Chemotherapy‐induced nausea and vomiting are common adverse effects associated with the use of cytotoxic chemotherapy drugs. This article evaluates differences in complete response rates and other antiemetic outcomes in dexamethasone‐spacing regimens.
Keywords: Nausea, Vomiting, Chemotherapy‐induced nausea and vomiting, Moderately emetogenic chemotherapy, Dexamethasone, Palonosetron
Abstract
Background.
A dexamethasone‐sparing regimen consisting of palonosetron plus 1‐day dexamethasone for the prevention of chemotherapy‐induced nausea and vomiting (CINV) has been studied previously. Here, we evaluate the noninferiority of the dexamethasone‐sparing regimen in overall antiemetic control using a meta‐analysis based on individual patient data (IPD).
Materials and Methods.
We conducted a systematic review for randomized trials reporting CINV outcomes for the comparison of palonosetron plus 1‐day dexamethasone (d1 arm) versus the same regimen followed by dexamethasone on days 2–3 after chemotherapy (d3 arm) in chemotherapy‐naïve adult patients undergoing either moderately emetogenic chemotherapy (MEC) or anthracycline plus cyclophosphamide (AC)‐containing chemotherapy. PubMed and MEDLINE were searched electronically. A manual search was also conducted. The primary endpoint was complete response (CR; no emesis and no rescue medication) in the overall 5‐day study period. The noninferiority margin was set at −8.0% (d1 arm−d3 arm).
Results.
Five studies (n = 1,194) were eligible for analysis and all IPD was collected. In the overall study period, the d1 arm showed noninferiority to the d3 arm for CR as well as complete control (pooled risk difference in CR rate − 1.5%, 95% confidence interval [CI] −7.1 to 4.0%, I2 = 0%; in complete control rate − 2.4%, 95% CI −7.7 to 2.9%, I2 = 0%). There was no significant interaction between dexamethasone regimen and risk factors (type of chemotherapy, sex, age, and alcohol consumption).
Conclusion.
This IPD meta‐analysis indicates that the dexamethasone‐sparing regimen is not associated with a significant loss in overall antiemetic control in patients undergoing MEC or AC‐containing chemotherapy, irrespective of known risk factors for CINV.
Implications for Practice.
Although dexamethasone in combination with other antiemetic agents has been used to prevent chemotherapy‐induced nausea and vomiting (CINV), it is of clinical importance to minimize total dose of dexamethasone in patients undergoing multiple cycles of emetogenic chemotherapy. This individual‐patient‐data meta‐analysis from five randomized controlled trials (1,194 patients) demonstrated a noninferiority of the dexamethasone‐sparing regimen for complete response and complete control of CINV. The outcomes were comparable across patients with different characteristics. These findings thus help physicians minimize use of the steroid and further reduce the burden of dexamethasone‐related side effects in patients undergoing multiple consecutive courses of emetogenic chemotherapy.
Introduction
Chemotherapy‐induced nausea and vomiting (CINV) is the most common nonhematological toxicity associated with the use of cytotoxic chemotherapy drugs, with incidence rates ranging around 70%–80% [1], [2], [3]. The optimal prevention of CINV remains a major clinical issue, which is directly related to adherence with chemotherapy and maintaining patient quality of life [1]. The guidelines developed by international societies recommend prophylaxis for CINV based on the emetogenicity of individual antineoplastic agents, which is classified into four categories as follows: highly emetogenic chemotherapy (HEC), moderately emetogenic chemotherapy (MEC), low emetogenic chemotherapy, and minimally emetogenic chemotherapy [4], [5], [6], [7], [8]. Whereas anthracyclines and cyclophosphamide are categorized as MEC, the combination of an anthracycline and cyclophosphamide (AC) has been recently reclassified as HEC. In the MEC setting, the cornerstone of antiemetic prophylaxis has long been a two‐drug regimen consisting of a 5‐hydroxytryptamine‐3 (5‐HT3) receptor antagonist and multiple‐day dexamethasone for the prevention of acute and delayed CINV [7], [9].
Palonosetron, a second‐generation 5‐HT3 receptor antagonist, is superior to the first‐generation antagonists in efficacy and safety, having greater binding affinity for the 5‐HT3 receptor and a longer elimination half‐life [10]. Additionally, palonosetron is the only agent in the class that is approved for the prevention of delayed CINV after MEC [10]. Dexamethasone synergistically enhances the antiemetic efficacy of 5‐HT3 receptor antagonists [11]. Although dexamethasone in combination with other antiemetic agents is recognized to be safe [12], [13], dexamethasone has been shown to cause moderate‐to‐severe adverse effects, such as insomnia, gastrointestinal symptoms, agitation, increased appetite, and weight gain, in patients receiving the steroid for delayed CINV [14]. Besides, dexamethasone could potentially deteriorate a patient's condition (e.g., diabetes, osteopenia/osteoporosis, and cataracts). Accordingly, it is of clinical importance to minimize the total dose of prophylactic dexamethasone in patients undergoing multiple cycles of emetogenic chemotherapy.
