan K, Law JB, Mehta JS, Yim EK. Micro- and nano-topography to enhance proliferation and sustain functional markers of donor-derived primary human corneal endothelial cells. Acta Biomater. 2015;19:138–148. doi: 10.1016/j.actbio.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen AT, Sathe SR, Yim EK. From nano to micro: topographical scale and its impact on cell adhesion, morphology and contact guidance. J Phys Condens Matter. 2016;28:183001. doi: 10.1088/0953-8984/28/18/183001. [DOI] [PubMed] [Google Scholar]
- 87.Nguyen AT, Mattiassi S, Loeblein M, Chin E, Ma D, Coquet P, Viasnoff V, Teo EH, Goh EL, Yim EK. Human Rett-derived neuronal progenitor cells in 3D graphene scaffold as an in vitro platform to study the effect of electrical stimulation on neuronal differentiation. Biomed Mater. 2018;13:034111. doi: 10.1088/1748-605X/aaaf2b. [DOI] [PubMed] [Google Scholar]
- 88.Norman JJ, Desai TA. Methods for fabrication of nanoscale topography for tissue engineering scaffolds. Ann Biomed Eng. 2006;34:89–101. doi: 10.1007/s10439-005-9005-4. [DOI] [PubMed] [Google Scholar]
- 89.Omar MH, Kerrisk Campbell M, Xiao X, Zhong Q, Brunken WJ, Miner JH, Greer CA, Koleske AJ. CNS neurons deposit laminin α5 to stabilize synapses. Cell Rep. 2017;21:1281–1292. doi: 10.1016/j.celrep.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pan F, Zhang M, Wu G, Lai Y, Greber B, Schöler HR, Chi L. Topographic effect on human induced pluripotent stem cells differentiation towards neuronal lineage. Biomaterials. 2013;34:8131–8139. doi: 10.1016/j.biomaterials.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 91.Qi L, Li N, Huang R, Song Q, Wang L, Zhang Q, Su R, Kong T, Tang M, Cheng G. The effects of topographical patterns and sizes on neural stem cell behavior. PLoS One. 2013;8:e59022. doi: 10.1371/journal.pone.0059022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Radner S, Banos C, Bachay G, Li YN, Hunter DD, Brunken WJ, Yee KT. β2 and γ3 laminins are critical cortical basement membrane components: Ablation of Lamb2 and Lamc3 genes disrupts cortical lamination and produces dysplasia. Dev Neurobiol. 2012;73:209–229. doi: 10.1002/dneu.22057. [DOI] [PubMed] [Google Scholar]
- 93.Rauch U. Extracellular matrix components associated with remodeling processes in brain. Cell Mol Life Sci CMLS. 2004;61:2031–2045. doi: 10.1007/s00018-004-4043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Recknor JB, Sakaguchi DS, Mallapragada SK. Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates. Biomaterials. 2006;27:4098–4108. doi: 10.1016/j.biomaterials.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 95.Sathe S, Chan QX, Jin J, Bernitt E, Döbereiner HG, Yim KE. Correlation and comparison of cortical and hippocampal neural progenitor morphology and differentiation through the use of micro- and nano-topographies. J Funct Biomater. 2017;8:E35. doi: 10.3390/jfb8030035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schuldiner M, Eiges R, Eden A, Yanuka O, Itskovitz-Eldor J, Goldstein RS, Benvenisty N. Induced neuronal differentiation of human embryonic stem cells. Brain Res. 2001;913:201–205. doi: 10.1016/s0006-8993(01)02776-7. [DOI] [PubMed] [Google Scholar]
- 98.Seppänen A, Suuronen T, Hofmann SC, Majamaa K, Alafuzoff I. Distribution of collagen XVII in the human brain. Brain Res. 2007;1158:50–56. doi: 10.1016/j.brainres.2007.04.073. [DOI] [PubMed] [Google Scholar]
- 99.Shellswell GB, Restall DJ, Duance VC, Bailey AJ. Identification and differential distribution of collagen types in the central and peripheral nervous systems. FEBS Lett. 1979;106:305–308. doi: 10.1016/0014-5793(79)80520-7. [DOI] [PubMed] [Google Scholar]
