This article focuses on the safety profile of palbociclib in combination with letrozole, evaluating the clinical patterns of hematologic adverse events in the PALOMA‐2 trial.
Keywords: Breast cancer, Hematologic, Neutropenia, Palbociclib, Safety
Abstract
Background.
PALOMA‐2 confirmed that first‐line palbociclib + letrozole improved progression‐free survival (hazard ratio, 0.58; 95% confidence interval, 0.46–0.72) in postmenopausal women with estrogen receptor–positive (ER+)/human epidermal growth factor receptor 2–negative (HER2−) advanced breast cancer (ABC). This analysis evaluated palbociclib‐associated hematologic adverse events (AEs) and provides insight on managing these AEs.
Materials and Methods.
Postmenopausal women with ER+/HER2− ABC were randomly assigned 2:1 to letrozole (2.5 mg daily continuously) plus oral palbociclib (125 mg daily; 3 weeks on/1 week off) or placebo. Safety assessments were performed at baseline, days 1 and 15 (first two cycles) and day 1 of subsequent cycles, and included white blood cell, platelet, and absolute neutrophil count (ANC).
Results.
PALOMA‐2 randomized 666 women to palbociclib + letrozole (n = 444) or placebo + letrozole (n = 222). Neutropenia was the most common AE (95.3%) with palbociclib (grade 3, 55.6%; grade 4, 11.5%) and was managed by dose modifications; progression‐free survival was similar between patients who experienced grade ≥ 3 neutropenia versus those who did not. Median (range) time to onset of neutropenia with palbociclib + letrozole was 15 (12–700) days (grade ≥ 3, 28.0 [12–854] days); median duration of each neutropenia episode grade ≥ 3 was 7.0 days. Asian ethnicity and low baseline ANC were associated with increased risk of grade 3/4 neutropenia with palbociclib (p < .001).
Conclusion.
Palbociclib + letrozole was generally well tolerated. Neutropenia, the most frequently reported AE in women with ER+/HER2− ABC, was mostly transient and manageable by dose modifications in patients who experienced grade ≥ 3 neutropenia, without appearing to compromise efficacy. (Pfizer; NCT01740427)
Implications for Practice.
Palbociclib demonstrated an acceptable safety profile in PALOMA‐2 in women with estrogen receptor–positive (ER+)/human epidermal growth factor receptor 2–negative (HER2−) advanced breast cancer (ABC) receiving first‐line palbociclib + letrozole. Although hematologic adverse events (AEs) are typically expected with anticancer therapies and are often clinically significant, palbociclib‐related hematologic AEs, particularly neutropenia (most frequent AE), were transient/manageable by dose reduction, interruption, or cycle delay, which is in contrast to the more profound neutropenia associated with chemotherapy. Palbociclib dose adjustments decreased hematologic AE severity without appearing to compromise efficacy, supporting palbociclib + letrozole as a first‐line treatment for ER+/HER2− ABC.
Introduction
Treatment guidelines recommend sequential endocrine therapy as first‐line therapy for hormone receptor–positive (HR+) advanced breast cancer (ABC) [1], [2], [3]. Although endocrine therapy is effective, a substantial proportion of women will develop endocrine resistance and, consequently, require alternative treatment options, including switching to a different endocrine therapy or another therapeutic approach [4]. Several targeted therapies, including the first‐in‐class cyclin‐dependent kinase 4/6 (CDK 4/6) inhibitor palbociclib (Ibrance; Pfizer Inc, New York, NY), have recently been approved for use in combination with endocrine therapy [5], [6], [7], [8], and guidelines include recommendations for adding a CDK 4/6 inhibitor in combination with endocrine therapy for the treatment of pre‐ and postmenopausal women with HR+/human epidermal growth factor receptor 2–negative (HER2−) ABC [1], [2], [3].
Palbociclib is a potent inhibitor of CDK 4/6 that blocks cell cycle progression from G1 to S phase and subsequent DNA synthesis [9], [10], [11]. Palbociclib is indicated for the treatment of HR+/HER2− advanced breast cancer as first‐line therapy in combination with an aromatase inhibitor and in the endocrine‐resistant setting in combination with fulvestrant [6], [12].
