Abstract
Coenzyme Q (CoQ) is an essential cofactor, primarily found in the mitochondrial inner membrane where it functions as an electron carrier in the respiratory chain, and as a lipophilic antioxidant. The redox state of the CoQ pool is the ratio of its oxidised (ubiquinone) and reduced (ubiquinol) forms, and is a key indicator of mitochondrial bioenergetic and antioxidant status. However, the role of CoQ redox state in vivo is poorly understood, because determining its value is technically challenging due to redox changes during isolation, extraction and analysis. To address these problems, we have developed a sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay that enables us to extract and analyse both the CoQ redox state and the magnitude of the CoQ pool with negligible changes to redox state from small amounts of tissue. This will enable the physiological and pathophysiological roles of the CoQ redox state to be investigated in vivo.
Keywords: CoQ10, CoQ9, Mass spectrometry, Redox state, Oxidative stress, Mitochondria, Coenzyme Q
Graphical abstract
Highlights
-
•
Sensitive LC-MS/MS assay to quantify CoQ redox state.
-
•
Changes in CoQ redox state can be measured in mitochondria, cells and in vivo.
-
•
CoQ redox state is stable during extraction and analysis.
1. Introduction
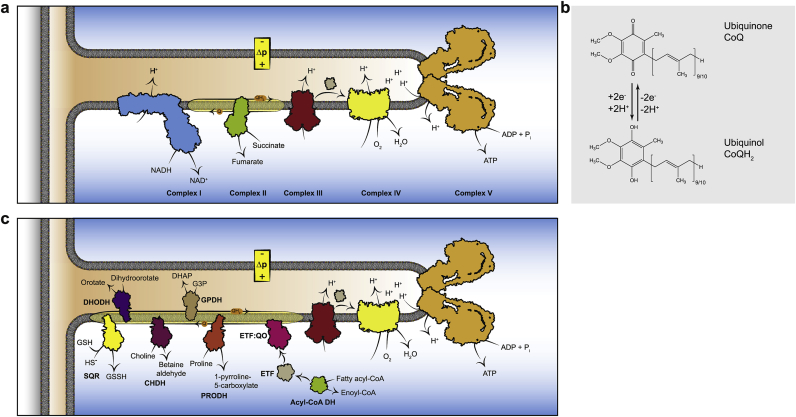
Coenzyme Q (CoQ) is a hydrophobic respiratory chain component within the mitochondrial inner membrane that is also found to a far lesser extent in other membranes [1] (Fig. 1a). The final steps of CoQ biosynthesis take place in mitochondria, where the redox active benzoquinone headgroup is linked to an isoprenyl chain in which the number of isoprenoid units varies with species: in yeast it is CoQ6, in rodents it is mainly CoQ9 with some CoQ10, while in humans CoQ10 predominates over CoQ9 [[2], [3], [4], [5], [1]]. Within the mitochondrial inner membrane CoQ collects electrons from dehydrogenases, notably complexes I and II (Fig. 1a), during which the ubiquinone form, CoQ, undergoes two-electron reduction to ubiquinol (CoQH2) (Fig. 1b). The CoQH2 is then reoxidised to CoQ at complex III to enable the respiratory chain to reduce O2 to water while pumping protons across the inner membrane to establish the protonmotive force (Δp) that drives ATP synthesis by oxidative phosphorylation [1,6] (Fig. 1a). In addition to complexes I and II, a number of other dehydrogenases also feed electrons into the CoQ pool from diverse metabolic pathways [1,7] (Fig. 1c). As well as its role in the respiratory chain, CoQH2 is also an important antioxidant within the mitochondrial inner membrane where it acts as a chain breaking antioxidant to prevent lipid peroxidation [[2], [3], [4], [5], [1]].
Fig. 1.
The CoQ pool in the mitochondrial inner membrane as essential link between metabolic pathways and cellular energy supply.
Various mitochondrial enzymes of diverse metabolic function feed electrons into the CoQ pool of the inner mitochondrial membrane. The electrons are then relayed to complex III of the ETC and thereby coupled to the maintenance of Δp and ATP production. a Complex I (NADH:Ubiquinone oxidoreductase) and complex II (succinate dehydrogenase) of the ETC chain are the canonical enzymes feeding electrons into the CoQ pool under normal conditions. The electrons are shuttled to complex III (ubiquinol:cytochrome c oxidoreductase) to be transferred onto cytochrome c and finally oxidised by complex IV (cytochrome c oxidase). By pumping protons from the matrix into the intermembrane space, complexes I, III & IV establish the mitochondrial protonmotive force (Δp), which is used for ATP synthesis by complex V (ATP-synthase) and various transport processes across the inner membrane. b The two electron carrier ubiquinone is a 1,4-benzoquinone, linked to an isoprene tail which in mammals consists of 9–10 isoprenyl subunits. Ubiquinone is reduced to ubiquinol by two electrons and acts as electron shuttle and antioxidant. c Numerous other mitochondrial oxidoreductases, such as SQR (hydrogen sulfide:ubiquinone oxidoreductase, catabolism of hydrogen sulphide), DHODH (dihydroorotate dehydrogenase, pyrimidine biosynthesis), CHDH (choline dehydrogenase, choline oxidation), G3PDH (glycerol 3-phosphate dehydrogenase, glycerol-3-phosphate shuttle), ETF-QO (electron-transferring-flavoprotein dehydrogenase, fatty acid oxidation) and PRODH (proline dehydrogenase, catabolism of proline) feed electrons into the mitochondrial CoQ pool. The electrons are funnelled by CoQ via complex III and cytochrome c to complex IV to reduce O2 to H2O.
Thus, the CoQ pool plays a central role in mitochondrial function and is the organelle's principal point of contact with many other metabolic pathways. Consequently CoQ deficiency contributes to mitochondrial dysfunction, disease and ageing [[8], [9], [10]]. The redox state of the CoQ pool is the ratio of its reduced (CoQH2) and oxidised (CoQ) forms, and is a key indicator of mitochondrial bioenergetic and antioxidant status. The CoQ redox state alters dynamically in response to its relative rates of reduction by dehydrogenases and oxidation by complex III or by reactive oxygen species (ROS). Changes in the CoQ redox state are central to mitochondrial redox signalling in oxygen sensing [11] and inflammation [12,13] and also to the tissue damage associated with ischaemia reperfusion injury [[14], [15], [16]]. Therefore, the CoQ redox state is central to health and disease and measuring it in vivo is vital.
While assessing the size of the CoQ pool in tissues in vivo is straightforward, measuring its redox state in vivo is technically challenging. This is mainly due to the difficulty of stabilising the redox state of the CoQ pool during isolation, extraction and analysis. In addition, for many analytical methods large amounts of material are required, limiting applicability. Methods based on liquid chromatography coupled to electrochemical detection were used extensively in the past to investigate CoQ levels and the CoQ redox state in biological samples [[17], [18], [19], [20]]. More recently several LC-MS/MS approaches for analysis of the CoQ redox state have been described [[17], [18], [19], [20], [21], [22], [23]]. In these protocols single-phase CoQ extraction with relatively polar alcohols such as methanol or propanol are used [17,[20], [21], [22], [23]]. However, as the samples change redox state during isolation and storage, samples have to be analysed rapidly and in small batches. Recently, an improved protocol was developed for the determination of the CoQ redox state in tissues by extraction into non-polar hexane followed by analysis in acidified ethanol [18]. While this limits oxidation, separate standard curves were still required for the two redox states, with the CoQH2 standard curve being a potential limitation due to oxidation of CoQH2 standards distorting the CoQ redox state. Here we describe a simplified two-phase extraction that utilises a single internal standard (IS) that generates a stable extract in which both the redox state of the CoQ pool and its amount can be determined. This will facilitate the analysis of both in vitro and in vivo models and expand our understanding of the role of the CoQ pool in health and disease.