Aapro et al. [15] reported that a dexamethasone‐sparing regimen consisting of palonosetron plus single‐dose dexamethasone (d1 arm) would not be inferior to the same regimen followed by additional dexamethasone doses on days 2 to 3 after chemotherapy initiation (d3 arm) regarding complete response (CR; no emesis and no rescue medication) rate. Two subsequent randomized phase III studies were performed with a similar objective, which also showed the noninferiority of the dexamethasone d1 arm for the prevention of CINV in patients undergoing a broad range of MEC regimens [16], [17]. However, because of a wide range of noninferiority margin (15%) and limited number of subjects in these three studies, evidence for the noninferiority of the dexamethasone d1 arm in patients with risk factors for CINV is insufficient. In addition, it is unclear whether well‐known risk factors for CINV, such as female sex, younger age, and history of alcohol consumption [11], modify the treatment effect on the antiemetic outcome [15], [16], [17].
We therefore performed a meta‐analysis of individual patient data (IPD) gathered from eligible studies identified in a systematic review of randomized control trials that had assessed the effect of reduction in dexamethasone total dose on CINV onset in patients receiving a prophylactic regimen of palonosetron and dexamethasone. We aimed to comprehensively evaluate any difference in CR rate and other antiemetic outcomes between the d1 arm and d3 arm as well as to explore any interaction between antiemetic regimen and predefined risk factors for CINV.
Materials and Methods
Study Protocol
First, we formed a meta‐analysis research group and contacted the principal investigators of the three aforementioned studies [15], [16], [17]. A protocol stipulating the research objective, selection criteria, search method, and analysis method was drawn up, and a systematic review was performed. The institutional review board at the University of Tokyo approved the study protocol (No. 11539).
Eligible studies were defined as randomized control trials of anti‐CINV palonosetron and dexamethasone combination therapies in chemotherapy‐naïve patients aged 18 years or above with malignant tumors in which regimens with and without dexamethasone dose reduction were compared. Considering the approval date for palonosetron, studies performed in or after the year of 2000 were regarded as eligible for inclusion. Studies involving nonchemotherapy treatments, such as radiotherapy and immunotherapy, were excluded. The PubMed and Ovid MEDLINE databases were searched electronically. Each database was searched for studies between January 2000 and November 2016 using the search filters without language restrictions (supplemental online Table 1).
In the event that any reviews or meta‐analyses were included in the search results, further searches were made to determine whether additional studies were eligible while checking the cited reports. Specialists in the research group also checked for any relevant studies that were not targeted in the electronic search. For studies with multiple publications, or where there was an overlap in the patients studied, the original study in question was included. All retrieved studies were refined and assessed independently for eligibility with the title and/or abstracts and the full text publication by two reviewers (one of whom was the author). We contacted the principal authors of the reports for each eligible study and acquired IPD from each study. All data were centrally reanalyzed and checked for inconsistencies.
Collected Data
Sex, age, primary tumor, chemotherapy regimen, performance status (PS), and alcohol consumption habit were considered as patient demographic characteristics. The presence or absence of both CR (no emesis and no rescue medication) and complete control (CC; defined as no emesis, no rescue medication, and no more than mild nausea) during the acute (within 24 hours of chemotherapy initiation), delayed (days 2–5 after chemotherapy initiation), and overall (days 1–5) study periods were considered as outcome variables. Those outcomes were based on a patient diary used to document the date and time of emetic episodes and use of rescue medication, as well as severity of nausea with a four‐point categorical scale (none; mild; moderate; severe) or an alternative assessment tool.
Endpoints
The primary efficacy endpoint was CR during the overall study period. Secondary endpoints were CR during the acute and delayed periods as well as CC during all study periods. In addition to the whole‐population analyses, subgroup analyses were performed for the following risk factors: type of chemotherapy (AC‐based HEC vs. MEC), age (younger than 60 years vs. 60 years or older), sex (male vs. female), habitual alcohol consumption (yes vs. no), and Eastern Cooperative Oncology Group PS (0 vs. 1 or higher).
Statistical Analyses
Data on the designs and characteristics of enrolled patients in the eligible studies were pooled. Continuous variables were summarized by mean and SD, and categorical variables were summarized by the number and proportion of subjects. Common risk differences of the primary and secondary endpoints were estimated through a one‐stage fixed effect model [18]. Heterogeneity on the risk difference between trials was assessed through the interaction test between study and treatment in the fixed effect model. Heterogeneity was also quantitatively assessed using the I2 statistic. Subgroup analyses were then performed by predefined risk factors, examining the interaction between each factor and treatment effects.
