The roles of radiotherapy and EGFR tyrosine kinase inhibitors are still under debate with regard to the treatment of patients with advanced EGFR mutant non‐small cell lung cancer and brain metastasis. This article evaluates the effects of these treatment options.
Keywords: Non‐small cell lung cancer, Brain metastases, Epidermal growth factor receptor, Cranial irradiation, Targeted therapies
Abstract
Background.
Immediate whole brain radiation (WBRT) has been the standard for patients with lung cancer with brain metastases. The study aims to evaluate the effect of immediate cranial irradiation in patients with epidermal growth factor receptor (EGFR) mutant lung cancer in the era of a new generation of EGFR inhibitors.
Materials and Methods.
Medical records of 198 patients with EGFR mutant non‐small cell lung cancer and brain metastases at initial metastatic diagnosis were reviewed. Patients were categorized into four groups: immediate WBRT, immediate cranial stereotactic radiosurgery (SRS), delayed radiation upon progression of cranial lesions (DRT), and never cranial irradiation (NRT). Overall survival (OS) and progression‐free survival related to EGFR inhibitors were analyzed.
Results.
The SRS group had the fewest brain metastases and fewest extracranial lesions, and the DRT and NRT groups had the smallest brain metastases. Median survival were 18.5, 55.7, 21.1, and 18.2 months for the WBRT, SRS, DRT, and NRT groups, respectively. Patients who had received EGFR T790M inhibitors survived longer (41.1 vs. 19.8 months). In multivariate analysis, the OS of patients in the SRS group was longer than that in the NRT group (adjusted hazard ratio [aHR]: 0.315). Patients who had fewer extracranial lesions and who had received EGFR T790M inhibitor treatments also survived longer (aHR: 0.442 and 0.357, respectively).
Conclusion.
Immediate stereotactic radiosurgery but not whole brain radiation was associated with longer survival. Because of patient heterogeneity and the introduction of EGFR T790M inhibitors, the timing and modality of cranial irradiation should be determined individually, and cranial irradiation may be omitted for selected patients.
Implications for Practice.
Immediate whole brain radiation has been the standard for patients with lung cancer with brain metastases. In this study, it was observed that, for patients with epidermal growth factor receptor (EGFR) mutant advanced lung cancer who had brain metastases, there was no difference in survival between patients who never received cranial irradiation and those who received whole brain radiation immediately. Patients who received immediate stereotactic radiosurgery or who had ever received EGFR T790M inhibitors survived longer. Patients who received immediate stereotactic radiosurgery have fewer brain metastases. These findings suggest that the timing and modality of cranial irradiation should be determined individually, and cranial irradiation may be omitted in selected patients.
摘要
背景。及时全脑放疗 (WBRT) 已成为发生脑转移的肺癌患者的标准疗法。在新一代表皮生长因子受体(EGFR)抑制剂问世的时代,本研究旨在评估及时全脑照射对EGFR突变型肺癌患者的疗效。
材料和方法。对 198 例EGFR突变型非小细胞肺癌和脑转移患者最初确诊发生转移的病历进行了回顾性分析。将患者分为四组:及时WBRT组、及时全脑立体定向放射外科治疗 (SRS) 组、对颅脑病灶进展实施延迟放射治疗 (DRT) 组及不实施全脑照射 (NRT) 组。我们对总生存期 (OS) 和无进展生存期与EGFR抑制剂的相关性进行了分析。
结果。SRS 组的脑转移和颅外病灶病例最少,DRT 和 NRT 组的脑转移病灶最小。WBRT、SRS、DRT 和 NRT 组的中位生存期分别为 18.5、55.7、21.1 及 18.2 个月。接受 EGFR T790M 抑制剂治疗的患者存活期较长(41.1 个月与 19.8 个月)。在多变量分析中,SRS 组患者的OS要长于 NRT 组 [校正风险比(aHR):0.315]。颅外病灶较少和接受 EGFR T790M 抑制剂治疗的患者生存期也较长(aHR:分别为 0.442 和 0.357)。
结论。及时立体定向放射外科治疗可延长生存期,而全脑放疗无法实现这一点。由于患者的异质性和 EGFR T790M 抑制剂的问世,全脑照射的时机和方式应因人而异,对于经选患者可无需接受全脑照射。
实践意义:及时全脑放疗已成为发生脑转移的肺癌患者的标准疗法。在本研究中发现,在发生脑转移的表皮生长因子 (EGFR) 突变型晚期肺癌患者中,从未接受全脑照射者与及时接受全脑放疗者在生存期方面无差异。接受及时立体定向放射外科治疗或曾接受 EGFR T790M 抑制剂治疗的患者生存期较长。接受及时立体定向放射外科治疗的患者脑转移的病灶较少。这些结果表明,全脑照射的时机和方式应因人而异,对于经选患者可无需接受全脑照射。
Introduction
Non‐small cell lung cancer (NSCLC), especially adenocarcinoma, is characterized by frequent mutation of oncogenic driver such as the epidermal growth factor receptor (EGFR) gene. Mutation of EGFR was observed in up to 50% of lung adenocarcinomas in East Asia [1]. EGFR tyrosine kinase inhibitors (TKIs) are widely used as first‐line therapies for patients with advanced EGFR mutant NSCLC [2], [3]. The EGFR T790M inhibitor osimertinib is superior to standard EGFR‐TKIs including gefitinib, erlotinib, or afatinib as first‐line therapy for patients with advanced EGFR mutant NSCLC in terms of progression‐free survival (PFS) [4].
