Abstract
ACh is a signaling molecule in the mammalian CNS, with well-documented influence over cognition and behavior. However, the nature of cholinergic signaling in the brain remains controversial, with ongoing debates focused on the spatial and temporal resolution of ACh activity. Generally, opposing views have embraced a dichotomy between transmission as slow and volume-mediated versus fast and synaptic. Here, we provide the perspective that ACh, like most other neurotransmitters, exhibits both fast and slow modes that are strongly determined by the anatomy of cholinergic fibers, the distribution and the signaling mechanisms of receptor subtypes, and the dynamics of ACh hydrolysis. Current methodological approaches remain limited in their ability to provide detailed analyses of these underlying factors. However, we believe that the continued development of novel technologies in combination with a more nuanced view of cholinergic activity will open critical new avenues to a better understanding of ACh in the brain.
Dual Perspectives Companion Paper: Forebrain Cholinergic Signaling: Wired and Phasic, Not Tonic, and Causing Behavior, by Martin Sarter and Cindy Lustig
Keywords: acetylcholine, neuromodulation, synaptic, extrasynaptic
Introduction
The earliest debates over the nature of neuronal communication focused on the spatial and temporal resolution necessary to effectively transmit information within the nervous system (Cowan and Kandel, 2001). The initial questions of chemical versus electrical transmission have largely given way to investigations of the means by which fast and spatially compartmentalized signaling is maintained via the release, reception, and clearance of a variety of neuroactive compounds. Indeed, fast “neurotransmitters,” such as glutamate and GABA, are often compared with “neuromodulators,” such as ACh, dopamine, and serotonin, which are thought to act slowly and with minimal spatial precision throughout the brain (Marder, 2012). However, many lines of evidence suggest that neuromodulators may also exhibit fast modes of signaling, giving rise to an ongoing controversy that spans groups studying a range of organisms with a diverse array of methodologies. In this perspective, we will discuss the experimental evidence for the nature of ACh signaling in the mammalian brain, focusing on the neocortex. We argue that existing views often posit a series of false dichotomies (e.g., fast vs slow, phasic vs tonic, synaptic vs nonsynaptic), as cholinergic activity operates over a range of spatial and temporal scales, reflecting its broad importance to nervous system function and behavior.
The discourse on spatial and temporal signaling by ACh has been ongoing since the first descriptions of central cholinergic anatomy and function (Mitchell, 1963; Kanai and Szerb, 1965; Phillis, 1968; Bigl et al., 1982; McKinney et al., 1983; Price and Stern, 1983; Descarries et al., 1997). Over time, there has been a merging and evolution of ideas that has resulted in an oversimplified dichotomy (Sarter et al., 2009; Yamasaki et al., 2010; Unal et al., 2012), often framed in terms of fast synaptic (or wired) transmission versus slow nonsynaptic (or volume) transmission. This view has its origins in a classification scheme that emerged based on observations of enkephalin signaling (Agnati et al., 1986) and was later applied to the cholinergic system (Descarries et al., 1997). Wired transmission occurs at synapses (Fig. 1, “synaptic”), yielding high fidelity signaling with a 1-to-1 relationship between sender and receiver. This mode is characterized by higher ligand concentration and lower receptor affinity. Volume transmission (Fig. 1, “Non-synaptic” and “Spillover”), on the other hand, is not constrained to cell-to-cell contact sites and relies more heavily on diffusion, including spillover from the synaptic cleft following presynaptic release. As a result, volume transmission may yield lower signaling fidelity, a 1-to-many sender–receiver relationship, and lower ligand concentrations that call for high receptor affinity. Since originally proposed, volume transmission as applied to the cholinergic system has become conflated with other models of diffuse signaling in which ACh is argued to mediate a tonic mode of communication arising from broadly collateralized axons whose putative release sites do not routinely make synapses. The result has been a literature that often attempts to determine whether ACh participates in fast or slow signaling. Fascinatingly, this rigid constraint has not been applied to other signaling molecules, despite evidence that most “fast” signaling molecules (e.g., GABA and glutamate) also exhibit slow modes of transmission.
Figure 1.
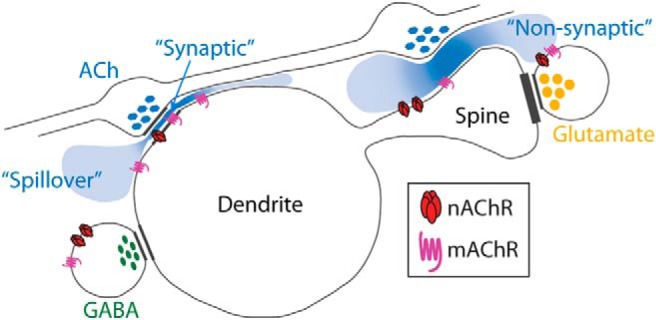
Spatial relationships in cortical cholinergic signaling. When cholinergic axons (blue, ACh) make synapses in the cortex (Synaptic), the postsynaptic element is usually a dendrite. Nicotinic and muscarinic cholinergic receptors (nAChR and mAChR, respectively) are observed on dendritic branches (Dendrite) and spines (Spine), and are also observed on noncholinergic axons (GABA, glutamate) where they regulate transmitter release. Cholinergic receptors can be activated synaptically or via volume transmission. The latter mode includes both diffusion outside the cleft after synaptic release (Spillover) or in the absence of a synapse (Non-synaptic).
