Abstract
Activation of the unfolded protein response in response to endoplasmic reticulum stress preserves cell viability and function under stressful conditions. Nevertheless, persistent, unresolvable activation of the unfolded protein response can trigger apoptosis to eliminate stressed cells. Recent studies show that the unfolded protein response plays an important role in the pathogenesis of various disorders of myelin, including multiples sclerosis, Charcot-Marie-Tooth disease, Pelizaeus-Merzbacher disease, vanishing white matter disease, spinal cord injury, tuberous sclerosis complex, and hypoxia-induced perinatal white matter injury. In this review we summarize the current literature on the unfolded protein response and the evidence for its role in the pathogenesis of myelin disorders.
Keywords: axon, ER, multiples sclerosis, myelin, oligodendrocyte, Schwann cell, spinal cord injury, UPR
Introduction
Endoplasmic reticulum (ER) is the cellular organelle in eukaryotic cells that is responsible for protein modification and folding, lipid biosynthesis, and maintaining cellular calcium homeostasis (Kaufman, 1999; Lin and Popko, 2009). Ribosomes on the cytosolic surface of the ER synthesize secretory and membrane proteins which translocate into the ER lumen via pores in the ER membrane. Protein modification and folding occurs in the ER lumen with the help of chaperones and enzymes. Properly folded proteins are transported to intracellular organelles, the plasma membrane, or extracellular space. Imbalance between protein translation and protein modification and folding leads to accumulation of unfolded or misfolded proteins in the ER lumen, resulting in ER stress and activation of the unfolded protein response (UPR). The UPR, which comprises three parallel signaling branches, pancreatic endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6α (ATF6α), restores ER homeostasis by facilitating protein folding, attenuating protein translation, and enhancing protein degradation (Marciniak and Ron, 2006; Walter and Ron, 2011; Hetz et al., 2015; Wang and Kaufman, 2016; Song et al., 2018; Almanza et al., 2019). Nevertheless, persistent, unresolvable activation of the UPR can trigger apoptosis programs to eliminate stressed cells (Tabas and Ron, 2011; Hetz and Papa, 2018).
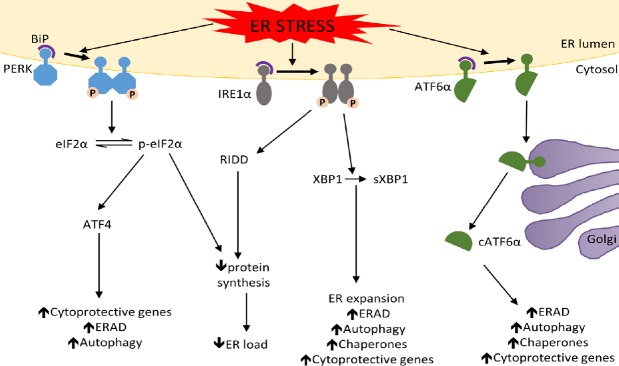
Under physiological conditions, immunoglobulin heavy chain binding protein (BiP), an ER chaperone, binds to the regulatory luminal domain of PERK, IRE1, and ATF6α and keeps them in an inactive state (Figure 1). During ER stress, unfolded or misfolded proteins compete for BiP binding, resulting in dissociation of BiP from each of the ER stress transducers (Marciniak and Ron, 2006; Walter and Ron, 2011; Hetz et al., 2015; Wang and Kaufman, 2016; Song et al., 2018; Almanza et al., 2019). The dissociation of BiP leads to oligomerization and autophosphorylation of PERK and IRE1. Phosphorylated PERK (p-PERK) inhibits global protein translation via the phosphorylation of the α subunit of eukaryotic translation initiation factor 2 (eIF2α); however, this also specifically heightens the translation of activating transcription factor 4 (ATF4). ATF4 stimulates the expression of genes that regulate autophagy, ER-associated degradation (ERAD), and cell viability. Phosphorylated eIF2α (p-eIF2α) level is tightly regulated by the protein phosphatase 1 (PP1) and growth arrest and DNA damage 34 (GADD34) complex which quickly dephosphorylates p-eIF2α, preventing detrimental long term global protein biosynthesis inhibition. Interestingly, GADD34 is upregulated by CAATT enhancer binding protein homologous protein (CHOP), a transcription factor whose expression is stimulated by ATF4, thus the PERK-eIF2α pathway is regulated via a tight autofeedback loop. Phosphorylation of IRE1 causes splicing of X-box binding protein 1 (XBP1) mRNA through its endoribonuclease activity. Spliced XBP1 (sXBP1) is a transcription factor that increases expression of chaperones and genes involved in ER biogenesis, autophagy, ERAD, and cell viability. Phosphorylated IRE1 can also reduce protein biosynthesis by enhancing degradation of certain mRNAs through regulated IRE1-dependent decay. Furthermore, by binding to certain adaptor proteins phosphorylated IRE1 can activate the JUN amino-terminal kinase and the apoptosis signal-regulating kinase 1. The dissociation of BiP allows ATF6α to transit to the Golgi complex. In the Golgi ATF6α is cleaved by Site-1 protease and Site-2 protease. Cleaved ATF6α (cATF6α) is a transcription factor that enhances the expression of chaperones and genes involved in autophagy, ERAD, and cell viability.
Figure 1.
The unfolded protein response (UPR).
Under normal conditions PERK, IRE1, and ATF6α are sequestered in an inactive state in the ER via their associations with BiP. BiP dissociates from the ER stress transducers in the presence of high levels of unfolded or misfolded proteins, leading to the UPR activation. The PERK pathway: Following BiP dissociation PERK becomes active through oligomerization and autophosphorylation. p-PERK then phosphorylates eIF2α which reduces ER load by decreasing global protein synthesis. p-eIF2α also preferentially stimulates the translation of ATF4, which enhances the expression of cytoprotective genes, autophagy-related genes, and ERAD-related genes. The IRE1 pathway: IRE1 becomes active after BiP dissociation through oligomerization and autophosphorylation. p-IRE1 splices XBP1 mRNA to generate sXBP1, a transcription factor that stimulates the expression of chaperones and genes involved in ER expansion, ERAD, autophagy, and cytoprotection. p-IRE1 also reduces ER load through the degradation of mRNA via RIDD. The ATF6α pathway: After BiP dissociation ATF6α transits to the Golgi complex where it is cleaved by the proteases S1P and S2P. cATF6α then migrates to the nucleus where is stimulates the expression of chaperons, autophagy-related genes, ERAD-related genes, and cytoprotective genes. “ ↑ ”: Increase; “ ↓ ”: decrease; ATF4: activating transcription factor 4; ATF6α: activating transcription factor 6α; BiP: immunoglobulin heavy chain binding protein; cATF6α: cleaved activating transcription factor 6α; eIF2α: α subunit of eukaryotic translation initiation factor 2; ER: endoplasmic reticulum; ERAD: endoplasmic reticulum-associated degradation; IRE1: inositol-requiring enzyme 1; p-eIF2α: phosphorylated α subunit of eukaryotic translation initiation factor 2; PERK: pancreatic endoplasmic reticulum kinase; p-IRE1: phosphorylated inositol-requiring enzyme 1; p-PERK: phosphorylated pancreatic endoplasmic reticulum kinase; RIDD: regulated IREI-dependent decay; SIP: Site-1 protease; S2P: Site-2 protease; sXBP1: spliced XBP1; XBP1: X-box binding protein 1.
Recent studies have identified a number of small chemical compounds that selectively modulate the activity of these three individual branches of the UPR (Hetz et al., 2013; Maly and Papa, 2014). GSK2606414 and GSK2656157 selectively bind to the eIF2α kinase domain of the PERK protein, suppress PERK-mediated eIF2α phosphorylation (Axten et al., 2012; Harding et al., 2012; Atkins et al., 2013). CCT020312, compound A, compound B, and compound C stimulate PERK-mediated eIF2α phosphorylation in the absence of ER stress (Stockwell et al., 2012; Xie et al. 2015). Salburinal is a selective inhibitor of the phosphatase complexes responsible for p-eIF2α dephosphorylation (Boyce et al., 2005). Guanabenz and Sephin1 selectively bind to GADD34 and inhibit GADD34/PP1 complex activity, diminishing p-eIF2α dephosphorylation (Tsaytler et al., 2011; Das et al., 2015). STF-083010, MKC-3946, and 4µ8c inhibit the endoribonuclease activity of IRE1 but not its ability to oligomerize and autophosphorylate (Papandreou et al., 2011; Mimura et al., 2012; Cross et al., 2012; Tam et al. 2014). KIRA3, KIRA, and KIRA6 impair IRE1 oligomerization, autophosphorylation, and endoribonuclease activity (Wang et al., 2012; Ghosh et al., 2014). 1NM-PP1 can activate the endoribonuclease activity of IRE1 in the absence of kinase autophosphorylation (Papa et al., 2003; Hollien et al., 2009). APY29 and sunitinib enhance IRE1 oligomerization, autophosphorylation, and endoribonuclease activity (Korennykh et al., 2009; Maly and Papa, 2014). Ceapins suppress ATF6α activation by selectively inhibiting its transport to the Golgi complex (Gallagher et al., 2016; Gallagher and Walter, 2016). Small molecules 147 and 263 activate ATF6α in the absence of ER stress (Plate et al., 2016).