The efficacy and safety of palbociclib as first‐line therapy in combination with letrozole in patients with endocrine receptor–positive (ER+)/HER2− ABC was initially demonstrated in the phase II PALOMA‐1 study [13] and subsequently confirmed in the phase III PALOMA‐2 study [14]. Palbociclib plus letrozole significantly prolonged median progression‐free survival (PFS) versus placebo plus letrozole in postmenopausal women with ER+/HER2– ABC (24.8 vs. 14.5 months; hazard ratio, 0.58; 95% confidence interval [CI], 0.46–0.72; 2‐sided p < .001; data cutoff, February 26, 2016). Although palbociclib significantly improved outcomes in PALOMA‐2, the incidence of myelotoxic events was higher with palbociclib compared with placebo plus letrozole (neutropenia, 79.5% vs. 6.3%; leukopenia, 39.0% vs. 2.3%; anemia, 24.1% vs. 9.0%; thrombocytopenia, 15.5% vs. 1.4%) [14].
Overall, the combination of palbociclib plus endocrine therapy has been shown to have a generally manageable safety profile in phase II and III clinical trials to date; however, these trials also reported higher rates of hematologic adverse events (AEs) versus letrozole or fulvestrant (monotherapy or with placebo). These hematologic AEs are expected given the mechanism of action of CDK 4/6 inhibitors [15] and are considered to be an on‐target, antiproliferative response in the treatment of breast cancer. Myelosuppressive events have consistently been shown with CDK 4/6 inhibitors in clinical trials [13], [15], [16], [17], [18]. Initial phase II clinical trial data show that palbociclib‐related hematologic AEs, including neutropenia, are mostly uncomplicated, transient, and generally of short duration [19]. In addition, a low incidence of febrile neutropenia was reported in patients receiving palbociclib (0.9%) [20]. Moreover, other analyses showed that these AEs have been successfully managed via dose reductions (without apparent loss of efficacy) [14], [20], with no differences in the rate of permanent treatment discontinuations between palbociclib and comparator treatment arms [14].
To better elucidate the safety profile of palbociclib used in combination with letrozole, the clinical patterns of hematologic AEs in PALOMA‐2 were evaluated over time, with an emphasis on neutropenia and the consequences of neutropenia (e.g., infection and dose modifications).
Materials and Methods
Patients
PALOMA‐2 enrolled postmenopausal women with histologically or cytologically confirmed ER+/HER2− ABC (locoregionally recurrent or metastatic) who had not received prior systemic anticancer therapy for ER+ advanced disease. Eligibility criteria (supplemental online Table 1) and study design have been previously published [14]. Disposition of patients is shown in supplemental online Figure 1. The protocol was approved by an institutional review board or independent ethics committee at each participating site. The study was conducted in accordance with the Declaration of Helsinki, and all patients provided written informed consent.
Study Design
PALOMA‐2 (ClinicalTrials.gov, NCT01740427) is an international, randomized, double‐blind, placebo‐controlled, phase III study designed to assess the efficacy and safety of palbociclib plus letrozole as first‐line therapy in postmenopausal women with ER+/HER2− ABC. The data cutoff for the current analyses was February 26, 2016, and is in line with the data cutoff from the primary paper [14]. The primary endpoint was investigator‐assessed PFS, defined as the time from randomization to radiologically confirmed disease progression (Response Evaluation Criteria in Solid Tumors, version 1.1 [RECIST] criteria) or death during the study. Secondary endpoints included a safety evaluation of patients treated with palbociclib plus letrozole or placebo plus letrozole.
Treatment
Patients were randomized in a 2:1 ratio to oral palbociclib 125 mg per day plus letrozole 2.5 mg per day or placebo plus letrozole 2.5 mg per day. Palbociclib was administered daily on a 4‐week cycle (3 weeks on, 1 week off); letrozole was administered continuously. Stratification factors include disease site (visceral or nonvisceral), disease‐free interval from end of (neo)adjuvant therapy (de novo metastatic, ≤12 or > 12 months), and prior (neo)adjuvant hormonal therapy (yes, no). “Visceral” disease referred to any lung (including pleura) and/or liver involvement, and “nonvisceral” disease referred to absence of lung (including pleura) and/or liver involvement. There was no stratification by country or region of the world. Granulocyte colony stimulating factor (G‐CSF) was not recommended per protocol but could be used in accordance with American Society of Clinical Oncology guidelines [1].