It was reported that around 40% of patients with lung cancer developed brain metastases at some point during their life. The survival of patients with lung cancer with brain metastases is shorter than that of those without brain metastases. Cranial irradiations including whole brain radiation and stereotactic surgery are among standard therapies in patients with brain metastases [5]. Systemic therapies with EGFR‐TKIs are safe and effective for the treatment of patients with EGFR mutant NSCLC with brain metastases. In a prospective observational study of patients with EGFR mutant lung cancer with brain metastases, gefitinib or erlotinib achieved a response rate of 83% and a median PFS of 6.6 months [6]. In a phase II trial of gefitinib in patients with EGFR mutant lung adenocarcinoma who had brain metastases, gefitinib alone without cranial irradiation resulted in an 87.8% response rate of brain metastases response, and the median PFS was 14.5 months [7].
For patients with advanced EGFR mutant NSCLC who have brain metastases, it is still conflicting whether cranial irradiation should be administrated immediately on diagnosis of brain metastases, in addition to the use of EGFR‐TKIs. Magnuson et al. demonstrated that the survival is longer for patients who received upfront cranial irradiation, especially stereotactic radiosurgery, than for patients who received deferral radiotherapy [8]. In contrast, Jiang et al. suggested worse survival in patients who received whole brain radiation plus EGFR inhibitors compared with those who received EGFR inhibitors alone [9]. With the progress of radiotherapy, such as stereotactic surgery, and the new generation of EGFR inhibitors such as osimertinib [10], [11], the roles of radiotherapy and EGFR‐TKI in patients with EGFR mutant NSCLC who have brain metastases are still under debate. This is a retrospective study to further define the role of cranial irradiation in the era of stereotactic surgery and EGFR‐T790M specific inhibitors.
Materials and Methods
Patients
We retrospectively reviewed the medical records of patients with NSCLC according to the following criteria: patients who had tumor tissue tested for the EGFR mutation from March 2012 to January 2016 at National Taiwan University Hospital (NTUH), patients who received anticancer therapies at NTUH, patients who had brain metastases and were diagnosed with advanced disease, and patients whose tumors carried the EGFR exon 19 deletion or the L858R mutation. Patients were excluded if their brain lesions were totally resected by surgical interventions. The study was approved by the Research Ethics Committee of NTUH.
Parameters
We recorded demographic data, tumor‐related features, and major anticancer treatments of the patients. Tumor‐related features included histological type, stage of tumor at diagnosis, and EGFR mutational status. Anticancer treatments included EGFR‐TKIs, chemotherapies, and radiotherapies such as whole brain radiation and stereotactic surgery. Whole brain radiation was generally performed at a dose of 30 Gy in 10 fractions. Image studies for evaluation of intracranial and/or extracranial lesions were generally conducted every 3 months. The data cutoff date for the analysis was October, 31, 2017.
Statistical Analysis
Comparisons of patient characteristics between groups were conducted using the chi‐squared test. Differences with respect to age between the groups were analyzed using Student's t test. Overall survival (OS) was defined as the period from diagnosis of brain metastasis to death or final follow‐up. PFS was defined as the period from diagnosis of brain metastasis to the first to occur of either systemic disease progression or death. Treatment response was evaluated according to the RECIST criteria version 1.1. Survival was estimated using the Kaplan‐Meier method. Time‐to‐intracranial progression was defined as the period from the diagnosis of brain metastasis to the development of intracranial progression, and the development of extracranial progression or death was regarded as censored data. Differences with respect to survival between groups were analyzed using the log‐rank test. Univariate and multivariate analyses were performed using the Cox proportional hazards regression model. Variables that were significantly associated with survival or PFS in the univariate analysis were included in the multivariate analysis. Age was a continuous variable, and other variables were nominal variables. All tests were two‐tailed, and a p value less than .05 was regarded as statistically significant. All analyses were conducted using SPSS for Windows software, version 19.0 (SPSS, Inc., Chicago, IL).