Myelin is a lipid-rich multilamellar sheath that wraps around axons in the central nervous system (CNS) and peripheral nervous system (PNS). The primary function of myelin is to facilitate conduction of the action potential along axons and to maintain axonal integrity (Chang et al., 2016; Tomassy et al., 2016). Myelinating cells, oligodendrocytes in the CNS and Schwann cells in the PNS, must produce enormous amounts of myelin proteins via the ER to assemble and maintain the myelin sheath (Pfeiffer et al., 1993; Anitei and Pfeiffer, 2006; Lin and Popko, 2009). The functional significance of the UPR in myelinating cells is highlighted by myelinating cell-specific BiP deficient mice that display severe myelinating cell loss and myelin loss in the CNS and PNS (Hussien et al., 2015). Moreover, it has been shown that ER stress and the UPR play a major role in various myelin disorders (Lin and Popko, 2009; Clayton and Popko, 2016; Volpi et al., 2017). In this review, we summarize the current understanding of the contribution of the UPR to the development of neurological diseases characterized by damage to the myelin sheath, such as multiple sclerosis (MS), Charcot-Marie-Tooth disease (CMT), Pelizaeus-Merzbacher disease (PMD), vanishing white matter disease (VWMD), spinal cord injury (SCI), tuberous sclerosis complex (TSC), and hypoxia-induced perinatal white matter injury (PWMI).
Search Strategy and Selection Criteria
PubMed search was conducted using the following key words: endoplasmic reticulum stress and myelin; unfolded protein response and myelin; endoplasmic reticulum stress and oligodendrocyte; endoplasmic reticulum stress and Schwann cell; unfolded protein response and oligodendrocyte; unfolded protein response and Schwann cell. The search was performed on March 10, 2019. Any papers related to the topic were selected, cited, and discussed in this review article.
Unfolded Protein Response in Multiple Sclerosis
MS and its animal model experimental autoimmune encephalomyelitis (EAE) are chronic inflammatory demyelinating and neurodegenerative diseases in the CNS (Lassmann and Bradl, 2017; Baecher-Allan et al., 2018; Reich et al., 2018). Myelin, oligodendrocytes, axons, and neurons are destroyed by inflammation in MS patients and EAE animals. It is well known that the UPR is activated in oligodendrocytes, T cells, macrophages/microglia, and astrocytes in MS and EAE (Lin and Popko, 2009; Stone and Lin, 2015; Way and Popko, 2016). Moreover, recent studies demonstrate activation of the UPR in neurons in MS and EAE (Ní Fhlathartaigh et al., 2013; Stone and Lin, 2015; Stone et al., 2019). Importantly, a large number of studies suggest that the UPR has a key role in regulating the viability of oligodendrocyte and axons in MS and EAE.
Several studies have demonstrated that activation of the PERK-eIF2α pathway protects mature oligodendrocytes and myelin against inflammation during EAE. Using transgenic mice which have temporally controlled interferon-γ (IFN-γ, an important pro-inflammatory cytokine in MS and EAE) expression in the CNS (Lin et al., 2004), a study shows that expression of IFN-γ in the CNS prior to EAE onset results in attenuation of disease severity and amelioration of EAE-induced oligodendrocyte loss, demyelination, and axon degeneration in the CNS (Lin et al., 2007). Interestingly, the beneficial effects of IFN-γ in EAE are associated with activation of the PERK-eIF2α pathway in oligodendrocytes and are diminished in PERK heterozygous deficient mice (Lin et al., 2007). Moreover, a report shows that EAE disease severity and EAE-induced oligodendrocyte loss, demyelination, and axon degeneration are significantly increased in mice with oligodendrocyte-specific PERK deficiency (Hussien et al., 2014). Additionally, PLP/Fv2E PERK mice which have controllable activation of the PERK-eIF2α pathway in oligodendrocytes has been generated (Lin et al., 2013). Fv2E PERK, an artificial PERK derivative whose activity is controlled by the dimerizer AP20187 and decoupled from ER stress (Lu et al., 2004), is specifically expressed in oligodendrocytes of PLP/Fv2E PERK mice. Treatment with a low dose of AP20187 induces moderate activation of PERK signaling selectively in oligodendrocytes of heterozygous PLP/Fv2E PERK mice, but does not affect the viability or function of oligodendrocytes (Lin et al., 2013, 2014a). Importantly, reports show that moderate PERK activation selectively in oligodendrocytes starting prior to EAE onset significantly attenuates EAE disease severity and ameliorates EAE-induced oligodendrocytes loss, demyelination, axon degeneration, and neuron loss in the CNS (Lin et al., 2013, Yue et al., 2019). Thus, these studies suggest the cytoprotective effects of PERK activation on mature oligodendrocytes in MS and EAE.
A number of studies have also demonstrated that activation of the PERK-eIF2α pathway promotes survival of (re)myelinating oligodendrocytes in immune-mediated demyelinating diseases. The presence of IFN-γ in the CNS induces ER stress and the UPR activation in myelinating oligodendrocytes via the JAK-STAT1 pathway, and results in myelinating oligodendrocyte apoptosis and myelin loss in the CNS of young, developing mice (Lin et al., 2005; Lin and Lin, 2010). Interestingly, PERK heterozygous deficiency exacerbates IFN-γ-induced myelinating oligodendrocyte apoptosis and myelin loss during developmental myelination (Lin et al., 2005). Conversely, a study shows that GADD34 inactivation in young, developing mice that express IFN-γ in the CNS results in enhanced activation of the PERK-eIF2α pathway in myelinating oligodendrocytes, and attenuation of IFN-γ-induced myelinating oligodendrocyte apoptosis and myelin loss (Lin et al., 2008). On the other hand, the presence of IFN-γ in the CNS induces remyelinating oligodendrocyte apoptosis and suppresses remyelination in the cuprizone model (Lin et al., 2006). Intriguingly, activation of the PERK-eIF2α pathway is associated with the detrimental effects of IFN-γ on remyelinating oligodendrocytes, and mice with PERK heterozygous deficiency have exacerbated IFN-γ-induced remyelinating oligodendrocyte apoptosis and remyelination failure in the cuprizone model (Lin et al., 2006). Additionally, using heterozygous PLP/Fv2E PERK mice treated with a low dose of AP20187, a report shows that moderate PERK activation selectively in oligodendrocytes provides protection to (re)myelinating oligodendrocytes and myelin from the harmful effects of IFN-γ in young, developing mice and in the cuprizone model (Lin et al., 2014a). Importantly, this report also shows that moderate PERK activation selectively in oligodendrocytes during the recovery stage of EAE facilitates oligodendrocyte regeneration and remyelination in EAE demyelination lesions (Lin et al., 2014a). Taken together, these studies imply the cytoprotective effects of PERK activation on remyelinating oligodendrocytes in MS and EAE.
Nevertheless, it remains elusive how the PERK-eIF2α pathway exerts cytoprotective effects on oligodendrocytes in MS and EAE. ATF4 is regarded as the master transcription factor of the PERK-eIF2α pathway (Marciniak and Ron, 2006; Walter and Ron, 2011; Wang and Kaufman, 2016). Surprisingly, a recent study shows that oligodendrocyte-specific ATF4 inactivation did not alter EAE disease severity or EAE-induced oligodendrocyte loss, demyelination, axon degeneration, or neuron loss in the CNS, although this study demonstrates activation of ATF4 in oligodendrocytes during EAE (Yue et al., 2019). In accordance with this study, a previous report shows that global deletion of CHOP, a major responsive gene of ATF4, does not affect EAE development (Deslauriers et al., 2011). Thus, it is unlikely that ATF4 is involved in the cytoprotective effects of the PERK-eIF2α pathway on oligodendrocytes during EAE. Conversely, the PERK-eIF2α pathway can activate another transcription factor, NF-κB, via inhibition of the translation of its inhibitor IκBα (Deng et al., 2004; Yue et al., 2018). In vitro and in vivo studies show that activation of the PERK-eIF2α pathway leads to activation of NF-κB in oligodendrocytes (Lin et al., 2012, 2013). Interestingly, a recent report shows that NF-κB activation protects oligodendrocytes against inflammation in immune-mediated demyelinating diseases (Stone et al., 2017). Therefore, it is possible that NF-κB activation accounts for the cytoprotective effects of the PERK-eIF2α pathway on oligodendrocytes in MS and EAE.
It is generally believed that neurodegeneration, particularly axon degeneration, is the major contributor to chronic disability in MS (Baecher-Allan et al., 2018; Reich et al., 2018). Interestingly, a recent study suggests the neuroprotective effects of PERK signaling in neurons in MS and EAE (Stone et al., 2019). Using a mouse model in which PERK is ubiquitously and selectively inactivated in neurons, the authors show that neuron-specific PERK inactivation impairs EAE resolution without affecting EAE initiation. Importantly, they show that neuron-specific PERK inactivation dramatically exacerbates axon degeneration and demyelination, but does not alter the numbers of oligodendrocytes or oligodendrocyte precursor cells (OPCs) in the lumbar spinal cord at the recovery stage of EAE. Moreover, neuron-specific PERK inactivation significantly but moderately increases neuron loss in the grey matter of CNS at the recovery stage of EAE (Stone et al., 2019). Additionally, the authors also generate a mouse model in which ATF4 is ubiquitously and selectively inactivated in neurons; however, they find that neuron-specific ATF4 inactivation has a minimal effect on EAE disease course or EAE-induced pathology, including neuron loss, axon degeneration, and demyelination in the CNS (Stone et al., 2019). Thus, these results suggest that ATF4 does not mediate the neuroprotective effects of PERK activation in neurons in MS and EAE.