Safety Assessment
Adverse event assessment included description of the event, duration, severity, timing, and seriousness of the AE and its relatedness to study treatment as reported by the investigator. Hematologic AEs were based on laboratory data and case report forms and summarized by worst grade severity. Neutropenia was determined based on laboratory tests of absolute neutrophil counts. Hematologic laboratory results were further verified for correct grading.
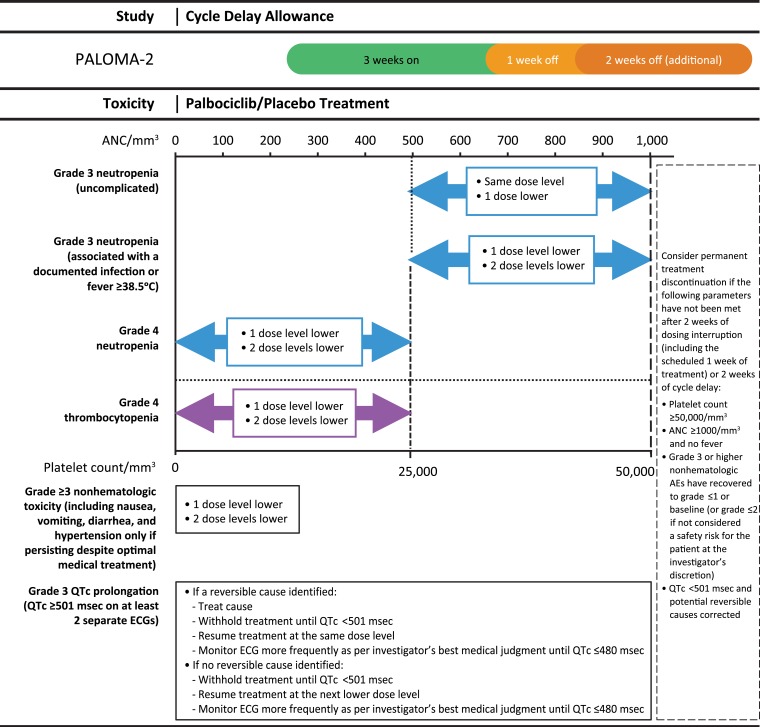
In the event of adverse reactions, dose reductions of palbociclib or placebo were permitted. Protocol‐required dose modifications for treatment‐related toxicities are shown in Figure 1. No dose adjustment for letrozole was permitted, but dosing interruptions were allowed. Discontinuations and dose reduction attributable to neutropenia and other hematologic AEs were based on data reported on case report forms.
Figure 1.
Dose modification scheme for managing palbociclib/placebo‐related toxicities.
Abbreviations: ANC, absolute neutrophil count; ECG, electrocardiogram; QTc, corrected QT.
Laboratory safety assessments were conducted at baseline, on days 1 and 15 of cycles 1 and 2, on day 1 of each subsequent cycle, and at end of treatment or withdrawal.
Data Analysis
Any patient who received ≥1 dose of study drug was included in the safety analyses. AEs reported by investigators were coded by the Medical Dictionary for Regulatory Activities (MedDRA) version 18.1, and AE severity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Descriptive statistics were used to summarize AE frequencies and the patterns of the AEs experienced by the patients. AE frequency was summarized based on the maximum toxicity grade for the patient during the treatment as reported by investigators. For AEs prespecified as being of clinical importance, the risk difference between the two treatment arms was calculated, along with their corresponding 95% CI, without adjustment for multiplicity. An overlap of at least 1 day was required for the analysis evaluating infection (any grade and grade 3/4) overlapping with neutropenia (any grade and grade 3/4).
The association between baseline characteristics and grade 3 or 4 neutropenia was examined for patients in the palbociclib treatment arm. Univariate analysis was used to assess the associations of these risk factors with neutropenia, which are presented as odds ratios with p values reported. A multivariate logistic analysis was conducted to evaluate potential risk factors for developing on‐treatment neutropenia. The covariate variables included in the multivariate logistic analysis were selected based on their clinical relevance and included prior chemotherapy status, previous radiotherapy status, age, baseline Eastern Cooperative Oncology Group performance status, number of disease sites, race, and baseline absolute neutrophil count.
