SUMMARY:
Cerebrovascular disease is a major source of mortality that commonly requires neurosurgical intervention. MR imaging is the preferred technique for imaging cerebrovascular structures, as well as regions of pathology that include microbleeds and ischemia. Advanced MR imaging sequences such as time-of-flight, susceptibility-weighted imaging, and 3D T2-weighted sequences have demonstrated excellent depiction of arterial and venous structures with and without contrast administration. While the advantages of 3T compared with 1.5T have been described, the role of ultra-high-field (7T) MR imaging in neurovascular imaging remains poorly understood. In the present review, we examine emerging neurosurgical applications of 7T MR imaging in vascular imaging of diverse conditions and discuss current limitations and future directions for this technique.
MR imaging at 7T is particularly beneficial to vascular imaging techniques. TOF angiography benefits from increased SNR and lengthening T1s at higher field strengths, allowing more effective tagging of flowing spins.1,2 SWI also benefits from increased SNR as well as the enhanced sensitivity of susceptibility effects at 7T.3,4 Vessel wall imaging techniques benefit in the same way as other T2-weighted sequences from increased SNR, allowing depiction of smaller structures such as the thickness of the vessel wall. MPRAGE also benefits from increased SNR, permitting smaller voxel volumes and higher-resolution imaging. The benefits along with the limitations of 7T MR imaging, including increased B0 and B1 inhomogeneity artifacts, should be examined to clarify the role of this emerging technology in the field of cerebrovascular imaging.
Here, we focus on the vascular components of neurologic protocols at 7T and their value to better diagnose and plan surgery, predict prognosis, and monitor treatment. We cover current primary neurosurgical applications for 7T vascular imaging and discuss areas for future development.
CLINICAL APPLICATIONS
Intracranial Tumors
Glioma.
High-grade gliomas are among the most vascularized malignant neoplasms, and angiogenesis is critical to their growth.5,6 There is interest in quantifying micro- and macrovascular properties of gliomas to predict tumor grade and better characterize microscopic infiltration (On-line Table 1). Moenninghoff et al7 found increasing microvascularity from low- to high-grade gliomas using 7T T2* MR imaging; 7T depicted susceptibility patterns that indicated microvascularity in 53% of patients compared with 33% using 1.5T MR imaging. While the authors attribute this difference to increased sensitivity of susceptibility contrast at 7T, there is a possibility that some of these findings could be false-positives due to increased conspicuity of imaging features imparted by a higher SNR at an ultra-high-field (UHF) strength. Differentiation of these potential false-positives from true clinical findings is an important area of future investigation.7 Paek et al8 also found that T2* 7T MR imaging provided superior depiction of glioma microvascularity compared with 1.5T MR imaging and reported gliomas with high intratumoral vasculature in 33.3% of cases at 7T compared with 12.5% at 1.5T. Christoforidis et al9 used UHF gradient-echo MR imaging to demonstrate that tumoral pseudoblush correlated histologically with foci of increased microvascularity and overall tumor grade. These studies suggest that 7T MR imaging may be useful in the noninvasive evaluation of tumor features that contribute to the World Health Organization grade, including microvascularity and necrosis, and could facilitate early stratification and risk assessment for patients with gliomas. However, multiple authors have also conceded that susceptibility artifacts near large air-tissue interfaces and the skull base could compromise the utility of 7T in some patients.8,10
MR imaging at 7T may also assist in identifying parenchymal areas with an increased likelihood of microscopic high-grade glioma infiltration, which is challenging at clinical field strengths and important for determining resection margins.11 Various imaging metrics, including those based on DWI, have been used to identify white matter infiltration by gliomas that cannot be resolved at lower field strengths. In addition, 7T SWI has proved superior in the delineation of venous structures.3 Grabner et al4 found that 7T SWI correctly predicted high-grade gliomas in 23.8% more cases than 3T SWI, suggesting that vasculature quantification at 7T may offer increased sensitivity for imaging glioblastoma multiforme preoperatively. These results concur with those of Moenninghoff et al,7 who also found higher tumor microvascularity in high-grade gliomas compared with low-grade lesions using 7T SWI.
Clinical applications of TOF angiography have been somewhat limited due to low spatial resolution attained at 1.5T and 3T; therefore, DSA remains the criterion standard angiographic technique. However, detectability of arterial structures using 7T TOF could be useful in noninvasive characterization of intratumoral vasculature. Advantages of TOF at 7T include increased SNR, longer T1 relaxation times augmenting vessel-tissue contrast, and inherently hyperintense arterial vasculature at higher field strengths overall.1,2 Radbruch et al12 demonstrated the feasibility of 7T TOF in imaging intratumoral vessels, reporting excellent delineation of vasculature in all 12 patients, even though tortuous and highly permeable arteries in glioblastoma multiforme traditionally challenge delineation by TOF. Furthermore, because gliomas tend to display greater angiogenesis with increasing grade, 7T TOF may be useful in noninvasive grading of gliomas;13 7T TOF angiography may also prove useful in monitoring the success of antiangiogenic agents in slowing tumor growth.14
Brain Metastases.
Early detection and delineation of brain metastases is critical in optimizing treatment planning. Historical strategies at conventional strengths, including higher dose contrast and magnetization transfer contrast imaging, have increased the detectability of enhancing metastases.15 Exploitation of susceptibility is another method to identify subtle lesions. Just as 3T demonstrates superior sensitivity for brain metastases compared with 1.5T,16 7T MR imaging demonstrates 20% more cerebral microhemorrhages on SWI compared with 1.5T, even though the quantity of brain metastases on T1-weighted MPRAGE is essentially equivalent.15 Because microscopic bleeding is common in brain metastases, 7T SWI may increase detection of very subtle metastatic disease. Direct comparison with 3 T SWI is warranted. Increased susceptibility at higher field strengths may augment identification of metastatic brain foci over conventional techniques.