Ioannidis et al. showed that the pooled risk difference of dexamethasone compared with placebo for CR was 16% (95% confidence interval [CI], 13%–19%) for the acute phase and 16% for the delayed phase [19]. In our analysis, the noninferiority margin for the primary endpoint was set to 8.0% (half of the 16% risk difference) of the between‐arm difference. Noninferiority was demonstrated when the lower boundary of the 95% CI of the risk difference was greater than the preset threshold (−8%). Heterogeneity was considered statistically significant in interaction testing when the p value was .10 or lower.
The analyses were performed with SAS statistical software (version 9.4; SAS Institute, Cary, NC). Forest plots were constructed with STATA statistical software (version 14.2; STATA Corp LP, College Station, TX).
Results
Listing of Eligible Studies
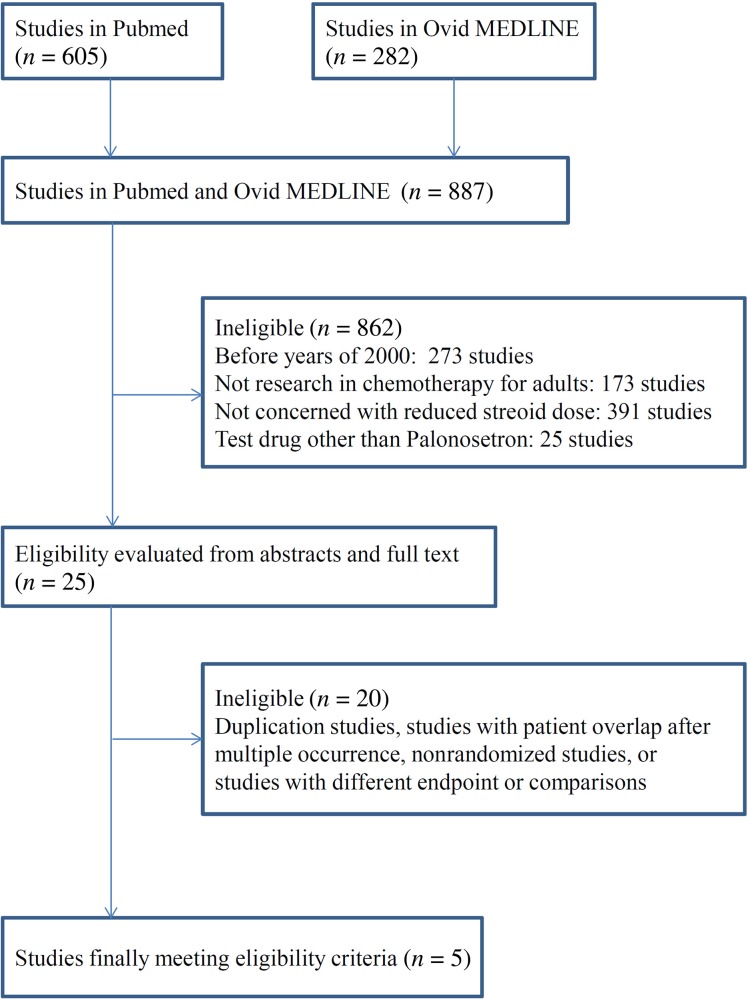
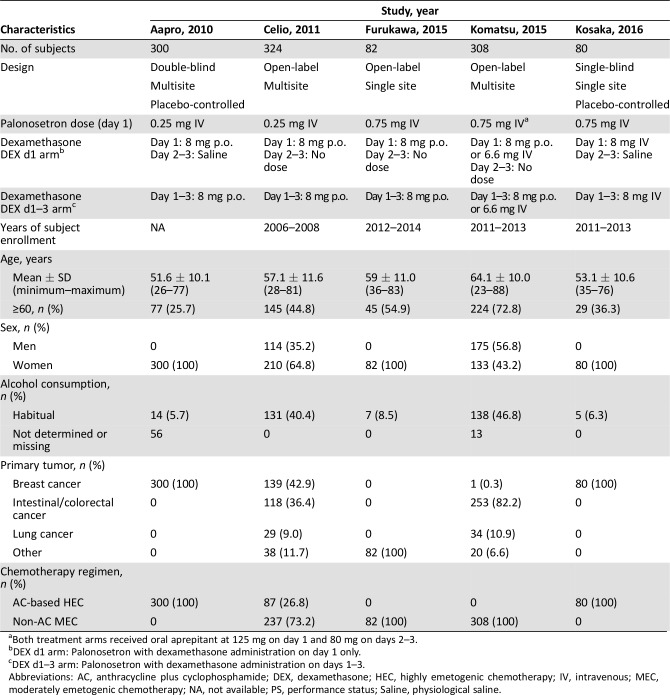
A total of five studies satisfied the eligibility criteria in the systematic review. All of these studies were phase II or III randomized controlled trials and had been published in peer‐reviewed journals. The PRISMA study selection diagram is shown in Figure 1. IPD were acquired from each of the five studies after contacting the principal investigator. An overview and characterization of each study along with patient characteristics is presented in Table 1. Twenty ineligible trials along with their reasons for exclusion are presented in supplemental online Table 2.