To evaluate the influence of palbociclib dose reduction, regardless of cause, on efficacy outcome PFS, landmark analyses with landmarks at 3, 6, and 9 months were conducted using the Kaplan‐Meier method. In the landmark analysis, patients whose treatment duration was shorter than the landmark time were excluded. Patients who experienced dose reduction before the landmark were assigned to the dose‐reduction group, whereas those not requiring a dose reduction before the landmark were assigned to the non–dose‐reduction group.
Results
Patient Population
A total of 666 patients were enrolled in PALOMA‐2 between February 28, 2013, and July 29, 2014 (palbociclib plus letrozole, n = 444; placebo plus letrozole, n = 222; supplemental online Fig. 1). Demographics and baseline characteristics were generally balanced across both treatment groups and have been previously published (supplemental online Table 2) [14].
The median (range) duration of treatment was substantially longer in the palbociclib plus letrozole arm compared with the placebo plus letrozole arm (19.8 [0.033–34.1] vs. 13.6 [0.33–35.2] months). In the palbociclib plus letrozole arm, the median daily dose of palbociclib was 125 mg, and the median relative dose intensity was 93.9% (range, 40.3–109.5).
Overall, 55.2% (n = 245) of patients receiving palbociclib plus letrozole and 72.5% (n = 161) of patients receiving placebo plus letrozole discontinued study treatment by the cutoff date for the current analyses. The majority of patients who permanently discontinued study treatment did so because of objective progressive disease (38.5% for palbociclib plus letrozole vs. 56.8% for placebo plus letrozole). The second leading cause of discontinuation from study treatment was the occurrence of an AE (7.4% for palbociclib plus letrozole vs. 4.5% for placebo plus letrozole). Overall, 1.4% of patients receiving palbociclib plus letrozole discontinued palbociclib because of a hematological AE, whereas 0.2% of patients discontinued both palbociclib and letrozole. Treatment‐related AEs associated with permanent discontinuation were reported in 5.6% of patients receiving palbociclib plus letrozole and 1.4% receiving placebo plus letrozole.
Safety Analysis
Hematologic‐Specific Adverse Events: Investigator Reported.
The overall incidence of AEs was comparable between the two treatment arms (palbociclib plus letrozole, 98.9%; placebo plus letrozole, 95.5%), with no unexpected AE findings for palbociclib (supplemental online Table 3). The most common all‐causality AE was neutropenia in the palbociclib plus letrozole arm (79.5%) and infection in the placebo plus letrozole arm (42.3%). Grades 3 and 4 neutropenia were reported in 249 (56.1%) and 46 (10.4%) patients, respectively, in the palbociclib arm and in 2 (0.9%) and 1 (0.5%) patients in the placebo arm. Febrile neutropenia was reported in 1.8% (n = 8; all non‐Asian) of patients in the palbociclib plus letrozole arm and did not result in permanent treatment discontinuation for any patient. Febrile neutropenia was not reported in the placebo plus letrozole arm. The incidence of serious AEs (SAEs; all causality, all cycles) was 19.6% in patients receiving palbociclib plus letrozole (supplemental online Table 4).
Neutropenia was also the most common treatment‐related AE reported in patients receiving palbociclib plus letrozole (78.4%). Treatment‐related serious AEs were reported in 5.4% (supplemental online Table 4) and 0.9% of the patients in the palbociclib plus letrozole and placebo plus letrozole arms, respectively (treatment‐related AEs and SAEs were AEs and SAEs assessed by the investigator as being related to treatment, possibly related to treatment, or unknown if related to treatment). Overall, the incidence of treatment‐related hematologic SAEs was low in patients receiving palbociclib plus letrozole (≤0.5%; supplemental online Table 4). In only one patient (palbociclib plus letrozole arm) was neutropenia assessed as a serious AE; the patient was initially hospitalized for cellulitis and subsequently developed grade 4 neutropenia during the hospitalization stay.