Glutamate: a canonical fast transmitter
To provide a framework and context for interpreting studies of cholinergic signaling, we briefly summarize our knowledge of a canonical “fast” neurotransmitter, glutamate, which also exerts slow “modulatory” effects. Glutamate is the major excitatory neurotransmitter in the cortex, where it is released from axons of pyramidal cells that exhibit extremely precise and topographic connectivity (Meldrum, 2000; Perin et al., 2011). Glutamate is packaged into presynaptic vesicles that fuse with the cell membrane following axonal invasion by an action potential and release their contents into the extracellular space over a few milliseconds (Acuna et al., 2014). The glutamatergic synaptic cleft is a small volume of extracellular space between the apposed presynaptic and postsynaptic membranes with a width of ∼20 nm (Harris and Weinberg, 2012). This small volume is spatially constrained by neuronal and non-neuronal structures, enabling large, rapid shifts in the local concentration of glutamate. This small volume is completely inaccessible to traditional electrophysiological or electrochemical probes, preventing direct assay of the glutamate concentration and kinetics in situ. However, studies using nonequilibrium dynamics of NMDA-type glutamate receptors in cultured neurons revealed that glutamate reaches a peak concentration of ∼1 mm and decays with a time constant of ∼1 ms at central synapses (Clements et al., 1992). This value contrasts sharply with estimates of submicromolar glutamate concentration obtained from microdialysis studies (Moussawi et al., 2011), illustrating the essential challenge to measuring transmitter concentration with exogenous probes that do not sample synaptic volumes. Ionotropic signals mediated by AMPA-, NMDA-, and kainate-type glutamate receptors occur over ∼1–100 ms (Dingledine et al., 1999). In contrast, metabotropic G-protein-coupled glutamate receptors are linked to a variety of downstream signaling pathways that gate membrane ion channels, regulate a vast milieu of biochemical signals, and influence gene expression with temporal scales ranging from hundreds of milliseconds to minutes or more (Niswender and Conn, 2010). Glutamatergic signaling is terminated primarily by reuptake of glutamate from the synaptic cleft via membrane transporters whose activity strongly shapes the spatiotemporal dynamics of signaling (Vandenberg and Ryan, 2013). Even under physiological conditions, “spillover” from the synaptic cleft is thought to activate extrasynaptic receptors (both ionotropic and metabotropic) that may provide a tonic mode of excitatory signaling. Together, these findings demonstrate that even a traditional “fast” neurotransmitter operates over several orders of spatial and temporal magnitude and illustrate the challenges to a straightforward classification of neuroactive molecules.
Do cholinergic axons make synapses?
A central issue in the debate over fast versus slow signaling for ACh has been the extent to which cholinergic signaling in the CNS makes use of the synaptic structures that facilitate fast chemical signaling. Using electron microscopy, a large number of studies have reported that some vesicle-containing varicosities (putative release sites) along cholinergic axons in the CNS do appear to make synapses, whereas others do not (De Lima and Singer, 1986; Aoki and Kabak, 1992; Umbriaco et al., 1994, 1995; Mrzljak et al., 1995; Smiley et al., 1997; Turrini et al., 2001; Muller et al., 2013). The controversy surrounds the proportion of release sites that make such contacts (Table 1).
Table 1.
Synaptic incidence for putative cholinergic varicositiesa
| Species | Brain region | Axonal marker | Method | Synaptic incidence (%) | Citation |
|---|---|---|---|---|---|
| Rat | Hippocampus | ChAT | Serial EM | 7 | Umbriaco et al., 1995 |
| Rat | Parietal cortex (all layers) | ChAT | Serial EM | 14 (mean over layers, range 10–21) | Umbriaco et al., 1994 |
| Cat | Primary visual cortex | ChAT | 2D sampling | 14 | De Lima and Singer, 1986 |
| Cat | Primary visual cortex | ChAT | 2D sampling | 21 | Aoki and Kabak, 1992 |
| Macaque | PFC, layers 2 and 3 | ChAT | Serial EM | 44 | Mrzljak et al., 1995 |
| Rat | Parietal cortex, layer 5 | vAChT | 2D sampling | 66 | Turrini et al., 2001 |
| Human | Anterior temporal lobe | ChAT | Serial EM | 67 | Smiley et al., 1997 |
| Rat | Amygdala | vAChT | Serial EM | 76 | Muller et al., 2013 |
aChAT, Choline acetyltransferase; vAChT, vesicular acetylcholine transporter; EM, electron microscopy.
Overall, the reported synaptic incidence (percentage of putative release sites forming synaptic contacts) varies from 7% (Umbriaco et al., 1995) to 76% (Muller et al., 2013). Thus, even at the high end of this distribution, one-fourth of cholinergic varicosities do not appear to make synapses and thus seem likely sources of a volume transmitted signal. The extent to which the differences reported across these studies represent bias, error, methodological differences, differences between brain areas, or species differences is unknown as comparative studies are rare. One alternative possibility is that all observed nonsynaptic varicosities are nonfunctional, meaning that they should not count as evidence for nonsynaptic transmission. In this case, the number of release sites in the rat hippocampus would drop from nearly 1 billion to 600,000 per volume of cortex under 1 mm2 of pia (Descarries et al., 2004), thus raising a new problem. With this low density of synapses, and if fast synaptic signaling is the only mode of operation, only an extremely small fraction of neurons would be expected to respond to ACh release, a conclusion at odds with the high rate of cholinergic sensitivity (Segal, 1978; Dannenberg et al., 2017). These observations thus support the conclusion that at least some neurons must respond to cholinergic signals that did not arrive via a direct synaptic contact. Whether there are other anatomical markers that could reliably be used to identify functional release sites is an open question.