Skull Base Tumors.
The skull base is anatomically complex. High-resolution imaging can contribute greatly to preoperative planning. de Rotte et al17 were the first to demonstrate the feasibility of pituitary adenoma imaging at 7T; however, their study did not report on vascular sequences. Barrett et al10 used a semiquantitative rating system to demonstrate superior visualization of internal carotid artery branches and vasculature within skull base tumors using 7T TOF, despite higher B0 and B1 inhomogeneity artifacts at 7T. It does appear that TOF could benefit surgical navigation to minimize vascular injury during endoscopic pituitary surgery (Fig 1). Additional limitations associated with 7T skull base imaging include artifacts from proximity to large air-tissue interfaces, such as the sphenoid sinus.17 Future technical development including advanced shimming and parallel transmit will be important to overcome local magnetic field inhomogeneity.
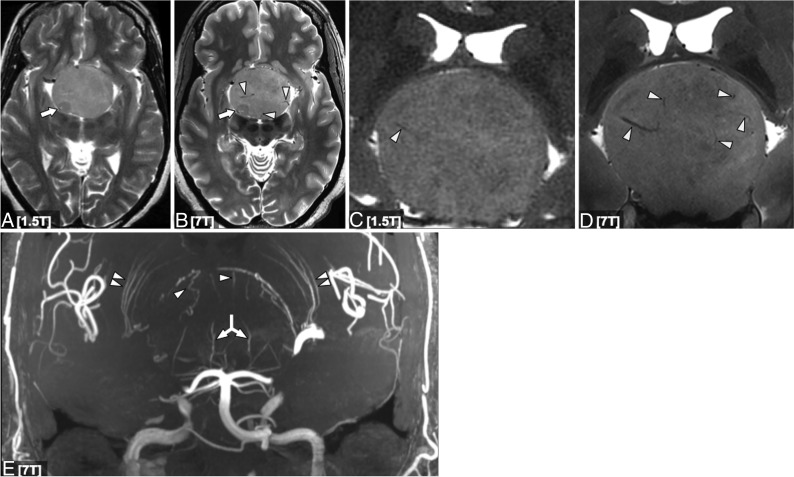
Fig 1.
Axial (A and B) and coronal (C and D) T2-weighted 1.5T (A and C) and 7T (B and D) images in a patient with a giant pituitary macroadenoma. C and D, Magnified approximately 250% compared with A and B. Although lesion margins are conspicuous at 1.5T, the 7T image provides a better definition of the internal architecture, including intrinsic adenoma vasculature (white arrowheads in B, C, and D). Both strengths show a nodule of slightly lower signal posterolaterally (arrows in A and B), but internal architecture is again better seen at 7T. Coronal MIP projection of a 7T time-of-flight MRA in the same patient (E) shows striate artery displacement (double arrowheads), as well as a recruited posterior circulation supply inferiorly (double-headed arrow) and additional vessels arising from the anterior cerebral artery superiorly (arrowheads).
Most important, no study has directly compared noncontrast 7T TOF with contrast-enhanced 3T in the detection of intratumoral and adjacent vascular structures. Such an analysis is warranted because increased visibility of arterial structures at 7T may ultimately limit contrast administration for some patients.
In their study of a homogeneous series of meningiomas at 7T, Song et al18 reported increased peri- and intratumoral vasculature and enhanced delineation of the tumor-brain interface in 4 patients with supratentorial meningiomas. Although the current diagnostic utility of conventional MR imaging for meningioma is already high, increased spatial resolution and SNR make 7T well-positioned to improve vascular characterization among these tumors.
Treatment Monitoring for Tumors.
Microhemorrhages are common sequelae following radiation therapy for intracranial neoplasms.19 Lupo et al20 reported high rates of microbleeds on 7T SWI among patients with glioma 2 years after radiation. Another study of 7T SWI in 113 patients having undergone focal radiation found a 100% incidence of at least 1 microbleed 2 years following radiation.21 Belliveau et al19 further demonstrated the increased ability of 7T to detect radiation-related cerebral microbleeds using SWI, apparent transverse relaxation, and quantitative susceptibility mapping. Bian et al23 found significantly more cerebral microbleeds in 7/10 patients with gliomas who underwent radiation therapy using 7T SWI compared with 3T SWI.22 However, when patients with 3 relatively inferior tumors were included, the effect between field strengths was not significant, suggesting that 7T SWI is more sensitive away from areas prone to inherent susceptibility artifacts.23 SWI at 7T, combined with tumor location consideration, may offer additional diagnostic value in early detection of radiation-induced microvascular damage.
In addition to imaging vascular damage from radiation therapy, there is growing interest in leveraging 7T vascular imaging to monitor the efficacy of drug therapy for aggressive intracranial neoplasms.24 Grabner et al24 used 7T SWI to study longitudinal glioma microvasculature changes during antiangiogenic therapy, concluding that 7T SWI is useful for antiangiogenic therapy monitoring in patients with advanced disease. The feasibility of high-resolution imaging of intratumoral arteries has also been demonstrated with 7T TOF12; however, its utility in assessing antiangiogenic efficacy has not yet been defined.
Dynamic contrast-enhanced imaging with 7T may also play an increasingly important role in the management of intracranial tumors and is a worthwhile area of technical development (On-line Appendix and On-line Fig 1).
Epilepsy
The benefits of 7T protocols for studying previously nonlesional epilepsy have recently been described.25 Vascular imaging sequences, in conjunction with structural MR imaging, hold promise in localizing cryptogenic seizure-onset zones in the setting of vascular lesions. It is estimated that up to 70% of symptomatic cavernomas cause seizures, approximately 40% of which are drug-resistant.26-28 Seizure freedom following cavernoma surgery is dependent on complete resection of the vascular malformation and metabolic products in the surrounding hemosiderin ring, and approximately 25% of patients with cavernoma-related epilepsy fail to achieve postoperative seizure freedom.29 The absence of an identifiable epileptogenic focus can disqualify certain patients from neurosurgery, necessitate invasive intracranial monitoring to localize seizure onset zone, and predispose patients who do progress to an operation to inferior postoperative outcomes.30 Advanced imaging tools may help clarify epileptogenesis and guide neurosurgical therapy.
