The authors evaluated whether, in acute ischemic stroke, iodine concentration within contrast-stained parenchyma compared with an internal reference in the superior sagittal sinus on dual-energy CT could predict subsequent intracerebral hemorrhage in 71 patients. Forty-three of 71 patients had parenchymal hyperdensity on initial dual-energy CT. The median relative iodine concentration compared with the superior sagittal sinus was significantly higher in those with subsequent intracerebral hemorrhage (137.9% versus 109.2%). They conclude that in dual-energy CT performed within 1 hour following thrombectomy that the relative iodine concentration within contrast-stained brain parenchyma compared with that in the superior sagittal sinus was a more reliable predictor of ICH compared with the absolute maximum iodine concentration.
Abstract
BACKGROUND AND PURPOSE:
Brain parenchymal hyperdensity on postthrombectomy CT in patients with acute stroke can be due to hemorrhage and/or contrast staining. We aimed to determine whether iodine concentration within contrast-stained parenchyma compared with an internal reference in the superior sagittal sinus on dual-energy CT could predict subsequent intracerebral hemorrhage.
MATERIALS AND METHODS:
Seventy-one patients with small infarct cores (ASPECTS ≥ 7) and good endovascular recanalization (modified TICI 2b or 3) for anterior circulation large-vessel occlusion were included. Brain parenchymal iodine concentration as per dual-energy CT and the percentage of contrast staining relative to the superior sagittal sinus were recorded and correlated with the development of intracerebral hemorrhage using Mann-Whitney U and Fisher exact tests.
RESULTS:
Forty-three of 71 patients had parenchymal hyperdensity on initial dual-energy CT. The median relative iodine concentration compared with the superior sagittal sinus was significantly higher in those with subsequent intracerebral hemorrhage (137.9% versus 109.2%, P = .007). By means of receiver operating characteristic analysis, a cutoff value of 100% (iodine concentration relative to the superior sagittal sinus) enabled identification of patients going on to develop intracerebral hemorrhage with 94.75% sensitivity, 43.4% specificity, and a likelihood ratio of 1.71.
CONCLUSIONS:
Within our cohort of patients, the relative percentage of iodine concentration at dual-energy CT compared with the superior sagittal sinus was a reliable predictor of intracerebral hemorrhage development and may be a useful imaging biomarker for risk stratification after endovascular treatment.
Despite advances in patient selection for reperfusion therapy for acute ischemic stroke, intracerebral hemorrhage (ICH) remains a serious potential complication.1,2 Clinical outcome post-thrombectomy is multifactorial. Despite good preprocedural ASPECTS, successful reperfusion post-thrombectomy does not always predict good clinical outcome. Immediately following endovascular treatment, parenchymal high density on NCCT is often seen, caused by disruption of the blood-brain barrier with secondary contrast staining3 and/or hemorrhage.4 It is difficult to differentiate contrast staining from hemorrhage at single-kilovolt(peak) energy CT because both abnormalities appear hyperdense.5,6 Dual-energy CT (DECT) has been shown effective in accurately differentiating parenchymal hemorrhage from contrast staining.3,7-9 The presence of parenchymal contrast staining on DECT immediately post-thrombectomy is associated with the development of ICH.3,7 A retrospective study by Bonatti et al7 of 85 patients undergoing mechanical thrombectomy showed that the presence of parenchymal hyperdensity with a maximum absolute iodine concentration of >1.35 mg/mL may identify patients developing intracerebral hemorrhage with 100% sensitivity and 67.6% specificity. Absolute iodine concentration, however, varies with patient height, weight, cardiac output, and renal function, as well as contrast medium factors, including concentration and volume of contrast administered and scan technique.10 A recent study demonstrated that normalization of iodine quantification to the aorta resulted in better separation of vascular and nonvascular renal lesions.11 We hypothesized that normalization of iodine concentration of brain parenchymal contrast staining on postthrombectomy DECT using the superior sagittal sinus (SSS) would reduce the variability in iodine quantification related to systematic differences in the timing/volume of contrast medium injection, as well as procedural and patient factors,12,13 and improve prediction of the presence of ICH on follow-up CT compared with absolute iodine concentration.
