Keywords: acute spinal cord injury, adenovirus, adenovirus gene IX, bone marrow mesenchymal stem cells, combined behavioral score scale, HIF-1a, nerve regeneration, nerve repair, Rhodiola rosea, Sry
Abstract
Rhodioloside has been shown to protect cells from hypoxia injury, and bone marrow mesenchymal stem cells have a good effect on tissue repair. To study the effects of rhodioloside and bone marrow mesenchymal stem cells on spinal cord injury, a rat model of spinal cord injury was established using the Infinite Horizons method. After establishing the model, the rats were randomly divided into five groups. Rats in the control group were intragastrically injected with phosphate buffered saline (PBS) (5 μL). PBS was injected at 6 equidistant points around 5 mm from the injury site and at a depth of 5 mm. Rats in the rhodioloside group were intragastrically injected with rhodioloside (5 g/kg) and intramuscularly injected with PBS. Rats in the mesenchymal stem cell (MSC) group were intramuscularly injected with PBS and intramuscularly with MSCs (8 × 106/mL in a 50-μL cell suspension). Rats in the Ad-HIF-MSC group were intragastrically injected with PBS and intramuscularly injected with HIF-1 adenovirus-infected MSCs. Rats in the rhodioloside + Ad-HIF-MSC group were intramuscularly injected with MSCs infected with the HIF-1 adenovirus and intragastrically injected with rhodioloside. One week after treatment, exercise recovery was evaluated with a modified combined behavioral score scale. Hematoxylin-eosin staining and Pischingert’s methylene blue staining were used to detect any histological or pathological changes in spinal cord tissue. Levels of adenovirus IX and Sry mRNA were detected by real-time quantitative polymerase chain reaction and used to determine the number of adenovirus and mesenchymal stem cells that were transfected into the spinal cord. Immunohistochemical staining was applied to detect HIF-1 protein levels in the spinal cord. The results showed that: (1) compared with the other groups, the rhodioloside + Ad-HIF-MSC group exhibited the highest combined behavioral score (P < 0.05), the most recovered tissue, and the greatest number of neurons, as indicated by Pischingert’s methylene blue staining. (2) Compared with the PBS group, HIF-1 protein expression was greater in the rhodioloside group (P < 0.05). (3) Compared with the Ad-HIF-MSC group, Sry mRNA levels were higher in the rhodioloside + Ad-HIF-MSC group (P < 0.05). These results confirm that rhodioloside combined with bone marrow mesenchymal stem cells can promote the recovery of spinal cord injury and activate the HIF-1 pathway to promote the survival of bone marrow mesenchymal stem cells and repair damaged neurons within spinal cord tissue. This experiment was approved by the Animal Ethics Committee of Gansu University of Traditional Chinese Medicine, China (approval No. 2015KYLL029) in June 2015.
Chinese Library Classification No. R453; R363; R364
Introduction
Spinal cord injury (SCI) refers to injuries to the spinal cord that are caused by trauma (Silva et al., 2014). Approximately 200,000 people are currently living with SCIs in the United States, and every year, approximately 12,000 to 20,000 new cases are estimated to occur. The rates range from 12 cases per million to nearly 60 cases per million in different countries (van den Berg et al., 2010; Selvarajah et al., 2014). Acute SCI can instantaneously change the function of every organ system, and primary mechanical trauma is usually followed by a series of secondary injuries, including ischemia, hypoxia, electrolyte disorders, edema, and loss of energy metabolism (Fan et al., 2013). Changes in injured and adjacent areas after the acute postinjury phase can cause progressive neuronal death. SCI is currently incurable, and treatment is limited to minimizing secondary complications and maximizing residual function through rehabilitation (Popa et al., 2010; Zhang et al., 2016). The demand for treatments that rescue and reactivate spinal cord systems and restore function after SCI is urgent, and new methods need to be developed as soon as possible.
Transplanted mesenchymal stem cells (MSCs) have shown the potential to be a treatment for patients with SCI (Zhou et al., 2013; García et al., 2019; Mukhamedshina et al., 2019). Human bone marrow-derived MSCs (hBMSCs) can survive, migrate, and integrate into the host tissue when they are transplanted into animal models of central nervous system injuries, including SCI (Lin et al., 2018). After being transplanted into the injured spinal cords of rats, hBMSCs are believed to transdifferentiate into cells of neural lineage and into endothelial cells, which then help restore spinal system functions (Diao et al., 2010). Moreover, MSCs can exert a protective effect through the production of antiapoptotic and trophic factors via immunomodulatory action. However, MSCs have no protective effects against further damage caused by ischemia and hypoxia. Thus, treatments are urgently needed to solve this problem. Hypoxia-inducible factor 1 (HIF-1) and Rhodiola rosea are two promising drugs.