2. Results
2.1. CoQ LC-MS/MS assay
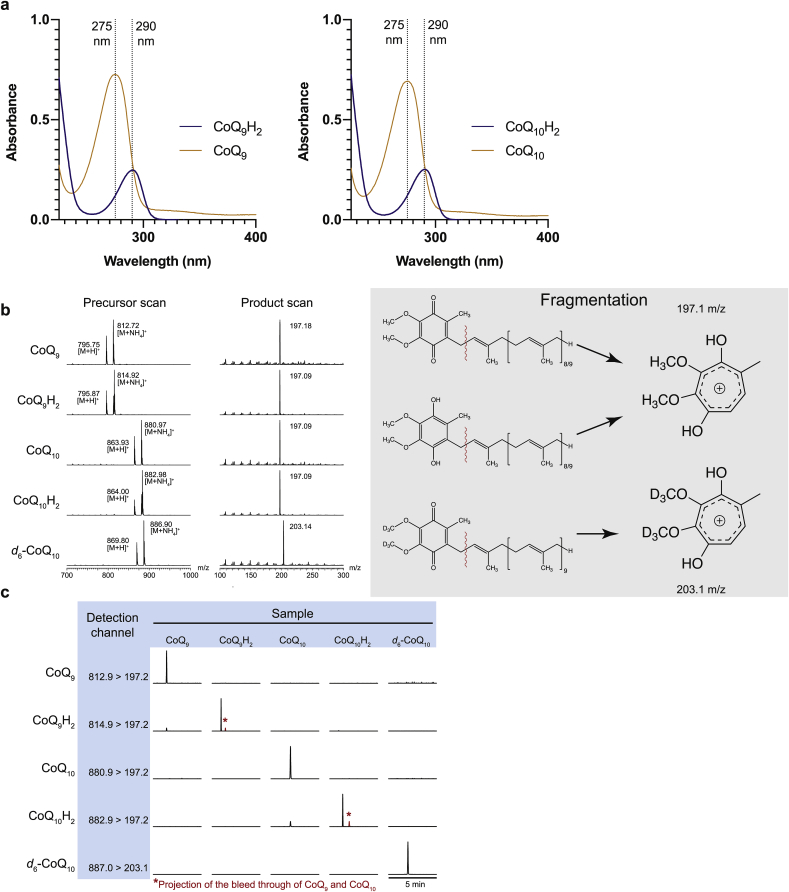
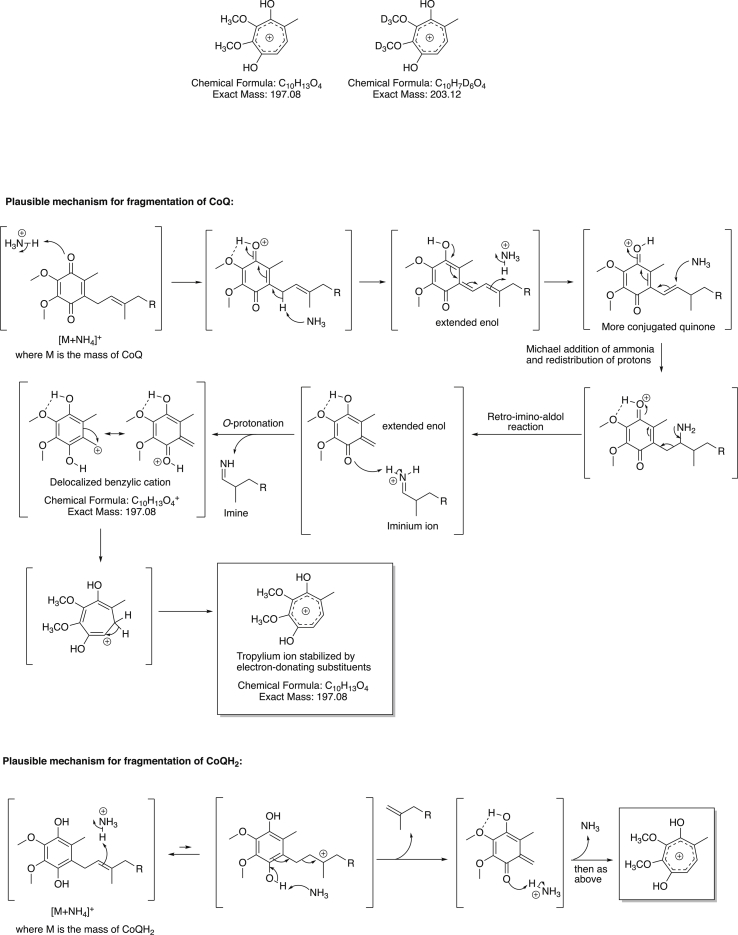
To establish CoQ redox state detection by LC-MS/MS, CoQ9/10, CoQ9/10H2 and d6-CoQ10 internal standard (IS) were prepared (Fig. 2a). Precursor scans of direct infusion of methanol/ammonium formate solutions of these compounds detected the H+ and NH4+ adducts that gave product ions at 197 m/z for both ubiquinone and ubiquinol, and at 203 m/z for the IS (Fig. 2b). We chose to use NH4+ adducts for LC-MS/MS analysis as they were more abundant, presumably due to higher concentrations of NH4+ compared to H+ within the buffer and because for both redox forms the H+ adducts showed the same mass for CoQ9 and CoQ10, making CoQ redox state analysis impossible. The proposed fragmentation mechanism is shown in Fig. S1. LC-MS/MS analysis of pure samples of each compound showed that the CoQ and CoQH2 were detected in the appropriate channel (Fig. 2c). While there was some bleed through of ubiquinone samples into the ubiquinol m/z channel, ubiquinone and ubiquinol were readily separated by LC (Fig. 2c). Hence, this LC-MS/MS analysis can easily separate the two redox forms, despite a mass difference of only 2.
Fig. 2.
Method development for the detection of CoQ9/10 and CoQ9/10H2 by LC-MS/MS
a The absorbance CoQ9 and CoQ10 were characterised by spectral scanning before and after reduction with NaBrH4. Upon reduction, the characteristic absorption maximum shifts from 275 to 290 nm b Representative precursor and product MS scans of CoQ9 and CoQ10 stocks and their reduced forms as well as the deuterated d6-CoQ10 internal standard. Precursor scans of the characteristic fragment products were performed. The product scans were performed by fragmenting the ammonium adduct precursors. Upon fragmentation the CoQ precursor loses its isoprene side chain and forms a tropylium ion. c Representative LC-MS/MS chromatograms showing the m/z transitions measured simultaneously for 0.5 pmol of CoQ9, CoQ10, CoQ9H2, CoQ10H2 and d6-CoQ10. Traces are normalised to the highest peak for each sample. CoQ9 and CoQ10 show bleed through into the transitions for CoQ9H2 and CoQ10H2, respectively. Bleed through of the CoQ9 and CoQ10 signals has been projected in red onto the CoQ9H2 and CoQ10H2 traces to show they are separated by LC. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.2. CoQ redox state determination
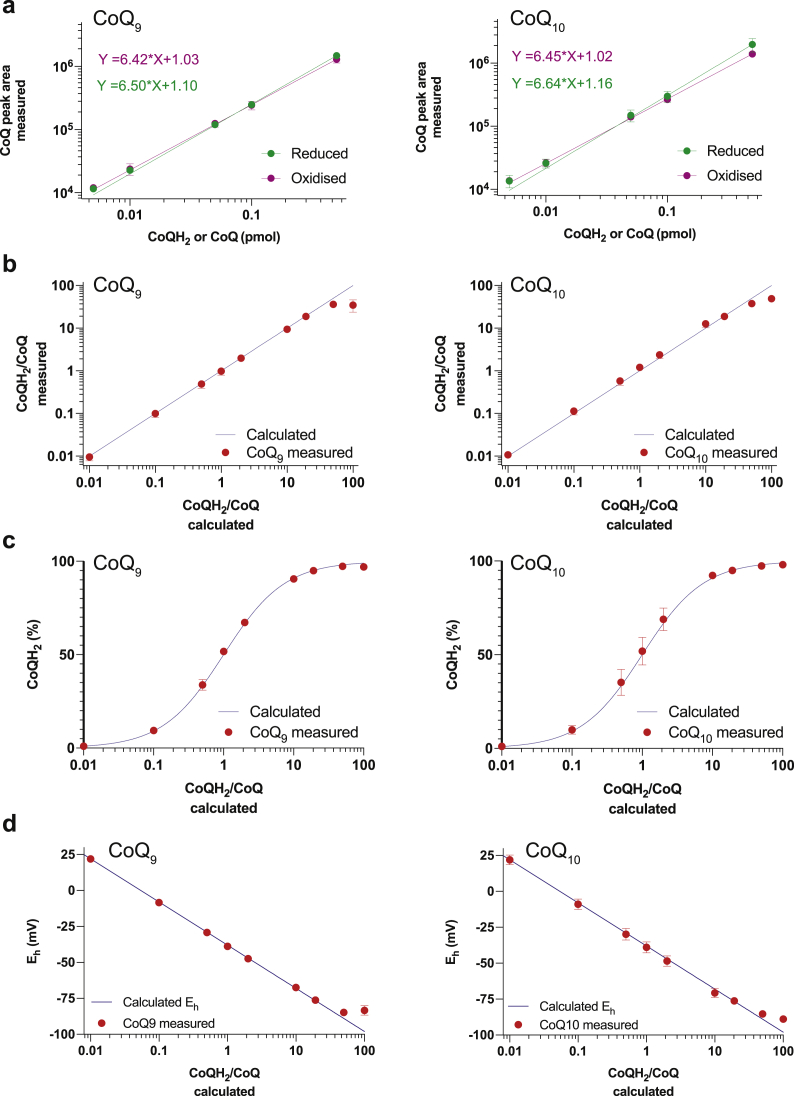
To determine the redox state of the CoQ pool it would be most convenient to measure the ratio of the mass spectrometer response to the two redox forms, to avoid having to construct standard curves for both redox forms of CoQ9 and CoQ10. For this to work the relative mass spectrometer response to CoQ and CoQH2 must be stable over a range of concentrations and ratios of both forms. To see if this was the case, the peak areas following analysis of equal amounts of CoQ and CoQH2 at a range of increasing total concentrations were compared (Fig. 3a). This analysis indicated that both redox forms of CoQ9 and CoQ10 had similar mass spectrometric responses. Furthermore, when samples containing a range of CoQH2/CoQ ratios were assessed the LC-MS/MS analysis also detected these ratios accurately (Fig. 3b). As the CoQH2/CoQ ratio also determines the % reduction of the CoQ pool, we replotted these data as % reduction (Fig. 3c) and also the reduction potential for the CoQ/CoQH2 redox couple [24] (Fig. 3d). Of note, for CoQH2/CoQ ratios of 100:1, in contrast to ratios ≤50:1, the measured value did not match that of the input sample, indicating that at very low CoQ reduction potentials some CoQH2 oxidation will inevitably occur. However, these highly reduced ratios are beyond the biologically relevant range. These analyses showed that measurement of the uncorrected ratio of the mass spectrometric response to both redox forms enables simultaneous and accurate determination of the redox states of both the CoQ9 and CoQ10 pools. We also wanted to investigate if CoQH2 has the potential to transfer electrons to CoQ following extraction, and thereby distort the measured ratios. To do this we mixed CoQ9H2 and CoQ10, or CoQ10H2 and CoQ9, in methanol containing 2 mM ammonium formate and incubated for 2 h at 37°C (Figs. S2a and b). This showed some slow oxidation of CoQH2, but this was not associated with direct reduction of the ubiquinone of the other species (Figs. S2a and b). This analysis showed that the oxidation of the ubiquinols was slow under our conditions, even at 37°C, and that this oxidation was not associated with ubiquinone reduction. Therefore, during cold storage of the extracts oxidation of CoQH2 will have minimal effect on the CoQH2/CoQ ratio.