Results
Patient Characteristics
In total, 679 patients with lung cancer tumors that carried the EGFR exon 19 deletion or the L858R mutation were identified. A total of 198 patients had brain metastases upon diagnosis of advanced disease. Patients were categorized into four groups according to the timing and method of the cranial therapies they received upon diagnosis of advanced disease: immediate whole brain radiation (WBRT group), immediate cranial stereotactic radiosurgery (SRS group), delayed radiation upon progression of cranial lesions (DRT group), and never cranial irradiation (NRT group). Table 1 presents the baseline characteristics of the 198 patients who received a diagnosis of advanced disease. Of the patients, 127 were women. Of the tumors, 190 were adenocarcinomas, and 102 and 96 tumors carried the EGFR L858R mutation and the exon 19 deletion, respectively. Forty‐nine (24.7%) patients had stage I–III disease on diagnosis and had received surgical resection of primary tumors or concurrent chemoradiations. Patients in the NRT group were older than those in the other three groups (p = .007 using the one‐way analysis of variance test). Relatively more patients in the SRS group than the other three groups were in the early stage of the disease at diagnosis (p = .007). No patients in the SRS group had more than four cranial lesions upon diagnosis of advanced disease (p < .001). Patients whose largest cranial tumors were smaller than or equal to 1 cm were more likely to be in the DRT or NRT groups (p < .001). Eleven (52.4%) patients in the SRS group did not have extracranial lesions on diagnosis of brain metastases, and the proportion was higher than those in the WBRT, DRT, and NRT groups (p < .001).
Table 1. Patient characteristics.
By chi‐squared test for all comparisons with the exception of age.
By t test.
Extracranial lesions: number of extracranial organs with cancers on diagnosis of brain metastases.
Abbreviations: DRT, delayed radiation upon progression of cranial lesions; NRT, never cranial irradiation; SRS, immediate cranial stereotactic radiosurgery; WBRT, immediate whole brain radiation.
Table 2 depicts the systemic anticancer therapies administered to patients during their lifespan. Of the patients, 195 (98.5%) had received at least one line of EGFR TKIs, and 101 (50.8%) had received at least one dose of chemotherapy. Three patients had not received any systemic anticancer therapies. Twenty‐seven patients had received at least one dose of EGFR T790M inhibitor during their lifetimes; 18 (66.7%) of them were enrolled in clinical trial of AZD9191, EGF816, CO‐1686, or HS‐10296, including 1 patient treated with AZD9291 as first‐line therapy. The SRS group had received more chemotherapy treatments than the NRT group (p = .017 using the chi‐squared test). All 27 patients in the DRT group received whole brain radiation as salvage radiotherapy to the cranial lesions
Table 2. Systemic anticancer therapies administered.
Abbreviations: DRT, delayed radiation upon progression of cranial lesions; EGFR, epidermal growth factor receptor; NRT, never cranial irradiation; SRS, immediate cranial stereotactic radiosurgery; TKI, tyrosine kinase inhibitor; WBRT, immediate whole brain radiation.
OS Analyses
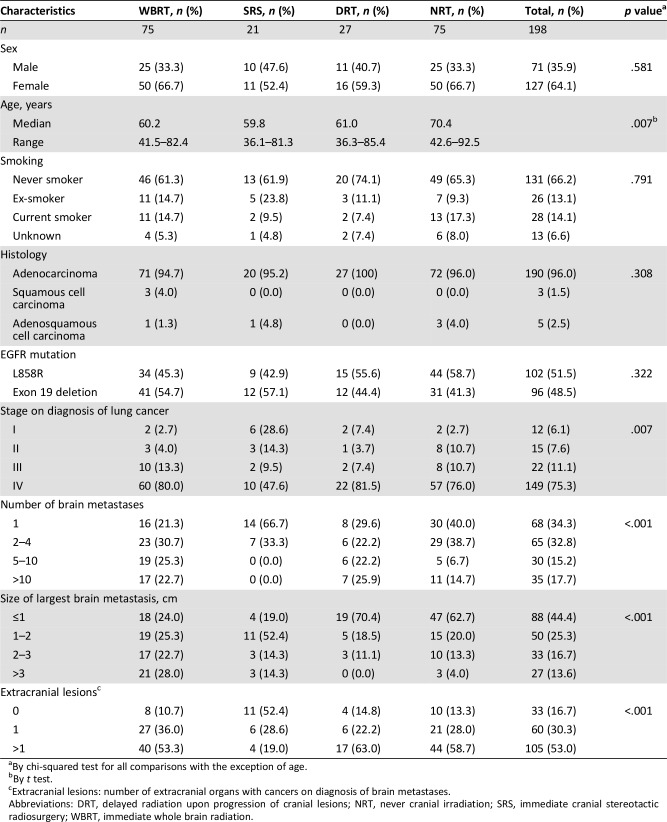
As of October 2017, the median survival time of the 198 patients was 20.6 months (95% confidence interval: 17.9–23.3 months) with a median follow‐up time of 19.0 months. The median survival times were 18.5 months, 55.7 months, 21.1 months, and 18.2 months for the WBRT, SRS, DRT, and NRT groups, respectively (p = .008 using the log‐rank test; Fig. 1A).
Figure 1.