Previous studies investigating the value of brain parenchymal contrast staining on postthrombectomy CT to predict subsequent ICH have focused on relatively heterogeneous cohorts of patients with varying degrees of infarction and reperfusion.7,14-17 It is known that patients with larger infarct cores are more likely to develop ICH.18,19 In patients with small infarct cores and good reperfusion, development of ICH may be less predictable. We therefore wanted to examine a more homogeneous cohort of patients and included only patients with small preprocedural infarcts (ASPECTS ≥ 7) and good endovascular recanalization (modified TICI score 2b or 3) to identify an imaging biomarker for ICH risk stratification that could aid antithrombotic and blood pressure strategy in the early posttreatment period in patients in whom development of ICH may otherwise be unexpected.20
MATERIALS AND METHODS
Patient Population
Approval was obtained from the institutional review board at Vancouver General Hospital, and the need for informed consent was waived for this retrospective study. Consecutive thrombectomy cases, scanned for anterior circulation large-vessel (distal ICA, MCA, and anterior cerebral artery) occlusion between January 2016 and June 2018 were reviewed. Inclusion criteria comprised endovascular treatment for anterior circulation large-vessel occlusion, preprocedural imaging with NCCT and CTA, small preprocedural infarct volume (ASPECTS ≥ 7), good endovascular recanalization (modified TICI 2b or 3), DECT performed within 1 hour after thrombectomy, and follow-up NCCT performed at 24 hours post-thrombectomy or earlier according to clinical necessity. Exclusion criteria were concomitant proximal ICA/common carotid artery occlusion and dissection, direct common carotid artery access, and poor image quality on DECT studies.
Imaging and Endovascular Treatment Protocols
Endovascular thrombectomy was performed on a biplane x-ray system, Allura Xper FD20/10 (Phillips Healthcare, Best, the Netherlands), by 1 of 5 interventional neuroradiologists/neurosurgeons with 10–20 years of experience in interventional neuroradiology. As per local procedures, endovascular treatment consisted of first-attempt aspiration with a 5MAX/ACE64/ACE68 reperfusion catheter (Penumbra, Almeda, California), second-line mechanical thrombectomy with a Solitaire Flow Restoration stent retriever (Covidien, Irvine, California),21-24 or a combination technique.
Noncontrast DECT head studies were performed on 1 of 2 dual-source CT scanners (Somatom Definition Flash and Somatom Force; Siemens, Erlangen, Germany) at 80 kV/Sn 140kV and 90 kV/Sn 150kV, respectively. Preoperative and follow-up CT head studies were performed at 120 kV on both scanners. See the On-line Table for additional scan parameters.
Image Analysis
Preprocedural NCCT head and CTA studies were evaluated by 2 radiologists with subspecialty training (D.B. and J.P.W.). The ASPECTS25 was recorded, and collateral status was assessed using the scoring system of Miteff et al.26
Endovascular procedural imaging was reviewed by an interventional neuroradiologist (A.R.) with >10 years of experience, and the modified TICI score was recorded for each case.
DECT studies were evaluated by the 2 readers (D.B. and J.P.W.). We used 3-mm-thick multiplanar reconstructions. Simulated 120-kV images representing a weighted average of the original low-kilovolt and high-kilovolt images were provided, and virtual noncontrast images and generated iodine overlay maps using a 3-material decomposition algorithm with commercially available software (syngo.via, Dual Energy CT brain hemorrhage application, Version VB20; Siemens) were generated.
