Abstract
India has got rich cultural inheritage in the forms of Ayurveda texts which are a rich and ample source of herbs, shrubs, trees and affluent in medicinally active phytoconstituents. Aconitum napellus is used for the cure of many ailments including rheumatoid arthritis, sciatica and gout. The present work attempts to evaluate the physicochemical and preliminary phytochemical studies on the tubers of Aconitum napellus along with its antidiabetic activity. The herbal standardization was carried out on the basis of organoleptic properties, physical characteristics and physicochemical properties. The body weight of ACON-I (1.25 mg/kg) and ACON-II (2.5 mg/kg) was recorded as 190.40 and 209.40 g, respectively, compared with 163.00 g in diabetic rats at day 28. The body weight of ACON-I and ACON-II was significantly increased compared with diabetic rats (p < 0.01). However, the body weight of ACON-I and ACON-II was decreased significantly (p < 0.01) compared with normal group (222.60 g). The blood glucose levels of diabetic rats and ACON-I group were recorded as 277.800 and 152.400 mg/dl, respectively, compared with 83.600 mg/dl in normal rats (p < 0.01). However, the HbA1c levels of diabetic rats and ACON-I group were recorded as 11.306 and 6.936% Hb, respectively, compared with 4.539% Hb in normal rats. The glucose and HbA1c levels of diabetic and ACON-I groups were significant compared with normal group (p < 0.01). The results of antidiabetic activity showed that the plant can be used as a potent source for the treatment of diabetes and its complications. The results of this work provided the referential information for the identification and standardization of Aconitum napellus along with its role as a hypoglycemic agent.
Keywords: Aconitum napellus, Aconitine, Shodhana, Physicochemical properties, Diabetes
Introduction
Diabetes is a metabolic disorder which is consequential to high blood glucose level, either because pancreas does not generate adequate amount of insulin or cells do not act in response to that insulin. The sedentary life style and obesity is the best known reason for diabetes. It becomes pandemic and the best known cause of mortality and morbidity (Leitner et al. 2017). Basically three types, i.e. type 1, type 2 and type 3 (gestational) of diabetes exist which occurs during pregnancy and engrosses threat mutually for the mother and child. The World Health Organization (WHO) states that there are 366 million people who are diagnosed with diabetes in 2011 and it will rise to 552 million by 2030. The estimated worldwide prevalence of diabetics in 2000 was 2.8% and it is projected to 5.4% in 2025 (Rao et al. 2010).
Aconite is being used in the traditional medicine of China (TCM) and of India (Ayurveda) (Mishra 2000; Heiner 2012). Aconite originates throughout the globe, but it is native to Asia, Europe and America (Dubey et al. 2012). It is widely found in Himalayas along with the costal districts of Orissa (Chhetree et al. 2010). Worldwide the plant Aconitum napellus (Fig. 1) is also called as Wolf’s bane, Monk’s blood, Monkshood, blue rocket and Friar’s cap. Its active ingredients are aconitine, benzoylaconitine, mesaconitine, isoaconitine, benzaconitine, aneopelline, eoline, napelline, ipaconitine and aconine (Kelly 1990; Sutan 2018). The roots of Aconitum are used as Ayurvedic medicine in India and its formulation products are included in the “Ayurvedic Pharmacopoeia and Ayurvedic formulary of India” (Rastogi 2011). Ethnomedically in TCM, it is used for the prevention of cold, general debility and ‘Yang’ deficiency. It is also used as an antidote for several poisonings (Singhuber et al. 2009). It is used for the management of different nervous disorders. Despite its toxicity, traditionally it has been used for the healing of facial palsy, joint pain, inflammation, gout, fever and pericarditis (Chang and Whitaker 2001). It is also used in folklore medicine for sciatica and rheumatism (Venkataraghavan and Sundaresan 1981). The roots are thermogenic, narcotic, anodyne, anti-inflammatory, diaphoretic, diuretic, expectorant, nerve tonic, stomachic, emmenagogue, antioxidant, anticholinesterase, aphrodisiac and sedative (Nadkarni 1976; Prajapati and Kumar 2003; Singh et al. 2012; Ahmad et al. 2017).
Fig. 1.