HIF-1 is the master regulator of responses to insufficient oxygen supply, and is critical in mediating the neuroprotective effects of heat acclimation in traumatic brain injury (Umschweif et al., 2013). At the early stages of SCI, overexpression of HIF-1 and its target genes might help induce hypoxia tolerance and regulate the vascularity of the injured spinal cord. HIF-1 is thought to protect hypoxic cells from apoptosis and necrosis under ischemic and anoxic conditions and to protect the nervous system from further damage (Yang et al., 2016). Rhodiola rosea is a widely used traditional Chinese medicine with a wide range of pharmacological functions, such as being an antioxidant, an anti-inflammatory, and anticancerous. Rhodiola rosea can also significantly reduce damage to the ultrastructure of rat organs caused by hypoxia and improve the body’s oxygen utilization coefficient and tolerance to hypoxia, which is related to improved expression of HIF-1 (Qi et al., 2015). Therefore, HIF-1 and Rhodiola rosea may play important roles in the recovery of SCI.
In the present study, a rat model of SCI was established to determine the effects of (Rho, the major component of Rhodiola rosea) on the recovery of SCI in rats, HIF-1expression, and the restoration of pathological spinal cord systems. We aimed to determine whether Rho combined with HIF-1-expressing BMSCs have a better effect in treating SCI than either Rho or BMSCs alone and to provide a viable method for potential clinical treatment in the future.
Materials and Methods
Cell culture
BMSCs were purchased from Chsurei Biotechnology (Jiangyin, China) and cultured in a humidified atmosphere of 95% air and 5% CO2 at 37°C using Dulbecco’s modified Eagle’s medium (Gibco, Shanghai, China) with 10% fetal bovine serum, 100 U/mL ampicillin, and 100 μg/mL streptomycin (Invitrogen, Shanghai, China).
Establishment of acute SCI rat models
A total of 120 specific-pathogen-free male adult Sprague-Dawley rats aged 4 weeks and weighing 300 ± 20 g were purchased from the Animal Center of Gansu University of Traditional Chinese Medicine, China [license No. SCXK (Gan) 2016-0006]. All experimental procedures and protocols were approved by the Experimental Animal Ethics Committee of Gansu University of Traditional Chinese Medicine, China (approval No. 2015KYLL029) in June 2015. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
A rat SCI model was established using an Infinite Horizons impactor-0400 Spinal Cord Impactor (PSI, Lexington, KY, USA) (Hubscher et al., 2006). Briefly, rats were anesthetized by intraperitoneal injection of pentobarbital sodium at 50 mg/kg and fixed on a stereotaxic apparatus (DL Naturegene, Beijing, China). Fur was sheared and rats were disinfected with 75% ethanol. An approximately 3 cm incision was made at the 12th rib. The skin was opened and the muscles around the spinous process were separated. The spinal cord was exposed by removing the T12–L1 vertebral plate and opening the spinal canal and spinal dura mater. The T11 and L2 vertebral bodies were fixed by forceps to ensure the location of the injury. The contusion was introduced with a Horizons spinal cord impactor at the spinal cord corresponding to the T12 spinous process. The striking force was 20.0 × 2.5 g·cm, and the contact time was 0.1 second. The muscles were realigned and the wound was closed. The rat body rapidly retracted and shivered, the surface of the local spinal cord rapidly suffered from edema and congestion, and the dura mater remained intact, indicating that the SCI model was successfully established. The body temperature was maintained at 36–37°C with an incandescent lamp and rectal thermometer monitoring throughout the operation. Rats were individually housed and given tetracycline (10 mg/kg, three times a day, orally) for 3 days.
BMSCs and infection with adenovirus
Third-passage cells were seeded in 10 cm flasks at a concentration of 1 × 104 cell/mL. After reaching 85% confluence, cells were infected with an adenovirus (Key Lab for Stem Cells and Gene Drugs, Gansu Province, Lanzhou, China) carrying HIF-1 at a multiplicity of infection (MOI) of 2 in the presence of 8 μg/mL of polybrene. The medium was changed 12 hours after adding the virus. The cells were collected 48 hours post-infection for transplantation.
Transplantation of BMSCs and Rho
The 120 SCI model rats were randomly divided into five groups. In the control group (n = 24), PBS was administered intramuscularly and intragastrically. In the MSCs group (n = 24), rats were injected with MSCs at 6 equidistant points about 5 mm from the injury site and at a depth of 5 mm, and PBS was administered intramuscularly and intragastrically. In the Ad-HIF-MSC group (n = 24), rats were intramuscularly injected with Ad-HIF-MSC (Key Lab for Stem Cells and Gene Drugs, Gansu Province, Lanzhou, China), and PBS was administered intragastrically. In the Rho group (n = 24), rats were intramuscularly injected with PBS and Rho (Chemical extract of Rhodiola; Tibet Military Rhodiola Development Center, Lhasa, China) was administered intragastrically by oral gavage. In the Rho + Ad-HIF-MSC group (n = 24), rats were intramuscularly injected with Ad-HIF-MSC and Rho was administered intragastrically. All interventions were carried out two days after establishing the SCI model. In total, 106 BMSCs were resuspended in 200 μL of PBS and Rho was intragastrically administered to rats by gavage at a dose of 5 mg/kg in 0.5 mL PBS for 6 hours after modeling. Rats were euthanized with a 10 mL intraperitoneal injection of 20% phenobarbital after processing was complete (Sigma, St. Louis, MO, USA).