Figure 1.
PRISMA diagram
Table 1. Eligible study characteristics and patient demographics.
Both treatment arms received oral aprepitant at 125 mg on day 1 and 80 mg on days 2–3.
DEX d1 arm: Palonosetron with dexamethasone administration on day 1 only.
DEX d1–3 arm: Palonosetron with dexamethasone administration on days 1–3.
Abbreviations: AC, anthracycline plus cyclophosphamide; DEX, dexamethasone; HEC, highly emetogenic chemotherapy; IV, intravenous; MEC, moderately emetogenic chemotherapy; NA, not available; PS, performance status; Saline, physiological saline.
The eligible studies were as follows: a study of patients receiving AC‐based HEC performed by Aapro et al. (Aapro study) [15]; a study of patients treated with a broad range of MEC regimens, including AC‐based HEC, performed by Celio et al. (Celio study) [16]; a study of patients receiving only MEC regimens performed by Komatsu at el. (Komatsu study) [17]; a study of carboplatin‐containing MEC performed by Furukawa et al. (Furukawa study) [20]; and a study of patients treated with AC‐based HEC performed by Kosaka et al. (Kosaka study) [21]. These five studies yielded an analysis population of 1,194 subjects. The subjects in the Aapro, Furukawa, and Kosaka studies were women with either breast cancer or gynecologic cancer. The subjects in the other two studies were chemotherapy‐naïve patients with a broad range of solid tumors. The intravenous dose of palonosetron was 0.25 mg in the Aapro and Celio studies, whereas the approved dose of palonosetron 0.75 mg was used in the other three studies. Dexamethasone dosage and administration varied widely between the studies because of the several different settings of CINV; however, there were few major differences. No bias in any patient characteristics was noted between the treatment arms in each study.
Primary Endpoint (CR Rate) Evaluation
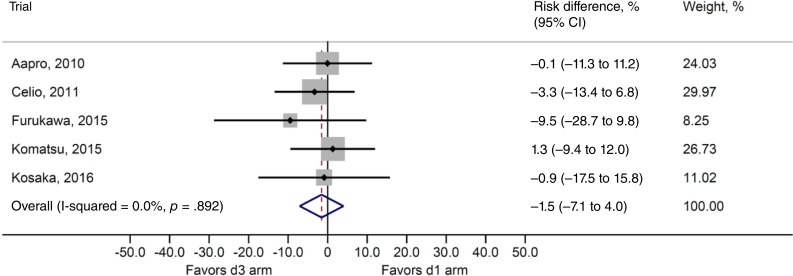
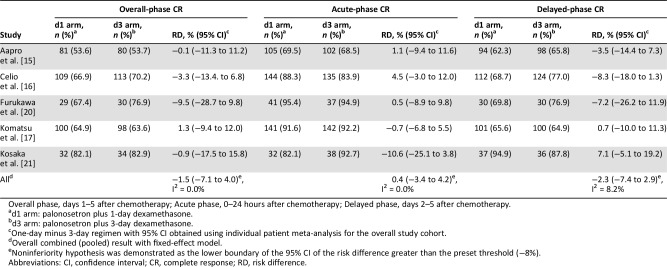
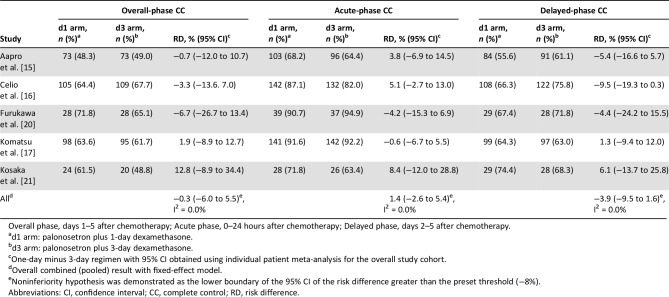
The risk difference between the two treatments for overall CR rate (primary efficacy endpoint) was −1.5% (95% CI, −7.1% to 4.0%; Fig. 2), and the noninferiority of the dexamethasone‐sparing regimen was demonstrated in this analysis. Heterogeneity testing yielded p = .892 and I2 = 0%, and interstudy heterogeneity could not be demonstrated. The risk differences for acute‐phase and delayed‐phase CR rates were 0.4% (95% CI, −3.4% to 4.2%) and −2.3% (95% CI, −7.4% to 2.9%), respectively, demonstrating noninferiority between treatments (Table 2). No statistically significant heterogeneity across the studies was noted among overall, acute‐phase, and delayed‐phase CR rates.
Figure 2.
Risk difference for complete response rate: Overall* and by study. d3 arm: Palonosetron plus 3‐day dexamethasone. d1 arm: Palonosetron plus 1‐day dexamethasone. *Overall combined (pooled) result with fixed‐effect model.