- 100.Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 2016;16:115. doi: 10.1038/nrd.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simitzi C, Ranella A, Stratakis E. Controlling the morphology and outgrowth of nerve and neuroglial cells: The effect of surface topography. Acta Biomater. 2017;51:21–52. doi: 10.1016/j.actbio.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 102.Sirko S, von Holst A, Wizenmann A, Götz M, Faissner A. Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development. 2007;134:2727. doi: 10.1242/dev.02871. [DOI] [PubMed] [Google Scholar]
- 103.Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795:44–54. doi: 10.1016/s0006-8993(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 104.Song L, Wang K, Li Y, Yang Y. Nanotopography promoted neuronal differentiation of human induced pluripotent stem cells. Colloids Surf B Biointerfaces. 2016;148:49–58. doi: 10.1016/j.colsurfb.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 105.Sridharan R, Reilly RB, Buckley CT. Decellularized grafts with axially aligned channels for peripheral nerve regeneration. J Mech Behav Biomed Mater. 2015;41:124–135. doi: 10.1016/j.jmbbm.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 106.Stukel JM, Willits RK. Mechanotransduction of neural cells through cell-substrate interactions. Tissue Eng Part B Rev. 2015;22:173–182. doi: 10.1089/ten.teb.2015.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sultana N, Mohd IH, Lim MM. Composite synthetic scaffolds for tissue engineering and regenerative medicine. Cham, Switzerland: Springer International Publishing; 2015. [Google Scholar]
- 108.Sun Y, Yong KMA, Villa-Diaz LG, Zhang X, Chen W, Philson R, Weng S, Xu H, Krebsbach PH, Fu J. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat Mater. 2014;13:599. doi: 10.1038/nmat3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tan KK, Tann JY, Sathe SR, Goh SH, Ma D, Goh EL, Yim EK. Enhanced differentiation of neural progenitor cells into neurons of the mesencephalic dopaminergic subtype on topographical patterns. Biomaterials. 2015;43:32–43. doi: 10.1016/j.biomaterials.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 110.Tan KK, Lim WWM, Chai C, Kukumberg M, Lim KL, Goh EL, Yim EK. Sequential application of discrete topographical patterns enhances derivation of functional mesencephalic dopaminergic neurons from human induced pluripotent stem cells. Sci Rep. 2018;8:9567. doi: 10.1038/s41598-018-27653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Teo BK, Tan GD, Yim EK. The synergistic effect of nanotopography and sustained dual release of hydrophobic and hydrophilic neurotrophic factors on human mesenchymal stem cell neuronal lineage commitment. Tissue Eng Part A. 2014;20:2151–2161. doi: 10.1089/ten.tea.2013.0382. [DOI] [PubMed] [Google Scholar]
- 112.Teo BK, Ankam S, Chan LY, Yim EK. Chapter 11 - Nanotopography/mechanical induction of stem-cell differentiation. In: Shivashankar GV, editor. Methods in Cell Biology. Academic Press; 2010. pp. 241–294. [DOI] [PubMed] [Google Scholar]
- 113.Teo BK, Wong ST, Lim CK, Kung TY, Yap CH, Ramagopal Y, Romer LH, Yim EK. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano. 2013;7:4785–4798. doi: 10.1021/nn304966z. [DOI] [PubMed] [Google Scholar]
- 114.Thomsen MS, Routhe LJ, Moos T. The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab. 2017;37:3300–3317. doi: 10.1177/0271678X17722436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tian Z, Zhao Q, Biswas S, Deng W. Methods of reactivation and reprogramming of neural stem cells for neural repair. Methods. 2018;133:3–20. doi: 10.1016/j.ymeth.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 116.Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience. 2013;236:55–65. doi: 10.1016/j.neuroscience.2012.12.066. [DOI] [PubMed] [Google Scholar]