Immunohistochemical staining
The transplanted spinal cord tissues were removed at 1, 2, and 3 weeks after transplantation, and HIF-1 protein expression in tissue slides was detected via immunohistochemistry. Slides were deparaffinized by washing them three times for 5 minutes in xylene and then rehydrating them by immersion three times in 100% alcohol (3 minutes each time), twice in 95% alcohol (3 minutes each time), and twice in 80% alcohol (3 minutes each time), followed by rinsing for 5 minutes in running distilled water. Endogenous peroxidase activity was quenched with freshly made 0.3% hydrogen peroxide. The antigen was heat retrieved in citrate buffer as follows: slides were immersed in 0.01 M citrate buffer and boiled in a microwave for 5 minutes. This heating process was repeated three times, and the samples were cooled down and rinsed three times with 0.01 M PBS for 3 minutes. Slides were blocked with normal rabbit serum at 37°C for 30 minutes before incubation with rabbit anti-HIF-1 (1:1000; PR-1053; Hopebiotech, Zhenjiang, China) overnight at 4°C. The slides were then rinsed with 0.01 M PBS three times (3 minutes each) before incubation with biotin-conjugated goat anti-rabbit IgG secondary antibody (1:8000), at 37°C for 30 minutes, and then rinsed with 0.01 M PBS three times (3 minutes each) followed by incubation with horseradish peroxidase-labeled streptavidin working solution at 37°C for 30 minutes. Slides were rinsed with PBS and then developed with 3,3′-diaminobenzidine (ZSGB-Bio, Beijing, China), followed by rinsing with tap water and counterstaining with hematoxylin. The slides were dehydrated with gradient alcohol, cleared with xylene, and mounted with neutral resin. The immunohistochemical results (i.e., protein expression) were observed by a microscope (CX23; Olympus, Tokyo, Japan). Semi-quantitative analysis and further comparative analyses were conducted using ImageJ 1.8 software (NIH, New York, USA).
Quantitative real-time polymerase chain reaction
mRNA expression levels for adenovirus gene IX and Sry were compared at different time points to detect the number of adenovirus and MSCs transferred to the spinal cord and their survival after transplantation, which reflected the relative levels of adenovirus and numbers of MSCs, respectively. Total RNA from rat spinal cord was extracted with the RNeasy Mini Kit (Qiagen, Shanghai, China) according to the manufacturer’s manual. First-strand cDNA was synthesized using a reverse transcription kit from Tiangen Bio (Beijing, China) according to the manufacturer’s protocol. Quantitative real-time polymerase chain reaction (RT-PCR) was performed with a quantitative PCR kit from TransGen (AQ131-01; Beijing, China) in a thermocycler (Eppendorf Mastercycler ep realplex) with the corresponding primers (Table 1). The reaction protocol consisted of 95°C for 3 minutes, followed by 40 cycles of 95°C for 30 seconds, 55°C for 20 seconds, and 72°C for 20 seconds. The relative mRNA levels were calculated by the 2–ΔΔCt method (Livak et al., 2013).
Table 1.
Primer sequences of the genes
| Gene | Sequence (5’–3’) | Product size (bp) |
|---|---|---|
| Sry | Forward: GCT GCA ATG GGA CAA CAA CC | 40 |
| Reverse: TTC TTG GAG GAC TGG TGT GC | ||
| Adenoviral gene IX | Forward: CGC GGG ATT GTG ACT GAC T | 41 |
| Reverse: GCC AAA AGA GCC GTC AAC TT | ||
| β-Actin | Forward: GTA AAG ACC TCT ATG CCA ACA | 41 |
| Reverse: GGA CTC ATC GTA CTC CTG CT |
Functional index and histological evaluation of SCI repair
Gross specimen observations, histological observations, and behavioral evaluations were performed at 1, 3, 6, and 12 weeks after transplantation. The evaluation criteria for neurological function were based on the Combined Behavioral Score Scale (Kerasidis et al., 1987). Evaluation categories included movement in open space, toe stretching, ground touching, reflex, posture change, ramp climbing, and swimming. The maximum score is 100 points for normal activity and 0 points for complete paralysis of the hind limbs.
Histological changes in the spinal cord after SCI were evaluated by hematoxylin-eosin staining (Beyotime, Shanghai, China). Stained sections can show the repair of tissue structure and the regeneration of blood vessels and neurons. The histology of the spinal cord was also examined by Pischingert’s methylene blue staining (Beyotime, Shanghai, China). Nissl bodies were dyed blue with methylene blue, which can reveal neuronal regeneration. The neuronal results were observed with an optical microscope (CX23; Olympus).
Statistical analysis
Measured data are expressed as the mean ± SD. Data were analyzed by SPSS 19.0 software (IBM Corp., Armonk, NY, USA). Two-way repeated measures analysis of variance (ANOVA) was used for comparison among groups and the Tukey’s post hoc test was used to identify specific group differences. P < 0.05 was considered statistically significant.