Furthermore, recent studies suggest that enhancing activation of the PERK-eIF2α pathway has therapeutic potential in MS patients. A study shows that treatment with Salburinal increases p-eIF2α level and ameliorates myelinating oligodendrocyte death and myelin loss in cultured hippocampal slices exposed to IFN-γ (Lin et al., 2008). Another study shows that treatment with Guanabenz elevates p-eIF2α level and protects myelinating oligodendrocytes from IFN-γ cytotoxicity in cultured cerebellar slices and in the CNS of young, developing IFN-γ-expressing mice (Way et al., 2015). Importantly, this study shows that treatment with Guanabenz increases p-eIF2α levels in oligodendrocytes, attenuates the severity of disease, and ameliorates oligodendrocyte death and demyelination in the CNS of EAE mice (Way et al., 2015). This study also shows that treatment with Guanabenz has no effect on the proliferation of T cell or on the production of cytokines in the peripheral immune system, but inhibits CD4 T cell activation in the CNS during EAE (Way et al., 2015). Moreover, a very recent study shows that Sephin1 treatment delays the disease onset, increases the levels of p-eIF2α, ATF4, and CHOP, and attenuates oligodendrocyte loss, demyelination, and axon degeneration in the CNS of EAE mice (Chen et al., 2019). Additionally, this report shows that combining treatment with Sephin1 and IFN-β further ameliorates the disease symptoms and attenuates oligodendrocyte loss and demyelination in the CNS of EAE mice (Chen et al., 2019). Thus, these studies imply therapeutic value of enhancing the PERK-eIF2α pathway in MS.
Although a report suggests activation of the IRE1-XBP1 pathway in MS lesions (Mháille et al., 2008), a number of studies show that this pathway is not activated in MS and EAE (Hussien et al., 2015; Stone and Lin, 2015; Stone et al, 2017; Falcão et al., 2018). Conversely, recent studies suggest that the ATF6α pathway is a player in MS and EAE. A very recent study shows that global deletion of ATF6α exacerbates IFN-γ-induced myelinating oligodendrocyte apoptosis and myelin loss in young, developing mice (Stone et al., 2018). This study also shows that global deletion of ATF6α increases the disease severity, aggravates oligodendrocyte loss and demyelination in the CNS, but does not affect inflammation in mice undergoing EAE (Stone et al., 2018). Interestingly, the harmful effects of ATF6α deficiency seen in IFN-γ-expressing mice and EAE mice are associated with the impaired expression of the chaperone BiP (a major ATF6α-responsive gene) in oligodendrocytes (Stone et al., 2018). In parallel to the above findings, a study shows that mice with heterozygous BiP deficiency specifically in oligodendrocytes have exacerbated EAE disease severity and increased EAE-induced oligodendrocyte death and demyelination in the CNS (Hussien et al., 2015). Thus, these data suggest the protective effects of the ATF6α-BiP pathway on oligodendrocytes in MS and EAE. Nevertheless, in contrast to the studies described above, another study shows that global deletion of ATF6α decreases EAE severity by impairing activation of microglia in the CNS (Ta et al., 2016). Therefore, it is important to use cell-specific conditional ATF6α deficient mouse models to further define the precise roles of ATF6α activation in oligodendrocytes and microglia in MS and EAE pathogenesis.
Unfolded Protein Response in Charcot-Marie-Tooth Disease
CMT is a group of PNS disorders that are caused by mutations in a number of distinct genes (Berger et al., 2006; Theocharopoulou and Vlamos, 2015). The duplication or mutation of peripheral myelin proteins accounts for the majority of demyelinating forms of CMT (CMT1). CMT1A is an autosomal dominant disease that results from a duplication or mutation of peripheral myelin protein 22 (PMP22) (Berger et al., 2006). Several studies suggest that ER stress participates in the pathogenesis of CMT1A. Trembler (Tr) or Trembler-J (Tr-J) mutation introduces a non-conservative amino acid change into the hydrophobic domains of PMP22. It has been shown that PMP22Tr or PMP22Tr-J is misfolded, associated with the ER chaperone Calnexin, and accumulated in the ER lumen of Schwann cells (Colby et al., 2000; Dickson et al., 2002). A study shows that treatment of PMP22Tr-J mice with Curcumin, a chemical compound that is able to stimulate the transport of misfolded proteins from the ER to the plasma membrane, significantly decreases apoptosis of Schwann cell and increases the size and number of myelinated axons in the sciatic nerves, resulting in improved motor performance (Khajavi et al., 2007). Another study shows that accumulation of PMP22Tr-J in the ER lumen disrupts ER homeostasis and leads to ER stress, resulting in increased expression of BiP, CHOP, ATF3, and sXBP1 in Schwann cells (Okamoto et al., 2013). Importantly, this study also shows that Curcumin treatment diminishes the induction of BiP, CHOP, and ATF3 in Schwann cells of the PMP22Tr-J mice (Okamoto et al., 2013). Taken together, these studies indicate that Curcumin treatment ameliorates Schwann cell apoptosis by relieving ER stress through attenuation of PMP22Tr-J accumulation in the ER lumen.
CMT1B is an autosomal dominant disease caused by a number of distinct point mutations in Myelin Protein Zero (P0) (Berger et al., 2006). Mutations in the gene encoding P0 have been shown to result in ER stress in Schwann cells. It has been shown that P0 deleted for S63 (P0S63del) causes retention of the mutant protein in the ER of Schwann cells due to disruption of the alignment of the hydrophobic residues in β strand C of P0, this leads to activation of all three branches of the UPR in the nerves of P0S63del mice, a mouse model of CMT1B (Wrabetz et al., 2006; Pennuto et al., 2008). Moreover, an in vitro study shows that many CMT1B-causing P0 mutations result in retention of mutant proteins in the ER and the UPR activation in RT4 Schwann cells (Bai et al., 2018)
It has also been demonstrated that the PERK-eIF2α-CHOP pathway influences the development of myelin abnormalities in P0S63del mice. Pennuto et al. show that CHOP is not detectable in Schwann cells from normal nerves, but is detectable in the nuclei of P0S63del Schwann cells (Pennuto et al., 2008). They cross P0S63del mice with CHOP null mice and find that CHOP deletion reduces the number of demyelinated fibers in P0S63del nerves and attenuates the behavioural and electrophysiological abnormalities in P0S63del mice (Pennuto et al., 2008). However, CHOP deletion has a minimal effect on Schwann cell apoptosis and hypomyelination in P0S63del nerves (Pennuto et al., 2008). Moreover, D’Antonio et al. (2013) shows that the level of GADD34 is increased in P0S63del nerves and that CHOP deletion diminishes P0S63del-induced GADD34 upregulation, resulting in an increased level of p-eIF2α. They further show that GADD34 deletion increases the level of p-eIF2α and ATF4, attenuates protein biosynthesis, and reduces the levels of cATF6 and sXBP1 in P0S63del nerves (D’Antonio et al., 2013). Importantly, they show that GADD34 deficiency attenuates hypomyelination in P0S63del nerves and ameliorates the behavioural and electrophysiological abnormalities in P0S63del mice (D’Antonio et al., 2013). Taken together, these two studies suggest that induction of GADD34, which functions as a detrimental effector of CHOP, reduces the level of p-eIF2α, and subsequently leads to increased expression of P0S63del and accumulation of P0S63del in the ER of Schwann cells, resulting in myelin abnormalities in P0S63del nerves. In contrast, using global PERK heterozygous deficient mice, Musner et al. (2016) show that heterozygous PERK deficiency attenuates the behavioural and electrophysiological abnormalities of P0S63del mice, reduces demyelination and onion bulbs in P0S63del nerves, but does not affect the degree of hypomyelination in P0S63del nerves. Heterozygous PERK deficiency reduces the level of p-eIF2α, but does not alter the levels of ATF4, CHOP, GADD34, cATF6α, or sXBP1 in P0S63del nerves (Musner et al., 2016). Similarly, using Schwann cell-specific PERK deficient mice, Sidoli et al. show that PERK deficiency specifically in Schwann cells attenuates hypomyelination in P0S63del nerves and ameliorates the behavioural and electrophysiological abnormalities in P0S63del mice (Sidoli et al., 2016). Schwann cell-specific PERK deficiency also reduces the level of p-eIF2α, but does not alter the levels of ATF4, CHOP, GADD34, cATF6α, or sXBP1 in P0S63del nerves (Sidoli et al., 2016). Collectively, these two studies suggest the detrimental effects of PERK activation in Schwann cells of P0S63del mice. Clearly, the paradoxical effects of the PERK-eIF2α-CHOP pathway on the pathogenesis of P0S63del mice warrant further investigation.
Furthermore, recent studies suggest that targeting the PERK-eIF2α pathway has therapeutic potential in CMT1B. Using dorsal root ganglia (DRG)-explant cultures, a study shows that myelination is dramatically reduced in S63del DRG explant cultures compared to wild type DRG explant cultures and that treatment with Salubrinal improved myelination in S63del DRG cultures (D’Antonio et al., 2013). This study also shows that treatment with Salubrinal reduces demyelinated fibers and onion bulbs in P0S63del nerves and attenuates the behavioural and electrophysiological abnormalities of P0S63del mice (D’Antonio et al., 2013). Moreover, another study shows that treatment with Sephin1 impairs the upregulation of CHOP and sXBP1 and rescues the myelination deficit in S63del DRG explant cultures (Das et al., 2015). Importantly, treating P0S63del mice with Sephin1 also impairs the upregulation of CHOP and sXBP1, rescues myelination deficit in the sciatic nerves, and attenuates their behavioural and electrophysiological abnormalities (Das et al., 2015).
The Unfolded Protein Response in Pelizaeus-Merzbacher Disease
PMD is a dysmyelinating disease of the CNS caused by mutations or increased expression of the X-linked proteolipid protein 1 (PLP1) gene. PMD has a large range of clinical severity, which is thought to depend on the nature on the PLP1 mutation (Gow and Lazzarini, 1996; Garbern 2007). The PLP1 gene encodes PLP and minor isoform DM20, which account for up to 50% of the total myelin protein in the CNS. Using cell cultures, post-mortem human tissues, and animal models carrying PLP mutations, previous studies show that mutant PLP proteins accumulate in the ER lumen, which causes ER stress and the UPR activation in oligodendrocytes (Southwood and Gow, 2001; Lin and Popko, 2009; Clayton and Popko, 2016). Moreover, using induced pluripotent stem cells from PMD patients, a report shows that induced pluripotent stem cells-derived oligodendrocytes displays accumulation of mutant PLP1 in the ER, activation of the UPR, and increased apoptosis (Numasawa-Kuroiwa et al., 2014).
