Identifying long‐term survival predictors in patients with metastatic pancreatic ductal adenocarcinoma would allow for individualized treatment decisions. This study identified patients surviving more than 18 months from diagnosis of metastasis, focusing on clinical and pathological factors that might affect survival.
Keywords: Pancreatic ductal adenocarcinoma, Long‐term survivors, Metastasis, Survival, Prognostic factors
Abstract
Background.
Metastatic pancreatic ductal adenocarcinoma (mPDAC) is an aggressive malignancy with a median overall survival (OS) of between 8 and 11 months. However, a significant number of patients experience a longer survival, more than 18 months. The aim of this study was to describe the “long‐term survivor” population and to evaluate clinical and pathological factors that might affect survival.
Materials and Methods.
All patients with mPDAC diagnosed in the Centre Leon Bérard (Lyon, France) between January 2010 and June 2015 and who survived more than 18 months were identified. They were compared with a control cohort matched on age, sex, performance status, stage at diagnosis, primary tumor localization, treatment, and liver metastasis. Their clinical features, treatment modalities, and outcomes were analyzed.
Results.
A total of 94 patients were included, 47 in each cohort. Both cohorts had identical characteristics as follows: women (51%), performance status ≤1 (95.7%), median age at diagnosis (60 years), and metastasis at diagnosis (83%). Median OS was 26.87 months (95% confidence interval [CI] 23–31.08) in the long‐term survivor group (LS group) and 9.79 months (95% CI 5.75–11.86) in the control group (C group). Potential factors of long‐term survival were explored with a logistic model (LS group vs. C group). Three factors were identified as significant prognostic factors in the univariate analysis: lymphopenia (odds ratio [OR] ref: yes = 0.26), neutrophil‐to‐lymphocyte ratio (NLR; OR ref >5 = 0.31), and peritoneal carcinomatosis (OR ref: yes = 0.40). NLR was the only remaining factor in our backward selection procedure.
Conclusion.
A significant subset of patients with mPDAC can achieve long‐term survival (≥18 months) in 2018. We identified low NLR as a significant prognostic factor associated with long‐term survival in mPDAC.
Implications for Practice.
Metastatic pancreatic ductal adenocarcinoma (mPDAC) is one of the most lethal types of cancer. A subset of patients with mPDAC can achieve long‐term survival (≥18 months) with a modern chemotherapy regimen, such as FOLFIRINOX or gemcitabine/nab‐paclitaxel. We identified low neutrophil‐to‐lymphocyte ratio (NLR) as a significant prognostic factor associated with long‐term survival in mPDAC. Prognostic factors such as NLR might allow accurate selection of patients with mPDAC in order to consider individual therapeutic approaches. NLR should be used as a stratification factor in clinical trials.
摘要
背景。转移性胰腺导管腺癌 (mPDAC) 是一种侵袭性恶性肿瘤,中位总生存期 (OS) 为 8 至 11 个月。然而,许多患者的生存期较长,超过 18 个月。本研究的目的是描述“长期生存者”群体,并评估可能影响生存期的临床和病理因素。
材料和方法。面向 2010 年 1 月至 2015 年 6 月期间在里昂贝拉德中心(Centre Leon Bérard,法国里昂)确诊为mPDAC且生存期超过 18 个月的所有患者。将这些患者与在年龄、性别、体力状态、诊断分期、原发肿瘤位置、治疗及肝转移方面相匹配的对照队列进行了对比。对他们的临床特征、治疗方式及预后进行了分析。
结果。共纳入 94 例患者,每个队列 47 例。两个队列的患者具有相同的特征,具体如下:女性 (51%)、体力状态≤1 (95.7%)、确诊时的中位年龄(60 岁),以及确诊时的转移率 (83%)。长期生存者组(LS 组)的中位OS为 26.87 个月 [95% 置信区间 (CI)23‐31.08],对照组(C 组)为 9.79 个月(95% 置信区间 5.75‐11.86)。通过利用逻辑模型,研究了影响长期生存的潜在因素(LS 组与 C 组)。在单变量分析中,确定了影响预后的三个重要因素:淋巴细胞减少 [优势比 (OR)ref: yes = 0.26]、中性粒细胞与淋巴细胞比(NLR;OR ref >5 = 0.31),以及腹膜癌(OR ref: yes = 0.40)。在我们的逆向选择过程中, NLR 是唯一剩下的因素。
结论。2018 年,mPDAC患者中的大部分人可实现长期生存(≥18 个月)。我们发现,较低的NLR是与mPDAC患者长期生存相关的一个重要预后因素。
实践意义:转移性胰腺导管腺癌 (mPDAC) 是最致命的癌症之一。通过现代化疗方案(例如 FOLFIRINOX 或吉西他滨 / 白蛋白结合型紫杉醇), mPDAC患者中的一部分人可实现长期生存(≥18 个月)。我们发现,较低的中性粒细胞与淋巴细胞比 (NLR) 是与mPDAC患者长期生存相关的一个重要预后因素。我们可利用NLR等影响预后的因素准确选择mPDAC患者,以考虑针对个体的治疗方法。在临床试验中,NLR应作为重要的分层因素。