Results
Rho and BMSCs temporarily up-regulate HIF-1α expression after acute SCI
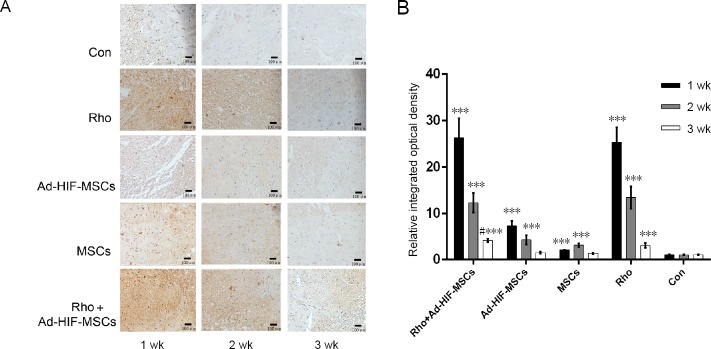
HIF expression in the spinal cord was detected by immunohistochemistry at 1, 2, and 3 weeks, and the expression levels were significantly higher than in the control or MSCs groups (P < 0.05). The Rho, Ad-HIF-MSC, and Rho + Ad-HIF-MSC groups exhibited significantly higher levels (P < 0.05) than control, and this decreased over time. HIF-1 expression was highest in the Rho + Ad-HIF-MSC group at 3 weeks (P < 0.05; Figure 1).
Figure 1.
Immunohistochemical staining of spinal cord tissue with an antibody against HIF-1 in each group at 1, 2, and 3 weeks after acute SCI.
(A) Immunohistochemical staining of spinal cord tissue (original magnification, 200×), scale bars: 100 μm. HIF-1 positive cells are dark brown. (B) Relative integrated optical density. ***P < 0.001, vs. control group; #P < 0.05, vs. Rho group. Data are expressed as the mean ± SD (n = 3; two-way repeated measures analysis of variance followed by Tukey’s post hoc test). Ad: Adenovirus; Con: control; HIF: hypoxia inducible factor; MSC: mesenchymal stem cell; Rho: rhodioloside.
Rho prolongs the survival of transplanted BMSCs
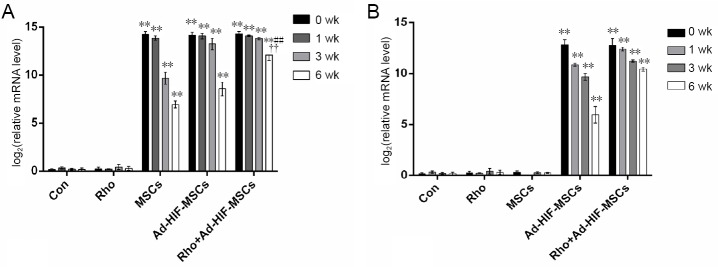
The successful transplantation of HIF-1-expressing adenovirus-infected BMSCs and their survival were assessed by detecting the adenovirus IX and Sry gene. Transcripts of the adenoviral gene Sry were undetectable in the spinal cords of rats in the control or Rho groups, but were highly expressed in the MSC, Ad-HIF-MSC, and Rho + Ad-HIF-MSC groups (P < 0.05; Figure 2A). Sry expression decreased with time, and was highest in the Rho + Ad-HIF-MSC group at 6 weeks (P < 0.05). Adenovirus gene IX was only expressed in the Ad-HIF-MSC and Rho + Ad-HIF-MSC groups, and was highest in the Rho + Ad-HIF-MSC group at 6 weeks (P < 0.05; Figure 2B).
Figure 2.
Relative mRNA levels of Sry (A) and adenoviral gene IX (B) in each group at 0, 1, 3, and 6 weeks.
**P < 0.01, vs. control group; #P < 0.05, ##P < 0.01, vs. Ad-HIF-MSC group; ††P < 0.01, vs. MSCs group. Data are expressed as the mean ± SD (n = 3; two-way repeated measures analysis of variance followed by Tukey’s post hoc test). Ad: Adenovirus; Con: control; HIF: hypoxia inducible factor; MSC: mesenchymal stem cell; Rho: rhodioloside.
Rho and BMSCs improve the recovery of locomotive activity after acute SCI
The recovery of locomotive activity was evaluated using the Combined Behavioral Score Scale at 1, 3, 6, and 12 weeks after acute SCI. Scores were higher in the Rho, MSC, Ad-HIF-MSC, and Rho + Ad-HIF-MSC groups than in the control group at each time point. The test results are shown in the Table 2. In the first week after surgery, the scores were significantly higher in the Ad-HIF-MSC and Rho + Ad-HIF-MSC groups than in the Rho or MSC groups (P < 0.05). However, scores for the Ad-HIF-MSC and Rho + Ad-HIF-MSC groups did not differ significantly (P > 0.05). At 3, 6, and 12 weeks after transplantation, scores were higher in the Rho + Ad-HIF-MSC group than in the Ad-HIF-MSC group at each time point (P > 0.05; Table 2).
Table 2.