Overall survival (OS). (A): Kaplan‐Meier curves of OS of patients in the SRS group (black solid line), WBRT group (black dashed line), DRT group (red solid line), and the NRT group (red dashed line). (B): Kaplan‐Meier curves of OS of patients who, at initial metastatic diagnosis, had 0 (black solid line), 1 (black dashed line), and more than 1 (red solid line) extracranial organs involved by cancers. Vertical lines indicate censored data.
Abbreviations: DRT, delayed radiation upon progression of cranial lesions; NRT, never cranial irradiation; SRS, immediate cranial stereotactic radiosurgery; WBRT, immediate whole brain radiation.
The survival times for patients with the EGFR exon 19 deletion and the L858R mutation were 20.6 and 20.6 months, respectively (p = .902). The survival times for patients treated with gefitinib, erlotinib, or afatinib as the first EGFR‐TKI were 20.5, 23.1, and 20.2 months, respectively (p = .878). We evaluated whether extracranial lesions may influence survival, and we observed difference in survival among patients who did not have extracranial lesion, who had one organ involved by cancers, and who had more than one organ involved by cancers (55.7, 28.4, and 15.3 months, respectively, p < .001; Fig. 1B). The median survival time for the 27 patients who had received EGFR T790M inhibitors was 41.1 months and was 19.8 months for the 171 patients who never had (p < .001).
PFS Associated with EGFR Inhibitors
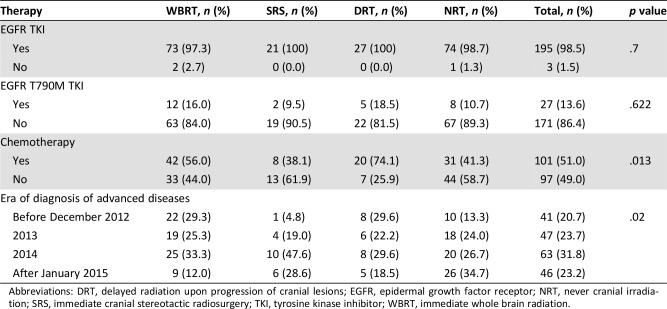
The median PFS to first‐line EGFR TKI of the 195 patients was 8.2 months. The median PFS were 6.9 months, 14.0 months, 7.9 months, and 8.5 months for the WBRT group, SRS group, DRT group, and NRT group, respectively (p = .001 by log‐rank test; Fig. 2A).
Figure 2.
Progression‐free survival (PFS). (A): Kaplan‐Meier curves of PFS related to epidermal growth factor receptor tyrosine kinase inhibitor of patients in the SRS group (black solid line), WBRT group (black dashed line), DRT group (red solid line), and the NRT group (red dashed line). (B): Time to intracranial progression of patients who received immediate cranial irradiation (SRS group and WBRT group, black dashed line) and patients who did not (DRT and NRT group, black solid line). Vertical lines indicate censored data.
Abbreviations: DRT, delayed radiation upon progression of cranial lesions; NRT, never cranial irradiation; SRS, immediate cranial stereotactic radiosurgery; WBRT, immediate whole brain radiation.
The median PFS were 7.9 and 8.5 months for patients whose tumors carried EGFR exon 19 deletion and L858R mutation, respectively (p = .844). The median PFS to gefitinib, erlotinib, and afatinib were 8.2, 8.2, and 8.2 months, respectively (p = .996). The median PFS were 13.4, 9.4, and 6.8 months for patients who did not have extracranial lesion, who had one organ involved by cancers, and who had more than one organ involved by cancers, respectively (p < .001).
On data cutoff, 147 patients had progression to EGFR inhibitors; 62 patients had intracranial progressions with or without extracranial progressions, and 85 patients had extracranial progressions only. Of 96 patients who received immediate cranial irradiation (the SRS and the WBRT groups), 18 had intracranial progression, whereas 44 out of 99 patients who did not received immediate cranial irradiation (the DRT and the NRT groups) had intracranial progressions. We evaluated whether immediate cranial irradiation may influence intracranial progression upon EGFR inhibitors. We observed significantly longer time‐to‐intracranial progression in patients who received immediate cranial irradiation (the SRS and the WBRT groups) than in those who did not (the DRT and the NRT groups). The median time‐to‐intracranial progression were 25.9 and 11.7 months, respectively (p < .001; Fig. 2B).