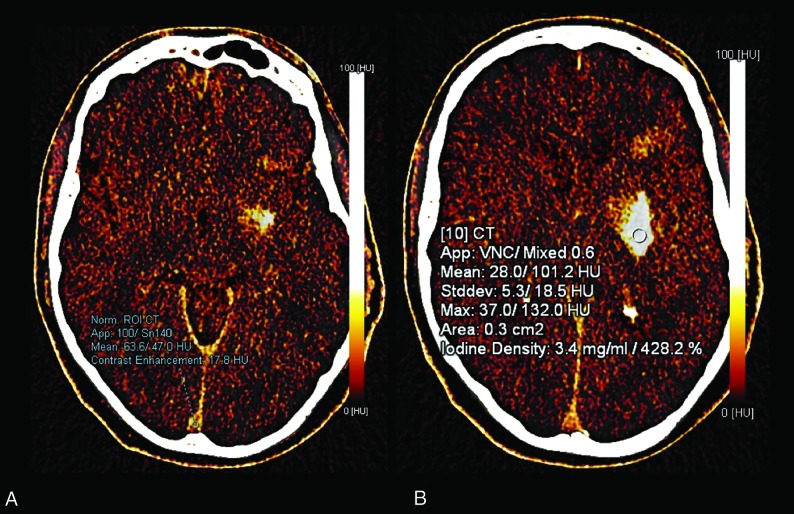
A small (0.1 cm2) ROI was placed on the SSS near the torcula to normalize the contrast. A 0.3-cm2 ROI was then placed subjectively in the area of most dense contrast staining (Fig 1). Iodine concentration (milligrams/milliliter) and percentage in relation to the SSS were recorded (Fig 1). The mean of the values recorded by the 2 readers was used for statistical analysis. The presence and location (parenchymal, subarachnoid, intraventricular) of hemorrhage and contrast staining were recorded on the virtual noncontrast and iodine overlay maps, respectively. Follow-up NCCT studies were evaluated for the development of intracerebral hemorrhage and recorded according to the European Cooperative Acute Stroke Study classification27 into hemorrhagic infarction and parenchymal hematoma.
Fig 1.
A, A small (0.1 cm2) ROI was placed in the superior sagittal sinus for normalization of contrast. B, In this case, the absolute maximum iodine concentration is 3.4 mg/mL and the relative concentration to the superior sagittal sinus is 428.2%.
Clinical and Radiologic Data Collection
Patient data including sex, age, NIHSS score at admission, site of vessel occlusion at preoperative CTA, preprocedural intravenous tPA administration (alteplase), number of passes, intra-arterial tPA administration, arterial stent delivery, puncture-to-recanalization time, time to recanalization, and oral antiplatelet/anticoagulation use before the procedure were recorded.
Statistical Analysis
The values of continuous variables are presented as mean or median with range as appropriate. Interobserver agreement was calculated according to intraclass correlation coefficient: A value below 0.50 indicates poor; between 0.5 and 0.75, moderate; between 0.75 and 0.90, good; and above 0.90, excellent reliability. Comparison between subgroups for continuous variables was performed using the Mann-Whitney U test. Categoric data were compared using the χ2 or Fisher exact tests as appropriate. The correlation between absolute iodine density and relative iodine concentrations was assessed using the Spearman rank correlation coefficient. A P value < .05 was considered statistically significant. Statistical analysis was performed using SPSS, Version 25.0 (IBM, Armonk, New York).
RESULTS
Patient Population
We identified 130 patients who received thrombectomy for acute anterior circulation stroke (76 men and 54 women) with a median age of 70 years (range, 18–97 years). Seventy-one of 130 patients with small preprocedural infarct volumes (ASPECTS ≥ 7) and good endovascular recanalization (modified TICI score 2b or 3) met the inclusion criteria (41 men and 30 women) with a median age of 70 years (range, 18–97 years). Patients with proximal ICA/common carotid artery occlusion (8/130) and dissection (11/130) and direct common carotid artery access and poor image quality on DECT studies (2/130) were excluded.
Pre- and Periprocedural Data
Patients had a baseline median NIHSS score of 17 (range, 6–29). Of the cases meeting the inclusion criteria, the median ASPECTS on initial preprocedural NCCT was 8 (range, 7–10). Intravenous tPA was administered to 45/71 (63.4%) patients. During endovascular treatment, 48 patients (67.6%) were treated with aspiration (a direct aspiration first pass technique) only; 2 (2.8%), with stent retriever only; and 21 (29.6%), with aspiration followed by stent retriever thrombectomy. The median number of aspiration attempts was 1 (range, 1–7). A carotid artery stent was placed in 2 patients. The final modified TICI score was 2b in 30 patients and 3 in 41 patients.