Fig. 3.
Determining and quantifying the ratio of CoQ and CoQH2 by LC-MS/MS
To validate the quantification of oxidised and reduced CoQ, CoQ9/10 and borohydride reduced CoQ9/10H2 stocks were mixed in predetermined ratios and measured by LC-MS/MS. a Detected peak areas of increasing amounts of CoQH2 and CoQ mixed in a 1:1 ratio. b The detected CoQH2/CoQ ratios were blotted against the expected theoretical CoQH2/CoQ ratios. c The proportion of CoQH2 was blotted against the expected theoretical CoQH2/CoQ ratios. d The redox potential of CoQH2/CoQ mixtures was calculated based on the detected CoQH2/CoQ ratios and was blotted against the expected theoretical CoQH2/CoQ ratios. The calculations are outlined in the methods section. Data are mean ± S.D. of 3 replicates.
2.3. CoQ9 and CoQ10 quantification
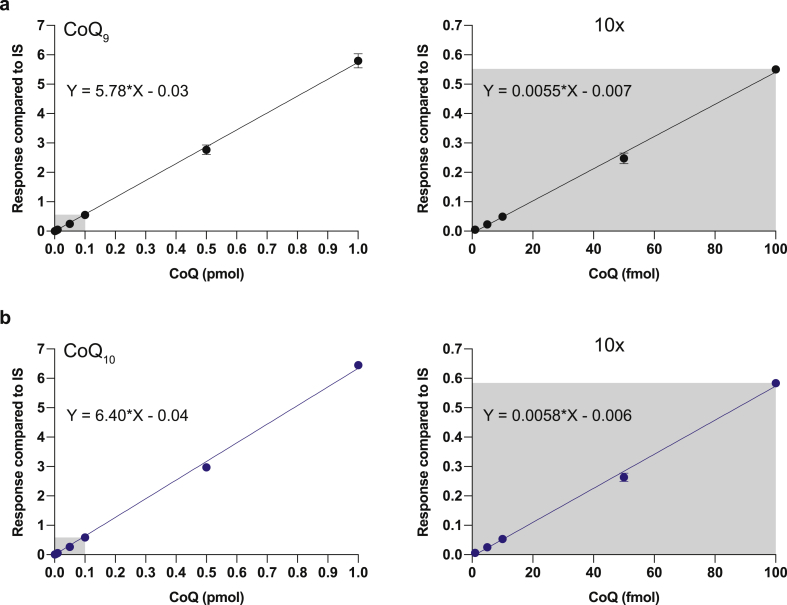
The above analysis showed that the CoQH2/CoQ redox ratio could be measured without the requirement for standard curves, simplifying the analysis and removing potential confounding factors of ubiquinol oxidation during standard curve preparation. However, it is also important to determine the sizes of the CoQ9 and CoQ10 pools. To do this we used d6-CoQ10 as an IS which was synthesised by base-catalysed exchange of the methoxy groups of CoQ10 dissolved in hexane-CD3OD. This generated pure compound that could be used as an internal standard. The two-stage preparation described previously [19] proved unnecessary. Using d6-CoQ10 as IS we constructed standard curves for CoQ9 and CoQ10 (Fig. 4a and b). This enables the total amounts of CoQ9 and CoQ10 in a sample to be determined, in parallel with measurement of their redox ratios.
Fig. 4.
Standard curve for the quantification of the CoQ pool size by LC-MS/MS
Representative standard curve or oxidised CoQ9 (a) and CoQ10 (b). The MS response of increasing concentrations of CoQ9 and CoQ10 was compared to 0.5 pmol of d6-CoQ10 (internal standard, IS). Data are mean ± S.D. of 3 replicates. The grey-shaded section of the standard curve on the left is expanded and replotted in the right panel.
2.4. Measurement of the CoQ redox state in mitochondria and cells
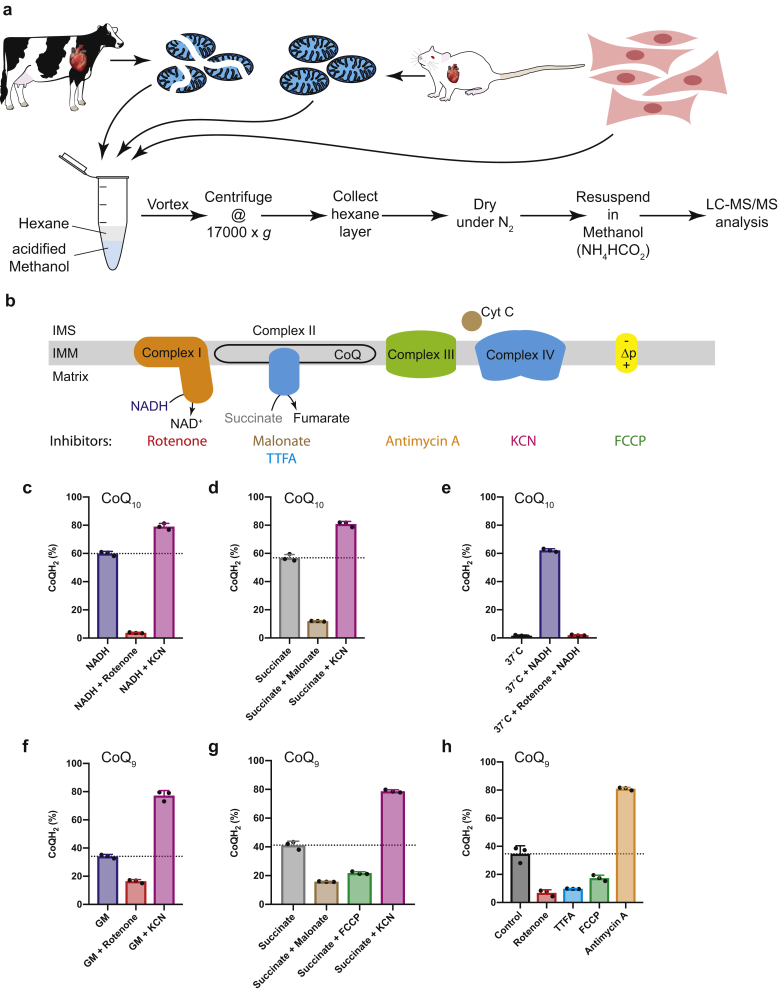
Next, we extended the CoQ extraction method to the analysis of isolated mitochondria and cells. For bovine heart mitochondrial membranes and isolated rat heart mitochondria we used a two-phase extraction with ice-cold hexane and acidified methanol (Fig. 5a). This precipitated proteins and retained many potential redox active polar metabolites in the aqueous-methanol phase, while acidification helped prevent oxidation of ubiquinol. Finally, extracting the CoQ pool into hexane generated a stable solution which was then processed for mass spectrometry.