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal tumor types, with a 5‐year overall survival (OS) rate of 8% and the third rank for mortality of all cancer types in Europe [1]. Furthermore, a recent analysis based on the Surveillance, Epidemiology, and End Results database and demographic shifts projected that pancreatic cancer will become the second leading cause of cancer‐related deaths before 2030 [2]. Data from the French digestive cancer registries show a dramatic increase in recent years, more marked in women than in men [3]. The currently recognized risk factors like tobacco or obesity [4] cannot explain this evolving epidemiology. Surgery is the only potentially curative approach for pancreatic cancer, but less than 20% of patients have resectable tumors at diagnosis [2], [5]. More than half of cases are diagnosed with metastases, for which the 5‐year survival rate is 2%–6% [5], [6], [7]. Efficacy of treatments for metastatic pancreatic cancer is limited, and patients often survive less than 12 months. For years, gemcitabine monotherapy has been the standard first‐line treatment option for metastatic PDAC (mPDAC) after it demonstrated superior clinical benefit compared with 5‐fluorouracil. Median OS for patients with mPDAC receiving gemcitabine monotherapy ranges from 5.7 to 6.8 months [8]. In the last few years, recent chemotherapy regimens were developed and associated with improved survival. In a phase III study, FOLFIRINOX was associated with statistically significant OS benefit (11.1 vs. 6.8 months, hazard ratio [HR] = 0.57, p < .001) but was also associated with increased toxicity [9]. In a recent phase III study, MPACT, nab‐paclitaxel associated with gemcitabine significantly improved OS compared with gemcitabine alone (8.7 vs. 6.6 months, HR = 0.72, p < .001) [10]. Some patients experience prolonged survival, more than 18 months, especially since these new chemotherapy regimens have been routinely used, as reported from the MPACT trial [8]. Recent retrospective studies reported that highly selected patients with oligometastatic PDAC could also potentially benefit from surgical resection, locoregional ablation techniques, or stereotaxic radiotherapy [11], [12]. Identifying long‐term survival predictors in patients with mPDAC would allow physicians to individualize treatment decisions and elaborate therapeutic strategies, including surgery and local treatment for the primary tumor and the metastases. The aim of this study was first to describe “long‐term survivors” and then to find clinical and pathological factors that might affect survival in metastatic PDAC.
Materials and Methods
Patients
We conducted a retrospective analysis of patients diagnosed in the Centre Leon Berard (Lyon, France) with metastatic pancreatic ductal adenocarcinoma between January 2010 and June 2015 and who survived more than 18 months. We first identified all patients who survived more than 18 months from the diagnosis of metastasis. Then we compared them with a control cohort of patients with mPDAC who survived less than 18 months who were matched for age, sex, performance status, stage at diagnosis, primary tumor localization, treatment, and liver metastasis. Data on clinical and biological features, treatment modality, and outcomes were extracted from patient files and analyzed.
Statistical Analysis
OS was defined as the time between the diagnosis of metastases and the date of death (from any cause), or censored at the date of latest follow‐up. Median follow‐up was calculated using reverse Kaplan‐Meier estimation.
A logistic regression analysis was used to evaluate prognostic factors of long‐term survivors (outcome [response] was defined as a binary variable: patients who survived more than 18 months vs. other). Probability modeled is patients who survived more than 18 months. The prespecified factors were weight loss, abdominal pain, icterus, grade, tumor size, lung metastases, peritoneal carcinomatosis, hemoglobin, lymphopenia (any grade), neutrophils, initial blood neutrophil‐to‐lymphocyte ratio (NLR), bilirubin, and CA19‐9, which were recorded at mPDAC diagnosis, before treatment initiation. We selected NLR greater than five as the cutoff value, as described in a recent publication on mPDAC [8].