Image Analysis
ICH Development: Imaging Findings.
Of the 28 patients with no hyperdensity on initial postthrombectomy DECT, none had ICH on follow-up CT. The remaining 43 patients demonstrated parenchymal hyperdensity, due to a combination of contrast staining and hemorrhage in 4/43 (9.3%) patients and due to contrast staining alone in 39/43 (90.7%) patients, according to virtual noncontrast image analysis. Of the latter, 17/39 (44%) developed subsequent ICH, with grade 1 or 2 ICH identified in 11/17 (65%) and grade 3 or 4 ICH identified in 6/17 (35%). Of those with contrast staining, 22/39 (56%) demonstrated resolution of hyperdensity without ICH on follow-up CT. Table 1 shows data on the correlation between DECT findings and ICH on follow-up imaging.
Table 1:
Findings on initial postprocedural DECT and follow-up
| DECT Findings | Follow-Up CT (1–7 days) |
Hemorrhage Grade |
|||||
|---|---|---|---|---|---|---|---|
| Overall (%) | No Hemorrhage (%) | Hemorrhage (%) | 1–2 (%) | 3–4 (%) | |||
| No hyperdensity | 28 (39.4) | 28 (39.4) | 0 (0) | 0 (0) | 0 (0) | ||
| Staining only | 39 (54.9) | 22 (30.9) | 17 (23.9) | 11 (15.5) | 6 (8.5) | ||
| Staining and hemorrhage | 4 (5.6) | 1 (1.4) | 3 (4.2) | 0 (0) | 3 (4.2) | ||
| Total | 71 (100.0) | 51 (71.8) | 20 (28.2) | 11 (15.5) | 9 (12.7) | ||
Absolute and Relative Iodine\Concentration on DECT.
Of the 39 patients with initial contrast staining, the median iodine concentration was 1.20 mg/mL (range, 0.6–8.90 mg/mL) in those with ICH on follow-up imaging and 0.90 mg/mL (range, 0.40–2.10 mg/mL) in those without ICH. Differences did not reach statistical significance (P = .183). The median iodine concentration in the brain parenchyma relative to the SSS was significantly higher in those who developed ICH (137.9%; range, 92.5%–1197.0%) compared with those who did not (109.2%; range, 25.4%–200.8%; P = .007) (Table 2). For iodine concentration relative to the SSS, a cutoff value of 100% enabled identification with 94.7% sensitivity and 43.4% specificity, with a likelihood ratio of 1.71. Significant correlation between the maximum absolute iodine concentration and relative iodine concentration was observed (Spearman rho = 0.761; 95% CI, 0.644–0.842).
Table 2:
Iodine staining values in cohorts without and with subsequent ICH
| Iodine Measurement Parameter | Follow-Up CT (Median) (IQR) |
||
|---|---|---|---|
| No ICH | ICH | P Value | |
| Absolute iodine concentration (mg/mL) | 0.90 (0.72) | 1.20 (1.00) | .183 (NS) |
| Relative iodine concentration compared with SSS (%) | 109.2 (97.1) | 137.9 (99.1) | .007 |
Note:—IQR indicates interquartile range; NS, not significant.
Examples of cases are provided in Figs 2 and 3.