Univariate and Multivariate Analysis
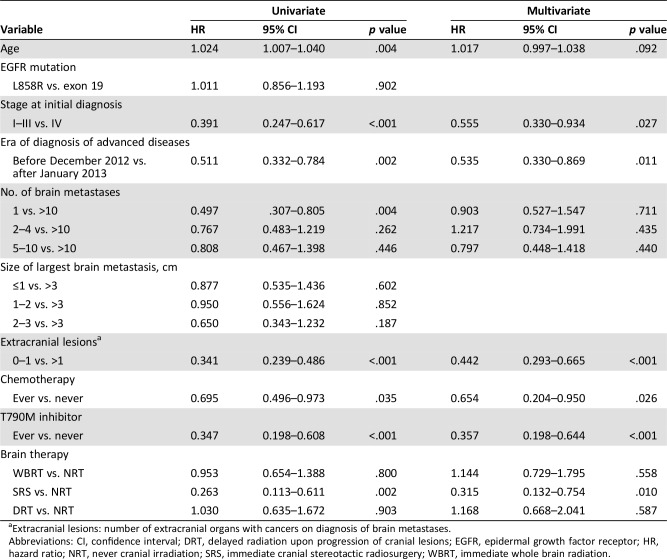
Univariate and multivariate analyses of survival are presented in Table 3. The results of the univariate analysis indicated that patients in the SRS group survived longer than those in the NRT group (hazard ratio [HR]: 0.263, p = .002). Other variables related to longer survival times included younger age at the time of diagnosis of advanced disease, disease at stage I–III at initial diagnosis, solitary brain metastasis upon diagnosis of advanced disease, absence or solitary extracranial organ involved by cancer, exposure to chemotherapies, and exposure to EGFR T790M inhibitors. The results of the multivariate analyses indicated that patients in the SRS group survived significantly longer than those in the NRT group (HR: 0.315, p = .010). Age at diagnosis of advanced disease as well as the number of brain metastases at diagnosis of advanced disease were not independently associated with survival. Fewer extracranial organ involved by cancer (HR: 0.442, p < .001) and the exposure to EGFR T790M inhibitors (HR: 0.357, p < .001) were strongly associated with longer survival.
Table 3. Univariate and multivariate analyses of survival.
Extracranial lesions: number of extracranial organs with cancers on diagnosis of brain metastases.
Abbreviations: CI, confidence interval; DRT, delayed radiation upon progression of cranial lesions; EGFR, epidermal growth factor receptor; HR, hazard ratio; NRT, never cranial irradiation; SRS, immediate cranial stereotactic radiosurgery; WBRT, immediate whole brain radiation.
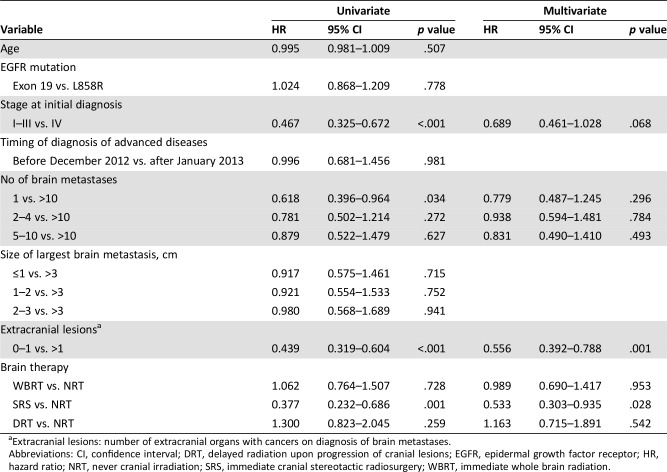
With respect to PFS and first‐line treatment with EGFR‐TKIs, patients in the SRS group had a longer PFS than those in the NRT group according to the results of the univariate analysis (HR: 0.377, p = .001; Table 4). An early disease stage at the initial diagnosis and solitary brain metastasis at the time of diagnosis of advanced disease were associated with longer PFS in the univariate analyses. In the multivariate analyses, belonging to the SRS group, having an early stage of disease at the initial diagnosis, and fewer extracranial organs involved by cancer were independently associated with longer PFS. Age at the time of diagnosis of advanced disease, the EGFR with the L858R mutation or the exon 19 deletion, and the size of the largest brain metastases were unrelated to PFS in both the univariate and multivariate analyses.
Table 4. Univariate and multivariate analyses of progression‐free survival times related to tyrosine kinase inhibitors.
Extracranial lesions: number of extracranial organs with cancers on diagnosis of brain metastases.
Abbreviations: CI, confidence interval; DRT, delayed radiation upon progression of cranial lesions; EGFR, epidermal growth factor receptor; HR, hazard ratio; NRT, never cranial irradiation; SRS, immediate cranial stereotactic radiosurgery; WBRT, immediate whole brain radiation.
Discussion
In the current study, we determined that for patients with EGFR mutant NSCLC and brain metastases upon diagnosis of advanced disease, those who received stereotactic radiosurgery upon diagnosis of advanced disease (the SRS group) survived significantly longer than those who did not receive therapy over cranial lesions (the NRT group), and the PFS time related to EGFR‐TKI treatment was also longer in the SRS group than in the NRT group. No difference in survival was observed between patients in the WBRT, DRT, and NRT groups. Patients who had fewer extracranial lesions and who had ever received treatment with EGFR T790M inhibitors also had relatively longer survival.