Exomorphic features of dried Aconitum napellus tubers
It has been reported that the unprocessed root of aconite produces fatal toxicities (Bisset 1981; Chan 2011, 2012). Different herbal medicines are subjected to the specific treatments before their utilization as materia medica in India and China. The processing techniques in Ayurveda (also known as Samskaras) include two different stages, i.e., the “Shodhana (purification or detoxification) and Bhaishajya kalpana (formulation methods)”. The Shodhana is carried out by the treatment of the drug with the urine and milk of the cow (Dhamanakar 1964; Mishra 2004; Sharma 2005; Shah et al. 2010; Belge and Belge 2012; Jaiswal et al. 2013).
In the ancient TCM, different techniques for the purification of aconite roots are available which include the processing of drug with mineral salt, water and decoction with water. Though Aconitum napellus is being used in the treatment of many ailments, no scientific evidence is being reported in the literature for the identification of the genuine sample. Therefore, the present work attempts to report necessary pharmacognostical and standardization parameters of Aconitum napellus tubers, which will help to identify the drug and to find out its beneficial role for the treatment of diabetes and its complications. The results obtained in this work would be useful in standardization, phytochemical screening and pharmacological evaluation of plant materials.
Materials and methods
Chemicals and plant material
Chloroform and other chemical used were of analytical grade which were procured from “Sigma Aldrich (St. Louis, MO, USA). The dried tubers of Aconitum napellus were obtained from the authenticated licensed shop from “Srinagar (Jammu and Kashmir, India)”. The authenticity of the sample was confirmed from the text report of “National Botanical Research Institute (Lucknow, India; Voucher No. NBRI/CIF/383/2013)”.
Extraction
The dried tuber of Aconitum napellus was extracted using chloroform as the solvent. The reflux assembly was placed on a water bath for about 6 h at ˂ 50 °C. The extraction was performed in triplicates. Finally, the extract was filtered and evaporated to a constant weight with the help of a “Rotary Evaporator (BUCHI Rotavapor R-205, Geneva, Switzerland)” at < 40 °C.
Morphological characters
The morphological characters of plants are used for identification and confirmation. The description is accompanied by its visual appearance and photographic images (Ali 2004; Ansari 2008).
Extractive value
The extractive value indicates the weight of extractable constituents present in the plant using various solvents, on the basis of increasing polarity to get the correct and dependable values. Usually petroleum ether and alcohol extracts are used for setting up the standard of herb. Fixed oil, resins and volatile substances are extracted into petroleum ether. The active constituents can be extracted into alcoholic extract. The extractive values are expressed in the form as described in Pharmacopoeia (IP 1996; Ansari 2008).
Cold extraction
The dried powder was macerated into different solvent on the basis of polarity. The drug was kept into closed flask for 24 h and stirred frequently at different time intervals. It was filtered and dried using rotary evaporator to a stable weight (IP 1996).
Hot extraction
The powdered drug (10 g) was filled in thimble and then placed in soxhlet apparatus using solvents like n-hexane, chloroform, alcohol and water and was used for the extraction. The filtrate was dried using rotary vacuum evaporator and the extractive value was recorded till constant weight (Ansari 2008).
Successive extraction
The coarsely dried powdered material (10 g) was successively extracted in a reflux assembly with different solvents on the basis of increasing polarity (n-hexane, chloroform, methanol and water). The extracts were evaporated to aridness using rota vapor till constant weight and the extractive values were recorded (Ansari 2008).
Ash value
Ash was produced after the drug was incinerated, and this ash contained organic matter for instance phosphates and carbonates. Heating causes thrashing of organic material in CO2 form which leaves inorganic components. When hydrochloric acid reacts with ash produces acid-insoluble ash which contains silica. The acid insoluble ash thereby indicates the contamination with earthly material. Inorganic elements can be detected by means of water soluble ash (IP 1996; Ansari 2008).
Total ash
The ground drug (2 g) was reduced to ashes in a silica crucible at < 450 °C in anticipation of free from carbon. For the estimation of total ash, the drug was cooled and weighed (IP 1996; Ansari 2008).
Acid insoluble ash
Ash was stewed for 5 min in dilute HCl (25 ml and 6 N). The insolvable matter was collected on an ashless filter paper, then cleansed using hot water and finally ignited in muffle furnace at a temperature beneath 450 °C to a constant weight (IP 1996; Ansari 2008).
Water soluble ash
Ash was dissolved in distilled water and the insoluble part was unruffled on a filter paper (ashless) and ignited at 450 °C to unvarying mass. Weight of soluble part was calculated by subtracting insoluble part weight to that of the total ash (IP 1996; Ansari 2008).