Abbreviation: CI, confidence interval.
Table 2. Risk differences for CR rates in each study period.
Overall phase, days 1–5 after chemotherapy; Acute phase, 0–24 hours after chemotherapy; Delayed phase, days 2–5 after chemotherapy.
d1 arm: palonosetron plus 1‐day dexamethasone.
d3 arm: palonosetron plus 3‐day dexamethasone.
One‐day minus 3‐day regimen with 95% CI obtained using individual patient meta‐analysis for the overall study cohort.
Overall combined (pooled) result with fixed‐effect model.
Noninferiority hypothesis was demonstrated as the lower boundary of the 95% CI of the risk difference greater than the preset threshold (−8%).
Abbreviations: CI, confidence interval; CR, complete response; RD, risk difference.
CC Rate Evaluation
In the Aapro study, daily nausea ratings were assessed by visual analog scale (VAS); 0 mm “no nausea” and 100 mm “as bad as it could be,” where complete control (CC) was defined as no emesis, no rescue medication, and no more than mild nausea (a VAS <25 mm). In the Furukawa study, CC was defined as no emetic episodes, no use of rescue medication, and no significant nausea (a score by Multinational Association of Supportive Care in Cancer Antiemesis Tool ≤3). In the other three studies, severity of nausea was recorded in a patient diary on a four‐point categorical Likert scale (0, none; 1, mild; 2, moderate; 3, severe). We thereby defined CC as no emesis, no rescue medication, and no more than mild nausea (no significant nausea in the Furukawa study) in the present study.
The between‐treatment risk difference for overall CC rate was −0.3% (95% CI, −6.0% to 5.5%), and the analysis also demonstrated the noninferiority of the dexamethasone‐sparing regimen for the secondary endpoint of CC (Table 3). Heterogeneity testing yielded results of p = .892 and I2 = 0.0%, and no interstudy heterogeneity could be shown. The risk differences for acute‐phase and delayed‐phase CC rates were 1.4% (95% CI, −2.6% to 5.4%) and − 3.9% (95% CI, −9.5% to 1.6%), respectively, showing no differences between treatments. No heterogeneity was found for all endpoints.
Table 3. Risk differences for CC rates in each study period.
Overall phase, days 1–5 after chemotherapy; Acute phase, 0–24 hours after chemotherapy; Delayed phase, days 2–5 after chemotherapy.
d1 arm: palonosetron plus 1‐day dexamethasone.
d3 arm: palonosetron plus 3‐day dexamethasone.
One‐day minus 3‐day regimen with 95% CI obtained using individual patient meta‐analysis for the overall study cohort.
Overall combined (pooled) result with fixed‐effect model.
Noninferiority hypothesis was demonstrated as the lower boundary of the 95% CI of the risk difference greater than the preset threshold (−8%).
Abbreviations: CI, confidence interval; CC, complete control; RD, risk difference.
Subgroup Analyses
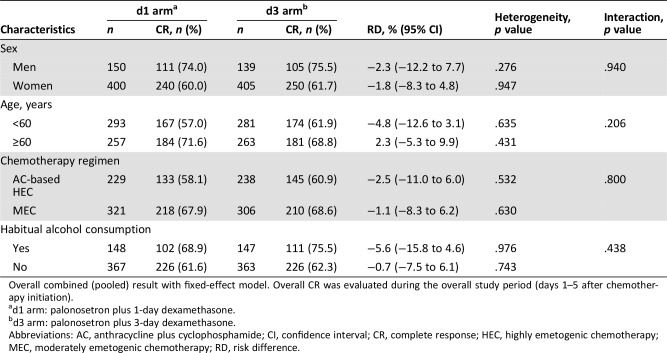
Subgroup analysis was not possible for PS, because this score was zero in nearly all subjects. Subgroup analyses were performed in patients categorized by sex, age (<60 and ≥60 years), type of chemotherapy (AC‐based HEC and MEC), and alcohol consumption habit. No statistically significant interactions between risk factors and antiemetic treatment were observed for the between‐treatment risk difference in CR rates during the overall study period (Table 4). No significant interactions between subgroups and treatment were observed for both acute‐phase and delayed‐phase CR rates as well as CC during all study periods (data not shown).
Table 4. Patient characteristic subgroup analysis and interaction for overall CR.
Overall combined (pooled) result with fixed‐effect model. Overall CR was evaluated during the overall study period (days 1–5 after chemotherapy initiation).
d1 arm: palonosetron plus 1‐day dexamethasone.
d3 arm: palonosetron plus 3‐day dexamethasone.
Abbreviations: AC, anthracycline plus cyclophosphamide; CI, confidence interval; CR, complete response; HEC, highly emetogenic chemotherapy; MEC, moderately emetogenic chemotherapy; RD, risk difference.