Variables with less than 10% missing value in univariate analyses and significant at a 10% level were included in a backward selection procedure to keep factors significant at a 5% level in the final logistic multivariate model. To note, the backward selection procedure eliminates the variable with the greatest p value at each step to obtain a final model containing only factors significant at a 5% level.
SAS version 9.4 (SAS Institute, Cary, NC) was used for all statistical analyses.
Results
Patients’ Characteristics
We identified 47 patients in the long‐term survivor (LS) group and matched them with 47 patients in the control (C) group. As patients were matched, both cohorts had identical characteristics as follows: women (51%), performance status ≤1 (95.7%), median age at diagnosis (60 years), and metastasis at diagnosis (83%; Table 1). Primary tumor site was head of pancreas in 20 patients (42.6%). Thirty‐two (68.1%) patients had liver metastases at diagnosis in both groups, 8 had lung metastases only at diagnosis (5 patients [10.6%] in the LS vs. 3 [6.4%] in the C group), and 24 had peritoneal carcinomatosis at diagnosis (8 [17%] in the LS vs. 16 [34%] in the C group).
Table 1. Patients’ characteristics at diagnosis.

Abbreviation: NLR, neutrophil‐to‐lymphocyte ratio.
Median baseline CA19‐9 was lower in the LS group (407; range 1–32,129) than in the C group (2,869; range 1–287,000). Median baseline albumin level was similar in both groups: 40 g/L (range 30–51) in the LS and 38 (range 23–48) in the C group. Lymphopenia status and NLR were significantly different between groups (lymphopenia Yes: 4 (9.3%) vs. 13 (28.3%), p = .031 and NLR >5: 7 (16.7%) vs. 18 (39.1%), p = .02 in the LS and C groups, respectively). Median lymphocyte blood level at diagnosis was 1.7 g/L (range 1–9) in the LS group versus 1.4 (range 0–4) in the C group (p = .005).
In our study, nine patients in the LS group were tested for microsatellite instability and/or breast cancer (BRCA) mutations: four patients for BRCA only (familial history of breast cancer), one patient for microsatellite instability (age <40 years at diagnosis), and three patients for both (familial history of digestive and breast cancer). Two patients had microsatellite instability and four had BRCA mutations (two with a BRCA1 mutation and two with a BRCA2 mutation).
Furthermore, somatic genomic profiling was performed for three patients in the C group and eight patients in the LS group. In the C group, all patients had KRAS mutations, two had TP53 mutations, one had MDM2 amplification, and one had deletion of CDKN2A, MACROD2, SMARCA4, TGFBR2, GADL1. In the LS group, seven patients had KRAS mutations, four had TP53 mutations, one had FGFR2 mutation, and one had TEK mutation.
Treatment Modalities
All patients received first‐line chemotherapy for metastatic disease, mostly FOLFIRINOX in both groups (39 patients, 83%). The median duration of the first‐line chemotherapy was 5.3 months (range 1–41) in the LS group and 4 months (range 0–10) in the C group. In the LS group, 37 patients (78.7%) discontinued their chemotherapy protocol after a fixed number of 12 cycles, versus 16 (34%) in the C group. The median chemotherapy‐free interval was 5 months (range 2–26) in the LS group versus 3 months (range 1–8) in the C group. For 10 patients (21.3%) in the LS group and 31 (67.4%) in the C group, first‐line chemotherapy was stopped because of progressive disease. More patients received second‐line chemotherapy in the LS group (45 vs. 35 in the C group), either fluorouracil (5FU) or gemcitabine based (Table 2). In the LS group, most patients received 5FU‐based polychemotherapy, FOLFIRINOX (20%) or FOLFIRI (20%), whereas in the control group, most patients received gemcitabine (57.1%).
Table 2. Second‐line chemotherapy.