- 117.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 118.Ushiki T, Ide C. Three-dimensional organization of the collagen fibrils in the rat sciatic nerve as revealed by transmission- and scanning electron microscopy. Cell Tissue Res. 1990;260:175–184. doi: 10.1007/BF00297503. [DOI] [PubMed] [Google Scholar]
- 119.Wang J, Ye R, Wei Y, Wang H, Xu X, Zhang F, Qu J, Zuo B, Zhang H. The effects of electrospun TSF nanofiber diameter and alignment on neuronal differentiation of human embryonic stem cells. J Biomed Mater Res A. 2012;100A:632–645. doi: 10.1002/jbm.a.33291. [DOI] [PubMed] [Google Scholar]
- 120.Weiss P. The problem of specificity in growth and development. Yale J Biol Med. 1947;19:235–278. [PMC free article] [PubMed] [Google Scholar]
- 121.Wieringa PA, Gonçalves de Pinho AR, Micera S, van Wezel RJA, Moroni L. Biomimetic architectures for peripheral nerve repair: a review of biofabrication strategies. Adv Healthc Mater. 2018;7:1701164. doi: 10.1002/adhm.201701164. [DOI] [PubMed] [Google Scholar]
- 122.Wong ST, Teo SK, Park S, Chiam KH, Yim EK. Anisotropic rigidity sensing on grating topography directs human mesenchymal stem cell elongation. Biomech Model Mechanobiol. 2014;13:27–39. doi: 10.1007/s10237-013-0483-2. [DOI] [PubMed] [Google Scholar]
- 123.Xie J, Willerth SM, Li X, Macewan MR, Rader A, Sakiyama-Elbert SE, Xia Y. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials. 2009;30:354–362. doi: 10.1016/j.biomaterials.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014a;13:645. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 126.Yang K, Jung K, Ko E, Kim J, Park KI, Kim J, Cho SW. Nanotopographical manipulation of focal adhesion formation for enhanced differentiation of human neural stem cells. ACS Appl Mater Interfaces. 2013;5:10529–10540. doi: 10.1021/am402156f. [DOI] [PubMed] [Google Scholar]
- 127.Yang K, Jung H, Lee HR, Lee JS, Kim SR, Song KY, Cheong E, Bang J, Im SG, Cho SW. Multiscale, hierarchically patterned topography for directing human neural stem cells into functional neurons. ACS Nano. 2014b;8:7809–7822. doi: 10.1021/nn501182f. [DOI] [PubMed] [Google Scholar]
- 128.Yim EK, Sheetz MP. Force-dependent cell signaling in stem cell differentiation. Stem Cell Res Ther. 2012;3:41. doi: 10.1186/scrt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yim EK, Pang SW, Leong KW. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res. 2007;313:1820–1829. doi: 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yim EK, Darling EM, Kulangara K, Guilak F, Leong KW. Nanotopography-induced changes in focal adhesions, cytoskeletal organization and mechanical properties of human mesenchymal stem cells. Biomaterials. 2010;31:1299–1306. doi: 10.1016/j.biomaterials.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yip AK, Nguyen AT, Rizwan M, Wong ST, Chiam KH, Yim EK. Anisotropic traction stresses and focal adhesion polarization mediates topography-induced cell elongation. Biomaterials. 2018;181:103–112. doi: 10.1016/j.biomaterials.2018.07.057. [DOI] [PubMed] [Google Scholar]
- 132.Yue B. Biology of the extracellular matrix: an overview. J Glaucoma. 2014;23:S20–S23. doi: 10.1097/IJG.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zeng Y, Wong ST, Teo SK, Leong KW, Chiam KH, Yim EK. Human mesenchymal stem cell basal membrane bending on gratings is dependent on both grating width and curvature. Sci Rep. 2018;8:6444. doi: 10.1038/s41598-018-24123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130:635–653. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.