Discussion
The present IPD‐based meta‐analysis demonstrated the noninferiority of the dexamethasone‐sparing regimen for both CR and CC with a margin of 8.0% for the prevention of CINV in patients undergoing either AC‐based HEC or MEC regimens. We also explored the impact of predefined risk factors for CINV on CR rates between the two antiemetic treatments. The subgroup analyses showed no major difference in antiemetic effect between the regimens during the overall study period. The results of this study provide strong evidence for a prophylactic regimen of palonosetron and 1‐day dexamethasone; these results further support that the use of dexamethasone may be decreased on the days after chemotherapy administration without the loss of antiemetic efficacy, at least when longer‐acting 5‐HT3 receptor antagonists are used.
Although previous Multinational Association of Supportive Care in Cancer (MASCC)/European Society for Medical Oncology (ESMO) guidelines (the 2010 MASCC guidelines) preferred palonosetron plus dexamethasone for the prophylaxis of acute nausea and vomiting, the 2016 MASCC guidelines explored a lack of evidence for the use of dexamethasone for days 2–3 routinely for prophylaxis for delayed CINV until their revision [7], [8]. To the best of our knowledge, there are no trials of the dexamethasone‐sparing strategy with older 5‐HT3 receptor antagonists in the setting of MEC. Ioannidis et al. [19] demonstrated the efficacy of concomitant dexamethasone treatment in a meta‐analysis of 32 studies, in which 23 of 32 studies included a 5‐HT3 receptor antagonist as an antiemetic. However, it should be noted that the 5‐HT3 receptor antagonists included were of an older generation than palonosetron because their meta‐analysis was conducted in 2000, before palonosetron was approved in 2003. Celio et al. [10] conducted a narrative review of the evidence about the efficacy and safety of palonosetron, but they did not examine the necessity for concomitant dexamethasone. The present study is the first to assess the clinical relevance of the dexamethasone‐sparing strategy in a systematic review with IPD meta‐analysis.
Subgroup analyses revealed no significant interaction between treatment and sex, age category, alcohol consumption habit, or chemotherapy regimen (AC‐based HEC vs. MEC). However, the study by Komatsu et al. found disparity between older and younger age subgroups in risk difference for CR rate [17]. Therefore, dexamethasone dose could be reduced irrespective of whether the patient is younger or older than 60 years. In addition to the risk factors discussed above (emetogenic potential of chemotherapy, sex, age, and alcohol consumption habit), other known risk factors should be noted, including rapid 5‐HT3 receptor antagonist metabolism, 5‐HT3 receptor polymorphism, history of motion sickness, anxiety, and fatigue [10]. We acknowledge that these other risk factors were not taken into consideration in the present study.
Effective approaches to reduce patients’ exposure to steroids remain among the hot topics in clinical research on CINV. Recent evidences from prospective studies highlighted a potentially detrimental impact of short‐term prophylactic dexamethasone in patients undergoing consecutive courses of emetogenic chemotherapy [22], [23]. Aapro et al. compared the efficacy of palonosetron when administered alone or in combination with 1‐day dexamethasone, in post hoc analyses of two studies on CINV in patients undergoing MEC; they reported that the proportion of nausea was lower in patients treated with the 1‐day dexamethasone regimen over a 5‐day period [24], [25]. However, it is difficult to clearly interpret these results because it involved a straightforward comparison of two studies without adopting appropriate endpoints, such as CR and CC, which were assessed in the present study. It is worth noting that the efficacy of a dexamethasone‐free prophylaxis with palonosetron for patients undergoing MEC regimens will be assessed in a noninferiority trial that is in progress in Japan [26].
The present study has some limitations. Current guidelines recommend a three‐drug prophylaxis consisting of a 5‐HT3 receptor antagonist, dexamethasone, and a neurokinin‐1 receptor antagonist (NK‐1RA) for prevention of CINV caused by AC‐based chemotherapy [8], [9], [27]; however, the combination of AC, currently classified as HEC [7], [8], [9], [27], was included in the MEC category in the period when the five studies were conducted. In that period, the relevant guidelines had been updated or revised multiple times [5], [6], [7], [8]. In the present study, only the Kosaka study evaluated the efficacy of the dexamethasone‐sparing strategy in patients with breast cancer treated with AC and receiving three‐drug prophylaxis for CINV [21]. Nevertheless, the study findings are consistent with the results of a recently published phase III trial that demonstrated a noninferiority difference between the three‐drug, dexamethasone‐sparing regimen and the control arm for the prevention of CINV caused by HEC regimens [28]. Approximately 400 patients were included in the study, but the majority (77%) of them were patients with breast cancer treated with AC. Also, international antiemetic guidelines still recommend a two‐drug prophylaxis consisting of palonosetron plus dexamethasone in patients with breast cancer treated with AC when an NK‐1RA is not available [8]. Our results support that the dexamethasone‐sparing regimen should represent the first‐choice prophylaxis for patients who do not receive NK‐1RA. Moreover, we could not carry out any clinically meaningful analysis of tolerability for the dexamethasone‐sparing regimen as the eligible studies provided only sparse information to reliably assess any between‐arm differences in the incidence of dexamethasone‐related side effects.