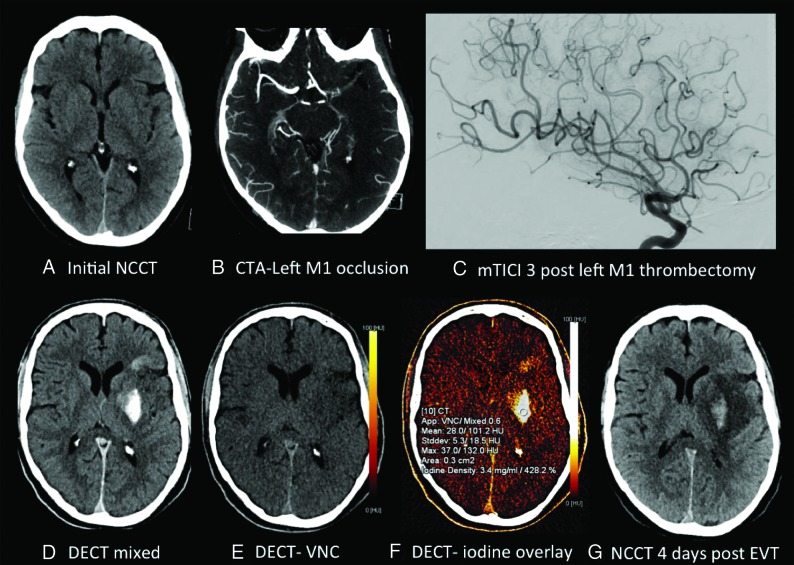
Fig 2.
A 59-year-old man with a preprocedural ASPECTS of 8 (A), left M1 occlusion (B), and modified TICI 3 reperfusion (C). Postthrombectomy DECT demonstrates parenchymal hyperdensity in the left basal ganglia and frontal lobe (D), without hemorrhage on the virtual noncontrast DECT (E) and consistent with contrast staining on the iodine overlay map (F), with maximum iodine concentration measuring 3.4 mg/mL and 428.2% relative to the SSS. NCCT performed 4 days postthrombectomy (G) demonstrates evolving left MCA infarction with development of grade 3 ICH involving the left lentiform nucleus.
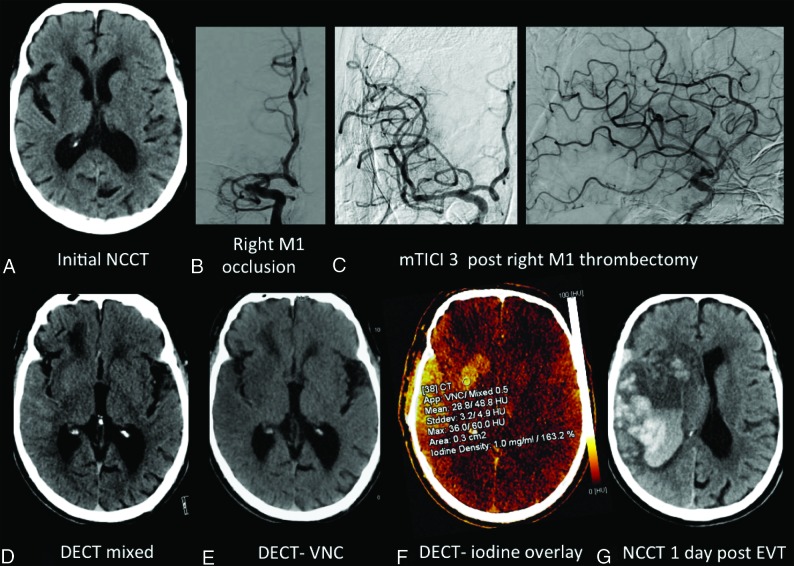
Fig 3.
An 86-year-old man with a preprocedural ASPECTS of 7 (A), right M1 occlusion (B), and modified TICI 3 reperfusion (C). Postthrombectomy DECT demonstrates parenchymal hyperdensity in the right basal ganglia and frontal and temporal lobes (D), without hemorrhage on the virtual noncontrast DECT (E) and consistent with contrast staining on the iodine overlay map (F), with maximum iodine concentration measuring 1.0 mg/mL and 163.2% relative to the SSS. NCCT performed 1 day post-thrombectomy (G) demonstrates frank hemorrhagic transformation with marked mass effect.
ICH Development: Clinical Parameters.
On preprocedural CT, the median ASPECTS was 8 in patients with ICH on follow-up imaging, and 8 in those without. There was no significant correlation between patient characteristics and subsequent ICH development: sex, age, site of vessel occlusion, revascularization technique, number of aspiration attempts, or number of stent retriever thrombectomy attempts.