Schlamann et al31 found a greater number of cavernomas using T2*-weighted gradient-echo imaging at 7T compared with 1.5T, consistent with enhanced susceptibility that is known to occur at higher fields. This finding is supported by a prior study that reported increased cavernoma detectability at 3T compared with 1.5T SWI.32 In a series of 37 patients with epilepsy with various etiologies who had negative findings on MR imaging at a lower field strength, small cavernomas were identified in 3 patients, 2 of which were likely related to epileptogenesis.33 Developmental venous anomalies were detected in 4 other patients using 7T SWI, 2 of which coincided with regions of electrographic abnormality, suggesting possible occult cavernomas.33 In another study of 11 patients with epilepsy with negative findings on MR imaging, 1 patient’s diagnosis changed from suspected focal cortical dysplasia to cavernoma based on 7T SWI.34 Examples of 7T SWI for polymicrogyria and cavernoma as epileptogenic foci are shown in Fig 2 and On-line Fig 2, respectively.
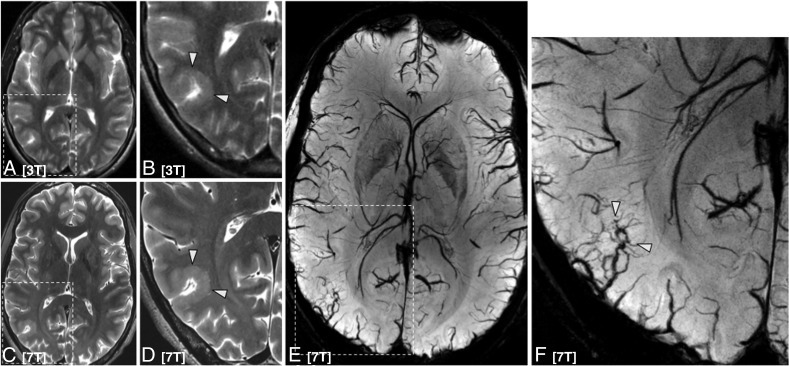
Fig 2.
A patient with epilepsy with a subtle right-sided parieto-occipital polymicrogyria faintly seen on axial T2-weighted imaging at 3T (A, magnified inset B) with improved characterization at 7T (C, magnified inset D). Internal architecture and heterogeneity are also much better appreciated at 7T. SWI 7T minimum-intensity-projection demonstrates a cluster of venous structures (arrowheads) associated with the polymicrogyria (E, magnified inset F).
While these studies provide compelling evidence to support the use of 7T in vascular epilepsy imaging, no study has directly compared cavernoma detectability between 3T and 7T SWI. Comparison of Engel and quality-of-life scores between patients who underwent preoperative 7T versus conventional MR imaging may clarify the role of 7T imaging in treating cavernoma-related epilepsy. Although the importance of hemosiderin ring excision in cavernoma surgery is controversial,35,36 7T MR imaging may facilitate a more precise definition of hemosiderin to optimize the resection of perilesional hemosiderin deposits.
Neurovascular Pathology
Aneurysm.
Intracranial aneurysms occur in approximately 3% of the general population. Rupture and subarachnoid hemorrhage are associated with considerable morbidity and mortality.37 While neurosurgical treatment for intracranial aneurysms, including surgical clipping and endovascular coiling, are effective therapies, these procedures are not without risk. Therefore, identifying patients with aneurysms who are at high risk for rupture is critical in identifying appropriate candidates for preventative treatment. Advantages of 7T vessel wall imaging are increased SNR, higher spatial resolution, and greater CSF suppression compared with 3T.38-40 However, 7T imaging is limited by artifacts caused by increased transmit field (B1) inhomogeneity. The superiority over lower-field MR imaging in vessel wall imaging remains inadequately understood.
Using gadolinium-enhanced MPRAGE 7T MR imaging, Sato et al41 reported 2 discrete aneurysm wall microstructures that are poorly resolved with lower-resolution MR imaging at lower field strengths: partial or complete enhancement of the inner wall (neovascularization) and outer wall (formation of the vasa vasorum). The latter pattern correlated histologically with vessel wall instability, suggesting that contrast-enhanced 7T MR imaging may be useful to characterize thrombosed intracranial aneurysms and could be a valuable clinical tool for determining rupture potential. Similarly, in comparing 1.5T and 7T TOF, Wrede et al2 reported that detectability and characterization of unruptured intracranial aneurysms were increased at 7T. In another study, 7T TOF provided delineation of unruptured intracranial aneurysms comparable with DSA, which is the current criterion standard. Such evidence suggests that 7T MR imaging may obviate ionizing radiation and iodinated contrast agent administration in the future.42 In a study of 21 saccular and 11 fusiform intracranial aneurysms using 0.4-mm isotropic 7T contrast-enhanced black-blood MR imaging, fusiform aneurysms exhibited superior wall enhancement due to increased resolution, image quality, and involvement of a larger surface than saccular aneurysms, reflecting differences in pathology.43
Imaging hemodynamics and quantifying forces within vessels are another important component of predicting aneurysm rupture risk. Blankena et al44 used a TSE-based vessel wall sequence and phase-contrast 7T MR imaging to demonstrate an inverse relationship between vessel wall thickness and wall shear stress. Other advanced imaging techniques, including volume pulsation quantification, rotational angiography, and 4D flow, have been explored as aneurysm-rupture risk predictors at lower-field MR imaging; however, their implementation at 7T remains limited. As a result of artifacts, UHF quantification of volume pulsation of unruptured cerebral aneurysms is not currently possible, even with the high spatial resolution of 7T data.45 Additional applications of 7T TOF imaging have included differentiation between cerebral aneurysms and infundibula.46
Despite these studies, it remains unclear whether 7T is more sensitive than lower-field MR imaging in detecting and characterizing cerebral aneurysms because there are relatively few direct comparison studies.