Conclusion
The present IPD meta‐analysis shows that the dexamethasone‐sparing strategy does not result in any significant loss in overall antiemetic control and indicates that multiple‐day dexamethasone dosing is an unnecessary component of antiemetic prophylaxis with palonosetron before the start of chemotherapy in patients undergoing single‐day MEC or AC‐based regimens. Our findings thus help physicians minimize use of the steroid and further reduce the burden of dexamethasone‐related side effects in patients undergoing multiple consecutive courses of emetogenic chemotherapy.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We thank Professor Yutaka Matsuyama of the Department of Biostatistics, School of Public Health, Graduate School of Medicine for his guidance in the preparation of this article as well as Kosuke Kashiwabara, Tomohiro Shinozaki, and Takeuchi Yoshinori, also of the Department of Biostatistics, for their support. We thank all the participants of these studies. We also thank Editage (www.editage.jp) for English language editing. This research was supported by AMED under Grant Number 19lk0201060h0004.
Contributed equally.
Author Contributions
Conception/design: Yuki Okada, Koji Oba, Naoto Furukawa, Yoshimasa Kosaka, Kenji Okita, Satoshi Yuki, Yoshito Komatsu, Luigi Celio, Matti Aapro
Provision of study material or patients: Yuki Okada, Koji Oba, Naoto Furukawa, Yoshimasa Kosaka, Kenji Okita, Satoshi Yuki, Yoshito Komatsu, Luigi Celio, Matti Aapro
Collection and/or assembly of data: Yuki Okada, Koji Oba, Naoto Furukawa, Yoshimasa Kosaka, Kenji Okita, Satoshi Yuki, Yoshito Komatsu, Luigi Celio, Matti Aapro
Data analysis and interpretation: Yuki Okada, Koji Oba, Naoto Furukawa, Yoshimasa Kosaka, Kenji Okita, Satoshi Yuki, Yoshito Komatsu, Luigi Celio, Matti Aapro
Manuscript writing: Yuki Okada, Koji Oba, Naoto Furukawa, Yoshimasa Kosaka, Kenji Okita, Satoshi Yuki, Yoshito Komatsu, Luigi Celio, Matti Aapro
Final approval of manuscript: Yuki Okada, Koji Oba, Naoto Furukawa, Yoshimasa Kosaka, Kenji Okita, Satoshi Yuki, Yoshito Komatsu, Luigi Celio, Matti Aapro
Disclosures
Koji Oba: Eisai, Chugai, Daiichi Sankyo, Asahi Kasei (H), Takeda Pharmaceutical Company, Ono Pharmaceutical, Co., Ltd. (C/A); Yoshito Komatsu: Taiho, Daiichi Sankyo, Chugai, Merck Sharp & Dohme, Eli Lilly and Company, Sanofi, Dainihon, Merck, Pfizer, Shionogi, Bayer (RF), Taiho, Daiichi Sankyo, Chugai, Merck Sharp & Dohme, Eli Lilly and Company, Sanofi, Dainihon, Merck, Yakult, Bayer, Pfizer (H); Matti Aapro: Helsinn (C/A, RF), Helsinn, Merck, Tesaro (SAB), Merck, Tesaro, Taiho, Mundipharma (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Aapro MS. Palonosetron as an anti‐emetic and anti‐nausea agent in oncology. Ther Clin Risk Manag 2007;3:1009–1020. [PMC free article] [PubMed] [Google Scholar]
- 2.Lindley CM, Hirsch JD, O'Neill CV et al. Quality of life consequences of chemotherapy‐induced emesis. Qual Life Res 1992;1:331–340. [DOI] [PubMed] [Google Scholar]
- 3.Morita S, Kobayashi K, Eguchi K et al. Influence of clinical parameters on quality of life during chemotherapy in patients with advanced non‐small cell lung cancer: Application of a general linear model. Jpn J Clin Oncol 2003;33:470–476. [DOI] [PubMed] [Google Scholar]
- 4.NCCN antiemesis practice guidelines. Oncology (Williston Park) 1997;11:57–89. [PubMed] [Google Scholar]
- 5.Berger MJ, Ettinger DS, Aston J et al. NCCN guidelines insights: Antiemesis, version 2.2017. J Natl Compr Cancer Netw 2017;15:883–893. [DOI] [PubMed] [Google Scholar]
- 6.Hesketh PJ, Bohlke K, Lyman GH et al. Antiemetics: American Society of Clinical Oncology focused guideline update. J Clin Oncol 2016;34:381–386. [DOI] [PubMed] [Google Scholar]
- 7.Roila F, Herrstedt J, Aapro M et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting: Results of the Perugia consensus conference. Ann Oncol 2010;21(suppl 5):v232–v243. [DOI] [PubMed] [Google Scholar]
- 8.Roila F, Molassiotis A, Herrstedt J et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27:v119–v133. [DOI] [PubMed] [Google Scholar]
- 9.Basch E, Prestrud AA, Hesketh PJ et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2011;29:4189–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celio L, Niger M, Ricchini F et al. Palonosetron in the prevention of chemotherapy‐induced nausea and vomiting: An evidence‐based review of safety, efficacy, and place in therapy. Core Evid 2015;10:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy‐induced nausea and vomiting. N Engl J Med 2016;374:1356–1367. [DOI] [PubMed] [Google Scholar]