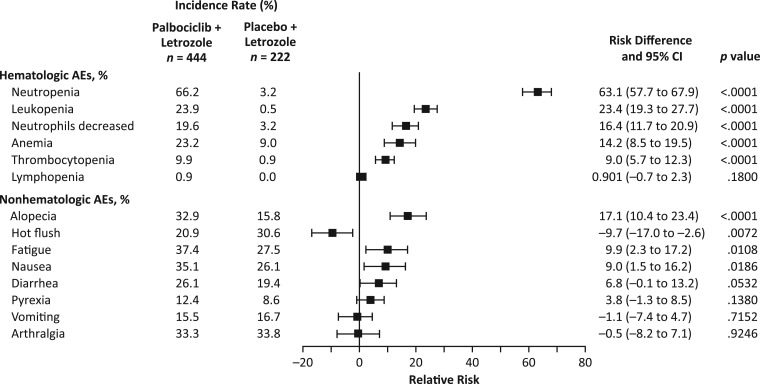
Figure 2 shows the risk difference between patients in the two treatment arms for selected hematologic and nonhematologic AEs. Patients treated with palbociclib plus letrozole had a significantly higher risk of experiencing AEs of neutropenia, leukopenia, neutrophil count decreased, anemia, and thrombocytopenia (all causality, any grade) versus placebo plus letrozole, as well as of experiencing the nonhematologic AEs of alopecia, fatigue, and nausea (all causality, any grade).
Figure 2.
Risk difference for prespecified adverse events of clinical importance (hematologic and nonhematologic: all causalities, all cycles, as‐treated population).
Abbreviations: AE, adverse event; CI, confidence interval.
Hematologic‐Specific Toxicities: Neutropenia, Anemia, and Thrombocytopenia: Assessment of Laboratory Data.
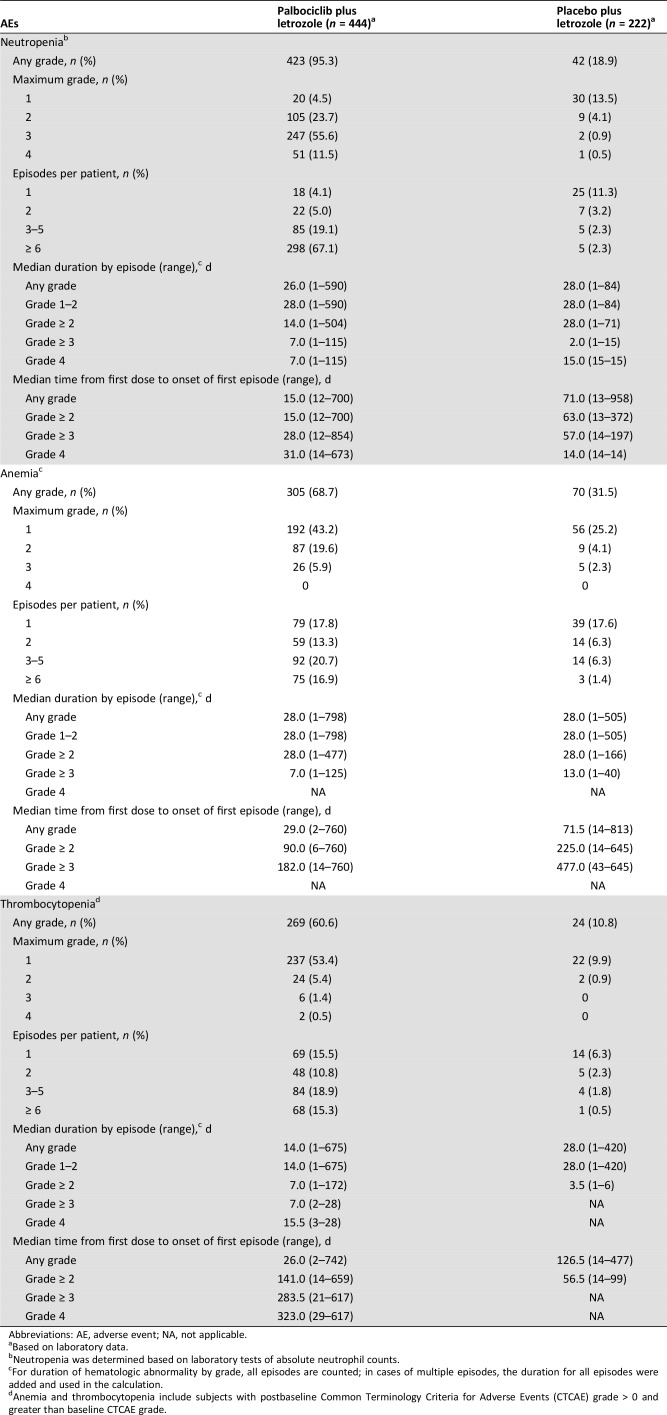
The frequency of any‐grade neutropenia was higher in the palbociclib plus letrozole arm versus the placebo plus letrozole arm (95.3% vs. 18.9%), with similar findings for grade 3/4 neutropenia (67.1% vs. 1.4%; Table 1). Median (range) time from first dose to first episode of any‐grade neutropenia was 15.0 (12–700) and 71.0 (13–958) days for palbociclib plus letrozole and placebo plus letrozole, respectively; time to grade ≥ 3 neutropenia was 28.0 (12–854) and 57.0 (14–197) days (supplemental online Fig. 2). Median (range) duration of a grade ≥ 3 neutropenia episode was 7 (1–115) days for patients receiving palbociclib plus letrozole and 2.0 (1–15) days for placebo plus letrozole.