Receptors define spatiotemporal signaling resolution
Cholinergic signaling occurs via ionotropic nicotinic and metabotropic muscarinic receptors (Picciotto et al., 2012; Higley and Picciotto, 2014). Functionally, nicotinic receptors are pentameric ion channels that are permeable to a variety of cations, including sodium, potassium, and calcium. The precise combination of receptor subunits defines the channel affinity, kinetics, and permeability (Albuquerque et al., 2009). In the neocortex and hippocampus, a variety of neurons express functional nicotinic receptors on both axonal and somatodendritic compartments (Picciotto et al., 2012). In acute brain slices, ACh released following electrical or optogenetic stimulation can evoke fast excitatory currents that enhance postsynaptic spike generation and presynaptic release probability (Jiang et al., 2014). Thus, it is clear that a fast mode of cholinergic signaling exists under some circumstances.
In contrast, muscarinic receptors are G-protein-coupled, either signaling via Gαq and phospholipase C activation (M1 type) or Gαi/o, and suppression of adenylate cyclase (M2 type) (Thiele, 2013). In both cases, signal transduction is inherently slower than typical fast transmission, requiring a number of downstream molecular cascades to produce functional consequences that can include gating ion channels, driving intracellular calcium release, or influencing gene transcription (Thiele, 2013). Moreover, given the intracellular diffusion of ACh-coupled signaling pathways, there is an inherent loss of spatial resolution with metabotropic activity. These cellular mechanisms give rise to multiple experimental observations of prolonged modulation of neuronal activity following relatively brief cholinergic stimulation (Cole and Nicoll, 1984; Alonso and Klink, 1993; Hasselmo and Fehlau, 2001). Thus, it is clear that a slower mode of cholinergic signaling also exists.
The functional activity of both receptor classes clearly depends critically on their spatial localization in the brain and their relationship to presynaptic release sites. Localization of receptors is generally done by immunohistochemistry, requiring rigorous controls to validate antibody specificity. Here, we limit our discussion to studies we feel meet a strict standard of reliability. Under the electron microscope, synapses can be classified as either Type I or Type II (Gray, 1959), more commonly referred as asymmetric (Fig. 1, “Glutamate”) and symmetric (Fig. 1, “GABA”), respectively (Colonnier, 1968). Cholinergic synapses are Type II/symmetric (De Lima and Singer, 1986; Aoki and Kabak, 1992; Umbriaco et al., 1994, 1995; Mrzljak et al., 1995; Smiley et al., 1997; Turrini et al., 2001; Muller et al., 2013); but intriguingly, in cortical areas 17 and 46 of macaque monkeys, the majority of both M1- and M2-type receptors are localized to asymmetric (glutamatergic) synapses formed on dendritic spines (with M1 most often postsynaptic and M2 most often presynaptic) (Mrzljak et al., 1993; Disney et al., 2006). A similar finding was reported for nicotinic receptors in area 17 of macaque, which are also preferentially localized to glutamatergic synapses on the presynaptic side (Disney et al., 2007). Interestingly, when they have been identified in proximity to a glutamatergic synapse, cholinergic axons are generally located on the opposite side of the spine head from the glutamatergic axon and cholinergic receptors (Fig. 1) (Aoki and Kabak, 1992; Disney et al., 2006), suggesting that diffusion of signal (either extracellularly or intracellularly) would be necessary for ACh to influence excitatory transmission.
In addition to postsynaptic modulation, ACh has been widely implicated in the control of presynaptic release from cortical neurons, at both glutamatergic and GABAergic synapses (Hasselmo, 1995, 2006; Thiele, 2013; Jiang et al., 2014). Immunoelectron microscopy in macaque monkeys has identified β2 subunit-containing nicotinic receptors on thalamocortical terminals (Disney et al., 2007) and M2-type receptors on intracortical and thalamocortical glutamatergic axons (Mrzljak et al., 1993, 1996; Disney et al., 2006). Similar ultrastructural observations on the localization of M2 receptors have been made for rat amygdala (Muller et al., 2013, 2016) and hippocampus (Rouse et al., 2000). These findings are supported by functional studies in the rat and mouse showing both nicotinic and muscarinic (M2-like) control of glutamatergic release probability through presynaptic receptors (Higley et al., 2009; Dasari and Gulledge, 2011; Urban-Ciecko et al., 2018). Nevertheless, cholinergic axons do not make axo-axonic synapses (De Lima and Singer, 1986; Aoki and Kabak, 1992, their Table 1; Umbriaco et al., 1994, 1995; Mrzljak et al., 1995, their Table 1; Descarries et al., 1997, their Table 2; Descarries et al., 2004; Smiley et al., 1997; Turrini et al., 2001; Muller et al., 2013, their Table 4), strongly suggesting that diffusion from distal ACh release sites must be required to activate these receptors.