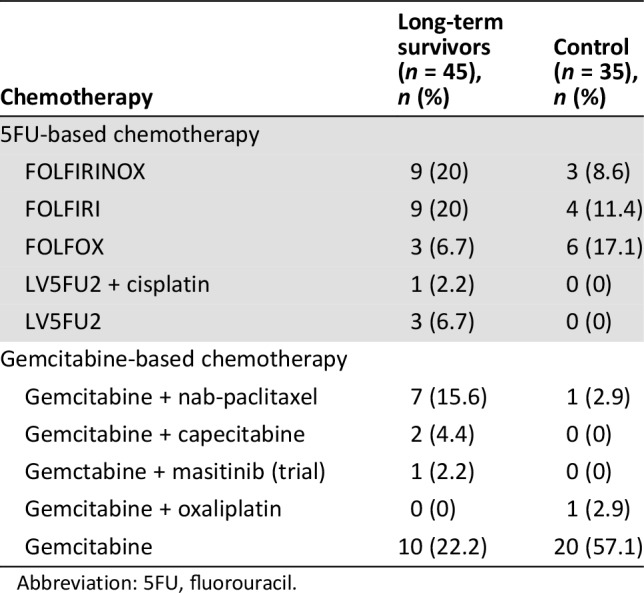
Abbreviation: 5FU, fluorouracil.
In the LS group, four patients (8.5%), who had complete radiological response on their metastatic sites after 6 months of induction chemotherapy, were treated with fluorouracil‐based chemoradiotherapy on primary pancreatic tumor.
Furthermore, two patients (LS group) had a surgical resection of their primary tumor and metastases. The first patient was a 40‐year‐old male patient with liver metastases only. He underwent surgical resection of both primary tumor and liver metastases. The second one was a 37‐year‐old female patient with BRCA2 mutation, who underwent surgical resection of primary tumor and lung metastases. In both cases, surgery was performed more than 2 years after mPDAC diagnosis (respectively 30 months and 4 years).
Biological and Radiological Assessment
On the first radiological evaluation, we observed a partial tumor response according to RECIST criteria 1.1 in 15 (31.9%) and 2 patients (4.3%) and stable disease in 31 (66%) and 26 patients (55.3%) in the LS and C groups, respectively. Progressive disease was observed in 1 patient (2.1%) in the LS group and 19 patients (40.4%) in the C group. The disease control rate at 8 weeks was 98% in the LS group versus 60% in the control group. The difference of response at 8 weeks (≤30% vs. >30%) between the LS and C groups was statistically significant (p < .001), although this parameter was not included in our logistic regression model because of unbalanced population: 15 patients (31.9%) in the LS group vs. 2 (4.3%) in the C group had significant tumor response (>30%) .
The CA19‐9 measurements at baseline and at 8 weeks (W8) from chemotherapy initiation were available for 38 patients in the LS group and 31 patients in the control group. A total of 26/38 (68.4%) and 16/31 (51.6%) presented a CA19‐9 decrease at W8, but this difference was not statistically significant.
Survival
Median OS was 26.87 months (95% confidence interval [CI] 23–31.08) in the LS group and 9.79 months (95% CI 5.75–11.86) in the C group.
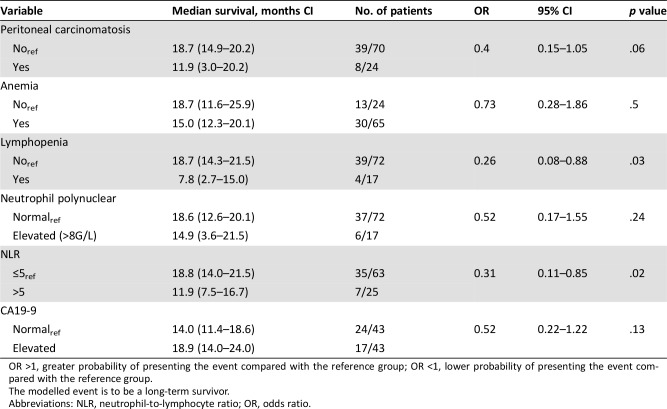
Potential factors of long‐term survival were explored with a logistic model (LS group vs. C group). Three factors were identified as significant prognostic factors in the univariate analysis (Table 3): Long‐term survivors are more likely to be patients without lymphopenia (odds ratio [OR] ref: yes = 0.26, 95% CI 0.08–0.88, p = .03), with NLR at diagnosis ≤5 (OR ref: >5 = 0.31, 95% CI 0.11–0.85, p = .02), and without peritoneal carcinomatosis (OR ref: yes = 0.40, 95% CI (0.15–1.05, p = .06). NLR was the only remaining factor in our backward selection procedure.