Table 1. Summary of key hematologic laboratory abnormalities.
Abbreviations: AE, adverse event; NA, not applicable.
Based on laboratory data.
Neutropenia was determined based on laboratory tests of absolute neutrophil counts.
For duration of hematologic abnormality by grade, all episodes are counted; in cases of multiple episodes, the duration for all episodes were added and used in the calculation.
Anemia and thrombocytopenia include subjects with postbaseline Common Terminology Criteria for Adverse Events (CTCAE) grade > 0 and greater than baseline CTCAE grade.
The frequency of grade 3/4 neutropenia was higher in earlier cycles compared with later cycles. For example, 56.1% of patients (n = 249) in the palbociclib plus letrozole arm had grade 3/4 neutropenia in the first four cycles, with 67.5% (n = 168) of these patients subsequently developing grade 3/4 neutropenia in later cycles. In addition, the percentage of patients with maximum grade 3 neutropenia in cycles 1–6 was 49.5% (n = 220), whereas only 3.6% (n = 8) of these patients developed grade 4 neutropenia after the first 6 cycles.
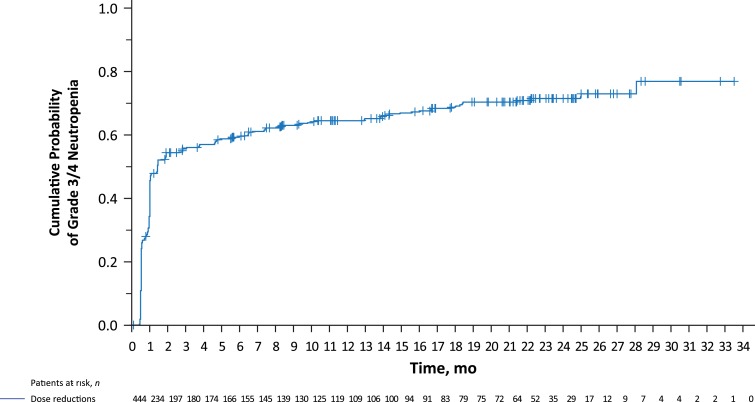
A Kaplan‐Meier plot showed that for the majority of patients, their first experience of grade 3 or 4 neutropenia occurred within the first 2 or 3 months of treatment (Fig. 3).
Figure 3.
Kaplan‐Meier curve showing cumulative incidences of grade 3/4 neutropenia (assessed by laboratory data) in patients treated with palbociclib plus letrozole.
Any‐grade anemia and thrombocytopenia occurred frequently in the palbociclib plus letrozole arm; however, the overall incidence was lower than that of neutropenia, and severity was generally mild to moderate. Median (range) time from first dose to the first episode of any‐grade anemia was 29.0 (2–760) days for palbociclib plus letrozole. Median time to onset of first grade ≥ 3 anemia episode was 182.0 (14–760) days (supplemental online Fig. 2) in the palbociclib plus letrozole arm. Median duration of a grade ≥ 3 episode of anemia was 7.0 (1–125) days. Median time from first dose to the first episode of any‐grade thrombocytopenia was 26.0 (2–742) days. Median time to onset of first grade ≥ 3 thrombocytopenia episode was 283.5 (21–617) days for patients receiving palbociclib plus letrozole (supplemental online Fig. 2); no patient in the placebo arm had grade ≥ 3 thrombocytopenia. Median duration of a grade ≥ 3 thrombocytopenia episode was 7.0 (2–28) days for patients treated with palbociclib plus letrozole. Of the two patients in the palbociclib group and three patients in the placebo group who reported an AE of bleeding (i.e., hematoma or hemorrhage), none experienced thrombocytopenia during the study.