Patients with EGFR mutant lung cancer and brain metastases were heterogeneous. No patients in the SRS group had more than four cranial lesions upon diagnosis of advanced disease, and patients in the SRS group had fewer extracranial lesions (Table 1; p < .001). Patients in the DRT or NRT groups were more likely to exhibit a largest cranial tumor smaller than or equal to 1 cm (Table 1; p < .001). Similar findings were also reported by Magnuson et al. [8]. In addition, patients in the NRT group were older than those in the other groups (p = .007). Despite patients in the SRS group surviving longer, it remains uncertain whether the longer survival is attributable to the effect of stereotactic surgery or to the heterogeneity of patient characteristics. Patients who did not receive immediate cranial irradiation, that is, the DRT and NRT groups, were heterogeneous as well. The survival of some patients in the NRT group may be short because the patient might be weak or the diseases were extensive so that the patients did not have the opportunities to receive cranial irradiation. On the other hand, some patients in the NRT group may achieve good response to EGFR inhibitors so that there was no indication for cranial irradiation. Patients in the DRT group achieved progressive disease, and they took the opportunities to receive cranial irradiation. The DRT group is a unique population of patients who did not receive immediate cranial irradiation and was in between the two extreme situations mentioned above. In brief, considering the heterogeneity of patients, the timing and modality of cranial irradiation should be determined individually.
The emergence of new second‐ or third‐generation EGFR‐TKIs may influence decisions regarding the timing and modality of cranial irradiation, especially with respect to drugs that can penetrate the blood‐brain barrier. The EGFR‐TKIs administered to patients in other studies that assessed patients with EGFR mutant NSCLC and brain metastases were mainly gefitinib or erlotinib [8], [9], [12], [13]. The penetration rates of gefitinib and erlotinib in the cerebrospinal fluid were 1.13% ± 0.36% and 2.77% ± 0.45%, respectively [14], and this limited cerebrospinal fluid penetration may limit the drugs’ effects on survival. Afatinib was reported to be safe and effective for the treatment of patients with EGFR mutant NSCLC and asymptomatic brain metastases [15]. In a phase III trial for patients with EGFR mutant NSCLC and multiple brain metastases, icotinib was associated with longer intracranial PFS times than chemotherapy plus whole brain radiation [16]. In an analysis of clinical outcome and genomic dynamics of patients with T790M‐positive advanced NSCLC who received osimertinib, Lin et al. observed higher probability of detectable circulating tumor DNA as well as shorter PFS and OS to osimertinib in patients who had brain metastases [17]. For drugs with better cerebrospinal fluid penetration, AZD3759 is a blood‐brain barrier‐penetrating EGFR inhibitor without anti‐EGFR T790M activity [18], and a phase I study demonstrated it was safe and clinically efficacious [19]. Goss et al. reported a 54% intracranial response rate to osimertinib in 50 patients with T790M‐positive advanced NSCLC who had progressed despite prior EGFR‐TKIs treatment [20]. Compared with gefitinib, erlotinib, or afatinib, osimertinib had better CNS efficacy in patients with asymptomatic brain metastases [10]. In the univariable and multivariable analyses conducted for this study, patients who had received at least one dose of EGFR T790M inhibitor independently survived longer. This warrants further investigation to re‐evaluate the effect of cranial irradiation in light of a new generation of EGFR‐TKIs.
In addition to the exposure of EGFR T790M inhibitors, other factors may contribute to the longer survival in patients who had received EGFR T790M inhibitors. First, many patients had received multiple lines of systemic anticancer therapies prior to EGFR T790M inhibitors, indicating that they should survive long enough so that they got the opportunity to receive EGFR T790M inhibitors. Second, the emergence of EGFR T790M mutation in the tumor per se may be prognostic, as Oxnard et al. reported that the prognosis of patients was better if their tumors had acquired EGFR T790M mutation [21]. Third, 18 (66.7%) of the 27 patients were enrolled in clinical trials of EGFR T790M inhibitors, and most were phase I and phase II clinical trials. These patients must have had good performance status and good organ function if they were enrolled in clinical trials.
The median survival time of the 198 patients in our study (20.6 months) was shorter than the survival time reported by Magnuson et al. (30 months) [8]. In a meta‐analysis of six randomized phase III trials of EGFR‐TKIs in patients with advanced EGFR mutant NSCLC, the median survival times were 25.8–26.0 months and the median PFS time related to EGFR‐TKIs was 11.0 months [3]. The survival times of patients with brain metastases tend to be shorter than those of patients without brain metastases; the PFS (8.2 months) and OS (20.6 months) times in our study were likely not the result of bias.