Average combined behavioral score in each group at different post-injury times
| Post injury (wk) | Control | MSCs | Rho | Ad-HIF-MSCs | Rho + Ad-HIF-MSCs |
|---|---|---|---|---|---|
| 1 | 22.14±1.36 | 29.15±1.93* | 30.38±3.96** | 35.67±1.82** | 36.28±4.26** |
| 3 | 36.19±1.52 | 45.2±3.56** | 62.52±4.52** | 57.34±3.64** | 67.39±5.68**## |
| 6 | 50.65±1.09 | 60.34±5.46** | 75.65±5.32** | 78.39±3.58** | 83.67±6.35**## |
| 12 | 53.39±1.63 | 67.56±6.38** | 81.27±7.58** | 83.36±5.21** | 94.61±8.35**## |
*P < 0.05, **P < 0.01, vs. control group; ##P < 0.01, vs. Ad-HIF-MSCs group. Data are expressed as the mean ± SD (n = 3; two-way repeated-measures analysis of variance followed by Tukey’s post hoc test). The value represents the effect of repairing acute spinal cord injury. The higher the value, the better the effect. Ad: Adenovirus; Con: control; HIF: hypoxia-inducible factor; MSCs: mesenchymal stem cells; Rho: rhodioloside.
Rho and BMSCs improve the neuronal pathology that results from acute SCI
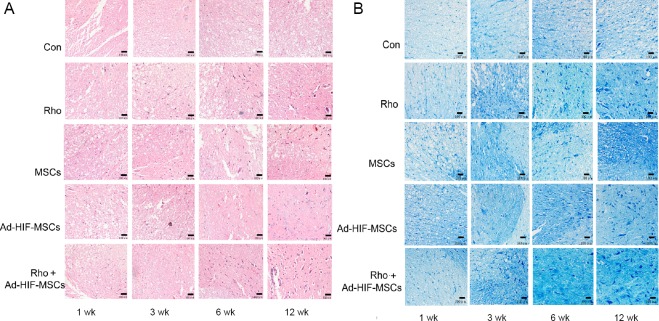
Histological changes confirmed the neuronal recovery after acute SCI. The spinal cord of rats receiving PBS gradually lost integrity, with increasing vacuole formation and neuronal cell death (Figure 3A). However, the spinal cord of rats receiving BMSC transplantation and/or Rho treatment had varying levels of tissue regeneration and neuronal growth, which resulted in healthier tissue structure, fewer cavities, more neurons, and hyperplastic tissue filling (Figure 3A). The best results were seen in the rats who received both Rho and the adenovirus-infected MSCs.
Figure 3.
Morphological changes in the rat spinal cord at 1, 3, 6 and 12 weeks after acute spinal cord injury.
(A) Hematoxylin-eosin staining of the spinal cord in the con, Rho, MSC, Ad-HIF-MSC, and Rho + Ad-HIF-MSC groups (original magnification, 200×): Blood vessels are reddish and the neurons are purple. Greater numbers of neurons and blood vessels were observed at 6 and 12 weeks in the con and Rho + Ad-HIF-MSC groups. (B) Pischingert’s methylene blue staining of the spinal cord in the con, Rho, MSC, Ad-HIF-MSC, and Rho + Ad-HIF-MSC groups (original magnification, 200×): Edema cavities are white and neuros are dark blue. There was no obvious repair in the con group. More neurons and less edema were visible in the Rho + Ad-HIF-MSC group at 12 weeks. Scale bars: 100 μm. Ad: Adenovirus; Con: control; HIF: hypoxia-inducible factor; MSC: mesenchymal stem cell; Rho: rhodioloside.
After acute SCI in the control group, spinal neurons experienced atrophy, degeneration, and necrosis with a reduction in Nissl bodies. Neurogenesis at the early stage was seldom observed, and the number of neurons in the damaged region of the spinal cord did not recover by 12 weeks post-SCI (Figure 3B). In contrast, rats that received Rho and/or the HIF-1-carrying adenovirus-infected MSCs, the number and cellular volume of neurons gradually recovered (Figure 3B).
Discussion
In recent years, transplant of MSCs in animal models of SCI has been shown to promote the recovery of spinal cord function (Sykova et al., 2006; Ozdemir et al., 2012; Chen et al., 2014). MSCs may promote recovery from SCI by remyelinating spared white matter tracts or inducing axonal regrowth. The earliest Bregman and Richardson studies showed that transplant of peripheral nerves and fetal cords can promote the regeneration of axons after SCI (Richardson et al., 1980; Bregman, 1987). MSCs can themselves synthesize growth factors and cytokines, thereby promoting neuronal survival and axonal growth, and most notably, reducing inflammatory responses and cavity formation. Hematoxylin-eosin staining showed that 3 weeks after MSC transplant, the number of cavities was greater in the control group than in the Rho group, and many hyperplastic tissues were observed in the spinal cords of the MSC group. At 12 weeks, the control group exhibited more empty cavities in the spinal cord tissue, more neuronal necrosis, darker colors, and more vacuoles near the transection than the Rho group. We did not observe any obvious cavities in the spinal cords of the MSC group. Small, bright colors and numerous neuronal fibers and cell bodies were visible in this group. In particular, the group treated with the combination of Rho and Ad-HIF-MSCs showed the most obvious results. However, the mechanism requires further analysis.