Beyond expression at presynaptic terminals or dendritic spines, muscarinic receptors in macaque area 17 are also found on nonsynaptic membranes (Disney et al., 2006), again suggesting that ACh signaling may occur outside of traditional synaptic connections. As noted above, localization of receptors traditionally depends on antibodies that can be difficult to validate for specificity. Recent efforts to transgenically label receptors with fluorescent markers present a new approach to localize these proteins and build on our current knowledge of cholinergic function in species for which transgenic methods are viable tool (Mikuni et al., 2016). However, species differences throughout the cholinergic system (for review, see Coppola and Disney, 2018), including the composition of the basal forebrain (Mesulam et al., 1983; Gritti et al., 1997; Raghanti et al., 2011), the laminar pattern of the cholinergic innervation of cortex (Avendaño et al., 1996; Raghanti et al., 2008), and the cortical and subcortical expression of nicotinic and muscarinic receptor subtypes (Wada et al., 1989; Marks et al., 1992; Séguéla et al., 1993; Quik et al., 2000; Disney and Reynolds, 2014; Coppola et al., 2016) call for careful interpretation of data across animal models.
AChE shapes spatiotemporal signaling of ACh
Debates over the spatial and temporal scale of cholinergic signaling also frequently focus on the rapid clearance of ACh from the extracellular space after release. ACh is broken down by one of the AChEs or (less commonly) by a butylcholinesterase (Massoulié et al., 1993). AChEs are a family of enzymes whose rate of ACh hydrolysis is usually limited only by substrate diffusion (Quinn, 1987). This efficiency, coupled with the argument that AChEs have effectively limitless capacity, has been used to suggest that no ACh molecules could escape the synaptic cleft, thus preventing the diffusion essential for volume transmission. There are some challenges to this interpretation. First, vertebrate AChEs are inhibited by their substrate (Alles and Hawes, 1940; Massoulié et al., 1993), with the rate of hydrolysis slowing at micromolar substrate concentrations (Alles and Hawes, 1940; Radić et al., 1993). If local levels of ACh approximate those of glutamate (millimolar concentration), AChE function may be severely limited. Second, when not inhibited by excess substrate, AChE hydrolysis remains limited by substrate diffusion. Thus, the local levels of AChE expression and patterns of subcellular localization together determine the distance between a release site and the nearest AChE molecule, consequently regulating the rate of hydrolysis. At the vertebrate neuromuscular junction, AChEs are densely expressed as membrane-anchored proteins on the basal lamina immediately surrounding ACh release sites. This arrangement may restrict ACh diffusion from the cleft, but even this conclusion remains controversial (Blotnick-Rubin and Anglister, 2018). However, there is no evidence for similar structural organization in the CNS, where AChE does not densely cluster around cholinergic varicosities (Dunant and Gisiger, 2017). Thus, the compartmentalization of ACh activity by AChE in the brain remains an open question.
Observing cholinergic signaling in vivo
A critical element in the debate over the interpretation of structural data for the cholinergic system surrounds the direct measurement of ACh levels in in functioning circuits. Attempts to measure ACh and its metabolites in vivo have primarily used either (1) microdialysis sampling with ex vivo liquid chromatography followed by electrochemistry or mass spectrometry or (2) in vivo electrochemistry. Both methods are limited by the size of the sampling probes and temporal resolution, resulting in significant challenges to inferring physiological modes of cholinergic signaling. Furthermore, early studies using microdialysis included AChE inhibitors in the dialysis perfusate to enhance signal detection, precluding conclusions about spatial or temporal scales of ACh activity (Ichikawa et al., 2000). More recent studies without AChE inhibition have suggested that resting extrasynaptic levels of ACh are very low, in the picomolar or femtomolar range. (Xu et al., 1991; Testylier and Dykes, 1996; Herzog et al., 2003). However, the smallest dialysis membranes are ∼1 mm long and, thus, lack sensitivity to highly localized extracellular domains. Furthermore, this approach is limited to a temporal resolution of several minutes.
Newer approaches using in vivo electrochemistry have substantially faster temporal resolution (potentially at a millisecond scale) and use a sampling surface that can be as small as 15 μm. In one version of this technique, a single enzyme (choline oxidase) is applied to the electrode and the measured molecule is actually choline, not ACh (Parikh et al., 2004). Experiments using this method have revealed transient signals in the neocortex in response to various behavioral cues with a duration in the range of seconds (Parikh et al., 2007, 2008; Teles-Grilo Ruivo et al., 2017). However, choline dynamics reflect the diffusion of ACh and rate of hydrolysis (influencing signal onset latency) as well as the diffusion of choline and reuptake by the choline transporter (influencing signal duration). Thus, choline transients can set an upper bound on the kinetics of the underlying ACh signal, but determining the spatiotemporal resolution of ACh activity from these measures is not possible. Furthermore, the low sampling rate used in these studies (usually 2–5 Hz) limits the detection of fast events.