Risk Factors for the Development of Grade 3/4 Neutropenia: Assessment of Laboratory Data.
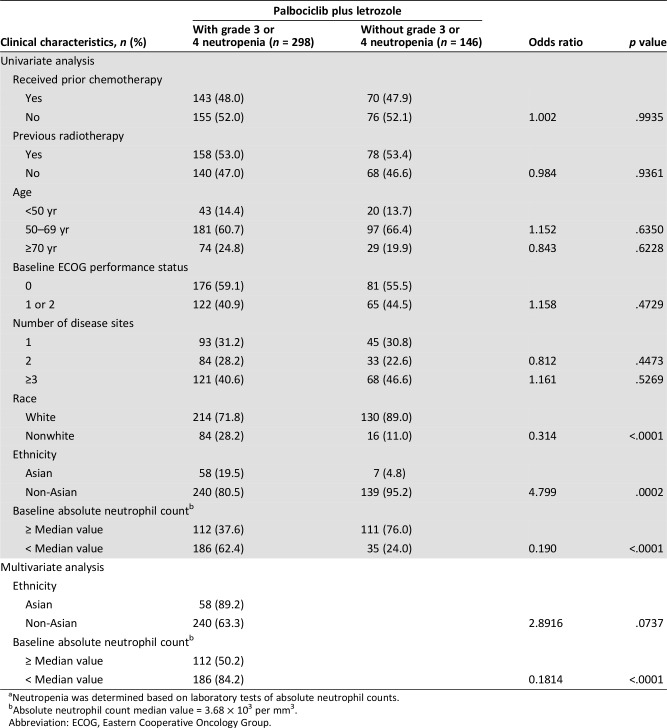
Univariate analysis of laboratory data identified nonwhite race (odds ratio, 0.314; p < .0001 vs. white), Asian ethnicity (odds ratio, 4.799; p = .0002 vs. non‐Asian), and baseline absolute neutrophil count <3.68 × 103/mm3 (odds ratio, 0.190; p < .0001) as potential risk factors for developing grade 3/4 neutropenia for patients receiving palbociclib plus letrozole (Table 2). No associations were identified between the risk of developing grade 3/4 neutropenia and prior chemotherapy or prior radiotherapy (Table 2).
Table 2. Risk of developing grade 3 or 4 neutropeniaa by clinical characteristics in patients receiving palbociclib plus letrozole.
Neutropenia was determined based on laboratory tests of absolute neutrophil counts.
Absolute neutrophil count median value = 3.68 × 103 per mm3.
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Multivariate analysis confirmed baseline absolute neutrophil count <3.68 × 103/mm3 (odds ratio, 0.1814; p < .0001) as a potential risk factor for developing grade 3/4 neutropenia for patients receiving palbociclib plus letrozole (Table 2).
Neutropenia‐Associated Infection.
To evaluate clinical outcomes that were potentially associated with neutropenia, we assessed the overlapping of infection and neutropenia (using laboratory data) of at least 1 day. The overlapping of infection and neutropenia occurred in 219 (51.8%) patients in the palbociclib plus letrozole arm, with the majority (96.5%) of infections being grade 1/2 in severity (supplemental online Fig. 3). Overlapping grade 3/4 infections occurred in only 15 (3.4%) patients with neutropenia (all grades). Among these 15 patients, grade 3/4 infections comprised grade 3 urinary tract infection (n = 4); cellulitis (n = 3); influenza (n = 2); appendicitis, breast cellulitis, bronchitis, viral gastroenteritis, pneumonia, and sinusitis (n = 1 each); and grade 4 urosepsis (n = 1). Among the 298 (67%) patients receiving palbociclib plus letrozole with grade 3/4 neutropenia, 93 (31.2%) had overlapping infections (all grades). Infections (all grades) occurring with an incidence of ≥5% in patients with grade 3/4 neutropenia included upper respiratory tract infection (19.4%), nasopharyngitis (18.3%), urinary tract infection (11.8%), oral herpes (10.8%), sinusitis (9.7%), conjunctivitis (6.5%), herpes zoster (6.5%), and rhinitis (5.4%). Six (2.0%) patients had an overlapping grade 3 infection (urinary tract infection, n = 2; breast cellulitis, cellulitis, influenza, and sinusitis, n = 1 each); no patient had a grade 4 infection.