To further comprehend the role of the UPR in the pathogenesis of PMD, Southwood et al. (2002) exploited a genetic approach by crossing PLP mutant mice with CHOP null mice. It is surprising that they find that CHOP deletion notably exacerbates the clinical phenotypes and oligodendrocyte apoptosis in PLP mutant mice (Southwood et al., 2002). CHOP is considered to be a pro-apoptotic transcription factor during ER stress (Tabas and Ron, 2011; Hetz and Papa, 2018). The cytoprotective effects of CHOP on oligodendrocytes of PLP mutant mice are strikingly contrasted to its pro-apoptotic functions in other cell types. To uncover the mechanisms through which CHOP provides protection to oligodendrocytes, Southwood et al. (2016) have generated a mouse model that constitutively overexpresses CHOP specifically in oligodendrocytes. They find that overexpression of CHOP specifically in oligodendrocytes has no effect on oligodendrocyte viability and myelin integrity in the CNS, and does not affect mouse behaviour or life span. Nevertheless, it remains to see whether overexpression of CHOP specifically in oligodendrocytes attenuates oligodendrocyte apoptosis and myelin abnormalities in PLP mutant mice. Moreover, a report shows that caspase-12, an ER resident caspase, is activated in oligodendrocytes in PLP mutant mice; however, deletion of caspase-12 does not have a significant effect on the clinical phenotypes, oligodendrocyte apoptosis, or myelin abnormalities in PLP mutant mice (Sharma and Gow, 2007). Additionally, another report shows that ATF3 is upregulated in oligodendrocytes in PLP mutant mice, but ATF3 deletion has a minimal effect on the apoptosis of oligodendrocytes in these animals (Sharma et al., 2007). Collectively, these studies suggest the potential role of the UPR in the pathogenesis of PMD. Further investigations that use mice with oligodendrocyte-specific deletion of PERK, ATF6α, or IRE1 to dissect their precise roles in oligodendrocytes of PLP mutant mice are warranted.
Interestingly, recent studies suggest that targeting the UPR may have therapeutic potential in PMD. A study shows that treatment with Ro 25–6981, a UPR modulator, enhances oligodendrocyte survival in human PMD patient induced pluripotent stem cells-derived cultures and Jimpy mice, a mouse model of PMD (Elitt et al., 2018). However, this study also shows that increasing oligodendrocyte survival induced by Ro 25–6981 treatment does not result in subsequent enhanced myelination in the CNS of Jimpy mice. Moreover, an in vitro study shows that treatment with 4-phenylbutyrate, a FDA approved drug and chemical chaperone that facilitates protein folding in the ER lumen, attenuates ER stress in an immortalized oligodendroglial cell line (158JP) transfected with a PLP mutation and reduces cell apoptosis (Wilding et al., 2018).
The Unfolded Protein Response in Vanishing White Matter Disease
VWMD is an inherited autosomal-recessive hypomyelination disorder caused by mutations in the genes encoding the five subunits of eIF2B which are ubiquitously expressed in all cell types (Bugiani et al., 2010; Lin, 2015). The brain white matter is predominantly affected in VWMD patients, exhibiting severe myelin loss and foamy oligodendrocytes. eIF2B acts as a guanine nucleotide exchange factor that promotes GDP release from its substrate eIF2, resulting in formation of the active eIF2-GTP complex. The eIF2-GTP complex then binds aminoacylated initiator methionyl tRNA, forming a ternary complex that is required for each protein translation initiation (Pavitt and Proud, 2009). While evidence suggests that mutations in eIF2B reduce its guanine nucleotide exchange factor activity, there is no strict correlation between the attenuated eIF2B activity and the severity of VWMD (Pavitt and Proud, 2009; Bugiani et al., 2010). Several knock-in mouse models that carry VWMD mutations have been generated. Although these mouse models show various degrees of attenuated eIF2B activity in cells, none of them develop the severe myelin abnormalities observed in VWMD patients (Geva et al., 2010; Dooves et al., 2016). Interestingly, a recent report shows that treatment with 2BAct, a small molecule eIF2B activator, restores the activity of eIF2B and eliminates minor myelin abnormalities in the CNS of mice carrying a VWMD mutation (Wong et al., 2019). Nevertheless, it remains mystery why and how mutations of ubiquitously expressed eIF2B selectively affect the white matter of CNS.
Several studies suggest that the PERK-eIF2α pathway is involved in oligodendrocyte dysfunction in VWMD. Using post-mortem samples, immunohistochemistry (IHC) analysis show that the levels of p-PERK, p-eIF2α, ATF4, and CHOP are increased in oligodendrocytes in the CNS of VWMD patients (van der Voorn et al., 2005; van Kollenburg et al., 2006). A report shows that primary fibroblasts cultured from individuals with VWMD respond normally to the pharmaceutical ER stress inducer thapsigargin, such as elevated p-eIF2α level and reduced global protein biosynthesis; however, induction of ATF4 is substantially increased in these cells compared to normal controls (Kantor et al., 2005). A study shows that ectopic expression of an eIF2B mutation derived from a VWMD patient in an oligodendroglial cell line results in increased expression of ATF4, GADD34, and BiP under basal condition and hyper-induction of these genes upon exposure to thapsigargin (Kantor et al., 2008). Moreover, a report shows that ectopic expression of eIF2B mutations in a human oligodendrocyte cell line leads to enhanced expression of ATF4 and increased apoptosis upon exposure to thapsigargin (Chen et al., 2015). Recently, Sekine et al. (2016) use the CRISPR-Cas9 approach to introduce the most severe known VWMD mutation, EIF2B4A391D into CHO cells. They find that the EIF2B4A391D mutation does not cause the UPR activation under basal condition, but leads to hyper-activation of the PERK-eIF2α pathway upon exposure to pharmaceutical ER stress inducers. Importantly, using homozygous PLP/Fv2E-PERK mice treated with a high dose of AP20187, a study shows that strong activation of PERK selectively in oligodendrocytes of young, developing mice inhibits eIF2B activity and reproduces the clinical and pathological features of VWMD in mice, including a tremoring phenotype, premature death, foamy oligodendrocytes, and severe myelin loss (Lin et al., 2014b). In contrast, strong activation of PERK selectively in oligodendrocytes of fully-myelinated adult mice also inhibits eIF2B activity, but despite this it has a minimal effect on oligodendrocytes and myelin (Lin et al., 2014b). This study raises the possibility that PERK activation in oligodendrocytes contributes to the pathogenesis of VWMD by synergizing with eIF2B mutations, leading to significantly inhibited protein synthesis in oligodendrocytes which impairs their cellular function (Lin, 2015). In contrast to human VWMD patients, mice carrying VWMD mutations display neither severe myelin abnormalities nor activation of the PERK-eIF2α pathway (Geva et al., 2010; Dooves et al., 2016). Thus, it is important to determine whether PERK activation facilitates oligodendrocyte dysfunction and myelin loss in mice carrying VWMD mutations.
The Unfolded Protein Response in Spinal Cord Injury
It is believed that the development of SCI is biphasic, a primary and secondary phase of injury (Rowland et al. 2008). The primary phase is caused by the initial mechanical injury, resulting in disruption of blood vessels, axons, and cell membranes. The secondary injury phase includes vascular dysfunction, inflammation, axon degeneration, oligodendrocyte death, and demyelination. Several lines of evidence have suggested that oligodendrocyte death and demyelination contribute significantly to neurological deficits in SCI patients (Siddiqui et al., 2015; Alizadeh and Karimi-Abdolrezaee, 2016). Data indicate that preventing demyelination and/or promoting remyelination represent important therapeutic targets for SCI treatments (Siddiqui et al., 2015; Alizadeh and Karimi-Abdolrezaee, 2016). Interestingly, activation of the UPR has been observed in oligodendrocytes in animal models of SCI. Several studies show that the mRNA levels of sXBP1, ATF4, CHOP, BiP, and GADD34 are significantly elevated in the injury epicenter as early as 6 hours post-SCI (Ohri et al., 2011; Fassbender et al., 2012). Moreover, double immunostaining analysis shows that the levels of p-eIF2α, ATF4, CHOP, and BiP are increased in oligodendrocytes in the injury epicentre (Ohri et al., 2011; Fassbender et al., 2012).
A number of studies show that the PERK-eIF2α-CHOP pathway modulates oligodendrocyte viability and myelin integrity in animal models of SCI. Using CHOP null mice, a study shows that CHOP deletion impairs the induction of sXBP1, ATF4, GADD34, and BiP in the injury epicenter, and leads to enhanced functional recovery after SCI (Ohri et al., 2011). Importantly, CHOP deletion attenuates oligodendrocyte apoptosis, ameliorates the reduction in myelin gene expression, and reduces myelin damage in the injury sites (Ohri et al., 2011). Similarly, a study shows that treatment with valproic acid, a nonselective histone deacetylase inhibitor and potential ER stress modulator, eliminates CHOP induction in the injury epicenter and reduces oligodendrocyte loss, myelin damage, and axon loss after SCI, which results in enhanced functional recovery (Penas et al., 2011). Moreover, Ohri et al. (2013) show that Salubrinal treatment increases the level of p-eIF2α, but decreases the levels of ATF4, GADD34, and BiP in the injury epicenter after SCI. Salubrinal treatment attenuates oligodendrocyte apoptosis, ameliorates the reduction of myelin gene expression, reduces myelin damage in the injury sites, and results in enhanced functional recovery after SCI (Ohri et al., 2013). Nevertheless, Ohri et al. also show that blockage of GADD34, via genetic mutation or treatment with its selective inhibitor Guanabenz, does not affect functional recovery after SCI (Ohri et al., 2014). Additionally, using ATF6α null mice, a recent study shows that the ATF6α pathway is not a major player in SCI. While an in vitro study shows that ATF6α deletion facilitates OPC apoptosis in response to the pharmaceutical ER stress inducers, ATF6α deletion has a minimal effect on functional recovery after SCI (Saraswat et al., 2018).