Atherosclerosis.
Advanced high-resolution imaging methods may be useful in predicting atherosclerotic plaque rupture, embolization, and stroke (Fig 3).47 Harteveld et al,48 who used 3D gadolinium-enhanced T1-weighted 7T MR imaging to quantify atherosclerotic lesion burden in patients with posterior cerebral ischemia, confirmed greater lesion burden in the posterior cerebral artery. These results suggest that 7T contrast-enhanced vessel wall imaging may aid in the association between intracranial vessel wall lesions and ischemic events. Direct comparison of vessel wall imaging in an elderly asymptomatic population confirmed greater vessel wall visibility and more lesions at 7T compared with 3T.49 These authors suggested that the conspicuity of vessel wall lesions in the proximal anterior cerebral and posterior cerebral arteries is optimally imaged with 7T MR imaging.50 While 7T has been used to precisely measure circle of Willis vessel wall thickness in symptomatic and asymptomatic patients ex vivo,50 a direct comparison between symptomatic and asymptomatic patients in vivo is required for more definitive clinical utility.
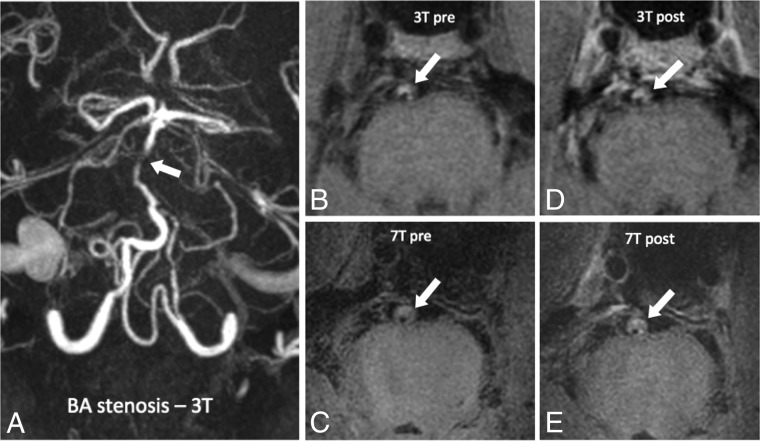
Fig 3.
A 73-year-old man with a posterior circulation stroke, an occluded left vertebral artery, and stenosis of the basilar artery. TOF angiography at 3T of a patient with basilar artery stenosis (white arrow, A). Pre- and postcontrast vessel wall imaging at 3T (B and C) and 7T (D and E) in a patient with basilar artery atherosclerotic plaque (white arrows indicate areas of stenosis).
A report on a cohort with mixed pathologies, including cerebral atherosclerotic lesions and aneurysms, compared vessel wall imaging with 3T and 7T T1-weighted sampling perfection with application-optimized contrasts by using different flip angle evolution (SPACE sequence; Siemens, Erlangen, Germany).51 MR imaging at 7T offered superior vessel wall characterization compared with 3T and greater visualization of the fibrous cap and lipid core among atherosclerotic plaques at 7T over 3T. Therefore, there may be a potential role of 7T MR imaging for diagnosis and risk stratification in intracranial vascular disease.51 By means of high-resolution 7T vessel wall imaging to determine the prevalence of vessel wall lesions, 96% of patients with vascular disease had at least 1 vessel wall lesion, nearly 3-fold the rate reported by the Atherosclerosis Risk in Communities Study at 3T.52,53 This discrepancy was attributed to differences in cohorts. Increased SNR at 7T MR imaging did contribute to higher detection of lesions. While these results suggest that 7T MR imaging may be useful in screening for intracranial vessel lesions and quantifying overall lesion burden, future studies are required to determine whether the smaller lesions detected only at UHF are clinically relevant.
Areas of wall thickening on imaging correspond to regions of advanced atherosclerosis with ex vivo 7T MR imaging.54 Additionally, 7T signal heterogeneity permitted spatial differentiation of constituent plaque components, including macrophages and collagen.54 Similar morphologic vessel wall properties at 3T and 7T, including vessel wall and luminal areas, were reported by others.55 However, 7T exhibited a significantly higher vessel wall SNR and contrast-to-noise ratio for both T1- and T2-weighted sequences.55 These results are similar to those of another carotid wall imaging study that reported significantly higher SNR at 7T compared with 3T, suggesting that 7T may be superior in diagnosing carotid vessel wall pathology.56 Majidi et al57 correlated in vitro intravascular sonography and 7T MR imaging findings with histology, concluding that 7T MR imaging is a reliable method of detecting burden within intracranial arteries.
Stroke.
MR imaging at 7T has been used to characterize infarct morphology.58 When similar stroke protocols at 3T and 7T in patients with subacute and chronic stroke were compared, higher spatial resolution at 7T revealed more subtle features of ischemic lesions and infarct morphology compared with lower-field MR imaging.58 Prior studies have successfully used 7T MR imaging to image occlusive changes in lenticulostriate arteries in acute59 and chronic60 stroke. Lenticulostriate arteries are difficult to image with conventional modalities due to their small size, but advances in 7T imaging have permitted excellent visualization at improved resolution with both T2-weighted and TOF imaging (On-line Fig 3).59-61 However, a direct comparison between 7T and lower field strengths for visualization of lenticulostriate artery features such as occlusion has not yet been quantified.
Moyamoya Vasculopathy.