Fig. 5.
Extracting CoQ from biological samples and modifying the CoQ redox state in mitochondrial membranes, mitochondria and cells.
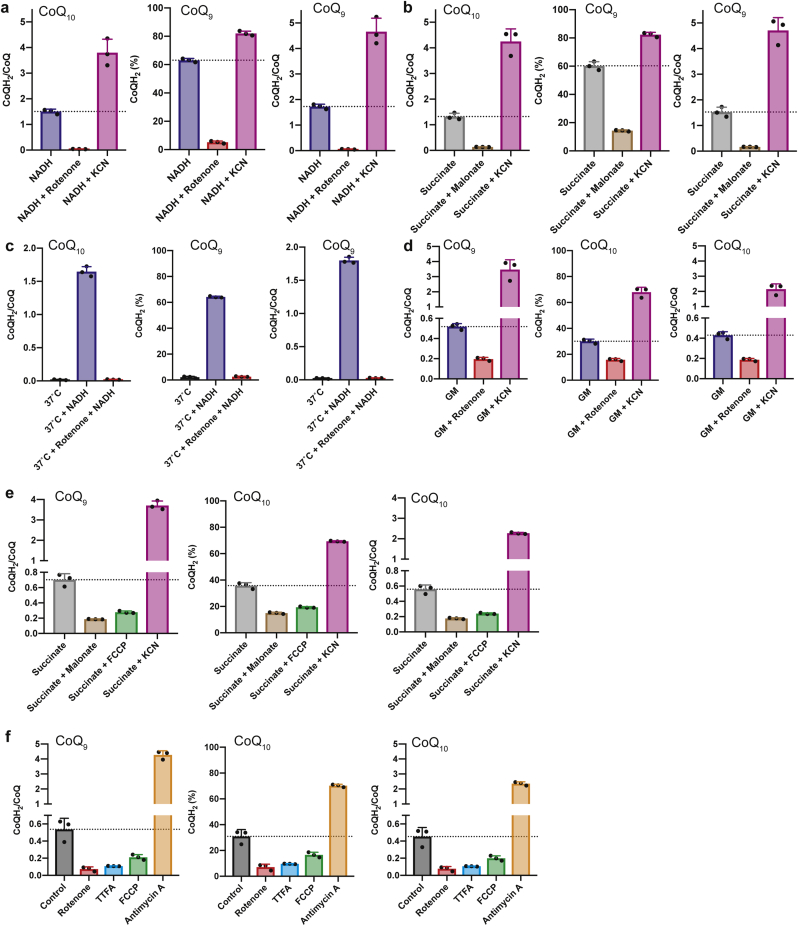
a A two-phase extraction with acidified methanol and hexane was used to extract CoQ from bovine mitochondrial membranes, mitochondria and cells. CoQ from incubations of biological samples was extracted by vortexing in extraction solution, phase separation by centrifugation and resuspending the extract in MS sample buffer (methanol with 2 mM ammonium formate). b Various inhibitors can be used to manipulate the mitochondrial CoQ redox state in biological samples. c-h Bovine heart mitochondrial membranes (BHMM), rat heart mitochondria (RHM) and C2C12 cells were incubated in KPi (BHMM), KCl (RHM) buffer or DMEM (cells) and different combinations of substrates and inhibitors were added before CoQ extraction and LC-MS/MS analysis of the CoQ redox state. All incubations were performed for 5 min at 37°C except otherwise indicated. CoQ redox state of: c BHMM incubated with NADH or NADH combined with rotenone or KCN. d BHMM incubated with succinate or succinate combined with malonate or KCN. e BHMM incubated for 20 min at 37°C. NADH or rotenone + NADH was added to indicated samples after 15 min f RHM incubated with glutamate + malate (GM) or GM combined with rotenone or KCN. g RHM incubated with succinate or succinate combined with malonate, FCCP or KCN. h C2C12 cells incubated in standard DMEM with FCCP, rotenone, TTFA or Antimycin A. For all experiments, data are represented as mean ± S.D. of 3 replicates. The proportion of CoQH2 is shown for the indicated most prevalent CoQ species.
To assess the efficacy of this procedure we measured the CoQ redox state in bovine mitochondrial membranes, which contain predominantly CoQ10, respiring on NADH or succinate in the presence of various inhibitors (Fig. 5b). Membranes respiring on NADH or succinate had a CoQ pool of about 56–60% reduced. With NADH the complex I inhibitor rotenone decreased this to ~4% reduced (Fig. 5c), while with succinate the complex II inhibitor malonate also maintained an oxidised CoQ pool of ~12% reduced (Fig. 5d). Addition of the complex IV inhibitor KCN led the CoQ pool to be ~80% reduced (Fig. 5c and d). Incubating membranes at 37°C in the absence of substrates led to complete oxidation of the CoQ pool, which can subsequently be reduced to ~60% by NADH (Fig. 5e).
We next examined isolated rat heart mitochondria, which contain mainly CoQ9. Mitochondria respiring on glutamate/malate to generate NADH led to reduction of the CoQ pool (Fig. 5f). The CoQ pool was oxidised by blocking complex I with rotenone and increased to ~77% reduction upon addition of KCN. Using succinate as a substrate also led to CoQ reduction that was decreased by malonate and increased by KCN. Addition of the uncoupler FCCP to abolish Δp and thereby stimulate respiration oxidised the CoQ pool (Fig. 5g).
To assess CoQ in a monolayer of C2C12 cells in culture we first rapidly washed the cell layer and then scraped the cells into cold PBS before extraction. As C2C12 cells are derived from mice, CoQ9 is the predominant form of CoQ. Cells showed a similar steady state CoQ redox state to isolated mitochondria, and this was oxidised by blocking complexes I and II with rotenone and TTFA, respectively (Fig. 5h). Uncoupling mitochondria with FCCP oxidises the CoQ pool due to increased respiration, and blocking complex III with antimycin led to reduction of the CoQ pool (Fig. 5h).
In all three systems, there was a dominant CoQ species, either CoQ9 or CoQ10, along with a far smaller amount of the other form. The redox state of the major form is reported above, but the method detected both forms accurately. In all cases the redox state of the major and minor CoQ species were the same, suggesting that both pools come to the same redox state (Figs. S3a–f).
2.5. Measurement of the CoQ redox state in vivo
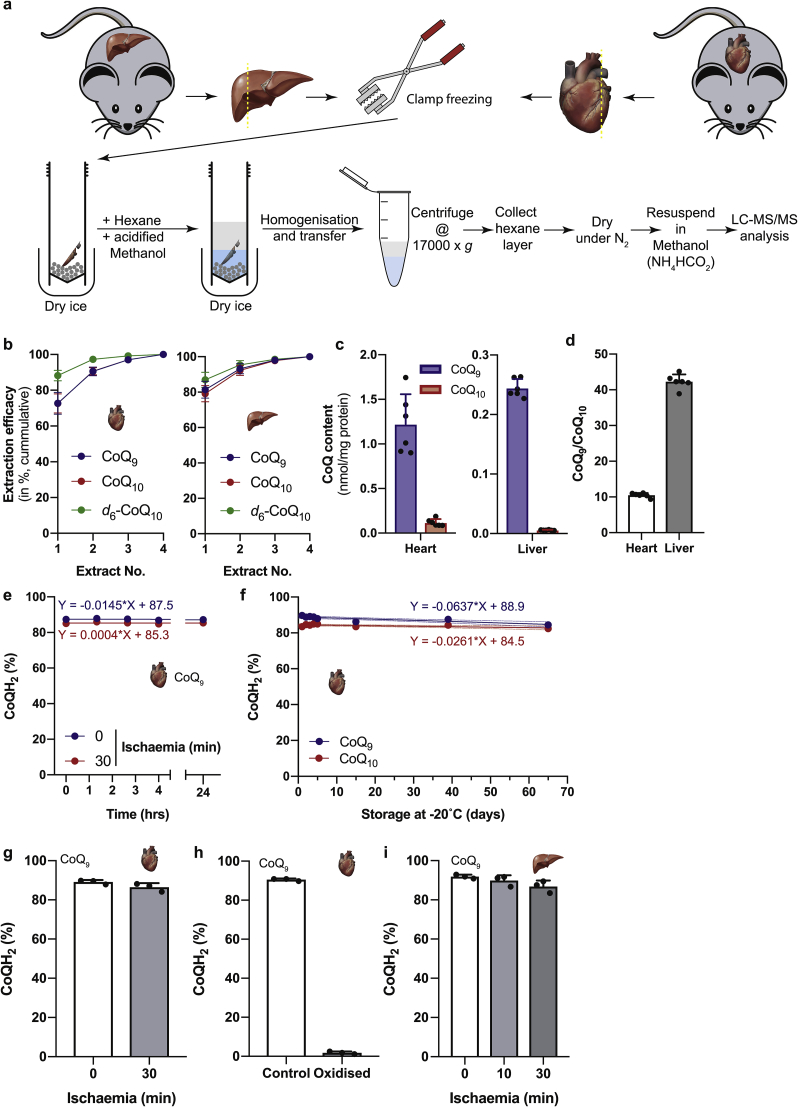
We next extended the two-phase extraction to assess CoQ redox state in vivo. To do this we focussed on the mouse heart and liver as these are important tissues in which the CoQ redox state is important in health and disease. In assessing tissues, it is vital to freeze the tissue as rapidly as possible so that the measured CoQ redox state accurately reflects that in vivo. To illustrate this, heart or liver tissue was excised either from terminally anaesthetized mice after thoracotomy, or after cervical dislocation and then rapidly frozen in a Wollenberger clamp at liquid nitrogen temperature. The frozen tissue was then homogenised in ice-cold acidified methanol and hexane and analysed by LC-MS/MS (Fig. 6a).