The Unfolded Protein Response in Tuberous Sclerosis Complex
TSC is a multisystem, autosomal-dominant disorder caused by mutations in genes encoding either TSC1 or TSC2 (Laplante and Sabatini, 2012). TSC often causes CNS dysfunctions, including cognitive impairment, epilepsy, and autism. In the CNS, one of major pathological feature of TSC is white matter abnormalities, including hypomyelination and oligodendrocyte loss (Griffiths et al., 1998; Krishnan et al., 2010). TSC1 and TSC2 form a heterodimeric protein complex to down-regulate the activity of mammalian-target-of-rapamycin. Several studies have shown that TSC1 deletion in oligodendrocytes induces mammalian-target-of-rapamycin activation and leads to oligodendrocyte death and hypomyelination in mice (Lebrun-Julien et al., 2014; Carson et al., 2015). Interestingly, a study shows the involvement of the PERK-eIF2α pathway in regulating oligodendrocyte viability in a mouse model of TSC (Jiang et al., 2016). The levels of p-PERK, p-eIF2α, CHOP, and GADD34 are significantly increased in oligodendrocytes of mice with oligodendrocyte-specific TSC1 deletion (Jiang et al., 2016). Importantly, treating these mice with Guanabenz results in increased p-eIF2α level in oligodendrocytes and reduced oligodendrocyte loss and myelin loss in the CNS (Jiang et al., 2016). Thus, these data suggest that mammalian-target-of-rapamycin activation induced by TSC1 deletion leads to excessive protein translation and ER stress in oligodendrocytes, resulting in oligodendrocyte death and myelin loss, and that activation of the PERK-eIF2α pathway preserves oligodendrocyte viability by attenuating ER stress through suppression of protein translation.
The Unfolded Protein Response in Hypoxia-Induced Perinatal White Matter Injury
PWMI is a myelin disorder which affects premature infants with a low-birth weight (born between 23 and 32 weeks of gestation), and is thought to be caused by hypoxia in the CNS due to underdeveloped neural vasculature and/or inefficient oxygenation from immature lungs (Deng, 2010; Back, 2015). Hypoxia predominantly affects OPCs in PWMI, inducing OPC death and inhibiting their ability to differentiate into myelinating oligodendrocytes, resulting in myelin loss and decreased white matter (Volpe, 2009; Back and Miller, 2014). It is well known that activation of the UPR in response to hypoxia preserves cell viability and function (Koumenis et al., 2007; Wouters and Koritzinsky, 2008). Surprisingly, a recent study shows that the UPR is not a major player in animal models of PWMI (Clayton et al., 2017). in vitro studies show that hypoxia activates the PERK-eIF2α pathway and suppresses primary mouse OPC differentiation, and that PERK deficiency diminishes hypoxia-induced activation of the PERK-eIF2α pathway in OPCs and increases their susceptibility to hypoxia (Clayton et al., 2017). In vivo studies, using two mouse models of PWMI, mild chronic hypoxia and severe acute hypoxia, shows that hypoxia elevates the level of p-eIF2α, but does not alter the expression of its downstream genes ATF4, CHOP, and GADD34 in OPCs (Clayton et al., 2017). Nevertheless, PERK deletion, GADD34 deletion, or CHOP deletion did not affect either mild chronic hypoxia- or severe acute hypoxia-induced PWMI (Clayton et al., 2017).
Therapeutic Potential and Future Directions
Accumulating evidence shows that the UPR is a promising therapeutic target for various diseases (Han and Kaufman, 2017; Hetz and Saxena, 2017), including myelin disorders (Table 1). Considerable progress has been made towards discovery of small chemical compounds that selectively modulate the activity of the three individual branches of the UPR (Hetz et al., 2013; Maly and Papa, 2014). Several studies have implied the therapeutic potential of these compounds in myelin disorders: 1) Salubrinal treatment is beneficial to animal models of MS, CMT1B, and SCI (Lin et al., 2008; D’Antonio et al., 2013; Ohri et al., 2013); 2) Guanabenz treatment is beneficial to animal models of MS and TSC (Way et al., 2015; Jiang et al., 2016); 3) Sephin1 treatment is beneficial to animal models of MS and CMT1B (Das et al., 2015; Chen et al., 2019). These studies are the basis for a clinical trial that tests the therapeutic potential of Guanabenz in MS patients (https://www.clinicaltrials.gov/ct2/show/NCT02423083). Moreover, orphan drug designation has been granted to Sepin1 for CMT1B treatment (http://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=449585). While therapeutic development of small chemical UPR modulators is promising, it is a formidable challenge to modulate the UPR for therapeutic purposes, without causing adverse effects.
Table 1.
The UPR is a promising therapeutic target for myelin disorders
| Disease | UPR pathway | Therapeutic target | Potential drug candidate |
|---|---|---|---|
| MS | PERK | PERK, GADD34 | PERK activators (e.g., CCT020312, compound A, compound B, and compound C), GADD34 inhibitors (e.g., Salburinal, Guanabenz and Sephin1) |
| ATF6α | ATF6α | ATF6α activators (e.g., Small molecules 147 and 263) | |
| CMT | PERK | GADD34 | GADD34 inhibitors (e.g., Salburinal, Guanabenz and Sephin1) |
| PMD | PERK | To be determined | To be determined |
| VWMD | PERK | PERK, eIF2B | PERK inhibitors (e.g., GSK2606414 and GSK2656157), eIF2B activators (e.g., 2BAct) |
| SCI | PERK | To be determined | To be determined |
| TSC | PERK | GADD34 | GADD34 inhibitors (e.g., Salburinal, Guanabenz and Sephin1) |
| PWMI | None | None | None |
ATF6α: Activating transcription factor 6α; CMT: Charcot–Marie–Tooth disease; eIF2α: α subunit of eukaryotic translation initiation factor 2; GADD34: growth arrest and DNA damage 34; MS: multiple sclerosis; PERK: pancreatic endoplasmic reticulum kinase; PMD; Pelizaeus– Merzbacher disease; PWMI: hypoxia-induced perinatal white matter injury; SCI: spinal cord injury; TSC: tuberous sclerosis complex; UPR: unfolded protein response; VWMD: vanishing white matter disease.
There are abundant studies on the effects of the PERK branch of the UPR in myelin disorders; however, their underlying mechanisms remain unknown and need further investigation. Moreover, the effects of the ATF6α and IRE1 branches on the pathogenesis of myelin disorders are still unclear. On the other hand, myelin proteins are synthesized, modified, and folded in the ER. Maintaining ER protein homeostasis is essential and necessary for the myelinating function of oligodendrocytes and Schwann cells. Nevertheless, the underlying mechanisms remain unexplored. The UPR is the primary mechanism that maintains ER protein homeostasis by facilitating protein folding, attenuating protein translation, and enhancing protein degradation. Therefore, it is important to assess the role of the UPR in maintaining ER protein homeostasis in oligodendrocytes and Schwann cells.
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
Financial support: This work was supported by grants from the National Institutes of Health (NS094151 and NS105689, both to WL).
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by grants from the National Institutes of Health (NS094151 and NS105689, both to WL).