Foreign matter
Herbal drugs are prepared using particular fraction of the plant and must be devoid from others. These other matter may be either due to fault collection. It may be a stone, residues and other parts of plant. For the accuracy purpose the drug must be free from foreign particles. The calculations are done in percentage (IP 1996; Ansari 2008).
Fluorescent analysis
The powdered plant material was mixed with different solvents and examined under UV light (254 nm and 366 nm) and day light (Ansari 2008).
Powdered drug reaction with different reagents
The powder drug and different reagents were treated with each other and the color shown by that treatment was noted (Ansari 2008).
Determination of pH
pH (10% solution): Drug (10 g) was accurately weighed and dissolved with water and then filtered. Standardized glass electrode was used to check the pH of filtrate (Ansari 2008).
pH (1% solution): Drug (1 g) was finally suspended in water to make a filtrate which is further used for the determination of pH.
Loss on drying
This parameter determines the amount of moisture as well as volatile components present in a particular sample. The pulverized drugs (10 g) were dried in hot air oven at 105 °C for 6 h and weighed. The process continues until two reading harmonized with each other (Ansari 2008).
Preliminary phytochemical screening
Powder was taken and tested for the presence of major chemical constituents such as sterols, tannins, flavonoids, proteins and amino acids, phenolics, carbohydrates, saponins and alkaloids (Ali et al. 2012; Kumar et al. 2019).
Experimental animals
Male Sprague–Dawley rats (weighing 200–250 g) were purchased from “Central Drug Research Institute (Lucknow, India)”. The animals were housed in the “Animal House Facility, Faculty of Pharmacy, Integral University (Lucknow, India)”. The animals were kept in polypropylene cages (5 animals/cage).
Experimental method
The citrate buffer was prepared by mixing 192 mg of citric acid in 10 ml of distilled water to make 0.1 M citric acid solution and 294 mg of trisodium citrate was also added in 10 ml of distilled water to prepare 0.1 M solution. Then, 4.5 ml of citric acid was assorted with 5.5 ml of trisodium citrate solution previously prepared. Finally the pH (4.5) was adjusted with citric acid. The antidiabetic activity of chloroform extract of Aconitum napellus was performed in rat models (Das et al. 2019; Naz et al. 2019). Streptozotocin (STZ) is frequently used drug to induce diabetes in animal models and hence STZ was used in the present work to induce diabetes in rats (Simon et al., 2018; Xu et al., 2018). STZ at the dose of 60 mg/kg i.p. was used to provoke the diabetes by freshly dissolving it in ice-cold citrate buffer (pH 4.5; 0.1 M). After 72 h, the blood sample was obtained from the tail vein of each rat and analyzed using “Alere G1 Glucometer (Waltham, MA, USA)”. The rats showing the glucose level ˃ 250 mg/dl were classified as diabetic which were used for further studies.
The accommodation of animals was standard laboratory condition and fed with pellet diet of the standard. The animals were grouped into five different groups (each group containing five rats). Group I served as nondiabetic/normal control (NC) which was treated with 0.5% w/v sodium carboxymethyl cellulose (SCMC). Group II served as diabetic control (DC) and groups III, IV and V served as diabetic treated with ACON-I, ACON-II and glibenclamide at a dose of 1.25, 2.5 and 10 mg/kg b.w. p.o., respectively. The treated group received plant extract and glibenclamide (standard) once daily morning in fasting state for 28 days by gastric intubation.
Parameters
Dose determination
The dose for the extract of Aconitum napellus was obtained from our previous work of acute toxicity studies (Shoaib et al. 2019).
Body weight
At the initial and final day of the treatment, body weight was measured using digital balance.
Blood glucose level and HbA1c
At the initial and final days of the treatment, blood glucose level was determined using tail’s vein blood by “ACCUCHEK, Roche, Germany”. The HbA1c was measured by the help of Biorad D10-HbA1c Analyzer; CAL-REMEDIES.
Histopathology
The pancreas from each group was taken and fixed using 10% v/v formalin solution for 48 h. Subsequently the tissue paraffin blocking was done and 5 μm thin sections were cut using regular rotary microtome. The sections were then mounted on slides and stained with hematoxylin and eosin (H and E). The stained slides were then observed and analyzed for the results.
Statistical evaluation
The values of antidiabetic evaluation are expressed as mean ± SEM. The values of physicochemical evaluation are expressed as mean ± SD. The statistical analysis was performed by Dunnett’s test using “GraphPad Prism software (version 6, GraphPad, San Diego, CA, USA)”. The statistical parameters were compared at either 1% or 5% level of significance.