The measured values of absolute iodine concentration and relative iodine concentration showed excellent agreement between the 2 readers; the intraclass correlation coefficient for absolute iodine concentration was 0.993 (P < .001), while the intraclass correlation coefficient for relative iodine concentration was 0.994 (P < .001).
DISCUSSION
ICH can be a devastating complication of revascularization treatment for acute ischemic stroke. The incidence of symptomatic ICH was 4.4% in the recent endovascular treatment randomized controlled trials.28 The presence and degree of brain parenchymal hyperdensity on head CTs obtained immediately after endovascular thrombectomy might predict which patients are at risk of developing ICH. Parenchymal hyperdensity is a common finding on postprocedural CT and may be due to hemorrhage and/or contrast staining due to breakdown of the blood-brain barrier at the site of ischemia,3 for which DECT allows differentiation. Contrast staining has also been previously shown to correlate moderately with final infarct volume and distribution.29
It was previously reported that a maximum iodine concentration of >1.35 mg/mL within the area of brain parenchymal contrast staining predicts development of ICH with 100% sensitivity and 67.5% specificity.7 In our dataset, there was no statistically significant difference in absolute iodine concentration between the cohort that developed ICH and the cohort that did not. This study shows that the relative iodine concentration compared with the SSS may be a better predictor of ICH development. In our study population, if iodine concentration in hyperdense brain areas was equal to or higher than the SSS, subsequent ICH development was predicted with 94.7% sensitivity and 43.4% specificity. Because parenchymal contrast staining resolved at 24 hours in most patients (22/39) with initial contrast staining, a cutoff value of 100% is associated with this relatively low specificity. However, having a high sensitivity for the prediction of ICH development is of more clinical importance in the early postprocedural phase. The ability to reliably predict ICH development in patients post-thrombectomy has clinical implications with regard to blood pressure control and antithrombotic strategy.20,30
During ischemia, endothelial impairment with disruption of the BBB occurs, allowing contrast to leak into the brain interstitium.3 The degree of endothelial damage likely correlates with the severity and duration of ischemia. Patients with the most profound endothelial damage are likely at risk of subsequent hemorrhage. While leakage of contrast/iodine staining is a surrogate marker of endothelial damage, the amount of contrast accumulating in ischemic brain tissue also depends on the concentration of iodine in the blood at the time of the CT scan, which varies according to the amount of contrast given before and during the intervention, blood volume, renal function, and so forth, which can be difficult to determine. Using the ratio of iodine in parenchyma versus blood in the SSS accounts for these interindividual differences and may explain why the relative value of iodine concentration in brain tissue may be a better predictor than absolute values. We chose the SSS because this is an easily identifiable constant anatomic structure that allows reproducible measurements of blood density.
This study has limitations due to its retrospective nature with a potential source for bias, because only patients with small infarcts and good reperfusion were included. Postprocedural DECT studies were performed on 2 different scanners. A recent phantom study demonstrated that iodine measurements are largely similar using different scanners and tube voltage pair combinations.31 However, slight differences might be possible and were not accounted for in our study. In addition, follow-up MR imaging was not routinely performed, and cases of petechial hemorrhage below the resolution of CT that could potentially be identified on MR imaging may have been missed and not included in the ICH cohort. ROIs within contrast-stained tissue were drawn subjectively and may not have reflected actual maximum iodine concentration; however, there was a high concordance among the 3 readers.
CONCLUSIONS
In this study, we sought an imaging biomarker of postthrombectomy ICH development in patients with acute stroke with small preprocedural infarcts and good recanalization. At dual-energy CT performed within 1 hour following thrombectomy, we found that the relative iodine concentration within contrast-stained brain parenchyma compared with that in the superior sagittal sinus was a more reliable predictor of ICH compared with the absolute maximum iodine concentration. A better understanding of risk for postprocedural hemorrhage can inform antithrombotic strategy, monitoring needs, and prognosis.