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Alizadeh A, Karimi-Abdolrezaee S. Microenvironmental regulation of oligodendrocyte replacement and remyelination in spinal cord injury. J Physiol. 2016;594:3539–3552. doi: 10.1113/JP270895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almanza A, Carlesso A, Chintha C, Creedican S, Doultsinos D, Leuzzi B, Luís A, McCarthy N, Montibeller L, More S, Papaioannou A, Püschel F, Sassano ML, Skoko J, Agostinis P, de Belleroche J, Eriksson LA, Fulda S, Gorman AM, Healy S, Kozlov A, Muñoz-Pinedo C, Rehm M, Chevet E, Samali A. Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J. 2019;286:241–278. doi: 10.1111/febs.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anitei M, Pfeiffer SE. Myelin biogenesis: sorting out protein trafficking. Curr Biol. 2006;16:R418–R421. doi: 10.1016/j.cub.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Atkins C, Liu Q, Minthorn E, Zhang SY, Figueroa DJ, Moss K, Stanley TB, Sanders B, Goetz A, Gaul N, Choudhry AE, Alsaid H, Jucker BM, Axten JM, Kumar R. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73:1993–2002. doi: 10.1158/0008-5472.CAN-12-3109. [DOI] [PubMed] [Google Scholar]
- 5.Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, Li WH, Heerding DA, Minthorn E, Mencken T, Atkins C, Liu Q, Rabindran S, Kumar R, Hong X, Goetz A, Stanley T, Taylor JD, Sigethy SD, Tomberlin GH, Hassell AM, Kahler KM, Shewchuk LM, Gampe RT. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2, 3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2, 3-d]pyrimidin-4-amin (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) J Med Chem. 2012;55:7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 6.Back SA. Brain injury in the preterm infant: new horizons for pathogenesis and prevention. Pediatr Neurol. 2015;53:185–192. doi: 10.1016/j.pediatrneurol.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Back SA, Miller SP. Brain injury in premature neonates: a primary cerebral dysmaturation disorder. Ann Neurol. 2014;75:469–486. doi: 10.1002/ana.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron. 2018;97:742–768. doi: 10.1016/j.neuron.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Bai Y, Wu X, Brennan KM, Wang DS, D’Antonio M, Moran J, Svaren J, Shy ME. Myelin protein zero mutations and the unfolded protein response in Charcot Marie Tooth disease type 1B. Ann Clin Transl Neurol. 2018;5:445–455. doi: 10.1002/acn3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger P, Niemann A, Suter U. Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease) Glia. 2006;54:243–257. doi: 10.1002/glia.20386. [DOI] [PubMed] [Google Scholar]
- 11.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 12.Bugiani M, Boor I, Powers JM, Scheper GC, van der Knaap MS. Leukoencephalopathy with vanishing white matter: a review. J Neuropatho Exp Neurol. 2010;69:987–996. doi: 10.1097/NEN.0b013e3181f2eafa. [DOI] [PubMed] [Google Scholar]
- 13.Carson RP, Kelm ND, West KL, Does MD, Fu C, Weaver G, McBrier E, Parker B, Grier M D, Ess KC. Hypomyelination following deletion of Tsc2 in oligodendrocyte precursors. Ann Clin Transl Neurol. 2015;2:1041–1054. doi: 10.1002/acn3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang KJ, Redmond SA, Chan JR. Remodeling myelination: implications for mechanisms of neural plasticity. Nat Neurosci. 2016;19:190–197. doi: 10.1038/nn.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen N, Jiang YW, Hao HJ, Ban TT, Gao K, Zhang ZB, Wang JM, Wu Y. Different eukaryotic initiation factor 2Bε mutations lead to various degrees of intolerance to the stress of endoplasmic reticulum in oligodendrocytes. Chin Med J (Engl) 2015;128:1772–1777. doi: 10.4103/0366-6999.159353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Podojil JR, Kunjamma RB, Jones J, Weiner M, Lin W, Miller SD, Popko B. Sephin1, which prolongs the integrated stress response, is a promising therapeutic for multiple sclerosis. Brain. 2019;142:344–361. doi: 10.1093/brain/awy322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clayton BL, Huang A, Kunjamma RB, Solanki A, Popko B. The integrated stress response in hypoxia-induced diffuse white matter injury. J Neurosci. 2017;37:7465–7480. doi: 10.1523/JNEUROSCI.2738-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clayton BLL, Popko B. Endoplasmic reticulum stress and the unfolded protein response in disorders of myelinating glia. Brain Res. 2016;1648:594–602. doi: 10.1016/j.brainres.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colby J, Nicholson R, Dickson KM, Orfali W, Naef R, Suter U, Snipes GJ. PMP22 carrying the trembler or trembler-J mutation is intracellularly retained in myelinating Schwann cells. Neurobiol Dis. 2000;7:561–573. doi: 10.1006/nbdi.2000.0323. [DOI] [PubMed] [Google Scholar]
- 20.Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, Silverman RH, Neubert TA, Baxendale IR, Ron D, Harding HP. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci U S A. 2012;109:E869–E878. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Antonio M, Musner N, Scapin C, Ungaro D, Del Carro U, Ron D, Feltri ML, Wrabetz L. Resetting translational homeostasis restores myelination in Charcot-Marie-Tooth disease type 1B mice. J Exp Med. 2013;210:821–838. doi: 10.1084/jem.20122005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das I, Krzyzosiak A, Schneider K, Wrabetz L, D’Antonio M, Barry N, Sigurdardottir A, Bertolotti A. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science. 2015;348:239–242. doi: 10.1126/science.aaa4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng W. Neurobiology of injury to the developing brain. Nat Rev Neurol. 2010;6:328–336. doi: 10.1038/nrneurol.2010.53. [DOI] [PubMed] [Google Scholar]
- 25.Deslauriers AM, Afkhami-Goli A, Paul AM, Bhat RK, Acharjee S, Ellestad KK, Noorbakhsh F, Michalak M, Power C. Neuroinflammation and endoplasmic reticulum stress are coregulated by crocin to prevent demyelination and neurodegeneration. J Immunol. 2011;187:4788–4799. doi: 10.4049/jimmunol.1004111. [DOI] [PubMed] [Google Scholar]
- 26.Dickson KM, Bergeron JJ, Shames I, Colby J, Nguyen DT, Chevet E, Thomas DY, Snipes GJ. Association of calnexin with mutant peripheral myelin protein-22 ex vivo: a basis for “gain-of-function” ER diseases. Proc Natl Acad Sci U S A. 2002;99:9852–9857. doi: 10.1073/pnas.152621799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dooves S, Bugiani M, Postma NL, Polder E, Land N, Horan ST, van Deijk AL, van de Kreeke A, Jacobs G, Vuong C, Klooster J, Kamermans M, Wortel J, Loos M, Wisse LE, Scheper GC, Abbink TE, Heine VM, van der Knaap MS. Astrocytes are central in the pathomechanisms of vanishing white matter. J Clin Invest. 2016;126:1512–1524. doi: 10.1172/JCI83908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elitt MS, Shick HE, Madhavan M, Allan KC, Clayton BLL, Weng C, Miller TE, Factor DC, Barbar L, Nawash BS, Nevin ZS, Lager AM, Li Y, Jin F, Adams DJ, Tesar PJ. Chemical screening identifies enhancers of mutant oligodendrocyte survival and unmasks a distinct pathological phase in Elizaeus-Merzbacher disease. Stem Cell Reports. 2018;11:711–726. doi: 10.1016/j.stemcr.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falcão AM, van Bruggen D, Marques S, Meijer M, Jäkel S, Agirre E, Samudyata, Floriddia EM, Vanichkina DP, Ffrench-Constant C, Williams A, Guerreiro-Cacais AO, Castelo-Branco G. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat Med. 2018;24:1837–1844. doi: 10.1038/s41591-018-0236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fassbender JM, Saraswat-Ohri S, Myers SA, Gruenthal MJ, Benton RL, Whittemore SR. Deletion of endoplasmic reticulum stress-induced CHOP protects microvasculature post-spinal cord injury. Curr Neurovasc Res. 2012;9:274–281. doi: 10.2174/156720212803530627. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher CM, Garri C, Cain EL, Ang KK, Wilson CG, Chen S, Hearn BR, Jaishankar P, Aranda-Diaz A, Arkin MR, Renslo AR, Walter P. Ceapins are a new class of unfolded protein response inhibitors, selectively targeting the ATF6α branch. Elife. 2016;5:e11878. doi: 10.7554/eLife.11878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallagher CM, Walter P. Ceapins inhibit ATF6α signaling by selectively preventing transport of ATF6α to the Golgi apparatus during ER stress. Elife. 2016;5:e11880. doi: 10.7554/eLife.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garbern JY. Pelizaeus-Merzbacher disease: Genetic and cellular pathogenesis. Cell Mol Life Sci. 2007;64:50–65. doi: 10.1007/s00018-006-6182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geva M, Cabilly Y, Assaf Y, Mindroul N, Marom L, Raini G, Pinchasi D, Elroy-Stein O. A mouse model for eukaryotic translation initiation factor 2B-leucodystrophy reveals abnormal development of brain white matter. Brain. 2010;133:2448–2461. doi: 10.1093/brain/awq180. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh R, Wang L, Wang ES, Perera BG, Igbaria A, Morita S, Prado K, Thamsen M, Caswell D, Macias H, Weiberth KF, Gliedt MJ, Alavi MV, Hari SB, Mitra AK, Bhhatarai B, Schürer SC, Snapp EL, Gould DB, German MS, Backes BJ, Maly DJ, Oakes SA, Papa FR. Allosteric inhibition of the IRE1α RNase preserves cell viability and function during endoplasmic reticulum stress. Cell. 2014;158:534–548. doi: 10.1016/j.cell.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gow A, Lazzarini RA. A cellular mechanism governing the severity of Pelizaeus-Merzbacher disease. Nat Genet. 1996;13:422–428. doi: 10.1038/ng0896-422. [DOI] [PubMed] [Google Scholar]
- 37.Griffiths PD, Bolton P, Verity C. White matter abnormalities in tuberous sclerosis complex. Acta Radiol. 1998;39:482–486. doi: 10.1080/02841859809172211. [DOI] [PubMed] [Google Scholar]