Fig. 6.
Extracting CoQ from tissues and determining the CoQ redox state.
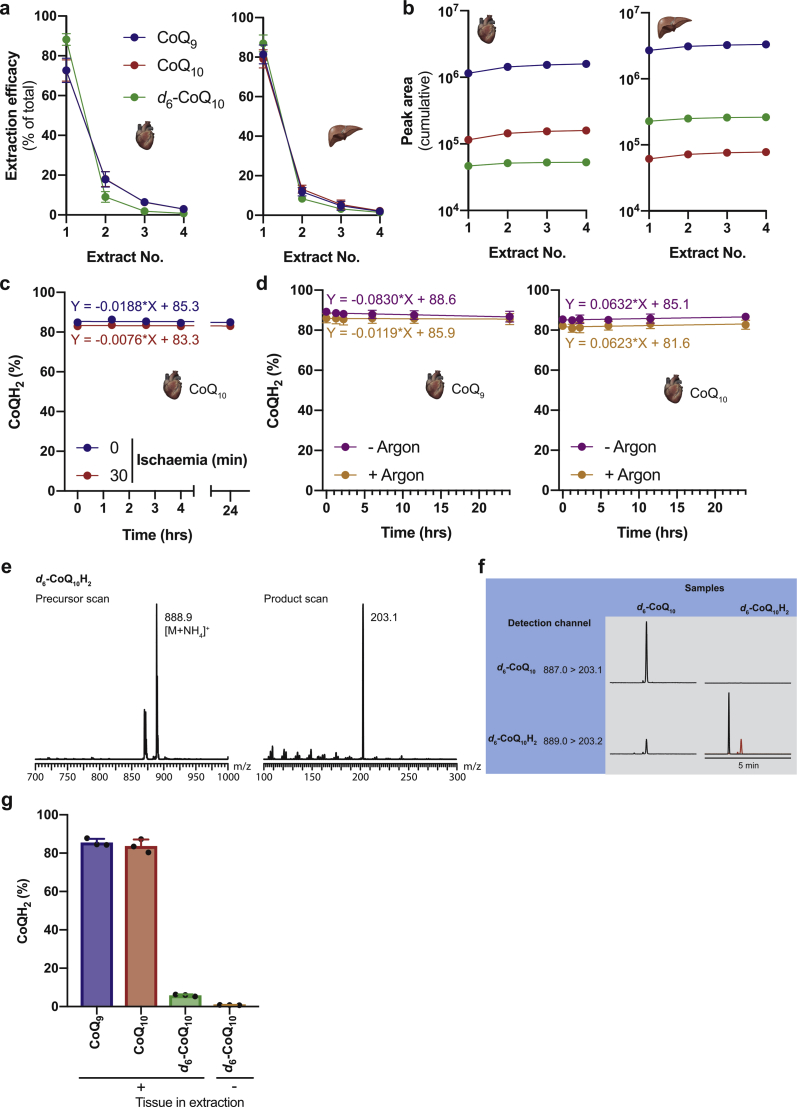
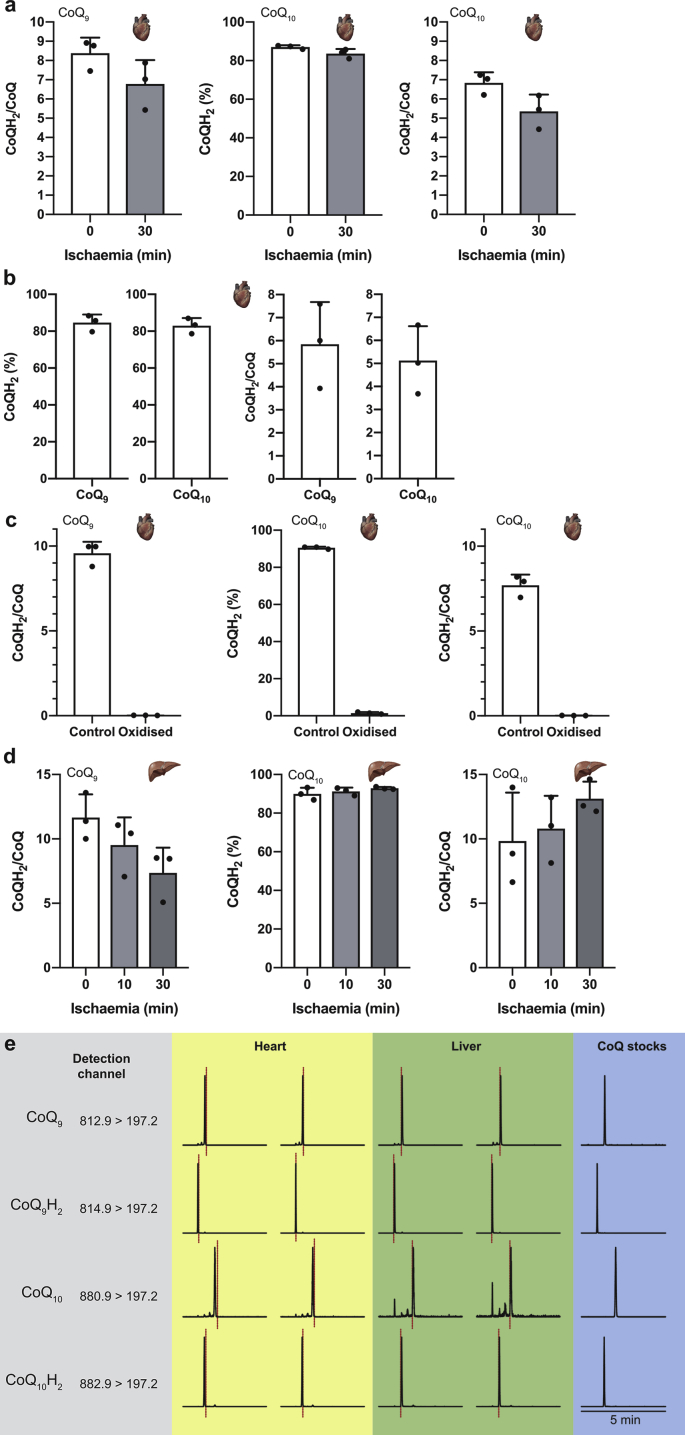
a A two-phase extraction with acidified methanol and hexane was used to extract CoQ from tissues. CoQ from clamp frozen tissue samples was extracted by homogenising in extraction solution, phase separation by centrifugation and resuspending the extract in MS sample buffer (methanol with 2 mM ammonium formate). b CoQ was extracted from mouse heart and liver tissue homogenates and the tissue homogenate was then reextracted. The peak areas for CoQ and CoQH2 were combined and the cumulative proportion combined with all previous extractions of the total is shown. Data are shown as mean ± S.D. of 6 different samples from 3 different animals. c The CoQ pool size of CoQ9 and CoQ10 in mouse heart and liver. Data are shown as mean ± S.D. of 6 different samples from 3 different animals. d The CoQ9/CoQ10 ratio in mouse heart and liver. Data are shown as mean ± S.D. of 6 different samples from 3 different animals. e CoQ redox state changes over time (at 8°C) during several consecutive LC-MS runs (~80 min per run per sample set; final run at 24 h) in CoQ extracts of control and ischaemic mouse hearts. The proportion of CoQH2 is represented as mean ± S.D. of 3 different hearts. f CoQ redox state changes during long term storage for up to 65 days (at −20°C) in CoQ extracts of control mouse hearts. The proportion of CoQH2 is represented as mean ± S.D. of 3 different hearts. g CoQ redox state of control and ischaemic mouse heart. The proportion of CoQH2 is represented as mean ± S.D. of 3 different hearts. h CoQ redox state of mouse heart tissue and tissue homogenates of the same hearts, after oxidising the CoQ pool in KPi buffer for 1 h at 37°C. The proportion of CoQH2 is represented as mean ± S.D. of 3 different hearts. i CoQ redox state of control and ischaemic (10 and 30 min) mouse liver. The proportion of CoQH2 is represented as mean ± S.D. of 3 different livers.