Results
Physicochemical characterization
Aconitum napellus is a perennial herbaceous plant having conical-shaped, tapering root, externally brown and internally white color with intolerable smell. The extractive values of Aconitum napellus tubers using four different solvents including n-hexane, chloroform, methanol and water are summarized in Table 1. Using cold percolation method, the extractive values were obtained in the range of 0.72–0.91% w/w using different solvents. However, using hot extraction method, the extractive values were recorded in the range of 1.99–2.25% w/w using different solvents. On the other hand, using successive extraction method, the extractive values were found in the range of 4.44–8.70% w/w using different solvents (Table 1). Overall, the maximum extractive value was obtained in chloroform extract (8.70% w/w) using successive extraction method. Therefore, chloroform extract of A. napellus was finally selected for antidiabetic activity in rats. The other physical constants such as total ash, acid insoluble ash, water soluble ash, foreign matter, loss on drying and pH of extract are summarized in Table 2. Total ash, acid insoluble and water soluble ash values values were obtained as 3.83, 4.83 and 5.16% w/w, respectively. Loss on drying was recorded as 0.44% w/w/. The pH of 1% and 10% aqueous solutions was obtained as 5.12 and 7.50, respectively. The results of fluorescent analysis using different solvents at day light and UV light (254 and 366 nm) are summarized in Table 3. Day light and UV light at 254 and 366 nm presented different fluorescent with different solvents. The results of powder drug reaction with different solvent are summarized in Table 4. The different color of powder was recorded with different solvents.
Table 1.
Extractive values of Aconitum napellus tubers using different solvents (n = 3)
| Solvents | Cold percolation (% w/w) ± SD | Hot extraction (% w/w) ± SD | Successive extraction (% w/w) ± SD |
|---|---|---|---|
| n-Hexane | 0.72 ± 0.01 | 1.99 ± 0.04 | 7.60 ± 0.08 |
| Chloroform | 0.91 ± 0.02 | 2.25 ± 0.05 | 8.70 ± 0.09 |
| Methanol | 0.89 ± 0.03 | 2.03 ± 0.04 | 4.44 ± 0.05 |
| Water | 0.84 ± 0.02 | 2.09 ± 0.03 | 6.91 ± 0.06 |
Table 2.
Physicochemical parameters of Aconitum napellus tubers (n = 3)
| Parameters | Values ± SD |
|---|---|
| Total ash | 3.83 ± 0.03 (% w/w) |
| Acid insoluble ash | 4.83 ± 0.05 (% w/w) |
| Water soluble ash | 5.16 ± 0.06 (% w/w) |
| Foreign matter | 0.20 ± 0.00 (% w/w) |
| Loss on drying | 0.44 ± 0.01 (% w/w) |
| pH of 1% aqueous solution | 5.12 ± 0.07 |
| pH of 10% aqueous solution | 7.50 ± 0.08 |
Table 3.
Florescent analysis of Aconitum napellus
| Solvent used | Day light | UV light (254 nm) | UV light (366 nm) |
|---|---|---|---|
| Benzene | Greenish Brown | Greenish black | Yellowish brown |
| Dist. water | Yellowish Brown | Green | Yellowish green |
| NaOH in water | Brownish yellow | Greenish yellow | Light yellow |
| NaOH in methanol | Blackish brown | Yellowish green | Blackish green |
| Chloroform | Pale yellow | Greenish black | Yellowish brown |
| Dil.HNO3 | Brownish green | Dark green | Yellowish brown |
| Acetone | Brownish yellow | Yellowish green | Yellow |
Table 4.
Powdered drug reaction with different reagents of Aconitum napellus
| Treatment | Observation |
|---|---|
| Powder as such | Yellowish brown |
| Conc. HCl | Yellow |
| Conc. HNO3 | Brownish Yellow |
| Conc. H2SO4 | Brown |
| Glacial acetic acid | Light yellow |
| Benzene | Brownish yellow |
| NaOH in methanol | Yellowish brown |
Qualitative phytochemical screening
In the phytochemical tests, we have found that there is a presence of sterols, glycosides, phenol and carbohydrate. The findings of chemical tests are summarized in Table 5.
Table 5.