Table 3. Univariate analysis.
OR >1, greater probability of presenting the event compared with the reference group; OR <1, lower probability of presenting the event compared with the reference group.
The modelled event is to be a long‐term survivor.
Abbreviations: NLR, neutrophil‐to‐lymphocyte ratio; OR, odds ratio.
Discussion
Pancreatic ductal adenocarcinoma is an aggressive tumor that ranks 11th in incidence but 4th in mortality. Median overall survival for patients with metastatic disease varies from 3 to 6 months without specific treatments and 8 to 11 months with chemotherapy. However, since the use of FOLFIRINOX and gemcitabine/nab‐paclitaxel regimens, we observe more and more patients experiencing a longer survival. In this retrospective study, we report our experience of treating patients with mPDAC and focus on those who survived more than 18 months from metastasis diagnosis.
Few cases of long‐term survivors in mPDAC have been published. The definition of long‐term survivor with mPDAC varies from one study to another: Goulart [5] and Inal [13] reported patients with mPDAC who survived more than 1 year, Soldic [14] reported one patient who survived more than 4 years, and Chue [15] and Oh [16] reported 1 and 11 patients, respectively, with mPDAc who survived more than 5 years. Here we chose 18 months based on the data reported in the study by Conroy et al. for patients treated with FOLFIRINOX. In that study, the median OS was 11 months (95% CI 9.0–13.1) and the overall survival rate at 18 months was 18.6% [9].
One hypothesis is that this difference in survival might be due to intrinsic differences in biology. Mismatch repair deficiency is a rare event in pancreatic adenocarcinoma (0.8% of patients) and has been associated with significantly prolonged survival time [17]. BRCA 1 and 2 mutations are also rare events in pancreatic adenocarcinoma, mostly found in the presence of a family history of the disease. Prolonged overall survival has been reported for patients with mPDAC harboring BRCA1 or BRCA2 mutations, especially for those receiving platinum‐based chemotherapy [18], [19], [20], [21]. In our study, two patients had microsatellite instability and four had BRCA mutations (two a BRCA1 mutation and two a BRCA2 mutation). As both of these anomalies have therapeutic impact (immunotherapy for patients with deficient mismatch repair status and poly ADP ribose polymerase inhibitors for patients with BRCA mutations), we think it should be tested in long‐term survivor patients with mPDAC if not performed initially.
Somatic genomic profiling was performed for three patients in the C group and eight patients in the LS group. Most patients had KRAS mutations, six had TP53 mutations, and few other mutations with unknown significance and therefore no therapeutic impact were identified.
Difference in survival might also have been related to differences in response to chemotherapy. In our study, most patients received FOLFIRINOX as first‐line chemotherapy (83%). We chose to match patients on chemotherapy type, given the differences in median survival reported with FOLFIRINOX and gemcitabine regimens. The aim was to identify factors that may predict longer survival. There were significantly more patients with partial responses in the LS group at 8 weeks. This parameter was not included in our logistic regression model because of unbalanced population. Furthermore, first‐line chemotherapy was mainly stopped because of tumor progression (67.4%) in the C group, whereas it was mainly discontinued after a fixed number of cycles in LS patients (78.7%) to limit toxicity, especially oxaliplatin‐induced sensitive neuropathy. The regression analysis identified three significant prognostic factors. LS patients are more likely to be patients without lymphopenia, with NLR at diagnosis ≤5, and without peritoneal carcinomatosis. Several studies have reported the prognostic value of lymphopenia in advanced cancer [21], [22]. It may be a biological mechanism stimulating tumor progression, in non‐Hodgkin lymphoma where immune suppression is clearly involved in tumor progression, and an increased risk of death due to treatment toxicity [22]. However, after our backward selection procedure, NLR remains the only independent significant prognostic factor in multivariate analysis.