Biases may complicate the interpretation of results. First, performance status is a known prognostic factor for survival in patients with advanced EGFR mutant NSCLC, although it is not necessarily prognostic for PFS to EGFR inhibitors [22]. However, the records of performance status in retrospective studies are often incomplete and less accurate. As performance status was not involved in the current study, we did not overemphasize the prognostic effect of variables that interfere with performance status, such as exposure to chemotherapies (Table 3). Next, we are cautious with respect to the conclusions that the stage at the time of diagnosis and the timing of diagnosis of advanced disease were determined to be prognostic in both univariate and multivariate analyses (Table 3). Patients with early‐stage diseases upon initial diagnosis received an imaging study every 3–6 months. The burden of tumor tended to be smaller with respect to the detection of recurrent diseases. The effect of disease stage at diagnosis may have resulted from selection bias. Finally, several patients received EGFR inhibitors in the era when EGFR testing was not available in regular practice, and the EGFR mutational status in their tumors was available several months after they started taking EGFR inhibitors. In univariate and multivariate analyses, we observed that patients who were diagnosed with and treated for advanced disease prior to December 2012 survived longer than those diagnosed thereafter (Table 3). As patients diagnosed prior to December 2012 had to survive long enough in order to have EGFR mutational status tested in their tumors, their long survival must be owing to selection bias.
There are prognostic scores to predict the survival of patients with NSCLC with brain metastases [23]. Common parameters include performance status, age, extracranial metastases, controlled primary tumor, and the number of brain metastases. In our study, age and the number of brain metastases were prognostic in univariate analysis but not multivariate analysis (Table 3). Because of the progress of radiotherapy including SRS as well as the aforementioned emergence of new EGFR inhibitors, prognostic factors used in current prognostic scoring, such as age and the number of brain metastases according to the Lung‐molGPA (graded prognostic assessment) score [24], may be less prognostic with respect to patients with EGFR mutant lung cancer and brain metastases. The prognostic scoring for such patients may require revision and separate criteria relative to scoring of patients with other lung cancers.
Conclusion
Performing stereotactic radiosurgery immediately upon diagnosis of brain metastases was associated with longer survival times in patients with EGFR mutant NSCLC. No difference was evident in the survival times among patients who received whole brain radiation, who received deferred radiation, and who did not receive cranial irradiation. Because of the heterogeneity of patients and the introduction of a new generation of EGFR‐TKIs, the timing and modality of cranial irradiation in patients with EGFR mutant lung cancer should be determined on an individual basis, and cranial irradiation may be omitted for selected patients.
Acknowledgments
We acknowledge Dr. Chao‐Chi Ho for his help in caring for patients.
Author Contributions
Conception/design: Jih‐Hsiang Lee, James Chih‐Hsin Yang
Provision of study material or patients: Jih‐Hsiang Lee, Feng‐Ming Hsu, Jin‐Shing Chen, Wei‐Yu Liao, Jin‐Yuan Shih, Chong‐Jen Yu, Kuan‐Yu Chen, Tzu‐Hsiu Tsai, James Chih‐Hsin Yang
Collection and/or assembly of data: Jih‐Hsiang Lee, Hsuan‐Yu Chen, James Chih‐Hsin Yang
Data analysis and interpretation: Jih‐Hsiang Lee, Hsuan‐Yu Chen, James Chih‐Hsin Yang
Manuscript writing: Jih‐Hsiang Lee, Hsuan‐Yu Chen, James Chih‐Hsin Yang
Final approval of manuscript: Jih‐Hsiang Lee, Hsuan‐Yu Chen, Feng‐Ming Hsu, Jin‐Shing Chen, Wei‐Yu Liao, Jin‐Yuan Shih, Chong‐Jen Yu, Kuan‐Yu Chen, Tzu‐Hsiu Tsai, James Chih‐Hsin Yang
Disclosures
Jin‐Yuan Shih: AstraZeneca, Roche, Boehringer Ingelheim, Eli Lilly and Company, Novartis, Merck Sharp & Dohme, Ono Pharmaceutical, Chugai, AbbVie, Bristol‐Myers Squibb (C/A); AstraZeneca, Roche, Boehringer Ingelheim, Eli Lilly and Company, Pfizer, Novartis, Merck Sharp & Dohme, Ono Pharmaceutical, Chugai, AbbVie, Bristol‐Myers Squibb (H); James Chih‐Hsin Yang: AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Bayer, Roche/Genentech, Chugai, Merck Sharp & Dohme, Pfizer, Novartis, Bristol‐Myers Squibb, Ono Pharmaceuticals (H); AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Bayer, Roche/Genentech, Chugai, Merck Sharp & Dohme, Pfizer, Novartis, Bristol‐Myers Squibb, Ono Pharmaceuticals, Blueprint Medicines, Takeda Pharmaceuticals, Hansoh Pharmaceuticals, Daiichi Sankyo, Yuhan Pharmaceuticals, Merrimack, Celgene, Merck Serono, Astellas (SAB). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Shi Y, Au JS, Thongprasert S et al. A prospective, molecular epidemiology study of EGF mutations in Asian patients with advanced non‐small‐cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CK, Brown C, Gralla RJ et al. Impact of EGFR inhibitor in non‐small cell lung cancer on progression‐free and overall survival: A meta‐analysis. J Natl Cancer Inst 2013;105:595–605. [DOI] [PubMed] [Google Scholar]