MR imaging at 7T has been applied to Moyamoya vasculopathy.62 Because revascularization surgery is a common treatment, high-resolution arterial imaging by DSA or CT angiography is used to plan surgery. However, 7T imaging may obviate contrast and radiation techniques. If one compared DSA, 3T MRA, and 7T MRA, all 3 were of diagnostic value, but 7T TOF was superior to 3T for detecting disease-specific small-vessel pathology.62 Furthermore, 7T and DSA provided similar results, despite motion artifacts being observed at 7T.62 While there were no significant differences between ICA diameters or ivy sign scores between field strengths, 7T exhibited higher sensitivity and specificity than 3T for detecting flow voids and provided superior depiction of slow-flowing blood within peripheral arteries.63 These preliminary studies using 7T MR imaging in characterizing abnormal vascular networks among patients with Moyamoya disease are promising and warrant further investigation.
Trigeminal Neuralgia.
Trigeminal neuralgia is another pathology in which 7T MR imaging may find use. Vascular compression of the trigeminal nerve is the most common etiology; TOF plays an important role in diagnosing neurovascular conflict.64 TOF at 7T facilitates increased visualization of first- and second-order arterial branches that are poorly depicted at 1.5T or 3T.65 While trigeminal neuralgia can present without vascular compression,64 some cases of MR imaging negative for trigeminal neuralgia may result from offending vessels that are too small to see by conventional imaging methods. Therefore, 7T TOF may be particularly useful for patients with trigeminal neuralgia in whom lower-field scanning fails to confirm vascular compression. Although studies have applied 7T MR imaging to trigeminal imaging,66 no 7T study has coupled high-resolution TOF with structural or diffusion MR imaging to characterize neurovascular compression. Fusion of structural and high-resolution TOF imaging in trigeminal neuralgia is possible (Fig 4). Therefore, studies applying these techniques in patients with trigeminal neuralgia with nondiagnostic clinical MR imaging may clarify the value of 7T TOF in the diagnosis of neuropathic pain.
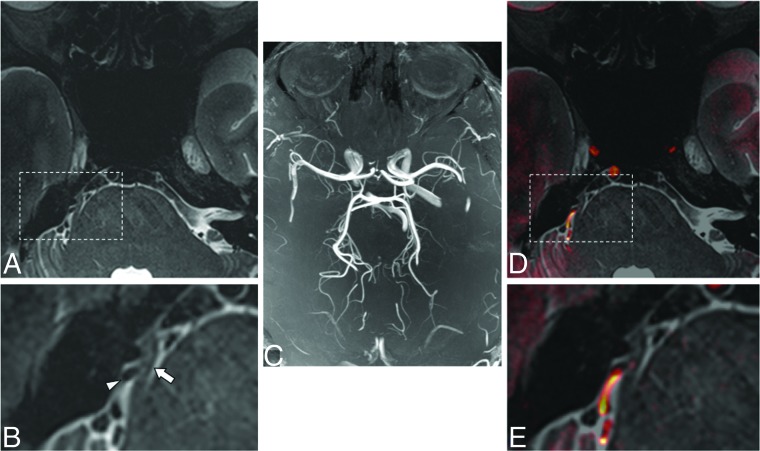
Fig 4.
Axial T2-weighted image at 7T of a patient with classic trigeminal neuralgia and associated right-sided neurovascular compression (arrow indicates right trigeminal nerve; arrowhead indicates artery in A and magnified inset B). There may be subtle hyperintensity within the nerve itself. Whole-brain maximum intensity time-of-flight projection at 7T (C) and fused gray-scale T2 and color-encoded TOF (D and E, at same locations as A and B) show orientation of the vessels and nerve and resultant neurovascular compression.
CONCLUSIONS
We described the emerging roles of vascular 7T MR imaging in neurosurgery and discussed current limitations of UHF neuroimaging. There has been considerable effort to apply vascular 7T MR imaging to conditions such as gliomas and vessel wall imaging, yet many unexplored applications of vascular imaging remain. The superior resolution and SNR of 7T compared with lower-field MR imaging in certain brain regions, coupled with technical developments that minimize susceptibility artifacts, are expected to expand the increasingly important role of 7T in the work-up of various neurosurgical diseases.
ABBREVIATION:
- UHF
ultra-high-field
This work was supported by National Institutes of Health R01 CA202911, Icahn School of Medicine Capital Campaign, and Translational and Molecular Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai.
Disclosures: Bradley N. Delman—UNRELATED: Consultancy: Bayer HealthCare; Payment for Lectures Including Service on Speakers Bureaus: Bayer HealthCare. Priti Balchandani—RELATED: Grant: National Institutes of Health*; UNRELATED: Employment: Icahn School of Medicine at Mount Sinai; Grants/Grants Pending: National Institutes of Health*; Patents (Planned, Pending or Issued): GE Healthcare, Siemens; Royalties: GE Healthcare, Siemens; OTHER RELATIONSHIPS: Dr Priti Balchandani (the Principal Investigator in this study) is a named inventor on patents relating to MRI and radiofrequency pulse design. The patents have been licensed to GE Healthcare, Siemens, and Philips International. Dr Balchandani receives royalty payments relating to these patents. Dr Balchandani is a named inventor on patents relating to Slice-Selective Adiabatic Magnetization T2-Preparation for efficient T2-weighted imaging at ultra-high-field strengths, Methods for Producing a Semi-Adiabatic Spectral-Spatial Spectroscopic Imaging Sequence and Devices Thereof, and Semi-Adiabatic Spectral-Spatial Spectroscopic Imaging. These patents have been filed through Mount Sinai Innovation Partners; they remain unlicensed, there is no discussion to license them in the near future, and there are, consequently, no royalties revolving around them. Raj Shrivastava—RELATED: Grant: National Institutes of Health R01 CA202911.* *Money paid to the institution.