Preliminary phytochemical screening of Aconitum napellus extract
| S.no | Tests | Extracts |
|---|---|---|
| 1 | Test for sterols | |
| Salkowaski test | + | |
| Libermann-Buchard’s test | + | |
| 2 | Tannins test | |
| Gelatin solution | − | |
| Catechin | − | |
| 3 | Flavonoids | |
| Shinoda test | + | |
| 4 | Test for protein and amino acid | |
| Ninhydrin test | − | |
| Biuret test | − | |
| Xanthoprotic test | − | |
| 5 | Glycosides test | |
| Killer killani | + | |
| Bontrager’s test | + | |
| Legal test | + | |
| Baljet test | + | |
| 6 | Phenolic test | |
| Ferric chloride | + | |
| Lead acetate | + | |
| Gelatin | + | |
| 7 | Carbohydrate test | |
| Fehling test | + | |
| Molish test | + | |
| Benedict test | + | |
| 8 | Saponin test | |
| Foam test | + | |
| 9 | Alkaloids Test | |
| Dragandroff | + | |
| Hagers | + | |
| Wagners | + | |
| Mayers | + |
Body weight
Daily oral administration of Aconitum napellus extract for 28 consecutive days at the dose of 1.25 (ACON-I) and 2.5 (ACON-II) mg/kg showed significant increase (p < 0.01) in body weight when compared to diabetic rats (Table 6). The body weight of ACON-I and ACON-II was recorded as 190.40 and 209.40 g, respectively, compared with 163.00 g in diabetic rats at day 28. However, the body weight of ACON-I and ACON-II was decreased significantly (p < 0.01) when compared with normal control group. The body weight of normal control group was recorded as 247.40 g at day 28. Glibenclamide at 10 mg/kg b.w., p.o. produced significant (p < 0.05) change in the weight gain of rats compared to their respective control (Table 6).
Table 6.
Effect of chloroform extract of Aconitum napellus on body weight in diabetic rats (n = 5)
| Group(s) | Body weight at day 0 (g) ± SEM | Body weight at day 28 (g) ± SEM |
|---|---|---|
| NC | 222.60 ± 8.553 | 247.40 ± 7.954 |
| DC | 224.40 ± 8.334 | 163.00 ± 10.469** |
| ACON-I | 229.80 ± 5.122 | 192.40 ± 3.970**,# |
| ACON-II | 225.20 ± 8.089 | 209.40 ± 8.171**,## |
| Glibenclamide | 234.80 ± 3.611 | 215.40 ± 5.845*,## |
All the values were expressed as mean ± SEM; where *denotes p < 0.05, **denotes p < 0.01 compared to NC, #denotes p < 0.05 and ##denotes p < 0.01 compared to DC
Blood glucose and HbA1c level
The animals of treated group (DC and ACON-I) showed significant increase (p < 0.01) in blood glucose and HbA1c levels when compared with NC group animals (Table 7). The blood glucose levels of DC and ACON-I group were recorded as 277.800 and 152.400 mg/dl, respectively, compared with 83.600 mg/dl in NC group. However, the HbA1c levels of DC and ACON-I group were recorded as 11.306 and 6.936% Hb, respectively, compared with 4.539% Hb in NC group. The standard control did not show any significance (p > 0.05) when compared with NC group while the ACON-II group showed less significance (p < 0.05) compared to NC group. The entire treated group showed significant decrease in blood glucose and HbA1c level when compared with group II, i.e., diabetic group (Table 7).
Table 7.
Effect of chloroform extract of Aconitum napellus on blood glucose level and HbA1C in diabetic rats (n = 5)
| Group(s) | Plasma glucose (mg/dl) ± SEM | HbA1C (% Hb) ± SEM |
|---|---|---|
| NC | 83.600 ± 2.502 | 4.539 ± 0.087 |
| DC | 277.800 ± 11.065** | 11.306 ± 0.3855** |
| ACON-I | 152.400 ± 16.070**,# | 6.936 ± 0.559**,## |
| ACON-II | 127.800 ± 4.465*,# | 6.079 ± 0.155*,## |
| Glibenclamide | 115.200 ± 5.704 ns,# | 5.641 ± 0.198ns,## |
All the values were expressed as mean ± SEM; where *denotes p < 0.05, **denotes p < 0.01 compared to NC, #denotes p < 0.05, ##denotes p < 0.01 and ns p > 0.05 compared to NC and DC
Histopathological changes
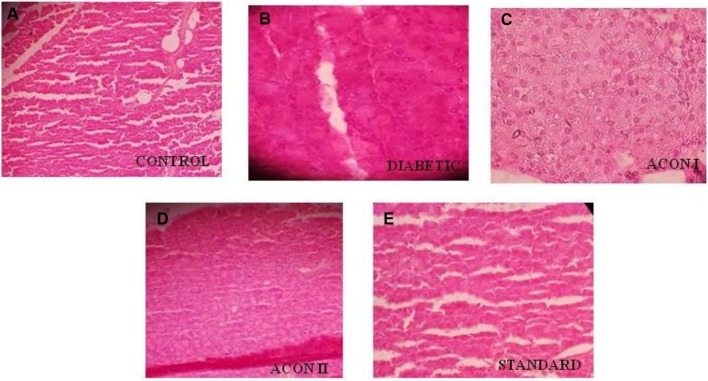
The pancreas was observed after H&E staining in all groups of animals and results are presented in Fig. 2. The control group (Fig. 2a) showed a normal proportion of AC and BC. In diabetic group (Fig. 2b), the proportion of these two (AC: BC) was not uniform. A decrease and disarranged islets cells were observed. The treatment of ACON I, II and glibenclamide (Fig. 2c–e, respectively) improved the proportion of AC and BC along with it changes the volume of islets cells as compared to control group.