This study found that MSC can be combined with cytokines or other cell-transplant treatments, such as the promotion of granulocyte colony-stimulating factor, to repair SCI. Granulocyte colony-stimulating factor has a role in injury repair. A study found that granulocyte colony-stimulating factor and autologous MSC transplantation into the SCI site markedly improved the recovery of behavioral functions in rats (Urdzikova et al., 2006). Tissue hypoxia is a common theme in acute SCI due to vascular system damage, which not only causes metabolic changes (increased lactate levels) (Okon et al., 2013), but also triggers hypoxic responses (increased HIF-1α levels and target genes) due to ischemia (Haque et al., 2017). Administration of HIF-1-carrying adenovirus into damaged rat spinal cord tissue after SCI increased the expression of Bcl-2 and VEGF, decreased the level of Bax, reduced apoptosis, and promoted the recovery of neurological function (Haque et al., 2017). In the current experiment, HIF-1 was used to help the injured spinal cord recover further. Our results showed that the spinal cord of rats that received HIF-1-expressing adenovirus-infected MSCs had greater levels of tissue regeneration and neuronal growth, as evidenced by Pischingert’s methylene blue staining and hematoxylin-eosin staining.
Rho can increase HIF-1 expression and the survival rate of MSCs, and might accelerate rat recovery from acute SCI by reducing neuronal injury and promoting neuronal regeneration. Therefore, Rho might be able to play a role in promoting recovery from SCI. The traditional Chinese medicine Rhodiola rosea has been used clinically to treat cardiovascular diseases, such as coronary heart disease, hypertension, and myocarditis. Studies have shown that this treatment can improve cardiac function, enhance cardiomyocyte aerobic metabolism, and promote angiogenesis (Ling and Liang, 2016). Rho is the primary active ingredient in Rhodiola rosea, and it has a beneficial effect on the proliferation and differentiation of MSCs in vitro and promotes the differentiation of rat MSCs into dopaminergic neurons (Zhang et al., 2012). Bai et al. (2014) found that Rho promoted the therapeutic effects of MSCs and improved the survival rate of MSCs. However, the mechanism through which it (and by extension Rhodiola rosea) achieves its specific and efficacious effects is unclear. The inhibitory effect of Rho on hypoxia-induced SCI may be through the activation of Akt phosphorylation, which induces stable expression of the HIF-1 pathway and further promotes the expression of VEGF. The results achieved in the current study demonstrated that Rho might act to up-regulate HIF-1 expression, whereas VEGF is a downstream target protein of HIF-1 (He et al., 2016). It is worth noting that although we detected HIF-1 between 1–3 weeks after the HIF-1 gene therapy, the regulation of hypoxia and ischemia in the spinal microenvironment can last longer than 3 weeks (Li et al., 2017). In hypoxic myocardium, HIF-1 and VEGF expression is further upregulated, which ultimately leads to proliferation of blood vessels in the myocardium, establishment of collateral circulation, and remodeling of the myocardium (Mihaljević et al., 2017). In addition, recent studies have confirmed that VEGF can inhibit apoptosis, and it has neuroprotective effects that directly inhibit apoptosis and stimulate neurogenesis (Peng et al., 2016; Luo et al., 2018).
Therefore, studies on MSCs combined with transplantation of cytokines or MSC for the treatment and repair of SCI have greatly increased our knowledge in recent years, but studies on the repair of SCI using MSCs modified with Chinese herbs combined with cytokines have not yet been reported. The current study primarily used the Chinese medicine Rhodiola rosea combined with cytokine-modified MSCs to examine the repair of SCI injury. This treatment involved the hypoxic-ischemic protective function of HIF-1α and the multi-directional differentiation potential of MSCs. No immunogenic advantages, combined with strong anti-hypoxic effects, and angiogenesis, were observed. Rhodiola rosea enhances HIF-1 expression, promotes MSCs survival, and ultimately improves the neuronal organization in the spinal cord after SCI injury in rats. Repair and functional recovery provide a potential therapeutic strategy for the treatment of SCI. This treatment could potentially provide scientifically feasible regenerative repair and functional reconstruction for multi-organ and multi-tissue injuries under hypobaric and ischemic conditions.
Although treatment with Rho and MSCs combined with some HIF-1 cytokines can accelerate the repair of SCI, there are still some problems that must be solved in practical research and clinical applications. (1) The specific mechanisms through which Rho combined with cytokine-modified MSCs accelerates SCI recovery, and the specific effects on SCI treatment are unclear. We did not examine the immune response of MSCs in this study. (2) The influencing factors and mechanisms through which Rho combined with HIF-1 cell transplantation affect SCI recovery have not been studied clearly. (3) At present, most studies are limited to animal experiments, while studies of clinical applications are less. However, we believe that with continuing SCI research, these problems will be resolved. The Chinese herbal medicine Rho could potentially open a new path for clinical treatment and rehabilitation of SCI.
In summary, Rho and MSCs might accelerate recovery from SCI by activating different pathways. A combination of Rho with MSCs show synergistic effects, as Rho can promote the survival and neuronal differentiation of MSCs through activation of the HIF-1 pathway.