An alternative electrochemical method involves applying two enzymes to the sensor, AChE and choline oxidase. With appropriate controls (Burmeister et al., 2008), these probes are suitable for detecting basal ACh concentrations and transient signals (Mattinson et al., 2011). In this case, basal extrasynaptic ACh concentration in rat PFC is reported to be 0.5–1.0 μm, and stimulation-induced transients of 5–7 μm could be evoked with a clearance time of several seconds (Mattinson et al., 2011). This suggests the presence of a nonsynaptic ACh signal, but its measured dynamics are orders of magnitude slower than canonical fast glutamatergic signaling. Of course, this may not reflect ACh activity in all physiologically relevant compartments, and low sampling rates (∼4 Hz) again limit conclusions about the underlying biology. Thus, availability of methods for inferring ACh activity in situ remains a major hurdle to be overcome, and open questions persist.
In conclusion, despite the identification of ACh as a neurotransmitter more than a century ago (Dale, 1914; Loewi, 1921), many open questions remain regarding cholinergic signaling, particularly in the CNS. A key step forward will be to develop a conceptual framework that promotes future investigation. As we have discussed, there is evidence to support both fast and slow modes of cholinergic signaling, occurring at both synaptic and extrasynaptic sites. While few studies have demonstrated dynamics of central ACh transmission that would be similar to that of the traditional fast transmitters (e.g., millimolar concentrations with millisecond kinetics), the methodologies for measuring cholinergic activity with necessary sensitivity in situ are lacking. Thus, the precise spatiotemporal limits of ACh remain poorly understood.
Given its age (in evolutionary terms) and the tendency for natural selection to operate via tinkering and repurposing (Jacob, 1977), particularly for neuromodulatory systems (Katz and Lillvis, 2014; Tamvacakis et al., 2018), it would be surprising if ACh did not share with other signaling molecules the capacity for fast and slow, tonic and phasic, synaptic and extrasynaptic modes of operation. Thus, ongoing research must focus on elaborating the cellular mechanisms that determine the diversity of cholinergic dynamics across a range of parameters, including species, brain region, cell type, and behavioral state. Several key questions remain unanswered. How far does ACh diffuse from a release site? What is the efficiency of AChE in situ? How do ionotropic and metabotropic signals interact to influence neuronal electrical and biochemical activity? Answers to these queries, and others in the same vein, will advance our understanding of ACh, and of other signaling molecules as well.
Footnotes
This work was supported by National Institutes of Health Grants MH099045, MH113852, and NS105640 to M.J.H. and Grant EY029663 to A.A.D., a Simons Foundation Research Grant to M.J.H., and a Whitehead Foundation Grant to A.A.D. We thank members of the A.A.D. and M.J.H. laboratories for suggestions in the preparation of this manuscript.
The authors declare no competing financial interests.
References
- Acuna C, Guo Q, Burré J, Sharma M, Sun J, Sudhof TC (2014) Microsecond dissection of neurotransmitter release: SNARE-complex assembly dictates speed and Ca(2)(+) sensitivity. Neuron 82:1088–1100. 10.1016/j.neuron.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F (1986) A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta Physiol Scand 128:201–207. 10.1111/j.1748-1716.1986.tb07967.x [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89:73–120. 10.1152/physrev.00015.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alles GA, Hawes RC (1940) Cholinesterases in the blood of man. J Biol Chem 133:375–390. [Google Scholar]
- Alonso A, Klink R (1993) Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J Neurophysiol 70:128–143. 10.1152/jn.1993.70.1.128 [DOI] [PubMed] [Google Scholar]
- Aoki C, Kabak S (1992) Cholinergic terminals in the cat visual cortex: ultrastructural basis for interaction with glutamate-immunoreactive neurons and other cells. Vis Neurosci 8:177–191. 10.1017/S0952523800002832 [DOI] [PubMed] [Google Scholar]
- Avendaño C, Umbriaco D, Dykes RW, Descarries L (1996) Acetylcholine innervation of sensory and motor neocortical areas in adult cat: a choline acetyltransferase immunohistochemical study. J Chem Neuroanat 11:113–130. 10.1016/0891-0618(96)00132-9 [DOI] [PubMed] [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL (1982) Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull 8:727–749. 10.1016/0361-9230(82)90101-0 [DOI] [PubMed] [Google Scholar]
- Blotnick-Rubin E, Anglister L (2018) Fine localization of acetylcholinesterase in the synaptic cleft of the vertebrate neuromuscular junction. Front Mol Neurosci 11:123. 10.3389/fnmol.2018.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister JJ, Pomerleau F, Huettl P, Gash CR, Werner CE, Bruno JP, Gerhardt GA (2008) Ceramic-based multisite microelectrode arrays for simultaneous measures of choline and acetylcholine in CNS. Biosens Bioelectron 23:1382–1389. 10.1016/j.bios.2007.12.013 [DOI] [PubMed] [Google Scholar]