We first determined how well this procedure extracted CoQ from the tissue (Figs. 6b, S4a,b). This was done by re-extracting the heart and liver tissue and seeing how much residual CoQ was extracted (Figs. 6b, S4a,b). This showed that the first extraction removed ~75–80% of the CoQ pool. While subsequent extractions could remove a bit more, it was decided not to include a second extraction routinely.
We next quantified the CoQ pool in heart and liver and found ~1.2 nmol CoQ9 and ~0.12 nmol CoQ10 per mg protein in heart (Fig. 6c). In the liver the CoQ pools are much smaller with ~250 pmol CoQ9 and ~6 pmol CoQ10 per mg protein (Fig. 6c). These values are consistent with the previously reported values [25,26]. Interestingly the CoQ9/CoQ10 ratio varied significantly between organs, being ~10 in heart and ~42 in liver (Fig. 6d).
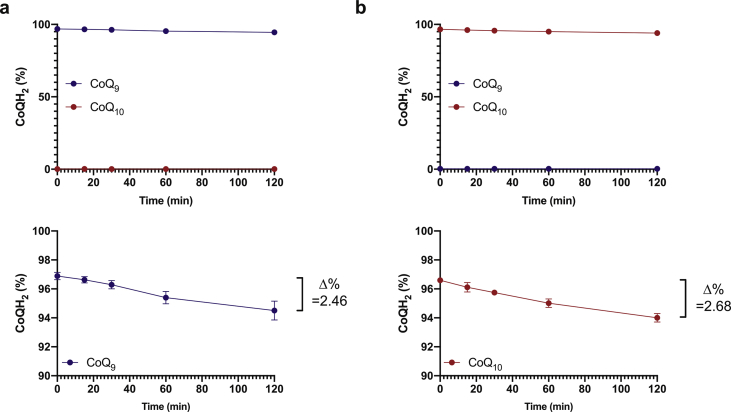
An important constraint in analysing the CoQ redox state is the oxidation of extracts by air before analysis. This often requires the rapid analysis of sample which are processed in small batches. To see if this was a concern, we reanalysed the CoQ redox state of samples stored in the LC-MS/MS autosampler at 8°C for up to 24 h in argon-flushed tubes. The CoQ redox state was stable for at least 24 h in the autosampler, confirming that large sample sets can be analysed in one run without artefactual oxidation (Figs. 6e and S4c). The CoQ redox state was stable without argon flushing (Fig. S4d). Nevertheless, we continued to flush samples with argon as a precaution. We next investigated the stability of the CoQ redox state of extracts during long-term storage at −20°C in argon-flushed tubes. This showed that the CoQ redox state was stable upon storage for up to at least 65 days (Fig. 6f). This allows to store and combine separate CoQ extractions in a single analysis run.
We noticed that CoQ extracts from mouse hearts were far more reduced than previously reported values (Fig. 6e) [18]. Consequently, we wanted to assess whether there was any artefactual reduction of CoQ during extraction. To do this we set up a detection protocol for d6-CoQ10 and d6-CoQ10H2 as described in section 2.1 (Figs. S4e and f). We then measured the reduction of d6-CoQ10 when it was spiked into the tissue extraction solution and detected ~6% of the added d6-CoQ10 as d6-CoQ10H2 in our extracts (Fig. S4g). In a mock extraction without tissue ~0.8% of the added d6-CoQ10 was converted to d6-CoQ10H2. No signal for d6-CoQ10 or d6-CoQ10H2 was detected in unspiked tissue extracts (Fig. S4g). Therefore, we conclude that a small amount of CoQ reduction can occur during extraction. However, completely oxidised d6-CoQ10 is thermodynamically much more prone to reduction than the already largely reduced CoQ pools found in vivo and even if the oxidised CoQ pool was underestimated by 5% this would have a minor effect on the overall redox state.
Next, we wanted to determine the CoQ redox state in clamp frozen heart. In mice killed by cervical dislocation the CoQ pool was highly reduced at 89% and remained at a similar redox state even after 30 min of warm ischaemia (Figs. 6g and S5a). To explore whether the tissue collection time from cervical dislocation to clamp-freezing has an effect on CoQ redox state, we next assessed the level of CoQ redox state in hearts obtained from terminally anaesthetized mice. In these mice the heart was excised directly after chest opening. This enabled a still-beating heart to be rapidly frozen and clamped. This gave a CoQH2% reduction of 85% (Fig. S5b), similar to that from hearts excised from animals after cervical dislocation. The redox state of the CoQ10 pool was also similar indicating that, as mentioned above, the two CoQ pools are bioenergetically equivalent (Figs. 6g, S5a,b). The CoQ pool can be fully oxidised by homogenising the heart in buffer and incubation at 37°C for 1 h (Figs. 6h and S5c). We also determined the CoQ redox state in freshly excised and ischaemic liver and found it to be highly reduced at 92% (Figs. 6i and S5d). As in the heart we also did not observe any significant changes in the CoQ redox state during ischaemia in liver (Figs. 6i and S5d). For all tested tissues the LC-MS/MS traces were clean and the retention times of the individual CoQ species matched those of pure CoQ (Fig. S5e).
3. Conclusions
Changes in the CoQ redox state have broad implications in the regulation of metabolic processes, respiration and mitochondrial ROS production. The difficulty in determining the CoQ redox state in biological samples and in vivo have been a significant impediment for the characterisation of CoQ function within mitochondria under physiological and pathophysiological conditions. Here we have developed a sensitive and comparable approach to assess the CoQ redox state by LC-MS in isolated mitochondria, cells and tissues. Applying this approach, we are able to detect CoQ and CoQH2 at equal sensitivity, allowing relative quantification without the need for a range of internal standards, an improvement on previous methods, which used standard curves for both CoQ and CoQH2. In parallel, the total CoQ pool size can be determined using only a standard curve of oxidised CoQ. In contrast to related approaches [18], we found that while the redox states of CoQ9 and CoQ10 can be manipulated with inhibitors and uncouplers they were essentially the same during normal conditions in a range of systems. Furthermore, we found that the CoQ redox state is highly (~90%) reduced in tissues in vivo, something that was not found in other studies, perhaps due to oxidation of the CoQ pool during extraction and processing [18]. Thus our method enables the redox state of the CoQ pool in vivo to be determined more accurately than hitherto. Our protocol opens up new possibilities to unravel the role of the CoQ redox state in vivo to understand the interplay between metabolism and bioenergetics in physiological and pathophysiological processes.
4. Materials and methods
4.1. Materials
All chemicals were purchased from Sigma unless otherwise stated. LC-MS grade Methanol was purchased from Fisher. All buffers, except LC-MS buffers, were stored over Chelex-100. Acidified solvents were prepared by addition of 0.1% (w/v) HCl.
4.2. Synthesis of d6-CoQ10
Sodium hydroxide (400 μL, 1 M in CD3OD) was added to a solution of CoQ10 (400 mg, 0.46 mmol, 1.0 eq) in hexane (5 mL) and d4-MeOD (4 mL). The solution was stirred at RT for 6 h then quenched with acetic acid. The solution was extracted into diethyl ether (~20 mL), washed with brine (2 × 75 mL), dried over sodium sulfate and concentrated under vacuum. The residue was purified by automated column chromatography using a 25g SNAP ultra cartridge on a Biotage Isolera using gradient elution from CH2Cl2:Hexane (0:100) to (80:20). The product was recrystallised from isopropanol-diethyl ether to give the labelled CoQ10 as an orange solid (162 mg, 40%). δH (400 MHz: CDCl3): 5.13–5.04 (9H, m, 9 × CH = ), 4.94 (1H, tq, J = 7.1 + 1.4 Hz, CH = ), 3.18 (2H, d, J = 7.0 Hz, CH2), 2.10–2.03 (16H, m, 8 × CH2), 2.01 (3H, s, CH3), 2.00–1.93 (20H, m, 10 × CH2), 1.74 (3H, s, CH3), 1.68 (3H, s, CH3), 1.60 (21H, broad s, 7 × CH3), 1.58 (3H, s, CH3). m/z (ESI): Found: 891.7120. C59H84D6NaO4 requires (M + Na) +, 891.7108.