ABBREVIATIONS:
- DECT
dual-energy CT
- ICH
intracerebral hemorrhage
- SSS
superior sagittal sinus
Footnotes
Disclosures: Heiko Schmiedeskamp—UNRELATED: Employment: Siemens. Thalia Field—UNRELATED: Grants/Grants Pending: Canadian Institutes of Health Research, Bayer Canada, Comments: site payments for recruitment (Canadian Institutes of Health Research) and in-kind study medication (Bayer Canada)*; Payment for Lectures Including Service on Speakers Bureaus: Servier Canada; Other: Michael Smith Foundation for Health Research, Vancouver Coastal Health Research Institute, Heart and Stroke Foundation of Canada, Comments: salary support awards.* * Money paid to the institution.
References
- 1.Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 1999;30:2280–84 10.1161/01.str.30.11.2280 [DOI] [PubMed] [Google Scholar]
- 2.Khatri P, Wechsler LR, Broderick JP. Intracranial hemorrhage associated with revascularization therapies. Stroke 2007;38:431–40 10.1161/01.STR.0000254524.23708.c9 [DOI] [PubMed] [Google Scholar]
- 3.Renu A, Amaro S, Laredo C, et al. Relevance of blood-brain barrier disruption after endovascular treatment of ischemic stroke: dual-energy computed tomographic study. Stroke 2015;46:673–79 10.1161/STROKEAHA.114.008147 [DOI] [PubMed] [Google Scholar]
- 4.Yedavalli V, Sammet S. Contrast extravasation versus hemorrhage after thrombectomy in patients with acute stroke. J Neuroimaging 2017;27:570–76 10.1111/jon.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang YM, Lee DH, Kim HS, et al. The fate of high-density lesions on the non-contrast CT obtained immediately after intra-arterial thrombolysis in ischemic stroke patients. Korean J Radiol 2006;7:221–28 10.3348/kjr.2006.7.4.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tijssen MP, Hofman PA, Stadler AA, et al. The role of dual energy CT in differentiating between brain haemorrhage and contrast medium after mechanical revascularisation in acute ischaemic stroke. Eur Radiol 2014;24:834–40 10.1007/s00330-013-3073-x [DOI] [PubMed] [Google Scholar]
- 7.Bonatti M, Lombardo F, Zamboni GA, et al. Iodine extravasation quantification on dual-energy CT of the brain performed after mechanical thrombectomy for acute ischemic stroke can predict hemorrhagic complications. AJNR Am J Neuroradiol 2018;39:441–47 10.3174/ajnr.A5513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu R, Padole A, Gupta R. Dual-energy computed tomographic applications for differentiation of intracranial hemorrhage, calcium, and iodine. Neuroimaging Clin N Am 2017;27:401–09 10.1016/j.nic.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 9.Van Hedent S, Hokamp NG, Laukamp KR, et al. Differentiation of hemorrhage from iodine using spectral detector CT: a phantom study. AJNR Am J Neuroradiol 2018;39:2205–10 10.3174/ajnr.A5872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010;256:32–61 10.1148/radiol.10090908 [DOI] [PubMed] [Google Scholar]
- 11.Patel BN, Vernuccio F, Meyer M, et al. Dual-energy CT material density iodine quantification for distinguishing vascular from nonvascular renal lesions: normalization reduces intermanufacturer threshold variability. AJR Am J Roentgenol 2019;212:366–76 10.2214/AJR.18.20115 [DOI] [PubMed] [Google Scholar]
- 12.Li X, Meng X, Ye Z. Iodine quantification to characterize primary lesions, metastatic and non-metastatic lymph nodes in lung cancers by dual energy computed tomography: an initial experience. Eur J Radiol 2016;85:1219–23 10.1016/j.ejrad.2016.03.030 [DOI] [PubMed] [Google Scholar]
- 13.Liang P, Ren XC, Gao JB, et al. Iodine concentration in spectral CT: assessment of prognostic determinants in patients with gastric adenocarcinoma. AJR Am J Roentgenol 2017;209:1033–38 10.2214/AJR.16.16895 [DOI] [PubMed] [Google Scholar]