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL (1992) The time course of glutamate in the synaptic cleft. Science 258:1498–1501. [DOI] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA (1984) Characterization of a slow cholinergic post-synaptic potential recorded in vitro from rat hippocampal pyramidal cells. J Physiol 352:173–188. 10.1113/jphysiol.1984.sp015285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnier M. (1968) Synaptic patterns on different cell types in the different laminae of the cat visual cortex: an electron microscope study. Brain Res 9:268–287. 10.1016/0006-8993(68)90234-5 [DOI] [PubMed] [Google Scholar]
- Coppola JJ, Disney AA (2018) Is there a canonical cortical circuit for the cholinergic system? Anatomical differences across common model systems. Front Neural Circuits 12:8. 10.3389/fncir.2018.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola JJ, Ward NJ, Jadi MP, Disney AA (2016) Modulatory compartments in cortex and local regulation of cholinergic tone. J Physiol Paris 110:3–9. 10.1016/j.jphysparis.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan WM, Kandel ER (2001) A brief history of synapses and synaptic transmission. In: Synapses (Cowan WM, Sudhof TC, Stevens CF, eds), pp 1–87. Baltimore: Johns Hopkins UP. [Google Scholar]
- Dale HH. (1914) The action of certain esters and ethers of choline and their relation to muscarine. J Pharmacol Exp Ther 6:147–190. [Google Scholar]
- Dannenberg H, Young K, Hasselmo M (2017) Modulation of hippocampal circuits by muscarinic and nicotinic receptors. Front Neural Circuits 11:102. 10.3389/fncir.2017.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Gulledge AT (2011) M1 and M4 receptors modulate hippocampal pyramidal neurons. J Neurophysiol 105:779–792. 10.1152/jn.00686.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lima AD, Singer W (1986) Cholinergic innervation of the cat striate cortex: a choline acetyltransferase immunocytochemical analysis. J Comp Neurol 250:324–338. 10.1002/cne.902500306 [DOI] [PubMed] [Google Scholar]
- Descarries L, Gisiger V, Steriade M (1997) Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol 53:603–625. 10.1016/S0301-0082(97)00050-6 [DOI] [PubMed] [Google Scholar]
- Descarries L, Mechawar N, Aznavour N, Watkins KC (2004) Structural determinants of the roles of acetylcholine in cerebral cortex. Prog Brain Res 145:45–58. 10.1016/S0079-6123(03)45002-4 [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51:7–61. [PubMed] [Google Scholar]
- Disney AA, Reynolds JH (2014) Expression of m1-type muscarinic acetylcholine receptors by parvalbumin-immunoreactive neurons in the primary visual cortex: a comparative study of rat, guinea pig, ferret, macaque, and human. J Comp Neurol 522:986–1003. 10.1002/cne.23456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney AA, Domakonda KV, Aoki C (2006) Differential expression of muscarinic acetylcholine receptors across excitatory and inhibitory cells in visual cortical areas V1 and V2 of the macaque monkey. J Comp Neurol 499:49–63. 10.1002/cne.21096 [DOI] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ (2007) Gain modulation by nicotine in macaque v1. Neuron 56:701–713. 10.1016/j.neuron.2007.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunant Y, Gisiger V (2017) Ultrafast and slow cholinergic transmission: different involvement of acetylcholinesterase molecular forms. Molecules 22:E1300. 10.3390/molecules22081300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG. (1959) Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat 93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE (1997) GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol 383:163–177. [DOI] [PubMed] [Google Scholar]
- Harris KM, Weinberg RJ (2012) Ultrastructure of synapses in the mammalian brain. Cold Spring Harb Perspect Biol 4:a005587. 10.1101/cshperspect.a005587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. (1995) Neuromodulation and cortical function: modeling the physiological basis of behavior. Behav Brain Res 67:1–27. 10.1016/0166-4328(94)00113-T [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. (2006) The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16:710–715. 10.1016/j.conb.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Fehlau BP (2001) Differences in time course of ACh and GABA modulation of excitatory synaptic potentials in slices of rat hippocampus. J Neurophysiol 86:1792–1802. 10.1152/jn.2001.86.4.1792 [DOI] [PubMed] [Google Scholar]
- Herzog CD, Nowak KA, Sarter M, Bruno JP (2003) Microdialysis without acetylcholinesterase inhibition reveals an age-related attenuation in stimulated cortical acetylcholine release. Neurobiol Aging 24:861–863. 10.1016/S0197-4580(02)00226-9 [DOI] [PubMed] [Google Scholar]
- Higley MJ, Picciotto MR (2014) Neuromodulation by acetylcholine: examples from schizophrenia and depression. Curr Opin Neurobiol 29:88–95. 10.1016/j.conb.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Soler-Llavina GJ, Sabatini BL (2009) Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nat Neurosci 12:1121–1128. 10.1038/nn.2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa J, Dai J, Meltzer HY (2000) Acetylcholinesterase inhibitors are neither necessary nor desirable for microdialysis studies of brain acetylcholine. Curr Separations 192:37–43. [Google Scholar]
- Jacob F. (1977) Evolution and tinkering. Science 196:1161–1166. 10.1126/science.860134 [DOI] [PubMed] [Google Scholar]