- 38.Han J, Kaufman RJ. Physiological/pathological ramifications of transcription factors in the unfolded protein response. Genes Dev. 2017;31:1417–1438. doi: 10.1101/gad.297374.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harding HP, Zyryanova AF, Ron D. Uncoupling proteostasis and development in vitro with a small molecule inhibitor of the pancreatic endoplasmic reticulum kinase, PERK. J Biol Chem. 2012;287:44338–44344. doi: 10.1074/jbc.M112.428987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hetz C, Chevet E, Oakes SA. Proteostasis control by the unfolded protein response. Nat Cell Biol. 2015;17:829–838. doi: 10.1038/ncb3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hetz C, Saxena S. ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol. 2017;13:477–491. doi: 10.1038/nrneurol.2017.99. [DOI] [PubMed] [Google Scholar]
- 42.Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12:703–719. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 43.Hetz C, Papa FR. The unfolded protein response and cell fate control. Mol Cell. 2018;69:169–181. doi: 10.1016/j.molcel.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 44.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hussien Y, Cavener DR, Popko B. Genetic inactivation of PERK signaling in mouse oligodendrocytes: normal developmental myelination with increased susceptibility to inflammatory demyelination. Glia. 2014;62:680–691. doi: 10.1002/glia.22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hussien Y, Podojil JR, Robinson AP, Lee AS, Miller SD, Popko B. ER chaperone BiP/GRP78 is required for myelinating cell survival and provides protection during experimental autoimmune encephalomyelitis. J Neurosci. 2015;35:15921–15933. doi: 10.1523/JNEUROSCI.0693-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang M, Liu L, He X, Wang H, Lin W, Wang H, Yoon SO, Wood TL, Lu QR. Regulation of PERK-eIF2α signalling by tuberous sclerosis complex-1 controls homoeostasis and survival of myelinating oligodendrocytes. Nat Commun. 2016;7:12185. doi: 10.1038/ncomms12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kantor L, Harding HP, Ron D, Schiffmann R, Kaneski CR, Kimball SR, Elroy-Stein O. Heightened stress response in primary fibroblasts expressing mutant eIF2B genes from CACH/VWM leukodystrophy patients. Hum Genet. 2005;118:99–106. doi: 10.1007/s00439-005-0024-x. [DOI] [PubMed] [Google Scholar]
- 49.Kantor L, Pinchasi D, Mintz M, Hathout Y, Vanderver A, Elroy-Stein O. A point mutation in translation initiation factor 2B leads to a continuous hyper stress state in oligodendroglial-derived cells. PLoS One. 2008;3:e3783. doi: 10.1371/journal.pone.0003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 51.Khajavi M, Shiga K, Wiszniewski W, He F, Shaw CA, Yan J, Wensel TG, Snipes GJ, Lupski JR. Oral curcumin mitigates the clinical and neuropathologic phenotype of the Trembler-J mouse: a potential therapy for inherited neuropathy. Am J Hum Genet. 2007;81:438–453. doi: 10.1086/519926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, Stroud RM, Walter P. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457:687–693. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koumenis C, Bi M, Ye J, Feldman D, Koong AC. Hypoxia and the unfolded protein response. Meth Enzymol. 2007;435:275–293. doi: 10.1016/S0076-6879(07)35014-3. [DOI] [PubMed] [Google Scholar]
- 54.Krishnan ML, Commowick O, Jeste SS, Weisenfeld N, Hans A, Gregas MC, Sahin M, Warfield SK. Diffusion features of white matter in tuberous sclerosis with tractography. Pediatr Neurol. 2010;42:101–106. doi: 10.1016/j.pediatrneurol.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lassmann H, Bradl M. Multiple sclerosis: experimental models and reality. 2017 doi: 10.1007/s00401-016-1631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lebrun-Julien F, Bachmann L, Norrmén C, Trötzmüller M, Köfeler H, Rüegg MA, Hall MN, Suter U. Balanced mTORC1 activity in oligodendrocytes is required for accurate CNS myelination. J Neurosci. 2014;34:8432–8448. doi: 10.1523/JNEUROSCI.1105-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin W. Impaired eIF2B activity in oligodendrocytes contributes to VWMD pathogenesis. Neural Regen Res. 2015;10:195–197. doi: 10.4103/1673-5374.152366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin W, Bailey SL, Ho H, Harding HP, Ron D, Miller SD, Popko B. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J Clin Invest. 2007;117:448–456. doi: 10.1172/JCI29571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin W, Harding HP, Ron D, Popko B. Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-gamma. J Cell Biol. 2005;169:603–612. doi: 10.1083/jcb.200502086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin W, Kemper A, Dupree JL, Harding HP, Ron D, Popko B. Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006;129:1306–1318. doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- 62.Lin W, Kemper A, McCarthy KD, Pytel P, Wang JP, Campbell IL, Utset MF, Popko B. Interferon-gamma induced medulloblastoma in the developing cerebellum. J Neurosci. 2004;24:10074–10083. doi: 10.1523/JNEUROSCI.2604-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin W, Kunkler PE, Harding HP, Ron D, Kraig RP, Popko B. Enhanced integrated stress response promotes myelinating oligodendrocyte survival in response to interferon-gamma. Am J Pathol. 2008;173:1508–1517. doi: 10.2353/ajpath.2008.080449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin W, Lin Y. Interferon-γ inhibits central nervous system myelination through both STAT1-dependent and STAT1-independent pathways. J Neurosci Res. 2010;88:2569–2577. doi: 10.1002/jnr.22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin W, Lin Y, Li J, Fenstermaker AG, Way SW, Clayton B, Jamison S, Harding HP, Ron D, Popko B. Oligodendrocyte-specific activation of PERK signaling protects mice against experimental autoimmune encephalomyelitis. J Neurosci. 2013;33:5980–5991. doi: 10.1523/JNEUROSCI.1636-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin W, Popko B. Endoplasmic reticulum stress in disorders of myelinating cells. Nat Neurosci. 2009;12:379–385. doi: 10.1038/nn.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin Y, Huang G, Jamison S, Li J, Harding HP, Ron D, Lin W. PERK activation preserves the viability and function of remyelinating oligodendrocytes in immune-mediated demyelinating diseases. Am J Pathol. 2014a;184:507–519. doi: 10.1016/j.ajpath.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin Y, Jamison S, Lin W. Interferon-γ activates nuclear factor-κ B in oligodendrocytes through a process mediated by the unfolded protein response. PLoS One. 2012;7:e36408. doi: 10.1371/journal.pone.0036408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin Y, Pang X, Huang G, Jamison S, Fang J, Harding HP, Ron D, Lin W. Impaired eukaryotic translation initiation factor 2B activity specifically in oligodendrocytes reproduces the pathology of vanishing white matter disease in mice. J Neurosci. 2014b;34:12182–12191. doi: 10.1523/JNEUROSCI.1373-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 2004;23:169–179. doi: 10.1038/sj.emboj.7600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maly DJ, Papa FR. Druggable sensors of the unfolded protein response. Nat Chem Biol. 2014;10:892–901. doi: 10.1038/nchembio.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 73.Mháille AN, McQuaid S, Windebank A, Cunnea P, McMahon J, Samali A, FitzGerald U. Increased expression of endoplasmic reticulum stress-related signaling pathway molecules in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2008;67:200–211. doi: 10.1097/NEN.0b013e318165b239. [DOI] [PubMed] [Google Scholar]
- 74.Mimura N, Fulciniti M, Gorgun G, Tai YT, Cirstea D, Santo L, Hu Y, Fabre C, Minami J, Ohguchi H, Kiziltepe T, Ikeda H, Kawano Y, French M, Blumenthal M, Tam V, Kertesz NL, Malyankar UM, Hokenson M, Pham T, Zeng Q, Patterson JB, Richardson PG, Munshi NC, Anderson KC. Blockade of XBP1 splicing by inhibition of IRE1α is a promising therapeutic option in multiple myeloma. Blood. 2012;119:5772–5781. doi: 10.1182/blood-2011-07-366633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Musner N, Sidoli M, Zambroni D, Del Carro U, Ungaro D, D’Antonio M, Feltri ML, Wrabetz L. Perk Ablation Ameliorates Myelination in S63del-Charcot-Marie-Tooth 1B Neuropathy. ASN Neuro. 2016 doi: 10.1177/1759091416642351. doi: 101177/1759091416642351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ní Fhlathartaigh M, McMahon J, Reynolds R, Connolly D, Higgins E, Counihan T, Fitzgerald U. Calreticulin and other components of endoplasmic reticulum stress in rat and human inflammatory demyelination. Acta Neuropathol Commun. 2013;1:37. doi: 10.1186/2051-5960-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Numasawa-Kuroiwa Y, Okada Y, Shibata S, Kishi N, Akamatsu W, Shoji M, Nakanishi A, Oyama M, Osaka H, Inoue K, Takahashi K, Yamanaka S, Kosaki K, Takahashi T, Okano H. Involvement of ER stress in dysmyelination of Pelizaeus-Merzbacher Disease with PLP1 missense mutations shown by iPSC-derived oligodendrocytes. Stem Cell Reports. 2014;2:648–661. doi: 10.1016/j.stemcr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohri SS, Hetman M, Whittemore SR. Restoring endoplasmic reticulum homeostasis improves functional recovery after spinal cord injury. Neurobiol Dis. 2013;58:29–37. doi: 10.1016/j.nbd.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ohri SS, Maddie MA, Zhao Y, Qiu MS, Hetman M, Whittemore SR. Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia. 2011;59:1489–1502. doi: 10.1002/glia.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohri SS, Mullins A, Hetman M, Whittemore SR. Inhibition of GADD34, the stress-inducible regulatory subunit of the endoplasmic reticulum stress response, does not enhance functional recovery after spinal cord injury. PLoS One. 2014;9:e109703. doi: 10.1371/journal.pone.0109703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okamoto Y, Pehlivan D, Wiszniewski W, Beck CR, Snipes GJ, Lupski JR, Khajavi M. Curcumin facilitates a transitory cellular stress response in Trembler-J mice. Hum Mol Genet. 2013;22:4698–4705. doi: 10.1093/hmg/ddt318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Papa FR, Zhang C, Shokat K, Walter P. Bypassing a kinase activity with an ATP-competitive drug. Science. 2003;302:1533–1537. doi: 10.1126/science.1090031. [DOI] [PubMed] [Google Scholar]
