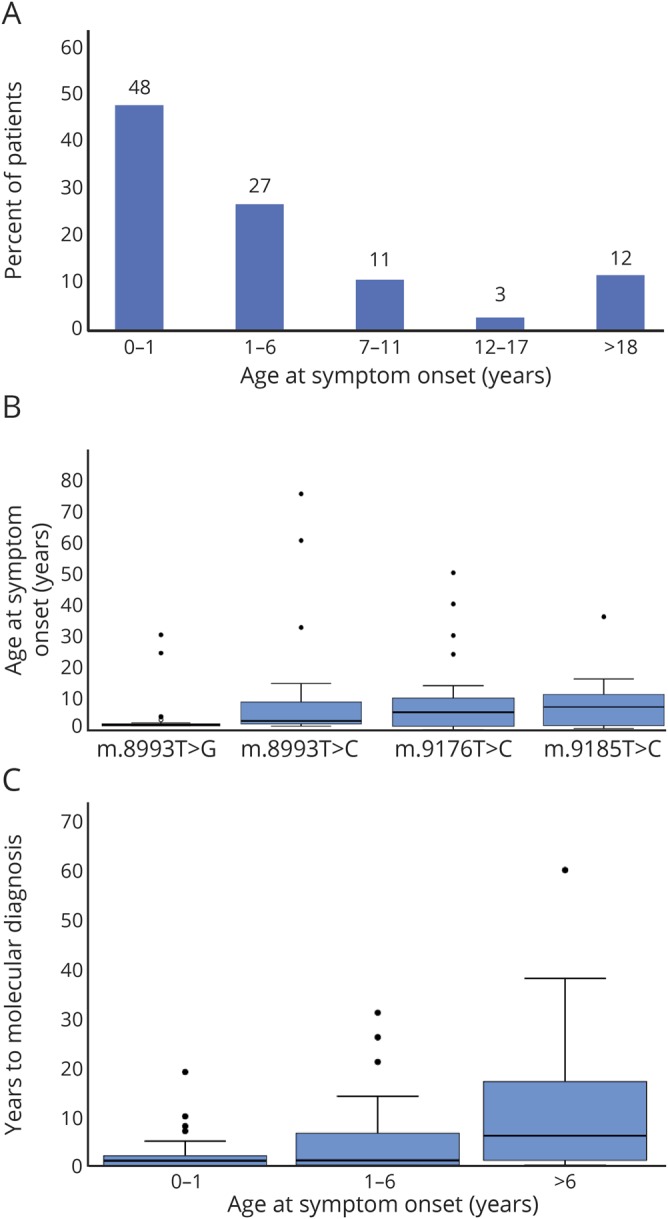
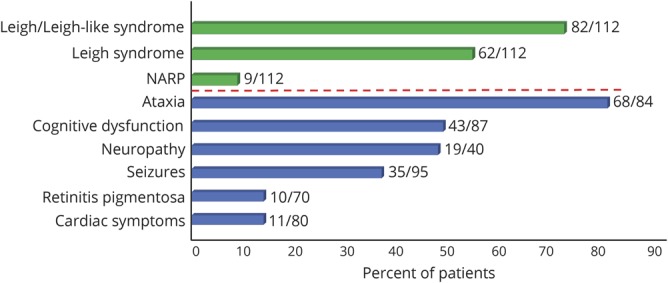
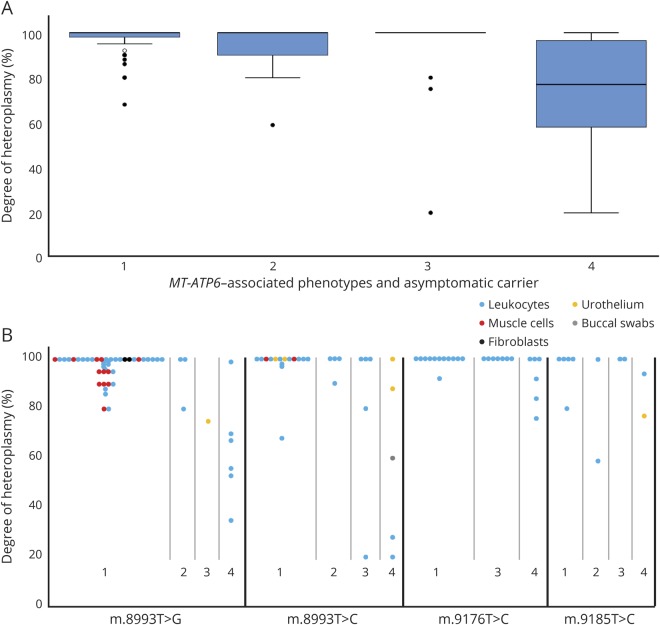
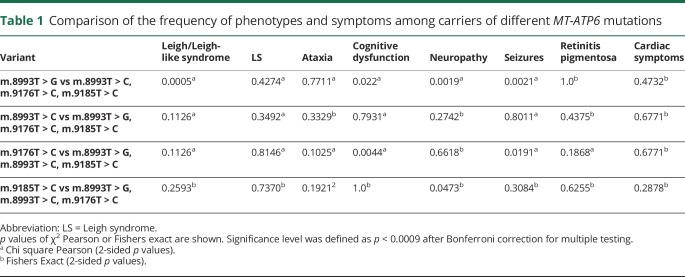
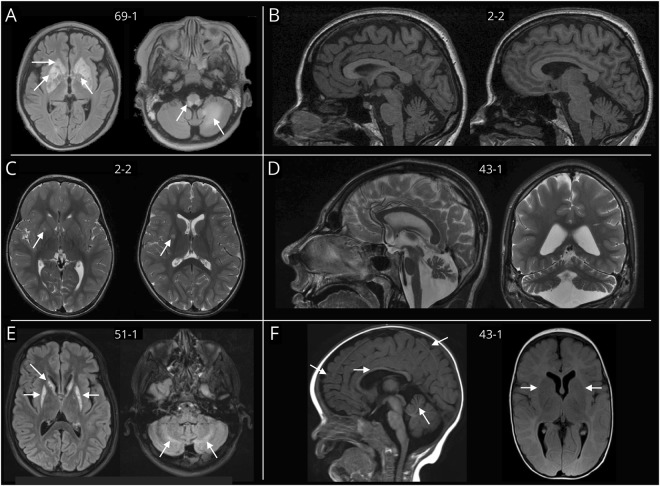
Claudia Stendel
Claudia Stendel, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,*,
Christiane Neuhofer
Christiane Neuhofer, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,*,
Elisa Floride
Elisa Floride, MD, PhD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,*,
Shi Yuqing
Shi Yuqing, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,*,
Rebecca D Ganetzky
Rebecca D Ganetzky, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Joohyun Park
Joohyun Park, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Peter Freisinger
Peter Freisinger, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Cornelia Kornblum
Cornelia Kornblum, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Stephanie Kleinle
Stephanie Kleinle, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Ludger Schöls
Ludger Schöls, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Felix Distelmaier
Felix Distelmaier, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Georg M Stettner
Georg M Stettner, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Boriana Büchner
Boriana Büchner, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Marni J Falk
Marni J Falk, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Johannes A Mayr
Johannes A Mayr, PhD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Matthis Synofzik
Matthis Synofzik, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Angela Abicht
Angela Abicht, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Tobias B Haack
Tobias B Haack, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Holger Prokisch
Holger Prokisch, PhD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Saskia B Wortmann
Saskia B Wortmann, MD, PhD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Kei Murayama
Kei Murayama, MD, PhD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,
Fang Fang
Fang Fang, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,*,
Thomas Klopstock
Thomas Klopstock, MD