Fig. 2.
Photomicrographs of rat pancreas; the control group (a) shows a normal proportion of acinar cells (AC) and beta cells (BC). In diabetic group (b) the proportion of these two (AC: BC) are not uniform. A decrease and disarranged islets cells were observed. The treatment of ACON I, II and glibenclamide (c, d and e, respectively) improves the proportion of AC and BC along with it changes the volume of islets cells as compared to control group
Discussion
Herbal medicines are prepared using different plant sources either in its original form or by after processing it with different constituents. In the traditional Indian system of medicine, there are number of herbal remedies having medicinal values but are not emerged in worldwide market due to lack of validated quality control procedures (Vadivel et al. 2018). This is done to compare the various factors at a glance (Rajakrishnan et al. 2016). The percentage of organic constituents can be determined by water soluble ash value and the acid insoluble ash, mainly gives the percentages of sand and impurities that remain insoluble in dil. HCl (Sreelekshmi 2014). There are a lot of chemical constituents present in tubers of Aconitum napellus in which alkaloid, terpenoid and flavonoids were reported (Srivastava et al. 2010). The presence of diterpenoid and steroidal alkaloids contents has also been reported (Shyaula 2011). The same was observed when we carried out different chemical tests using powder of Aconitum napellus tubers along with the presence of sterols, glycosides, phenol and carbohydrate (Table 5). The phenolic compounds have been reported to have strong antioxidant efficacies which play a significant role in managing various chronic disorders such as diabetes mellitus (Adefegha and Oboh 2013). Aconitum napellus is a poisonous plant which can be used after the detoxification or purification process. It is a great source of phenol and antioxidants. One of our studies which were on the toxicity and its pharmacokinetics of dried Aconitum napellus tubers showed that at particular dose, this plant did not produce any sign of toxicity (Shoaib et al. 2019). Further investigation was carried out to access the antidiabetic activity of the dried tuber extract on serum insulin level in STZ-induced diabetic animal.
Conclusions
From the results of this study, it can be concluded that the present study on Aconitum napellus tubers can serve as an important source of information to ascertain the identity and to determine the quality and purity of plant material available in the market. This article is a step to characterize the drug chemically and the presence of various chemical constituents in the plant Aconitum napellus which may be a potential cause of treatment of various disorders. The quality of the plant can be estimated by determining the physical parameters which could be used effectively for the identification of the drug. These investigations are of great importance for carrying out the revalidation and estimation of its other pharmacological activities.
Acknowledgements
Authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding the research through the research group project number RGP-202.
Author contributions
AS: Methodology. MMSB: Funding acquisition. HHS: Formal analysis. RKD: Methodology. MB: Formal analysis. MK: Methodology. B: Formal analysis. FS: Project administrator.
Compliance with Ethical Standards
Conflict of interest
We declare that we have no conflict of interest associated with this manuscript.
Ethical approval
Ethical clearance was gained from the “Institutional Animal Ethical Committee (IAEC) (Approval No: IU/IAEC/16/27) Integral University, Lucknow, India”.