References
- 1.Balchandani P, Naidich TP. Ultra-high-field MR neuroimaging. AJNR Am J Neuroradiol 2015;36:1204–15 10.3174/ajnr.A4180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wrede KH, Dammann P, Mönninghoff C, et al. . Non-enhanced MR imaging of cerebral aneurysms: 7 Tesla versus 1.5 Tesla. PLoS One 2014;9:e84562 10.1371/journal.pone.0084562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deistung A, Rauscher A, Sedlacik J, et al. . Susceptibility weighted imaging at ultra high magnetic field strengths: theoretical considerations and experimental results. Magn Reson Med 2008;60:1155–68 10.1002/mrm.21754 [DOI] [PubMed] [Google Scholar]
- 4.Grabner G, Kiesel B, Wöhrer A, et al. . Local image variance of 7 Tesla SWI is a new technique for preoperative characterization of diffusely infiltrating gliomas: correlation with tumour grade and IDH1 mutational status. Eur Radiol 2017;27:1556–67 10.1007/s00330-016-4451-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brat DJ, Van Meir EG. Glomeruloid microvascular proliferation orchestrated by VPF/VEGF: a new world of angiogenesis research. Am J Pathol 2001;158:789–96 10.1016/S0002-9440(10)64025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brem S. The role of vascular proliferation in the growth of brain tumors. Clin Neurosurg 1976;23:440–43 [DOI] [PubMed] [Google Scholar]
- 7.Moenninghoff C, Maderwald S, Theysohn JM, et al. . Imaging of adult astrocytic brain tumours with 7T MRI: preliminary results. Eur Radiol 2010;20:704–13 10.1007/s00330-009-1592-2 [DOI] [PubMed] [Google Scholar]
- 8.Paek SL, Chung YS, Paek SH, et al. . Early experience of pre- and post-contrast 7.0T MRI in brain tumors. J Korean Med Sci 2013;28:1362–72 10.3346/jkms.2013.28.9.1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christoforidis GA, Yang M, Abduljalil A, et al. . “Tumoral pseudoblush” identified within gliomas at high-spatial-resolution ultrahigh-field-strength gradient-echo MR imaging corresponds to microvascularity at stereotactic biopsy. Radiology 2012;264:210–17 10.1148/radiol.12110799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett TF, Dyvorne HA, Padormo F, et al. . First application of 7-T magnetic resonance imaging in endoscopic endonasal surgery of skull base tumors. World Neurosurg 2017;103:600–10 10.1016/j.wneu.2017.03.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regnery S, Knowles BR, Paech D, et al. . High-resolution FLAIR MRI at 7 Tesla for treatment planning in glioblastoma patients. Radiother Oncol 2019;130:180–84 10.1016/j.radonc.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 12.Radbruch A, Eidel O, Wiestler B, et al. . Quantification of tumor vessels in glioblastoma patients using time-of-flight angiography at 7 Tesla: a feasibility study. PLoS One 2014;9:e110727 10.1371/journal.pone.0110727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008;359:492–507 10.1056/NEJMra0708126 [DOI] [PubMed] [Google Scholar]
- 14.Emblem KE, Mouridsen K, Bjornerud A, et al. . Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat Med 2013;19:1178 10.1038/nm.3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monninghoff C, Maderwald S, Theysohn JM, et al. . Imaging of brain metastases of bronchial carcinomas with 7T MRI: initial results. Rofo 2010;182:764–72 10.1055/s-0029-1245440 [DOI] [PubMed] [Google Scholar]
- 16.Nobauer-Huhmann IM, Ba-Ssalamah A, Mlynarik V, et al. . Magnetic resonance imaging contrast enhancement of brain tumors at 3 Tesla versus 1.5 Tesla. Invest Radiol 2002;37:114–19 10.1097/00004424-200203000-00003 [DOI] [PubMed] [Google Scholar]
- 17.de Rotte AA, van der Kolk AG, Rutgers D, et al. . Feasibility of high-resolution pituitary MRI at 7.0 Tesla. Eur Radiol 2014;24:2005–11 10.1007/s00330-014-3230-x [DOI] [PubMed] [Google Scholar]
- 18.Song SW, Son YD, Cho Z-H, et al. . Experience with 7.0 T MRI in patients with supratentorial meningiomas. J Korean Neurosurg Soc 2016;59:405–09 10.3340/jkns.2016.59.4.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belliveau JG, Bauman GS, Tay KY, et al. . Initial investigation into microbleeds and white matter signal changes following radiotherapy for low-grade and benign brain tumors using ultra-high-field MRI techniques. AJNR Am J Neuroradiol 2017;38:2251–56 10.3174/ajnr.A5395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lupo JM, Chuang CF, Chang SM, et al. . 7-Tesla susceptibility-weighted imaging to assess the effects of radiotherapy on normal-appearing brain in patients with glioma. Int J Radiat Oncol Biol Phys 2012;82:e493–500 10.1016/j.ijrobp.2011.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison MA, Hess CP, Clarke JL, et al. . Risk factors of radiotherapy-induced cerebral microbleeds and serial analysis of their size compared with white matter changes: a 7T MRI study in 113 adult patients with brain tumors. J Magn Reson Imaging 2019;50:868–77 10.1002/jmri.26651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nandigam RN, Viswanathan A, Delgado P, et al. . MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol 2009;30:338–43 10.3174/ajnr.A1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bian W, Hess CP, Chang SM, et al. . Susceptibility-weighted MR imaging of radiation therapy-induced cerebral microbleeds in patients with glioma: a comparison between 3T and 7T. Neuroradiology 2014;56:91–96 10.1007/s00234-013-1297-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grabner G, Nobauer I, Elandt K, et al. . Longitudinal brain imaging of five malignant glioma patients treated with bevacizumab using susceptibility-weighted magnetic resonance imaging at 7T. Magn Reson Imaging 2012;30:139–47 10.1016/j.mri.