- 83.Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, Solow-Cordero DE, Bouley DM, Offner F, Niwa M, Koong AC. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pavitt GD, Proud CG. Protein synthesis and its control in neuronal cells with a focus on vanishing white matter disease. Biochem Soc Trans. 2009;37:1298–1310. doi: 10.1042/BST0371298. [DOI] [PubMed] [Google Scholar]
- 85.Penas C, Verdú E, Asensio-Pinilla E, Guzmán-Lenis MS, Herrando-Grabulosa M, Navarro X, Casas C. Valproate reduces CHOP levels and preserves oligodendrocytes and axons after spinal cord injury. Neuroscience. 2011;178:33–44. doi: 10.1016/j.neuroscience.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 86.Pennuto M, Tinelli E, Malaguti M, Del Carro U, D’Antonio M, Ron D, Quattrini A, Feltri ML, Wrabetz L. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron. 2008;57:393–405. doi: 10.1016/j.neuron.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- 88.Plate L, Cooley CB, Chen JJ, Paxman RJ, Gallagher CM, Madoux F, Genereux JC, Dobbs W, Garza D, Spicer TP, Scampavia L, Brown SJ, Rosen H, Powers ET, Walter P, Hodder P, Wiseman RL, Kelly JW. Small molecule proteostasis regulators that reprogram the ER to reduce extracellular protein aggregation. Elife. 2016;20:5. doi: 10.7554/eLife.15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 91.Saraswat Ohri S, Mullins A, Hetman M, Whittemore SR. Activating transcription factor-6α deletion modulates the endoplasmic reticulum stress response after spinal cord injury but does not affect locomotor recovery. J Neurotrauma. 2018;35:486–491. doi: 10.1089/neu.2015.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sekine Y, Zyryanova A, Crespillo-Casado A, Amin-Wetzel N, Harding HP, Ron D. Paradoxical sensitivity to an integrated stress response blocking mutation in vanishing white matter cells. PLoS One. 2016;11:e0166278. doi: 10.1371/journal.pone.0166278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharma R, Gow A. Minimal role for caspase 12 in the unfolded protein response in oligodendrocytes in vivo. J Neurochem. 2007;101:889–897. doi: 10.1111/j.1471-4159.2007.04541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharma R, Jiang H, Zhong L, Tseng J, Gow A. Minimal role for activating transcription factor 3 in the oligodendrocyte unfolded protein response in vivo. J Neurochem. 2007;102:1703–1712. doi: 10.1111/j.1471-4159.2007.04646.x. [DOI] [PubMed] [Google Scholar]
- 95.Siddiqui AM, Khazaei M, Fehlings MG. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog Brain Res. 2015;218:15–54. doi: 10.1016/bs.pbr.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 96.Sidoli M, Musner N, Silvestri N, Ungaro D, D’Antonio M, Cavener DR, Feltri ML, Wrabetz L. Ablation of perk in Schwann cells improves myelination in the S63del charcot-marie-tooth 1B mouse. J Neurosci. 2016;36:11350–11361. doi: 10.1523/JNEUROSCI.1637-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song S, Tan J, Miao Y, Zhang Q. Crosstalk of ER stress-mediated autophagy and ER-phagy: Involvement of UPR and the core autophagy machinery. J Cell Physiol. 2018;233:3867–3874. doi: 10.1002/jcp.26137. [DOI] [PubMed] [Google Scholar]
- 98.Southwood C, Gow A. Molecular pathways of oligodendrocyte apoptosis revealed by mutations in the proteolipid protein gene. Microsc Res Tech. 2001;52:700–708. doi: 10.1002/jemt.1054. [DOI] [PubMed] [Google Scholar]
- 99.Southwood CM, Garbern J, Jiang W, Gow A. The unfolded protein response modulates disease severity in Pelizaeus-Merzbacher disease. Neuron. 2002;36:585–596. doi: 10.1016/s0896-6273(02)01045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Southwood CM, Fykkolodziej B, Maheras KJ, Garshott DM, Estill M, Fribley AM, Gow A. Overexpression of CHOP in myelinating cells does not confer a significant phenotype under normal or metabolic stress conditions. J Neurosci. 2016;36:6803–6819. doi: 10.1523/JNEUROSCI.1118-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stockwell SR, Platt G, Barrie SE, Zoumpoulidou G, Te Poele RH, Aherne GW, Wilson SC, Sheldrake P, McDonald E, Venet M, Soudy C, Elustondo F, Rigoreau L, Blagg J, Workman P, Garrett MD, Mittnacht S. Mechanism-based screen for G1/S checkpoint activators identifies a selective activator of EIF2AK3/PERK signalling. PLoS One. 2012;7:e28568. doi: 10.1371/journal.pone.0028568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stone S, Jamison S, Yue Y, Durose W, Schmidt-Ullrich R, Lin W. NF-κB activation protects oligodendrocytes against inflammation. J Neurosci. 2017;37:9332–9344. doi: 10.1523/JNEUROSCI.1608-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stone S, Lin W. The unfolded protein response in multiple sclerosis. Front Neurosci. 2015;9:264. doi: 10.3389/fnins.2015.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stone S, Wu S, Jamison S, Durose W, Pallais JP, Lin W. Activating transcription factor 6α deficiency exacerbates oligodendrocyte death and myelin damage in immune-mediated demyelinating diseases. Glia. 2018;66:1331–1345. doi: 10.1002/glia.23307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stone S, Yue Y, Stanojlovic M, Wu S, Karsenty G, Lin W. Neuron-specific PERK inactivation exacerbates neurodegeneration during experimental autoimmune encephalomyelitis. JCI Insight. 2019 doi: 10.1172/jci.insight.124232. doi: 101172/jciinsight124232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ta HM, Le TM, Ishii H, Takarada-Iemata M, Hattori T, Hashida K, Yamamoto Y, Mori K, Takahashi R, Kitao Y, Hori O. Atf6α deficiency suppresses microglial activation and ameliorates pathology of experimental autoimmune encephalomyelitis. J Neurochem. 2016;139:1124–1137. doi: 10.1111/jnc.13714. [DOI] [PubMed] [Google Scholar]
- 107.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tam AB, Koong AC, Niwa M. Ire1 has distinct catalytic mechanisms for XBP1/HAC1 splicing and RIDD. Cell Rep. 2014;9:850–858. doi: 10.1016/j.celrep.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Theocharopoulou G, Vlamos P. Modeling protein misfolding in charcot-marie-tooth disease. Adv Exp Med Biol. 2015;820:91–102. doi: 10.1007/978-3-319-09012-2_7. [DOI] [PubMed] [Google Scholar]
- 110.Tomassy GS, Dershowitz LB, Arlotta P. Diversity matters: A revised guide to myelination. Trends Cell Biol. 2016;26:135–147. doi: 10.1016/j.tcb.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–94. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- 112.van der Voorn JP, van Kollenburg B, Bertrand G, Van Haren K, Scheper GC, Powers JM, van der Knaap MS. The unfolded protein response in vanishing white matter disease. J Neuropathol Exp Neurol. 2005;64:770–775. doi: 10.1097/01.jnen.0000178446.41595.3a. [DOI] [PubMed] [Google Scholar]
- 113.van Kollenburg B, van Dijk J, Garbern J, Thomas AA, Scheper GC, Powers JM, van der Knaap MS. Glia-specific activation of all pathways of the unfolded protein response in vanishing white matter disease. J Neuropathol Exp Neurol. 2006;65:707–715. doi: 10.1097/01.jnen.0000228201.27539.50. [DOI] [PubMed] [Google Scholar]
- 114.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Volpi VG, Touvier T, D’Antonio M. Endoplasmic reticulum protein quality control failure in myelin disorders. Front Mol Neurosci. 2017;9:162. doi: 10.3389/fnmol.2016.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 117.Wang L, Perera BG, Hari SB, Bhhatarai B, Backes BJ, Seeliger MA, Schürer SC, Oakes SA, Papa FR, Maly DJ. Divergent allosteric control of the IRE1α endoribonuclease using kinase inhibitors. Nat Chem Biol. 2012;8:982–989. doi: 10.1038/nchembio.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–335. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
- 119.Way SW, Podojil JR, Clayton BL, Zaremba A, Collins TL, Kunjamma RB, Robinson AP, Brugarolas P, Miller RH, Miller SD, Popko B. Pharmaceutical integrated stress response enhancement protects oligodendrocytes and provides a potential multiple sclerosis therapeutic. Nat Commun. 2015;6:6532. doi: 10.1038/ncomms7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Way SW, Popko B. Harnessing the integrated stress response for the treatment of multiple sclerosis. Lancet Neurol. 2016;15:434–443. doi: 10.1016/S1474-4422(15)00381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilding AS, Patte-Mensah C, Taleb O, Brun S, Kemmel V, Mensah-Nyagan AG. Protective effect of 4-Phenylbutyrate against proteolipid protein mutation-induced endoplasmic reticulum stress and oligodendroglial cell death. Neurochem Int. 2018;118:185–194. doi: 10.1016/j.neuint.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 122.Wong YL, LeBon L, Basso AM, Kohlhaas KL, Nikkel AL, Robb HM, Donnelly-Roberts DL, Prakash J, Swensen AM, Rubinstein ND, Krishnan S, McAllister FE, Haste NV, O’Brien JJ, Roy M, Ireland A, Frost JM, Shi L, Riedmaier S, Martin K, Dart MJ, Sidrauski C. eIF2B activator prevents neurological defects caused by a chronic integrated stress response. Elife. 2019 doi: 10.7554/eLife.42940. doi: 107554/eLife42940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 124.Wrabetz L, D’Antonio M, Pennuto M, Dati G, Tinelli E, Fratta P, Previtali S, Imperiale D, Zielasek J, Toyka K, Avila RL, Kirschner DA, Messing A, Feltri ML, Quattrini A. Different intracellular pathomechanisms produce diverse Myelin Protein Zero neuropathies in transgenic mice. J Neurosci. 2006;26:2358–2368. doi: 10.1523/JNEUROSCI.3819-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xie W, Pariollaud M, Wixted WE, Chitnis N, Fornwald J, Truong M, Pao C, Liu Y, Ames RS, Callahan J, Solari R, Sanchez Y, Diehl A, Li H. Identification and characterization of PERK activators by phenotypic screening and their effects on NRF2 activation. PLoS One. 2015;10:e0119738. doi: 10.1371/journal.pone.0119738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yue Y, Stanojlovic M, Lin Y, Karsenty G, Lin W. Oligodendrocyte-specific ATF4 inactivation does not influence the development of EAE. J Neuroinflammation. 2019;16:23. doi: 10.1186/s12974-019-1415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yue Y, Stone S, Lin W. Role of nuclear factor κB in multiple sclerosis and experimental autoimmune encephalomyelitis. Neural Regen Res. 2018;13:1507–1515. doi: 10.4103/1673-5374.237109. [DOI] [PMC free article] [PubMed] [Google Scholar]