- Jiang L, López-Hernández GY, Lederman J, Talmage DA, Role LW (2014) Optogenetic studies of nicotinic contributions to cholinergic signaling in the central nervous system. Rev Neurosci 25:755–771. 10.1515/revneuro-2014-0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai T, Szerb JC (1965) Mesencephalic reticular activating system and cortical acetylcholine output. Nature 205:80–82. 10.1038/205080b0 [DOI] [PubMed] [Google Scholar]
- Katz PS, Lillvis JL (2014) Reconciling the deep homology of neuromodulation with the evolution of behavior. Curr Opin Neurobiol 29:39–47. 10.1016/j.conb.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Loewi O. (1921) Uber humorale ubertragbarkeit der herznervenwirkung: I. Pflugers Arch 214:678–688. [Google Scholar]
- Marder E. (2012) Neuromodulation of neuronal circuits: back to the future. Neuron 76:1–11. 10.1016/j.neuron.2012.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC (1992) Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci 12:2765–2784. 10.1523/JNEUROSCI.12-07-02765.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoulié J, Pezzementi L, Bon S, Krejci E, Vallette FM (1993) Molecular and cellular biology of cholinesterases. Prog Neurobiol 41:31–91. 10.1016/0301-0082(93)90040-Y [DOI] [PubMed] [Google Scholar]
- Mattinson CE, Burmeister JJ, Quintero JE, Pomerleau F, Huettl P, Gerhardt GA (2011) Tonic and phasic release of glutamate and acetylcholine neurotransmission in sub-regions of the rat prefrontal cortex using enzyme-based microelectrode arrays. J Neurosci Methods 202:199–208. 10.1016/j.jneumeth.2011.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney M, Coyle JT, Hedreen JC (1983) Topographic analysis of the innervation of the rat neocortex and hippocampus by the basal forebrain cholinergic system. J Comp Neurol 217:103–121. 10.1002/cne.902170109 [DOI] [PubMed] [Google Scholar]
- Meldrum BS. (2000) Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 130:1007S–1015S. 10.1093/jn/130.4.1007S [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH (1983) Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol 214:170–197. 10.1002/cne.902140206 [DOI] [PubMed] [Google Scholar]
- Mikuni T, Nishiyama J, Sun Y, Kamasawa N, Yasuda R (2016) High-throughput, high-resolution mapping of protein localization in mammalian brain by in vivo genome editing. Cell 165:1803–1817. 10.1016/j.cell.2016.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF. (1963) The spontaneous and evoked release of acetylcholine from the cerebral cortex. J Physiol 165:98–116. 10.1113/jphysiol.1963.sp007045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Riegel A, Nair S, Kalivas PW (2011) Extracellular glutamate: functional compartments operate in different concentration ranges. Front Syst Neurosci 5:94. 10.3389/fnsys.2011.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Levey AI, Goldman-Rakic PS (1993) Association of m1 and m2 muscarinic receptor proteins with asymmetric synapses in the primate cerebral cortex: morphological evidence for cholinergic modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A 90:5194–5198. 10.1073/pnas.90.11.5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Pappy M, Leranth C, Goldman-Rakic PS (1995) Cholinergic synaptic circuitry in the macaque prefrontal cortex. J Comp Neurol 357:603–617. 10.1002/cne.903570409 [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Levey AI, Rakic P (1996) Selective expression of m2 muscarinic receptor in the parvocellular channel of the primate visual cortex. Proc Natl Acad Sci U S A 93:7337–7340. 10.1073/pnas.93.14.7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, Zaric V, McDonald AJ (2013) Muscarinic cholinergic receptor M1 in the rat basolateral amygdala: ultrastructural localization and synaptic relationships to cholinergic axons. J Comp Neurol 521:1743–1759. 10.1002/cne.23254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, Zaric V, Mott DD, McDonald AJ (2016) Localization of the M2 muscarinic cholinergic receptor in dendrites, cholinergic terminals, and noncholinergic terminals in the rat basolateral amygdala: an ultrastructural analysis. J Comp Neurol 524:2400–2417. 10.1002/cne.23959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50:295–322. 10.1146/annurev.pharmtox.011008.145533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Pomerleau F, Huettl P, Gerhardt GA, Sarter M, Bruno JP (2004) Rapid assessment of in vivo cholinergic transmission by amperometric detection of changes in extracellular choline levels. Eur J Neurosci 20:1545–1554. 10.1111/j.1460-9568.2004.03614.x [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M (2007) Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron 56:141–154. 10.1016/j.neuron.2007.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Man K, Decker MW, Sarter M (2008) Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. J Neurosci 28:3769–3780. 10.1523/JNEUROSCI.5251-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin R, Berger TK, Markram H (2011) A synaptic organizing principle for cortical neuronal groups. Proc Natl Acad Sci U S A 108:5419–5424. 10.1073/pnas.1016051108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis JW. (1968) Acetylcholine release from the cerebral cortex: its role in cortical arousal. Brain Res 7:378–389. 10.1016/0006-8993(68)90004-8 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, Mineur YS (2012) Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76:116–129. 10.1016/j.neuron.2012.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Stern R (1983) Individual cells in the nucleus basalis-diagonal band complex have restricted axonal projections to the cerebral cortex in the rat. Brain Res 269:352–356. 10.1016/0006-8993(83)90145-2 [DOI] [PubMed] [Google Scholar]