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 25.Rondinoni C, Magnun C, Vallota da Silva A, et al. . Epilepsy under the scope of ultra-high field MRI. Epilepsy Behav 2019. July 10. [Epub ahead of print] 10.1016/j.yebeh.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 26.Bertalanffy H, Benes L, Miyazawa T, et al. . Cerebral cavernomas in the adult. Review of the literature and analysis of 72 surgically treated patients. Neurosurg Rev 2002;25:1–53; discussion 54–55 [DOI] [PubMed] [Google Scholar]
- 27.Rosenow F, Alonso-Vanegas MA, Baumgartner C, et al. . Cavernoma-Related Epilepsy: Review and Recommendations for Management—Report of the Surgical Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2013;54:2025–35 10.1111/epi.12402 [DOI] [PubMed] [Google Scholar]
- 28.Ryvlin P, Mauguière F, Sindou M, et al. . Interictal cerebral metabolism and epilepsy in cavernous angiomas. Brain 1995;118:677–87 10.1093/brain/118.3.677 [DOI] [PubMed] [Google Scholar]
- 29.Englot DJ, Han SJ, Lawton MT, et al. . Predictors of seizure freedom in the surgical treatment of supratentorial cavernous malformations. J Neurosurg 2011;115:1169–74 10.3171/2011.7.JNS11536 [DOI] [PubMed] [Google Scholar]
- 30.Leeman-Markowski B. Review of MRI-negative epilepsy. JAMA Neurol 2016;73:1377 10.1001/jamaneurol.2016.3698 [DOI] [Google Scholar]
- 31.Schlamann M, Maderwald S, Becker W, et al. . Cerebral cavernous hemangiomas at 7 Tesla: initial experience. Acad Radiology 2010;17:3–6 10.1016/j.acra.2009.10.001 [DOI] [PubMed] [Google Scholar]
- 32.Pinker K, Stavrou I, Szomolanyi P, et al. . Improved preoperative evaluation of cerebral cavernomas by high-field, high-resolution susceptibility-weighted magnetic resonance imaging at 3 Tesla: comparison with standard (1.5 T) magnetic resonance imaging and correlation with histopathological findings: preliminary results. Invest Radiol 2007;42:346–51 10.1097/01.rli.0000262744.85397.fc [DOI] [PubMed] [Google Scholar]
- 33.Feldman RE, Delman BN, Pawha PS, et al. . 7T MRI in epilepsy patients with previously normal clinical MRI exams compared against healthy controls. PLoS One 2019;14:e0213642 10.1371/journal.pone.0213642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colon AJ, van Osch MJ, Buijs M, et al. . Detection superiority of 7 T MRI protocol in patients with epilepsy and suspected focal cortical dysplasia. Acta Neurol Belg 2016;116:259–69 10.1007/s13760-016-0662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumann CR, Schuknecht B, Lo Russo G, et al. . Seizure outcome after resection of cavernous malformations is better when surrounding hemosiderin-stained brain also is removed. Epilepsia 2006;47:563–66 10.1111/j.1528-1167.2006.00468.x [DOI] [PubMed] [Google Scholar]
- 36.Yeon JY, Kim JS, Choi SJ, et al. . Supratentorial cavernous angiomas presenting with seizures: surgical outcomes in 60 consecutive patients. Seizure 2009;18:14–20 10.1016/j.seizure.2008.05.010 [DOI] [PubMed] [Google Scholar]
- 37.Vlak MH, Algra A, Brandenburg R, et al. . Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 2011;10:626–36 10.1016/S1474-4422(11)70109-0 [DOI] [PubMed] [Google Scholar]
- 38.Umutlu L, Theysohn N, Maderwald S, et al. . 7 Tesla MPRAGE imaging of the intracranial arterial vasculature: nonenhanced versus contrast-enhanced. Acad Radiol 2013;20:628–34 10.1016/j.acra.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 39.Vergouwen MDI, Backes D, van der Schaaf IC, et al. . Gadolinium enhancement of the aneurysm wall in unruptured intracranial aneurysms is associated with an increased risk of aneurysm instability: a follow-up study. AJNR Am J Neuroradiol 2019;40:1112 10.3174/ajnr.A6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Kolk AG, Hendrikse J, Brundel M, et al. . Multi-sequence whole-brain intracranial vessel wall imaging at 7.0 tesla. Eur Radiol 2013;23:2996–3004 10.1007/s00330-013-2905-z [DOI] [PubMed] [Google Scholar]
- 41.Sato T, Matsushige T, Chen B, et al. . Wall contrast enhancement of thrombosed intracranial aneurysms at 7T MRI. AJNR Am J Neuroradiol 2019;40:1106–11 10.3174/ajnr.A6084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wrede KH, Matsushige T, Goericke SL, et al. . Non-enhanced magnetic resonance imaging of unruptured intracranial aneurysms at 7 Tesla: comparison with digital subtraction angiography. Eur Radiol 2017;27:354–64 10.1007/s00330-016-4323-5 [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Zhang Z, Zhu C, et al. . Wall enhancement of intracranial saccular and fusiform aneurysms may differ in intensity and extension: a pilot study using 7-T high-resolution black-blood MRI. Eur Radiol 2019. June 19. [Epub ahead of print] 10.1007/s00330-019-06275-9 [DOI] [PubMed] [Google Scholar]