References
- Adefegha SA, Oboh G. Phytochemistry and mode of action of some tropical spices in the management of type-2 diabetes and hypertension. Afr J Pharm Pharmacol. 2013;7:332–346. doi: 10.5897/AJPPX12.014. [DOI] [Google Scholar]
- Ahmad H, Ahmad S, Shah SAA, Latif A, Ali M, Khan FA, Tahir MN, Shaheen F, Wadood A, Ahmad M. Antioxidant and anticholinesterase potential of diterpenoid alkaloids from Aconitum heterophyllum. Bioorg Med Chem. 2017;25:3368–3376. doi: 10.1016/j.bmc.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Ali M (2004) Evaluation of drugs: textbook of pharmacognosy, pp. 52–58. CBS Publishers, Delhi
- Ali B, Mujeeb M, Aeri V, Mir SR, Faiyazuddin M, Shakeel F. Anti-inflammatory and antioxidant activity of Ficus carica Linn. leaves. Nat Prod Res. 2012;26:460–465. doi: 10.1080/14786419.2010.488236. [DOI] [PubMed] [Google Scholar]
- Ansari SH (2008) Essential of pharmacognosy. Standardization of crude drugs, pp. 575–596. Birla Publication Private Limited, Delhi
- Belge RS, Belge AR. Ayurvedic shodhana treatments and their applied aspect with special reference to loha. IOSR J Pharm Biol Sci. 2012;2:45–49. [Google Scholar]
- Bisset NG. Arrow poisons in China. Part II. Aconitum–botany, chemistry, and pharmacology. J Ethnopharmacol. 1981;4:247–336. doi: 10.1016/0378-8741(81)90001-5. [DOI] [PubMed] [Google Scholar]
- Chan TY. Causes and prevention of herb-induced aconite poisonins in Asia. Hum Exp Toxicol. 2011;30:2023–2026. doi: 10.1177/0960327111407224. [DOI] [PubMed] [Google Scholar]
- Chan TY. Aconite poisoning following the percutaneous absorption of aconitum alkaloids. Foren Sci Int. 2012;223:25–27. doi: 10.1016/j.forsciint.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Chang LK, Whitaker DC. The impact of herbal medicines on dermatologic surgery. Dermatol Surg. 2001;27:759–763. doi: 10.1046/j.1524-4725.2001.01089.x. [DOI] [PubMed] [Google Scholar]
- Chhetree RR, Dash GK, Mondal S, Acharyya S. Studies on the hypoglycaemic activity of Aconitum napellus l. roots. Drug Invent Today. 2010;2:343–346. [Google Scholar]
- Das SK, Samantaray D, Sahoo SK, Pradhan SK, Samanta L, Thatoi H. Bioactivity guided isolation of antidiabetic and antioxidant compound from Xylocarpus granatum J. Koenig bark. 3 Biotech. 2019;9:E198. doi: 10.1007/s13205-019-1711-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamanakar PV. Ayurvediya aushadhikarana. Mumbai: Dhootpapeshwar Prakashan; 1964. [Google Scholar]
- Dubey N, Dubey N, Mehta R. Development and validation of selective high-performance liquid chromatographic method using photo diode array detection for estimation of aconitine in polyherbal ayurvedic taila preparations. Chromatogr Res Int. 2012;2012:E157916. doi: 10.1155/2012/157916. [DOI] [Google Scholar]
- Heiner F. The flagship remedy of Chinese medicine: reflections on the toxicity and safety of aconite. Am J Chin Med. 2012;100:36–41. [Google Scholar]
- Indian Pharmacopoeia (1996) Vol 2, Appendix 3.37–3.44. Controller of Publications, Delhi
- Jaiswal Y, Liang Z, Yong P, Chen H, Zhao Z. A comparative study on the traditional Indian Shodhana and Chinese processing methods for aconite roots by characterization, and determination of the major components. Chem Cent J. 2013;7:E169. doi: 10.1186/1752-153X-7-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP. Aconite poisoning. Med J Aust. 1990;153:499. doi: 10.5694/j.1326-5377.1990.tb126164.x. [DOI] [PubMed] [Google Scholar]
- Kumar V, Shriram V, Bhagat R, Khare T, Kapse S, Kadoo N. Phytochemical profile, anti-oxidant, anti-inflammatory, and anti-proliferative activities Pogostemon deccanensis essential oils. 3 Biotech. 2019;9(1):E31. doi: 10.1007/s13205-018-1560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner DR, Frühbeck G, Yumuk V, Schindler K, Micic D, Woodward E, Toplak H. Obesity and type 2 diabetes: two diseases with a need for combined treatment strategies-EASO can lead the way. Obes Facts. 2017;10:483–492. doi: 10.1159/000480525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SN. Rasendra chintamani. India: Chaukhamba Orientalia; 2000. [Google Scholar]