NLR is a biomarker of systemic inflammatory response [23] and has been identified as an important prognostic factor for a number of malignancies, including colorectal cancer, renal cell carcinoma, and non‐small cell lung cancer [8], [24]. Elevated NLR at diagnosis has been strongly associated with worse progression‐free survival and OS [25]. The inflammatory response plays an important role in cancer development and progression [24]. The exact mechanism that explains the poor survival outcomes for patients with elevated blood NLR has not been clearly determined. It may reflect a high level of circulating cytokine including transforming growth factor β, which enhances tumor progression by favoring immunosuppression, angiogenesis, and peri‐tumoral stroma formation [24]. In our study, initial NLR was significantly lower in LS patients with mPDAC. Those results are consistent with previous studies as the prognosis role of the NLR has been demonstrated in PDAC in both early [26], [27], [28] and advanced stages [8], [23], [24], [25], [29]. NLR should be considered a stratification factor in further clinical trials in order to determine whether patients with high NLR at diagnosis could benefit from intensified treatment or, on the contrary, should only require palliative treatment.
Finally, differences in management might impact survival. In the LS cohort, two patients (4.3%) underwent surgical resection compared with none in the control group, and four patients (8.5%) received radiotherapy in the LS group compared with three (6.4%) in the control group. Because of our limited sample, we cannot establish any conclusion on this hypothesis in our study. Few studies have evaluated the role of surgery in mPDAC with partly conflicting results [11]. However, recent studies have reported that selected patients with oligometastatic PDAC may potentially benefit from surgical resection with an acceptable morbidity [11], [12]. This might be related to improvement in surgical technique and in selection of appropriate candidates. Ablation techniques such as thermo‐ablative approaches (radiofrequency ablation, cryoablation) or stereotactic body radiation therapy could also be therapeutic alternatives in patients with oligometastatic PDAC [12]. However, surgery and local ablation techniques have only been evaluated in retrospective studies, and their benefit must be confirmed with prospective trials.
Conclusion
Metastatic pancreatic ductal adenocarcinoma is one of the most lethal types of cancer, with a very high mortality‐to‐incidence ratio. Chemotherapy regimens such as FOLFIRINOX or gemcitabine‐nab‐paclitaxel have been established as standard of care and offer improvement in survival, but prognosis remains poor. A subset of patients with mPDAC can achieve long‐term survival (≥18 months) in 2018. We identified low NLR as a significant prognostic factor associated with long‐term survival in mPDAC. Prognostic factors such as NLR might allow accurate selection of patients with metastatic pancreatic ductal adenocarcinoma in order to consider individual therapeutic approaches. NLR should be used as a stratification factor in clinical trials.
Acknowledgments
We thank Helen Boyle, M.D., for providing writing assistance.
Author Contributions
Conception/design: Pauline Rochefort, Audrey Lardy‐Cleaud, Christelle de la Fouchardière
Provision of study material or patients: Pauline Rochefort, Matthieu Sarabi, Françoise Desseigne, Anne Cattey‐Javouhey, Christelle de la Fouchardière
Collection and/or assembly of data: Pauline Rochefort, Matthieu Sarabi, Françoise Desseigne, Anne Cattey‐Javouhey, Christelle de la Fouchardière
Data analysis and interpretation: Pauline Rochefort, Audrey Lardy‐Cleaud, Matthieu Sarabi, Françoise Desseigne, Anne Cattey‐Javouhey, Christelle de la Fouchardière
Manuscript writing: Pauline Rochefort, Audrey Lardy‐Cleaud, Christelle de la Fouchardière
Final approval of manuscript: Pauline Rochefort, Audrey Lardy‐Cleaud, Matthieu Sarabi, Françoise Desseigne, Anne Cattey‐Javouhey, Christelle de la Fouchardière
Disclosures
The authors indicated no financial relationships.