- 44.Blankena R, Kleinloog R, Verweij BH, et al. . Thinner regions of intracranial aneurysm wall correlate with regions of higher wall shear stress: a 7T MRI study. AJNR Am J Neuroradiol 2016;37:1310–17 10.3174/ajnr.A4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleinloog R, Zwanenburg JJ, Schermers B, et al. . Quantification of intracranial aneurysm volume pulsation with 7T MRI. AJNR Am J Neuroradiol 2018;39:713–19 10.3174/ajnr.A5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wermer MJ, van Walderveen MA, Garpebring A, et al. . 7 Tesla MRA for the differentiation between intracranial aneurysms and infundibula. Magn Reson Imaging 2017;37:16–20 10.1016/j.mri.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 47.Arenillas JF. Intracranial atherosclerosis: current concepts. Stroke 2011;42:(1 Suppl):S20–23 10.1161/STROKEAHA.110.597278 [DOI] [PubMed] [Google Scholar]
- 48.Harteveld AA, van der Kolk AG, van der Worp HB, et al. . Detecting intracranial vessel wall lesions with 7T-magnetic resonance imaging: patients with posterior circulation ischemia versus healthy controls. Stroke 2017;48:2601–04 10.1161/STROKEAHA.017868 [DOI] [PubMed] [Google Scholar]
- 49.Harteveld AA, van der Kolk AG, van der Worp HB, et al. . High-resolution intracranial vessel wall MRI in an elderly asymptomatic population: comparison of 3T and 7T. Eur Radiol 2017;27:1585–95 10.1007/s00330-016-4483-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harteveld AA, Denswil NP, Van Hecke W, et al. . Ex vivo vessel wall thickness measurements of the human circle of Willis using 7T MRI. Atherosclerosis 2018;273:106–14 10.1016/j.atherosclerosis.2018.04.023 [DOI] [PubMed] [Google Scholar]
- 51.Zhu C, Haraldsson H, Tian B, et al. . High resolution imaging of the intracranial vessel wall at 3 and 7 T using 3D fast spin echo MRI. MAGMA 2016;29:559–70 10.1007/s10334-016-0531-x [DOI] [PubMed] [Google Scholar]
- 52.Zwartbol MH, van der Kolk AG, Ghaznawi R, et al. . Intracranial vessel wall lesions on 7T MRI (magnetic resonance imaging). Stroke 2019;50:88–94 10.1161/STROKEAHA.118.022509 [DOI] [PubMed] [Google Scholar]
- 53.Qiao Y, Suri FK, Zhang Y, et al. . Racial differences in prevalence and risk for intracranial atherosclerosis in a US community-based population. JAMA Cardiol 2017;2:1341–48 10.1001/jamacardio.2017.4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Kolk AG, Zwanenburg JJ, Denswil NP, et al. . Imaging the intracranial atherosclerotic vessel wall using 7T MRI: initial comparison with histopathology. AJNR Am J Neuroradiol 2015;36:694–701 10.3174/ajnr.A4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kroner ES, van Schinkel LD, Versluis MJ, et al. . Ultrahigh-field 7-T magnetic resonance carotid vessel wall imaging: initial experience in comparison with 3-T field strength. Invest Radiol 2012;47:697–704 10.1097/RLI.0b013e31826dc174 [DOI] [PubMed] [Google Scholar]
- 56.Koning W, de Rotte AA, Bluemink JJ, et al. . MRI of the carotid artery at 7 Tesla: quantitative comparison with 3 Tesla. J Magn Reson Imaging 2015;41:773–80 10.1002/jmri.24601 [DOI] [PubMed] [Google Scholar]
- 57.Majidi S, Sein J, Watanabe M, et al. . Intracranial-derived atherosclerosis assessment: an in vitro comparison between virtual histology by intravascular ultrasonography, 7T MRI, and histopathologic findings. AJNR Am J Neuroradiol 2013;34:2259–64 10.3174/ajnr.A3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Madai VI, von Samson-Himmelstjerna FC, Bauer M, et al. . Ultrahigh-field MRI in human ischemic stroke: a 7 Tesla study. PLoS One 2012;7:e37631 10.1371/journal.pone.0037631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyazawa H, Natori T, Kameda H, et al. . Detecting lenticulostriate artery lesions in patients with acute ischemic stroke using high-resolution MRA at 7T. Int J Stroke 2019;14:290–97 10.1177/1747493018806163 [DOI] [PubMed] [Google Scholar]
- 60.Kang CK, Park CA, Park CW, et al. . Lenticulostriate arteries in chronic stroke patients visualised by 7T magnetic resonance angiography. Int J Stroke 2010;5:374–80 10.1111/j.1747-4949.2010.00464.x [DOI] [PubMed] [Google Scholar]
- 61.Cho ZH, Kang CK, Han JY, et al. . Observation of the lenticulostriate arteries in the human brain in vivo using 7.0T MR angiography. Stroke 2008;39:1604–06 10.1161/STROKEAHA.107.508002 [DOI] [PubMed] [Google Scholar]
- 62.Dengler NF, Madai VI, Wuerfel J, et al. . Moyamoya vessel pathology imaged by ultra-high-field magnetic resonance imaging at 7.0 T. J Stroke Cerebrovasc Dis 2016;25:1544–51 10.1016/j.jstrokecerebrovasdis.2016.01.041 [DOI] [PubMed] [Google Scholar]
- 63.Oh BH, Moon HC, Baek HM, et al. . Comparison of 7T and 3T MRI in patients with Moyamoya disease. Magn Reson Imaging 2017;37:134–38 10.1016/j.mri.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 64.Lee A, McCartney S, Burbidge C, et al. . Trigeminal neuralgia occurs and recurs in the absence of neurovascular compression. J Neurosurg 2014;120:1048–54 10.3171/2014.1.JNS131410 [DOI] [PubMed] [Google Scholar]
- 65.Heverhagen JT, Bourekas E, Sammet S, et al. . Time-of-flight magnetic resonance angiography at 7 Tesla. Invest Radiol 2008;43:568–73 10.1097/RLI.0b013e31817e9b2c [DOI] [PubMed] [Google Scholar]
- 66.Moon HC, You ST, Baek HM, et al. . 7.0 Tesla MRI tractography in patients with trigeminal neuralgia. Magn Reson Imaging 2018;54:265–70 10.1016/j.mri.2017.12.033 [DOI] [PubMed] [Google Scholar]