4.3. Calculation of CoQ reduction potential
The CoQ reduction potential was calculated using the following equation, assuming a matrix pH of 7.7 [24]. Eh for the CoQ/CoQH2 couple in mV is:
4.4. Preparation of mitochondria and mitochondrial membranes
Bovine heart mitochondrial membranes were kindly provided by Dr Hiran Prag and Prof Judy Hirst (MRC MBU) and were prepared as described previously [27]. Female Wistar rats (Charles River) of 10–12 weeks of age were killed by stunning, followed by cervical dislocation. Rat hearts were homogenised in STEB (250 mM sucrose, 5 mM Tris-HCl, 1 mM EGTA, 0.1% fatty acid free BSA, pH 7.4, 4°C). Mitochondria were isolated by differential centrifugation (2x 700×g for 5 min, 2 × 5,500×g for 10 min) at 4°C and the protein content was determined by the bicinchoninic acid assay using the Pierce™ BCA Protein assay kit with bovine serum albumin as a standard.
4.5. Mouse heart and liver experiments
All mouse experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and the University of Cambridge Animal Welfare policy (Project license 70/8238). Male mice (8–10 weeks; C57BL/6J; Charles River Laboratories) were terminally anaesthetized with sodium pentobarbital injected intraperitoneally and ventilated with O2. Core temperature was monitored continuously via a rectal probe. Alternatively, mice were culled by cervical dislocation. A sternal thoracotomy was performed (heart) or the abdomen was opened (liver) and the organ was rapidly excised and then frozen with a Wollenberger clamp cooled in liquid nitrogen followed by storage at −70°C. Ischaemia was induced by excising the organ and keeping it in the abdomen or chest of the warmed mouse for indicated lengths. Organs were then frozen and stored as described.
4.6. Reduction of ubiquinone
Ubiquinones (CoQ9, CoQ10 and d6-CoQ10) were reduced to ubiquinols using sodium borohydride. All glassware was rinsed in hexane and dried under nitrogen before use. CoQ9 or CoQ10 were made up to 10 mM in hexane, then diluted 1:10 in hexane. Solid sodium borohydride was added (2 mg/100 μl) to the CoQ dilution followed by addition of methanol (to 5% v/v), vortexing for 3 min and incubation in the dark for 5 min. The reaction was stopped by addition of 1 vol acidified H2O with vortexing for 1 min followed by centrifugation (1,500×g, 5 min at 4°C). The CoQ-containing upper hexane layer was transferred to a glass vial, overlaid with argon and stored at −20°C. UV scanning spectra confirmed reduction and enabled the concentration to be calculated: ε290 = 4.1 mM−1cm−1 (CoQ9H2), 4.0 mM−1cm−1 (CoQ10H2); ε275 = 14.7 mM−1cm−1 (CoQ9), 14.6 mM−1cm−1 (CoQ10) [28].
4.7. Mass spectrometry
The mass spectrometric fragmentation patterns for the ammonium adducts of each CoQ CoQH2 compound were determined by direct infusion of 10 μM of the compound in 2 mM ammonium formate in methanol at 2 μl/min into a triple quadrupole mass spectrometer (Waters Xevo TQ-S). Electrospray ionisation in positive ion mode was used as published previously with the following settings [29]: capillary voltage – 1.7 kV; cone voltage – 30 V; ion source temperature – 100°C; collision energy – 22 V. Nitrogen and argon were used as the curtain and the collision gases, respectively.
4.8. Liquid chromatography tandem mass spectrometry
LC-MS/MS analyses were carried out using an I-Class Acquity LC system attached to a Xevo TQ-S triple quadrupole mass spectrometer (Waters) using published parameters [29]. Samples were kept at 8°C prior to injection by the autosampler of 2–10 μl into a 15 μl flow-through needle and RP-HPLC at 45°C using an Acquity C18 column (2.1 × 50 mm, 1.7 μM; Waters). Mobile phase was isocratic 2 mM ammonium formate in methanol run at 0.8 ml/min over 5 min. For MS analysis, electrospray ionisation in positive ion mode was used as described above. Multiple reaction monitoring in positive ion mode was used for compound detection. Transitions used for quantification were: CoQ9, 812.9 > 197.2; CoQ10, 880.9 > 197.2; CoQ9H2, 814.9 > 197.2; CoQ10H2, 882.9 > 197.2; d6-CoQ10, 887.0 > 203.1; d6-CoQ10H2, 889.0 > 203.1. Standard curves were prepared using known amounts of CoQ, which were spiked with d6-CoQ10 (IS). Standards and samples were quantified using MassLynx 4.1 software to determine the peak area for CoQ9, CoQ10, CoQ9H2, CoQ10H2, d6-CoQ10 and d6-CoQ10H2, and the standard curves used to determine the total amount of CoQ present in samples.
4.9. Mitochondrial incubations
Mitochondrial membrane or rat heart mitochondria were incubated at 15 μg protein in 100 μl KPi buffer (50 mM KPi, pH 7.8) or KCl buffer (120 mM KCl, 10 mM HEPES, 1 mM EGTA. pH 7.2), respectively. Substrates and inhibitors were added at the following concentrations where indicated: 1 mM NADH, 1 mM succinate, 1 mM glutamate/malate, 0.5 μM carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP), 0.5 μM rotenone, 1 mM malonate, 1 mM KCN. Samples were incubated with shaking for 5 min or 20 min at 37°C.
4.10. Cell incubations
C2C12 mouse myoblast cells (ATCC, UK) were cultured at 37°C under a humidified atmosphere of 5% CO2 in Dulbecco's modified Eagles's medium (DMEM/4.5 g/l glucose + GlutaMAX, Gibco, UK) supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 mg/ml streptomycin. Cells (100,000) were plated 24 h prior to experiment in 6-well plates with 2 ml medium. For experiments inhibitors were added at the following concentrations: 1 μM rotenone, 1 mM theonoyltrifluoroacetone (TTFA), 5 μM FCCP, 5 μM Antimycin A. Cells were then incubated for 5 min at 37°C, washed with PBS and scraped in 200 μl fresh PBS on ice prior to CoQ extraction.
4.11. CoQ extraction
To extract CoQ from mitochondrial membranes and mitochondria, incubations of 100 μl containing 15 μg protein were added to ice cold extraction solution (200 μl acidified methanol and 200 μl hexane) followed by vortexing. To extract CoQ from cells, the cell suspension in 200 μl PBS was transferred to ice cold extraction solution (200 μl acidified methanol and 300 μl of hexane) followed by vortexing. To extract CoQ from tissues, 5 mg frozen tissue was weighed into cooled lysis tubes (Precellys, CK-14) on dry ice. Then a mixture of 250 μl ice-cold acidified methanol and 250 μl hexane was added, and tissue was homogenised in the bullet blender (Bertin Instruments, Precellys 24) at setting 6500 during 15 s (CK14 (1.4 mm) beads). The homogenate was transferred to fresh 1.5 ml Eppendorf tubes. In all cases the upper, CoQ-containing hexane layer was separated by centrifugation (5 min, 17,000×g, 4°C) and then dried down in 1 ml glass mass spectrometry vials (186005663CV, Waters) under a stream of N2. Dried samples were then resuspended in methanol containing 2 mM ammonium formate and diluted (heart 1:10, liver not dilution: CoQ10 or 1:3 dilution CoQ9), overlaid with argon and stored at −20°C until analysis. The extracts were stable under storage for up to at least 15 days.
4.12. CoQ extraction for CoQ pool size determination
To extract CoQ from tissues, 5 mg frozen tissue was weighed into cooled lysis tubes (Precellys, CK-14) on dry ice. Then 150 μl KPi buffer (50 mM KPi, pH 7.8) was added and tissue was homogenised in the bullet blender (Bertin Instruments, Precellys 24) at setting 6500 during 15 s (CK14 (1.4 mm) beads). CoQ was extracted from 100 μl of the homogenate by adding a mixture of 250 μl ice-cold acidified methanol and 250 μl hexane and vortexing. The upper, CoQ-containing hexane layer was separated by centrifugation (5 min, 17,000×g, 4°C) and then dried down in 1 ml glass mass spectrometry vials (186005663CV, Waters) under a stream of N2. Dried samples were then resuspended in methanol containing 2 mM ammonium formate. Heart tissue was diluted 1:10 (20 μl in 170 μl methanol containing 2 mM ammonium formate and 10 μl of 5 μM d6-CoQ10 internal standard), while liver homogenate was either not diluted (CoQ10 analysis) or diluted 1:3 (CoQ9 analysis). Samples were overlaid with argon and stored at −20°C until analysis.