- Quik M, Polonskaya Y, Gillespie A, Jakowec M, Lloyd GK, Langston JW (2000) Localization of nicotinic receptor subunit mRNAs in monkey brain by in situ hybridization. J Comp Neurol 425:58–69. [DOI] [PubMed] [Google Scholar]
- Quinn DM. (1987) Acetylcholinesterase: enzyme structure, reaction dynamics and virtual transition states. Chem Rev 87:955–979. 10.1021/cr00081a005 [DOI] [Google Scholar]
- Radić Z, Pickering NA, Vellom DC, Camp S, Taylor P (1993) Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase inhibitors. Biochemistry 32:12074–12084. 10.1021/bi00096a018 [DOI] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC (2008) Cholinergic innervation of the frontal cortex: differences among humans, chimpanzees, and macaque monkeys. J Comp Neurol 506:409–424. 10.1002/cne.21546 [DOI] [PubMed] [Google Scholar]
- Raghanti MA, Simic G, Watson S, Stimpson CD, Hof PR, Sherwood CC (2011) Comparative analysis of the nucleus basalis of Meynert among primates. Neuroscience 184:1–15. 10.1016/j.neuroscience.2011.04.008 [DOI] [PubMed] [Google Scholar]
- Rouse ST, Edmunds SM, Yi H, Gilmor ML, Levey AI (2000) Localization of M(2) muscarinic acetylcholine receptor protein in cholinergic and non-cholinergic terminals in rat hippocampus. Neurosci Lett 284:182–186. 10.1016/S0304-3940(00)01011-9 [DOI] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM (2009) Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat Rev Neurosci 10:383–390. 10.1038/nrn2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. (1978) The acetylcholine receptor in the rat hippocampus; nicotinic, muscarinic or both? Neuropharmacology 17:619–623. 10.1016/0028-3908(78)90157-0 [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW (1993) Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci 13:596–604. 10.1523/JNEUROSCI.13-02-00596.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Morrell F, Mesulam MM (1997) Cholinergic synapses in human cerebral cortex: an ultrastructural study in serial sections. Exp Neurol 144:361–368. 10.1006/exnr.1997.6413 [DOI] [PubMed] [Google Scholar]
- Tamvacakis AN, Senatore A, Katz PS (2018) Single neuron serotonin receptor subtype gene expression correlates with behaviour within and across three molluscan species. Proc Biol Sci 285:20180791. 10.1098/rspb.2018.0791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles-Grilo Ruivo LM, Baker KL, Conway MW, Kinsley PJ, Gilmour G, Phillips KG, Isaac JT, Lowry JP, Mellor JR (2017) Coordinated acetylcholine release in prefrontal cortex and hippocampus is associated with arousal and reward on distinct timescales. Cell Rep 18:905–917. 10.1016/j.celrep.2016.12.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testylier G, Dykes RW (1996) Acetylcholine release from frontal cortex in the waking rat measured by microdialysis without acetylcholinesterase inhibitors: effects of diisopropylfluorophosphate. Brain Res 740:307–315. 10.1016/S0006-8993(96)00893-1 [DOI] [PubMed] [Google Scholar]
- Thiele A. (2013) Muscarinic signaling in the brain. Annu Rev Neurosci 36:271–294. 10.1146/annurev-neuro-062012-170433 [DOI] [PubMed] [Google Scholar]
- Turrini P, Casu MA, Wong TP, De Koninck Y, Ribeiro-da-Silva A, Cuello AC (2001) Cholinergic nerve terminals establish classical synapses in the rat cerebral cortex: synaptic pattern and age-related atrophy. Neuroscience 105:277–285. 10.1016/S0306-4522(01)00172-5 [DOI] [PubMed] [Google Scholar]
- Umbriaco D, Watkins KC, Descarries L, Cozzari C, Hartman BK (1994) Ultrastructural and morphometric features of the acetylcholine innervation in adult rat parietal cortex: an electron microscopic study in serial sections. J Comp Neurol 348:351–373. 10.1002/cne.903480304 [DOI] [PubMed] [Google Scholar]
- Umbriaco D, Garcia S, Beaulieu C, Descarries L (1995) Relational features of acetylcholine, noradrenaline, serotonin and GABA axon terminals in the stratum radiatum of adult rat hippocampus (CA1). Hippocampus 5:605–620. 10.1002/hipo.450050611 [DOI] [PubMed] [Google Scholar]
- Unal CT, Golowasch JP, Zaborszky L (2012) Adult mouse basal forebrain harbors two distinct cholinergic populations defined by their electrophysiology. Front Behav Neurosci 6:21. 10.3389/fnbeh.2012.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban-Ciecko J, Jouhanneau JS, Myal SE, Poulet JF, Barth AL (2018) Precisely timed nicotinic activation drives SST inhibition in neocortical circuits. Neuron 97:611–625.e5. 10.1016/j.neuron.2018.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg RJ, Ryan RM (2013) Mechanisms of glutamate transport. Physiol Rev 93:1621–1657. 10.1152/physrev.00007.2013 [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW (1989) Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol 284:314–335. 10.1002/cne.902840212 [DOI] [PubMed] [Google Scholar]
- Xu M, Nakamura Y, Yamamoto T, Natori K, Irie T, Utsumi H, Kato T (1991) Determination of basal acetylcholine release in vivo by rat brain dialysis with a U-shaped cannula: effect of SM-10888, a putative therapeutic drug for Alzheimer's disease. Neurosci Lett 123:179–182. 10.1016/0304-3940(91)90925-J [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Matsui M, Watanabe M (2010) Preferential localization of muscarinic M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J Neurosci 30:4408–4418. 10.1523/JNEUROSCI.5719-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