Acknowledgments:
We are very grateful to Jie Li, Ru-Ru Jiang and Xin Li from Gansu University of Chinese Medicine, China for their help in modifications to the later part of the article.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was financially supported by the National High Technology Research and Development Program of China (863 Program), No. 2015CB755400 (to XQH). The funding source had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: All experimental procedures and protocols were approved by the Experimental Animal Ethics Committee of Gansu University of Traditional Chinese Medicine of China (approval No. 2015KYLL029) in June 2015.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the National High Technology Research and Development Program of China (863 Program), No. 2015CB755400 (to XQH).
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Phillips A, de Souza M, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Bai H, Wang CB, Ma XH, Wei YP, Xi R, Zhao Q, Zhang Q. Effects of salidroside on proliferation of bone marrow mesenchymal stem cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014;22:1072–1077. doi: 10.7534/j.issn.1009-2137.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 2.Bregman BS. Spinal cord transplants permit the growth of serotonergic axons across the site of neonatal spinal cord transection. Brain Res. 1987;431:265–279. doi: 10.1016/0165-3806(87)90214-8. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Cui X, Wu Z, Jia L, Yu Y, Zhou Q, Hu X, Xu W, Luo D, Liu J, Xiao J, Yan Q, Cheng L. Transplantation of bone marrow mesenchymal stem cells pretreated with valproic acid in rats with an acute spinal cord injury. Biosci Trends. 2014;8:111–119. doi: 10.5582/bst.8.111. [DOI] [PubMed] [Google Scholar]
- 4.Chen MH, Ren QX, Yang WF, Chen XL, Lu C, Sun J. Influences of HIF-lalpha on Bax/Bcl-2 and VEGF expressions in rats with spinal cord injury. Int J Clin Exp Pathol. 2013;6:2312–2322. [PMC free article] [PubMed] [Google Scholar]
- 5.Diao Y, Ma L, Meng F. Transplantation of bone marrow mesenchymal stem cells promotes functional recovery of the injured rats spinal cord. Zhongguo Yike Daxue Xuebao. 2010;1:7–9. [Google Scholar]
- 6.Fan H, Liu X, Tang HB, Xiao P, Wang YZ, Ju G. Protective effects of Batroxobin on spinal cord injury in rats. Neurosci Bull. 2013;29:501–508. doi: 10.1007/s12264-013-1354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García E, Rodríguez-Barrera R, Buzoianu-Anguiano V, Flores-Romero A, Malagón-Axotla E, Guerrero-Godinez M, De la Cruz-Castillo E, Castillo-Carvajal L, Rivas-Gonzalez M, Santiago-Tovar P, Morales I, Borlongan C, Ibarra A. Use of a combination strategy to improve neuroprotection and neuroregeneration in a rat model of acute spinal cord injury. Neural Regen Res. 2019;14:1060–1068. doi: 10.4103/1673-5374.250627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haque A, Capone M, Matzelle D, Cox A, Banik NL. Targeting enolase in reducing secondary damage in acute spinal cord injury in rats. Neurochem Res. 2017;42:2777–2787. doi: 10.1007/s11064-017-2291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Z, Chen AY, Rojanasakul Y, Rankin GO, Chen YC. Gallic acid, a phenolic compound, exerts anti-angiogenic effects via the PTEN/AKT/HIF-1alpha/VEGF signaling pathway in ovarian cancer cells. Oncol Rep. 2016;35:291–297. doi: 10.3892/or.2015.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hubscher CH, Armstrong JE, Johnson JR. Effects of spinal cord injury on the rat estrous cycle. Brain Res. 2006;1100:118–124. doi: 10.1016/j.brainres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Kerasidis H, Wrathall JR, Gale K. Behavioral assessment of functional deficit in rats with contusive spinal cord injury. J Neurosci Methods. 1987;20:167–179. doi: 10.1016/0165-0270(87)90048-3. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Lucas-Osma AM, Black S, Bandet MV, Stephens MJ, Vavrek R, Sanelli L, Fenrich KK, Di Narzo AF, Dracheva S, Winship IR, Fouad K, Bennett DJ. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat Med. 2017;23:733–741. doi: 10.1038/nm.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin L, Lin H, Bai S, Zheng L, Zhang X. Bone marrow mesenchymal stem cells (BMSCs) improved functional recovery of spinal cord injury partly by promoting axonal regeneration. Neurochem Int. 2018;115:80–84. doi: 10.1016/j.neuint.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Ling M, Liang E. Effects of Rhodioloside and nimodipine in cerebral vasospasm induced by subarachnoid hemorrhage. Zhongguo Xunzheng Xinxueguanyixue Zazhi. 2016;5:691–693. [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2013;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Luo X, Gu S, Zhang Y, Zhang J. Kinsenoside ameliorates oxidative stress-induced RPE cell apoptosis and inhibits angiogenesis via Erk/p38/NF-kappaB/VEGF signaling. Front Pharmacol. 2018;9:240–247. doi: 10.3389/fphar.2018.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mihaljević Z, Ćosić A, Bilić-Dujmušić N, Prenek L, Engelmann P, Baus-Lončar M, Drenjančević I. Protein expression of HIF-1 alpha, VEGF and cyclooxygenases in cerebral blood vessels of Sprague-Dawley rats on a short-term high salt diet. Acta Physiol. 2017;30:221–229. [Google Scholar]