- 12.Grunberg SM. Antiemetic activity of corticosteroids in patients receiving cancer chemotherapy: Dosing, efficacy, and tolerability analysis. Ann Oncol 2007;18:233–240. [DOI] [PubMed] [Google Scholar]
- 13.Jordan K, Kasper C, Schmoll HJ. Chemotherapy‐induced nausea and vomiting: Current and new standards in the antiemetic prophylaxis and treatment. Eur J Cancer 2005;41:199–205. [DOI] [PubMed] [Google Scholar]
- 14.Vardy J, Chiew KS, Galica J et al. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer 2006;94:1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aapro M, Fabi A, Nolè F et al. Double‐blind, randomised, controlled study of the efficacy and tolerability of palonosetron plus dexamethasone for 1 day with or without dexamethasone on days 2 and 3 in the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy. Ann Oncol 2010;21:1083–1088. [DOI] [PubMed] [Google Scholar]
- 16.Celio L, Frustaci S, Denaro A et al. Palonosetron in combination with 1‐day versus 3‐day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: A randomized, multicenter, phase III trial. Support Care Cancer 2011;19:1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komatsu Y, Okita K, Yuki S et al. Open‐label, randomized, comparative, phase III study on effects of reducing steroid use in combination with Palonosetron. Cancer Sci 2015;106:891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burke DL, Ensor J, Riley RD. Meta‐analysis using individual participant data: One‐stage and two‐stage approaches, and why they may differ. Stat Med 2017;36:855–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ioannidis JP, Hesketh PJ, Lau J. Contribution of dexamethasone to control of chemotherapy‐induced nausea and vomiting: A meta‐analysis of randomized evidence. J Clin Oncol 2000;18:3409–3422. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa N, Kanayama S, Tanase Y et al. Palonosetron in combination with 1‐day versus 3‐day dexamethasone to prevent nausea and vomiting in patients receiving paclitaxel and carboplatin. Support Care Cancer 2015;23:3317–3322. [DOI] [PubMed] [Google Scholar]
- 21.Kosaka Y, Tanino H, Sengoku N et al. Phase II randomized, controlled trial of 1 day versus 3 days of dexamethasone combined with palonosetron and aprepitant to prevent nausea and vomiting in Japanese breast cancer patients receiving anthracycline‐based chemotherapy. Support Care Cancer 2016;24:1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han HS, Park JC, Park SY et al. A prospective multicenter study evaluating secondary adrenal suppression after antiemetic dexamethasone therapy in cancer patients receiving chemotherapy: A Korean South West Oncology Group study. The Oncologist 2015;20:1432–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura M, Ishiguro A, Muranaka T et al. A prospective observational study on effect of short‐term periodic steroid premedication on bone metabolism in gastrointestinal cancer (ESPRESSO‐01). The Oncologist 2017;22:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajdenberg J, Grote T, Yee L et al. Infusion of palonosetron plus dexamethasone for the prevention of chemotherapy‐induced nausea and vomiting. J Support Oncol 2006;4:467–471. [PubMed] [Google Scholar]
- 25.Decker GM, DeMeyer ES, Kisko DL. Measuring the maintenance of daily life activities using the functional living index‐emesis (FLIE) in patients receiving moderately emetogenic chemotherapy. J Support Oncol 2006;4:35–41. [PubMed] [Google Scholar]
- 26.Suzuki K, Yamanaka T, Hashimoto H et al. Randomized, double‐blind, phase III trial of palonosetron versus granisetron in the triplet regimen for preventing chemotherapy‐induced nausea and vomiting after highly emetogenic chemotherapy: TRIPLE study. Ann Oncol 2016;27:1601–1606. [DOI] [PubMed] [Google Scholar]
- 27.Hesketh PJ, Kris MG, Basch E et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017;35:3240–3261. [DOI] [PubMed] [Google Scholar]
- 28.Ito Y, Tsuda T, Minatogawa H et al. Placebo‐controlled, double‐blinded phase III study comparing dexamethasone on day 1 with dexamethasone on days 1 to 3 with combined neurokinin‐1 receptor antagonist and palonosetron in high‐emetogenic chemotherapy. J Clin Oncol 2018;36:1000–1006. [DOI] [PubMed] [Google Scholar]