Acknowledgements
Work in the MPM laboratory was supported by the Medical Research Council UK (MC_U105663142) and by a Wellcome Trust Investigator award (110159/Z/15/Z) to MPM. Work in the RCH laboratory was supported by a Wellcome Trust Investigator award (110158/Z/15/Z). AM received funding from the Swedish Research Council.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.freeradbiomed.2019.11.028. Chemical characterization data files can be found at http://dx.doi.org/10.5525/gla.researchdata.924.
Abbreviations
- Δp
mitochondrial protonmotive force
- CoQ10
coenzyme Q10
- CoQ10H2
reduced Q10
- CoQ9
coenzyme Q9
- CoQ9H2
reduced Q9
- ETC
electron transfer chain
- IS
internal standard
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- MRM
multiple reaction monitoring
- RET
reverse electron transfer
- ROS
reactive oxygen species
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
References
- 1.Wang Y., Hekimi S. Understanding ubiquinone. Trends Cell Biol. 2016;26:367–378. doi: 10.1016/j.tcb.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Stefely J.A., Pagliarini D.J. Biochemistry of mitochondrial coenzyme Q biosynthesis. Trends Biochem. Sci. 2017;42:824–843. doi: 10.1016/j.tibs.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcázar-Fabra M., Navas P., Brea-Calvo G. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim. Biophys. Acta Bioenerg. 2016;1857:1073–1078. doi: 10.1016/j.bbabio.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Bentinger M., Tekle M., Dallner G. Coenzyme Q – biosynthesis and functions. Biochem. Biophys. Res. Commun. 2010;396:74–79. doi: 10.1016/j.bbrc.2010.02.147. [DOI] [PubMed] [Google Scholar]
- 5.Turunen M., Olsson J., Dallner G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta Biomembr. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Rich P.R., Maréchal A. The mitochondrial respiratory chain. Essays Biochem. 2010;47:1–23. doi: 10.1042/bse0470001. [DOI] [PubMed] [Google Scholar]
- 7.Landry A.P., Ballou D.P., Banerjee R. Modulation of catalytic promiscuity during hydrogen sulfide oxidation. ACS Chem. Biol. 2018;13:1651–1658. doi: 10.1021/acschembio.8b00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernández-Camacho J.D., Bernier M., López-Lluch G., Navas P. Coenzyme Q10 supplementation in aging and disease. Front. Physiol. 2018;9:44. doi: 10.3389/fphys.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luna‐Sánchez M., Hidalgo‐Gutiérrez A., Hildebrandt T.M., Chaves‐Serrano J., Barriocanal‐Casado E., Santos‐Fandila Á., Romero M., Sayed R.K., Duarte J., Prokisch H., Schuelke M., Distelmaier F., Escames G., Acuña‐Castroviejo D., López L.C. CoQ deficiency causes disruption of mitochondrial sulfide oxidation, a new pathomechanism associated with this syndrome. EMBO Mol. Med. 2017;9:78–95. doi: 10.15252/emmm.201606345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alcázar-Fabra M., Trevisson E., Brea-Calvo G. Clinical syndromes associated with Coenzyme Q10 deficiency. Essays Biochem. 2018;62:377–398. doi: 10.1042/EBC20170107. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Agüera M.C., Gao L., González-Rodríguez P., Pintado C.O., Arias-Mayenco I., García-Flores P., García-Pergañeda A., Pascual A., Ortega-Sáenz P., López-Barneo J. Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metabol. 2015;22:825–837. doi: 10.1016/j.cmet.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Ryan D.G., Murphy M.P., Frezza C., Prag H.A., Chouchani E.T., O'Neill L.A., Mills E.L. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat. Metab. 2019;1:16–33. doi: 10.1038/s42255-018-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills E.L., Kelly B., Logan A., Costa A.S.H., Varma M., Bryant C.E., Tourlomousis P., Däbritz J.H.M., Gottlieb E., Latorre I., Corr S.C., McManus G., Ryan D., Jacobs H.T., Szibor M., Xavier R.J., Braun T., Frezza C., Murphy M.P., O'Neill L.A. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–470. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chouchani E.T., Pell V.R., James A.M., Work L.M., Saeb-Parsy K., Frezza C., Krieg T., Murphy M.P. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metabol. 2016;23:254–263. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Chouchani E.T., Pell V.R., Gaude E., Aksentijević D., Sundier S.Y., Robb E.L., Logan A., Nadtochiy S.M., Ord E.N.J., Smith A.C., Eyassu F., Shirley R., Hu C.-H., Dare A.J., James A.M., Rogatti S., Hartley R.C., Eaton S., Costa A.S.H., Brookes P.S., Davidson S.M., Duchen M.R., Saeb-Parsy K., Shattock M.J., Robinson A.J., Work L.M., Frezza C., Krieg T., Murphy M.P. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barshop B.A., Gangoiti J.A. Analysis of coenzyme Q in human blood and tissues. Mitochondrion. 2007;7:89–93. doi: 10.1016/j.mito.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Pandey R., Riley C.L., Mills E.M., Tiziani S. Highly sensitive and selective determination of redox states of coenzymes Q9 and Q10 in mice tissues: application of orbitrap mass spectrometry. Anal. Chim. Acta. 2018;1011:68–76. doi: 10.1016/j.aca.2018.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duberley K.E.C., Hargreaves I.P., Chaiwatanasirikul K.-A., Heales S.J.R., Land J.M., Rahman S., Mills K., Eaton S. Coenzyme Q10 quantification in muscle, fibroblasts and cerebrospinal fluid by liquid chromatography/tandem mass spectrometry using a novel deuterated internal standard. Rapid Commun. Mass Spectrom. 2013;27:924–930. doi: 10.1002/rcm.6529. [DOI] [PubMed] [Google Scholar]
- 20.Schou-Pedersen A.M.V., Schemeth D., Lykkesfeldt J. Determination of reduced and oxidized coenzyme Q10 in canine plasma and heart tissue by HPLC-ECD: comparison with LC-MS/MS quantification. Antioxidants. 2019;8:253. doi: 10.3390/antiox8080253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Z., Li S., Guan X., Schmitt-Kopplin P., Lin S., Cai Z. Rapid assessment of the coenzyme Q10 redox state using ultrahigh performance liquid chromatography tandem mass spectrometry. Analyst. 2014;139:5600–5604. doi: 10.1039/c4an00760c. [DOI] [PubMed] [Google Scholar]
- 22.Claessens A.J., Yeung C.K., Risler L.J., Phillips B.R., Himmelfarb J., Shen D.D. Rapid and sensitive analysis of reduced and oxidized coenzyme Q10 in human plasma by ultra performance liquid chromatography-tandem mass spectrometry and application to studies in healthy human subjects. Ann. Clin. Biochem. 2016;53:265–273. doi: 10.1177/0004563215593097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teshima K., Kondo T. Analytical method for ubiquinone-9 and ubiquinone-10 in rat tissues by liquid chromatography/turbo ion spray tandem mass spectrometry with 1-alkylamine as an additive to the mobile phase. Anal. Biochem. 2005;338:12–19. doi: 10.1016/j.ab.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Robb E.L., Hall A.R., Prime T.A., Eaton S., Szibor M., Viscomi C., James A.M., Murphy M.P. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 2018;293:9869–9879. doi: 10.1074/jbc.RA118.003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sohal R.S., Kamzalov S., Sumien N., Ferguson M., Rebrin I., Heinrich K.R., Forster M.J. Effect of coenzyme Q10 intake on endogenous coenzyme Q content, mitochondrial electron transport chain, antioxidative defenses, and life span of mice. Free Radic. Biol. Med. 2006;40:480–487. doi: 10.1016/j.freeradbiomed.2005.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sohal R.S., Forster M.J. Coenzyme Q, oxidative stress and aging. Mitochondrion. 2007;7:103–111. doi: 10.1016/j.mito.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharpley M.S., Shannon R.J., Draghi F., Hirst J. Interactions between phospholipids and NADH:ubiquinone oxidoreductase (complex I) from bovine mitochondria. Biochemistry. 2006;45:241–248. doi: 10.1021/bi051809x. [DOI] [PubMed] [Google Scholar]
- 28.Rebrin I., Kamzalov S., Sohal Rajindar S. Tissue bioavailability and detection of coenzyme Q. Quinones Quinone Enzym. Part A. 2004:138–145. doi: 10.1016/S0076-6879(04)78009-X. Academic Press. [DOI] [PubMed] [Google Scholar]
- 29.Hahn S.H., Kerfoot S., Vasta V. Assay to measure oxidized and reduced forms of CoQ by LC–MS/MS. Humana Press. 2012:169–179. doi: 10.1007/978-1-61779-504-6_11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.