- 18.Mukhamedshina YO, Gracheva OA, Mukhutdinova DM, Chelyshev YA, Rizvanov AA. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural Regen Res. 2019;14:227–237. doi: 10.4103/1673-5374.244778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okon EB, Streijger F, Lee JH, Anderson LM, Russell AK, Kwon BK. Intraparenchymal microdialysis after acute spinal cord injury reveals differential metabolic responses to contusive versus compressive mechanisms of injury. J Neurotrauma. 2013;30:1564–1576. doi: 10.1089/neu.2013.2956. [DOI] [PubMed] [Google Scholar]
- 20.Ozdemir M, Attar A, Kuzu I, Ayten M, Ozgencil E, Bozkurt M, Dalva K, Uckan D, Kilic E, Sancak T, Kanpolat Y, Beksac M. Stem cell therapy in spinal cord injury: in vivo and postmortem tracking of bone marrow mononuclear or mesenchymal stem cells. Stem Cell Rev. 2012;8:953–962. doi: 10.1007/s12015-012-9376-5. [DOI] [PubMed] [Google Scholar]
- 21.Peng N, Gao S, Guo X, Wang G, Cheng C, Li M, Liu K. Silencing of VEGF inhibits human osteosarcoma angiogenesis and promotes cell apoptosis via VEGF/PI3K/AKT signaling pathway. Am J Transl Res. 2016;8:1005–1015. [PMC free article] [PubMed] [Google Scholar]
- 22.Popa C, Popa F, Grigorean VT, Onose G, Sandu AM, Popescu M, Burnei G, Strambu V, Sinescu C. Vascular dysfunctions following spinal cord injury. J Med Life. 2010;3:275–285. [PMC free article] [PubMed] [Google Scholar]
- 23.Qi YJ, Cui S, Lu DX, Yang YZ, Luo Y, Ma L, Ma Y, Wuren T, Chang R, Qi L, Ben BJ, Han J, Ge RL. Effects of the aqueous extract of a Tibetan herb, Rhodiola algida var. tangutica on proliferation and HIF-1alpha, HIF-2alpha expression in MCF-7 cells under hypoxic condition in vitro. Cancer Cell Int. 2015;15:81. doi: 10.1186/s12935-015-0225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- 25.Selvarajah S, Hammond ER, Haider AH, Abularrage CJ, Becker D, Dhiman N, Hyder O, Gupta D, Black JH, 3rd, Schneider EB. The burden of acute traumatic spinal cord injury among adults in the united states: an update. J Neurotrauma. 2014;31:228–238. doi: 10.1089/neu.2013.3098. [DOI] [PubMed] [Google Scholar]
- 26.Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Sykova E, Jendelova P, Urdzikova L, Lesny P, Hejcl A. Bone marrow stem cells and polymer hydrogels--two strategies for spinal cord injury repair. Cell Mol Neurobiol. 2006;26:1113–1129. doi: 10.1007/s10571-006-9007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umschweif G, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E. Hypoxia-inducible factor 1 is essential for spontaneous recovery from traumatic brain injury and is a key mediator of heat acclimation induced neuroprotection. J Cereb Blood Flow Metab. 2013;33:524–531. doi: 10.1038/jcbfm.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urdzikova L, Jendelova P, Glogarova K, Burian M, Hajek M, Sykova E. Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma. 2006;23:1379–1391. doi: 10.1089/neu.2006.23.1379. [DOI] [PubMed] [Google Scholar]
- 30.van den Berg ME, Castellote JM, Mahillo-Fernandez I, de Pedro-Cuesta J. Incidence of spinal cord injury worldwide: a systematic review. Neuroepidemiology. 2010;34:184–192. doi: 10.1159/000279335. [DOI] [PubMed] [Google Scholar]
- 31.Yang G, Wang W, Zheng X, Lei WL. The effect of controlled hypotension on the expression of HIF-1α and caspase-3 in spinal cord anterior horn neurons of different segments. Anat Res. 2016;38:351–354. [Google Scholar]
- 32.Zhang M, Zhao H, Li Z, Yang Y, Wen Y, Dong J, Zhang Q, Ge B. Effect of salidroside on rat bone marrow mesenchymal stem cells differentiation into cholinergic nerve cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26:158–165. [PubMed] [Google Scholar]
- 33.Zhang Y, Yang J, Zhang P, Liu T, Xu J, Fan Z, Shen Y, Li W, Zhang H. Calcitonin gene-related peptide is a key factor in the homing of transplanted human MSCs to sites of spinal cord injury. Sci Rep. 2016;6:27724. doi: 10.1038/srep27724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z, Chen Y, Zhang H, Min S, Yu B, He B, Jin A. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy. 2013;15:434–448. doi: 10.1016/j.jcyt.2012.11.015. [DOI] [PubMed] [Google Scholar]