References
- 1.Malvezzi M, Carioli G, Bertuccio P et al. European cancer mortality predictions for the year 2018 with focus on colorectal cancer. Ann Oncol 2018;29:1016–1022. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 3.Jooste V, Dejardin O, Bouvier V et al. Pancreatic cancer: Wait times from presentation to treatment and survival in a population‐based study. Int J Cancer 2016;139:1073–1080. [DOI] [PubMed] [Google Scholar]
- 4.Korc M, Jeon CY, Edderkaoui M et al. Tobacco and alcohol as risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol 2017;31:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goulart BH, Clark JW, Lauwers GY et al. Long term survivors with metastatic pancreatic adenocarcinoma treated with gemcitabine: A retrospective analysis. J Hematol Oncol 2009;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 7.Sohal DPS, Mangu PB, Khorana AA et al. Metastatic pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016;34:2784–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein D, El‐Maraghi RH, Hammel P et al. nab‐Paclitaxel plus gemcitabine for metastatic pancreatic cancer: Long‐term survival from a phase III trial. J Natl Cancer Inst 2015;107. [DOI] [PubMed] [Google Scholar]
- 9.Conroy T, Desseigne F, Ychou M et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 10.Von Hoff DD, Ervin T, Arena FP et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackert T, Niesen W, Hinz U et al. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol 2017;43:358–363. [DOI] [PubMed] [Google Scholar]
- 12.Renz BW, Boeck S, Roeder F et al. Oligometastatic disease in pancreatic cancer – How to proceed? Visc Med 2017;33:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inal A, Ciltas A, Yildiz R et al. Long term survivors with metastatic pancreatic cancer treated with gemcitabine alone or plus cisplatin: A retrospective analysis of an Anatolian Society of Medical Oncology multicenter study. Asian Pac J Cancer Prev 2012;13:1841–1844. [DOI] [PubMed] [Google Scholar]
- 14.Soldic Z, Zitnjak D, Bolanca A et al. Long‐time survival of a patient with metastatic pancreatic cancer: A case report. Case Rep Oncol 2011;4:367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chue BM. Five‐year survival of metastatic pancreatic carcinoma: A study of courage and hope. Gastrointest Cancer Res 2009;3:208–211. [PMC free article] [PubMed] [Google Scholar]
- 16.Oh SY, Edwards A, Mandelson MT et al. Rare long‐term survivors of pancreatic adenocarcinoma without curative resection. World J Gastroenterol 2015;21:13574–13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu ZI, Shia J, Stadler ZK et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: Challenges and recommendations. Clin Cancer Res 2018;24:1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pihlak R, Weaver JMJ, Valle JW et al. Advances in molecular profiling and categorisation of pancreatic adenocarcinoma and the implications for therapy. Cancers (Basel) 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kowalewski A, Szylberg Ł, Saganek M et al. Emerging strategies in BRCA‐positive pancreatic cancer. J Cancer Res Clin Oncol 2018;144:1503–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golan T, Kanji ZS, Epelbaum R et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 2014;111:1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kou F, Lu Z, Li J et al. Pretreatment lymphopenia is an easily detectable predictive and prognostic marker in patients with metastatic esophagus squamous cell carcinoma receiving first‐line chemotherapy. Cancer Med 2016;5:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray‐Coquard I, Cropet C, Van Glabbeke M et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res 2009;69:5383–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Formica V, Morelli C, Ferroni P et al. Neutrophil/lymphocyte ratio helps select metastatic pancreatic cancer patients benefitting from oxaliplatin. Cancer Biomark 2016;17:335–345. [DOI] [PubMed] [Google Scholar]
- 24.Mei Z, Shi L, Wang B et al. Prognostic role of pretreatment blood neutrophil‐to‐lymphocyte ratio in advanced cancer survivors: A systematic review and meta‐analysis of 66 cohort studies. Cancer Treat Rev 2017;58:1–13. [DOI] [PubMed] [Google Scholar]
- 25.Piciucchi M, Stigliano S, Archibugi L et al. The neutrophil/lymphocyte ratio at diagnosis is significantly associated with survival in metastatic pancreatic cancer patients. Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang JJ, Hu ZG, Shi WX et al. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: A meta‐analysis. World J Gastroenterol 2015;21:2807–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcea G, Ladwa N, Neal CP et al. Preoperative neutrophil‐to‐lymphocyte ratio (NLR) is associated with reduced disease‐free survival following curative resection of pancreatic adenocarcinoma. World J Surg 2011;35:868–872. [DOI] [PubMed] [Google Scholar]
- 28.Bhatti I, Peacock O, Lloyd G et al. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: Neutrophil‐lymphocyte versus platelet‐lymphocyte ratio. Am J Surg 2010;200:197–203. [DOI] [PubMed] [Google Scholar]
- 29.Dogan M, Algin E, Guven ZT et al. Neutrophil‐lymphocyte ratio, platelet‐lymphocyte ratio, neutrophil‐platelet score and prognostic nutritional index: Do they have prognostic significance in metastatic pancreas cancer? Curr Med Res Opin 2017:1–7. [DOI] [PubMed] [Google Scholar]