- 14.Parrilla G, Garcia-Villalba B, Espinosa de Rueda M, et al. Hemorrhage/contrast staining areas after mechanical intra-arterial thrombectomy in acute ischemic stroke: imaging findings and clinical significance. AJNR Am J Neuroradiol 2012;33:1791–96 10.3174/ajnr.A3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu C, Zhou Y, Zhang R, et al. Metallic hyperdensity sign on noncontrast CT immediately after mechanical thrombectomy predicts parenchymal hemorrhage in patients with acute large-artery occlusion. AJNR Am J Neuroradiol 2019;40:661–67 10.3174/ajnr.A6008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta R, Phan CM, Leidecker C, et al. Evaluation of dual-energy CT for differentiating intracerebral hemorrhage from iodinated contrast material staining. Radiology 2010;257:205–11 10.1148/radiol.10091806 [DOI] [PubMed] [Google Scholar]
- 17.Phan CM, Yoo AJ, Hirsch JA, et al. Differentiation of hemorrhage from iodinated contrast in different intracranial compartments using dual-energy head CT. AJNR Am J Neuroradiol 2012;33:1088–94 10.3174/ajnr.A2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan S, Wang D, Liu M, et al. Frequency and predictors of spontaneous hemorrhagic transformation in ischemic stroke and its association with prognosis. J Neurol 2014;261:905–12 10.1007/s00415-014-7297-8 [DOI] [PubMed] [Google Scholar]
- 19.Terruso V, D’Amelio M, Di Benedetto N, et al. Frequency and determinants for hemorrhagic transformation of cerebral infarction. Neuroepidemiology 2009;33:261–65 10.1159/000229781 [DOI] [PubMed] [Google Scholar]
- 20.Jadhav AP, Molyneaux BJ, Hill MD, et al. Care of the post-thrombectomy patient. Stroke 2018;49:2801–07 10.1161/STROKEAHA.118.021640 [DOI] [PubMed] [Google Scholar]
- 21.Lapergue B, Blanc R, Guedin P, et al. A direct aspiration, first pass technique (ADAPT) versus stent retrievers for acute stroke therapy: an observational comparative study. AJNR Am J Neuroradiol 2016;37:1860–65 10.3174/ajnr.A4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massari F, Henninger N, Lozano JD, et al. ARTS (aspiration-retriever technique for stroke): initial clinical experience. Interv Neuroradiol 2016;22:325–32 10.1177/1591019916632369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romano DG, Cioni S, Leonini S, et al. Manual thromboaspiration technique as a first approach for endovascular stroke treatment: a single-center experience. Interv Neuroradiol 2016;22:529–34 10.1177/1591019916653256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romano DG, Cioni S, Vinci SL, et al. Thromboaspiration technique as first approach for endovascular treatment of acute ischemic stroke: initial experience at nine Italian stroke centers. J Neurointerv Surg 2017;9:6–10 10.1136/neurintsurg-2016-012298 [DOI] [PubMed] [Google Scholar]
- 25.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group—Alberta Stroke Programme Early CT Score. Lancet 2000;355:1670–74 [DOI] [PubMed] [Google Scholar]
- 26.Miteff F, Levi CR, Bateman GA, et al. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain 2009;132:2231–38 10.1093/brain/awp155 [DOI] [PubMed] [Google Scholar]
- 27.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II): Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245–51 10.1016/S0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 28.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 29.Djurdjevic T, Rehwald R, Knoflach M, et al. Prediction of infarction development after endovascular stroke therapy with dual-energy computed tomography. Eur Radiol 2017;27:907–17 10.1007/s00330-016-4412-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitt JR, Trillanes M, Hemphill JC 3rd.. Management of blood pressure during and after recanalization therapy for acute ischemic stroke. Front Neurol 2019;10:138 10.3389/fneur.2019.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobsen MC, Schellingerhout D, Wood CA, et al. Intermanufacturer comparison of dual-energy CT iodine quantification and monochromatic attenuation: a phantom study. Radiology 2018;287:224–34 10.1148/radiol.2017170896 [DOI] [PubMed] [Google Scholar]