1From the Department of Neurology (C.S.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE), Munich, Germany; Institute of Human Genetics (C.N.), Department of Medical Genetics, University of Göttingen, Germany; Department of Pediatrics (E.F.), Salzburg State Hospitals (SALK) and Paracelsus Medical University; Division of Clinical Genetics Salzburg State Hospitals and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (S.Y., F.F.), Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China; Mitochondrial Medicine Frontier Program (R.D.G., M.J.F.), Children's Hospital of Philadelphia; Division of Human Genetics (R.D.G.), Department of Pediatrics, University of Pennsylvania Perelman School of Medicine Philadelphia; Department of Pediatrics (R.D.G.), Perelman School of Medicine, University of Pennsylvania; Institute of Medical Genetics and Applied Genomics (J.P.), University of Tübingen, Germany; Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research (J.P.), University of Tübingen, Germany; Children's Hospital (P.F.), Klinikum Reutlingen, Reutlingen; Department of Neurology (C.K.), University Hospital Bonn; Medical Genetic Center (S.K.), Munich; Department of Neurodegeneration (L.S., M.S.), Hertie Institute for Clinical Brain Research, University of Tübingen; German Center for Neurodegenerative Diseases (DZNE) (L.S.), Tübingen; Department of General Pediatrics (F.D.), Neonatology and Pediatric Cardiology, University Children's Hospital, Heinrich-Heine University, Duesseldorf, Germany; Division of Pediatric Neurology (G.M.S.), University Children's Hospital Zurich, Switzerland; Department of Neurology (B.B.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Department of Pediatrics (J.A.M.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Department of Neurology (A.A.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich, Germany; Medical Genetic Center (A.A.), Munich; Institute of Medical Genetics and Applied Genomics (T.B.H.), Tübingen, Germany; Institute of Human Genetics (H.P.), Technische Universität München, Munich, Germany; Institute of Human Genetics (H.P.), Helmholtz Center Munich, Neuherberg, Germany; Department of Pediatrics (S.B.W.), Salzburg State Hospitals (SALK) and Paracelsus Medical University, Salzburg, Austria; Institute of Human Genetics, Technische Universität München, Munich, Germany; Institute of Human Genetics (S.B.W.), Helmholtz Center Munich, Neuherberg, Germany; Center for Medical Genetics (K.M.), and Department of Metabolism, Chiba Children's Hospital, Chiba, Japan; and Department of Neurology (T.K.), Friedrich-Baur-Institute, Ludwig-Maximilians-University Munich; German Center for Neurodegenerative Diseases (DZNE) (T.K.), Munich; Munich Cluster for Systems Neurology (SyNergy) (T.K.), Ludwig Maximilians University Munich, Germany.
1,*,✉;
on behalf of the ATP6 Study Group1