- 3.Lee CK, Davies L, Wu YL et al. Gefitinib or erlotinib vs chemotherapy for EGFR mutation‐positive lung cancer: Individual patient data meta‐analysis of overall survival. J Natl Cancer Inst 2017;109 [DOI] [PubMed] [Google Scholar]
- 4.Soria JC, Ohe Y, Vansteenkiste J et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med 2018;378:113–125. [DOI] [PubMed] [Google Scholar]
- 5.Loganadane G, Hendriks L, Le Pechoux C et al. The current role of whole brain radiation therapy in non‐small cell lung cancer patients. J Thorac Oncol 2017;12:1467–1477. [DOI] [PubMed] [Google Scholar]
- 6.Park SJ, Kim HT, Lee DH et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non‐small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer 2012;77:556–560. [DOI] [PubMed] [Google Scholar]
- 7.Iuchi T, Shingyoji M, Sakaida T et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR‐mutant lung adenocarcinoma. Lung Cancer 2013;82:282–287. [DOI] [PubMed] [Google Scholar]
- 8.Magnuson WJ, Lester‐Coll NH, Wu AJ et al. Management of brain metastases in tyrosine kinase inhibitor‐naive epidermal growth factor receptor‐mutant non‐small‐cell lung cancer: A retrospective multi‐institutional analysis. J Clin Oncol 2017;35:1070–1077. [DOI] [PubMed] [Google Scholar]
- 9.Jiang T, Su C, Li X et al. EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases. J Thorac Oncol 2016;11:1718–1728. [DOI] [PubMed] [Google Scholar]
- 10.Reungwetwattana T, Nakagawa K, Cho BC et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR‐mutated advanced non‐small‐cell lung cancer. J Clin Oncol 2018;36:3290–3297. [DOI] [PubMed] [Google Scholar]
- 11.Ballard P, Yates JW, Yang Z et al. Preclinical comparison of osimertinib with other EGFR‐TKIs in EGFR‐mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016;22:5130–5140. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao SH, Chou YT, Lin SE et al. Brain metastases in patients with non‐small cell lung cancer: The role of mutated‐EGFRs with an exon 19 deletion or L858R point mutation in cancer cell dissemination. Oncotarget 2017;8:53405–53418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Q, Sun Y, Cui Y et al. Clinical outcome of tyrosine kinase inhibitors alone or combined with radiotherapy for brain metastases from epidermal growth factor receptor (EGFR) mutant non small cell lung cancer (NSCLC). Oncotarget 2017;8:13304–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Togashi Y, Masago K, Masuda S et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non‐small cell lung cancer. Cancer Chemother Pharmacol 2012;70:399–405. [DOI] [PubMed] [Google Scholar]
- 15.Schuler M, Wu YL, Hirsh V et al. First‐line afatinib versus chemotherapy in patients with non‐small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol 2016;11:380–390. [DOI] [PubMed] [Google Scholar]
- 16.Yang JJ, Zhou C, Huang Y et al. Icotinib versus whole‐brain irradiation in patients with EGFR‐mutant non‐small‐cell lung cancer and multiple brain metastases (BRAIN): A multicentre, phase 3, open‐label, parallel, randomised controlled trial. Lancet Respir Med 2017;5:707–716. [DOI] [PubMed] [Google Scholar]
- 17.Lin CC, Shih JY, Yu CJ et al. Outcomes in patients with non‐small‐cell lung cancer and acquired Thr790Met mutation treated with osimertinib: A genomic study. Lancet Respir Med 2018;6:107–116. [DOI] [PubMed] [Google Scholar]
- 18.Yang Z, Guo Q, Wang Y et al. AZD3759, a BBB‐penetrating EGFR inhibitor for the treatment of EGFR mutant NSCLC with CNS metastases. Sci Transl Med 2016;8:368ra172. [DOI] [PubMed] [Google Scholar]
- 19.Ahn MJ, Kim DW, Cho BC et al. Activity and safety of AZD3759 in EGFR‐mutant non‐small‐cell lung cancer with CNS metastases (BLOOM): A phase 1, open‐label, dose‐escalation and dose‐expansion study. Lancet Respir Med 2017;5:891–902. [DOI] [PubMed] [Google Scholar]
- 20.Goss G, Tsai CM, Shepherd FA et al. CNS response to osimertinib in patients with T790M‐positive advanced NSCLC: Pooled data from two phase II trials. Ann Oncol 2018;29:687–693. [DOI] [PubMed] [Google Scholar]
- 21.Oxnard GR, Arcila ME, Sima CS et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR‐mutant lung cancer: Distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosell R, Moran T, Queralt C et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958–967. [DOI] [PubMed] [Google Scholar]
- 23.Dawe DE, Greenspoon JN, Ellis PM. Brain metastases in non‐small‐cell lung cancer. Clin Lung Cancer 2014;15:249–257. [DOI] [PubMed] [Google Scholar]
- 24.Sperduto PW, Yang TJ, Beal K et al. Estimating survival in patients with lung cancer and brain metastases: An update of the graded prognostic assessment for lung cancer using molecular markers (Lung‐molGPA). JAMA Oncol 2017;3:827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]