- Mishra LC. Scientific basis for Ayurvedic therapies. Washington, DC: CRC Press; 2004. [Google Scholar]
- Nadkarni KM (1976) Indian Materia Medica, vo1. 1, p. 28. Bombay popular Prakashan Pvt. Ltd., Bombay
- Naz D, Muhamad A, Zeb A, Shah I. In vitro and in vivo antidiabetic properties of phenolic antioxidants from Sedum adenotrichum. Front Nutr. 2019;6:E177. doi: 10.3389/fnut.2019.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapati DN, Kumar U (2003) Agro’s dictionary of medicinal plants, p. 7, published by Dr. Updesh Purohit for agrobios (Jodhpur, India)
- Rajakrishnan R, Lekshmi R, Samuel D. Analytical standards for the root tubers of Ativisha-Aconitum heterophyllum Wall. ex Royle. Int J Sci Res Publ. 2016;6:531–534. [Google Scholar]
- Rao MU, Sreenivasulu M, Chengaiah B, Reddy KJ, Chetty CM. Herbal medicines for diabetes mellitus: a review. Int J PharmTech Res. 2010;2:1883–1892. [Google Scholar]
- Rastogi S. A review of aconite (Vatsanabha) usage in Ayurvedic formulations: traditional views and their references. Spatula. 2011;1:233–244. doi: 10.5455/spatula.20111220112858. [DOI] [Google Scholar]
- Shah RK, Kenjale RD, Shah DP, Sathaye S, Kaur H. Evaluation of cardiotoxicity of shodhit and ashodhit samples of aconite root. Int J Pharmacol Biol Sci. 2010;4:65–68. [Google Scholar]
- Sharma PV. Caraka samhita (English translation) Varanasi: Chowkhamba Orientalia; 2005. [Google Scholar]
- Shoaib Ambreen, Badruddeen, Siddiqui Hefazat Hussain, Dixit Rakesh Kumar, Akhtar Juber. Aconitum Napellus: Detoxification and Acute Toxicity Investigation Followed by Sub-Acute Toxicity and Bioavailability Assessment of Highest and Lowest LD50 Extract. Journal of Biologically Active Products from Nature. 2019;9(2):108–119. doi: 10.1080/22311866.2019.1605931. [DOI] [Google Scholar]
- Shyaula SL. Phytochemicals, traditional uses and processing of Aconitum species in Nepal. J Sci Technol. 2011;12:171–178. [Google Scholar]
- Simon JP, Baskaran UL, Basha SK, Ramalingam G, Prince SV. Evidence of antidiabetic activity of Spirulina fusiformis against streptozotocin-induced diabetic Wistar albino rats. 3 Biotech. 2018;8:E129. doi: 10.1007/s13205-018-1156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Vinod M, Iyer SK, Khare G, Sharwan G, Larokar YK. Aconite: a pharmacological update. Int J Res Pharm Sci. 2012;3:242–246. [Google Scholar]
- Singhuber J, Zhu M, Prinz S, Kopp B. Aconitum in traditional Chinese medicine—a valuable drug or an unpredictable risk. J Ethanopharmacol. 2009;126:18–30. doi: 10.1016/j.jep.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Sreelekshmi RV. Standardization of Acconitum ferrox. Int J Sci Eng Res. 2014;5:483. [Google Scholar]
- Srivastava N, Sharma V, Kamal B, Jadon VS. Aconitum: Need for sustainable exploitation (with special reference to Uttarakhand) Int J Green Pharm. 2010;4:220–228. doi: 10.4103/0973-8258.74129. [DOI] [Google Scholar]
- Sutan NA. Phytochemical constituents and biological properties of extracts from Aconitum sp.—a short review. Curr Trends Nat Sci. 2018;7:28–39. [Google Scholar]
- Vadivel V, Ravichandran N, Brindha P, Gopal A, Kumaravelu C. Microscopic, phytochemical, HPTLC, GC–MS and NIRS methods to differentiate herbal adulterants: pepper and papaya seeds. J Herb Med. 2018;11:36–45. doi: 10.1016/j.hermed.2018.01.004. [DOI] [Google Scholar]
- Venkataraghavan S, Sundaresan TP. A short note on contraceptive in Ayurveda. J Plant Sci Res. 1981;2:39. [Google Scholar]
- Xu Y, Zhao Y, Sui Y, Lei XJ. Protective effect of Pterocarpus marsupium bark extracts against cataract through the inhibition of aldose reductase activity in streptozotocin-induced diabetic male albino rats. 3 Biotech. 2018;8:E188. doi: 10.1007/s13205-018-1210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]