Granulocyte colony stimulating factor could be administered to patients with treatment‐emergent neutropenia. A total of 10.7% of patients in the palbociclib plus letrozole arm received G‐CSF (8.6%, 1.6%, and 0.5% of patients received filgrastim, pegfilgrastim, and lenograstim, respectively).
Effect of Dose Reduction and Neutropenia Severity on Efficacy Endpoints
Permanent treatment discontinuation from the study as a result of either all‐causality neutropenia (n = 5 [1.1%]) or decreased neutrophil count (n = 2 [0.5%]) was observed in patients receiving palbociclib plus letrozole (Table 3). Febrile neutropenia, leukopenia, anemia, and thrombocytopenia did not result in permanent discontinuation of study treatment in either treatment arm. Patients receiving palbociclib plus letrozole had an increased frequency of temporary study treatment discontinuation because of neutropenia (54.7% vs. 0.9%), decreased neutrophil count (13.3% vs. 0.5%), febrile neutropenia (1.6% vs. 0%), or thrombocytopenia (1.1% vs. 0.5%) compared with those receiving placebo plus letrozole.
Table 3. Hematologic AEsa associated with permanent or temporary treatment discontinuations and dose reductions.
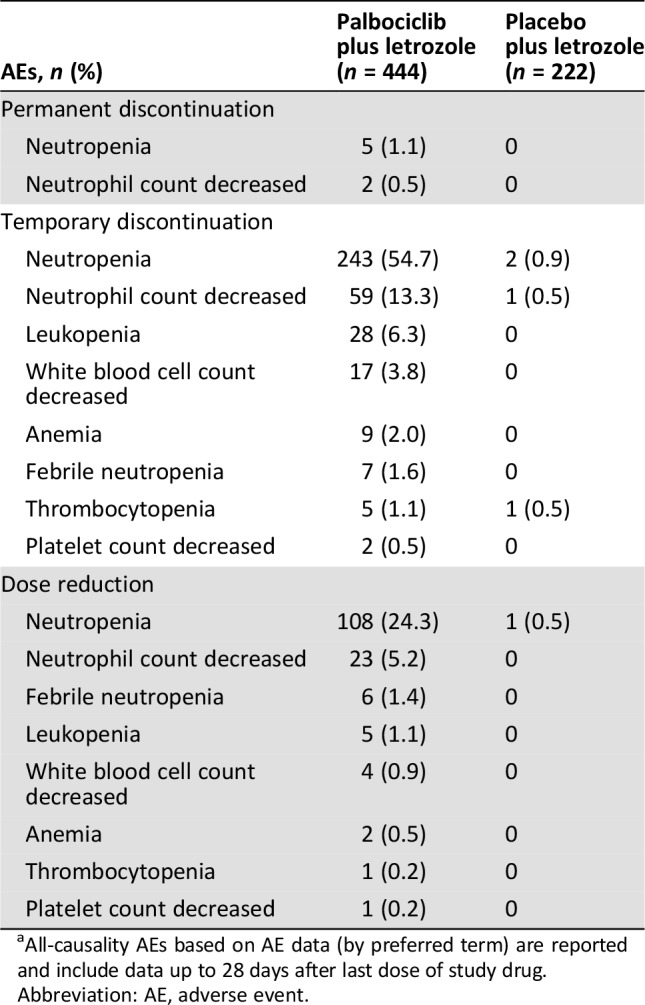
All‐causality AEs based on AE data (by preferred term) are reported and include data up to 28 days after last dose of study drug.
Abbreviation: AE, adverse event.
Among patients with all‐causality AEs associated with dose reduction in the palbociclib plus letrozole arm, 67.5% (108 of 160 patients) had a dose reduction associated with neutropenia (24.3% of patients in the palbociclib plus letrozole arm vs. 0.5% of patients in the placebo plus letrozole arm; Table 3), 14.4% (23 of 160 patients) had a dose reduction associated with decreased neutrophil count (5.2% in the palbociclib arm vs. 0% in the placebo arm), and 3.8% (6 of 160 patients) had a dose reduction associated with febrile neutropenia (1.4% in the palbociclib arm vs. 0% in the placebo arm). Although the study protocol defined the toxicities for which dose reductions were to be made to manage the toxicity, it should be noted that some reductions were not necessarily due to toxicity findings.
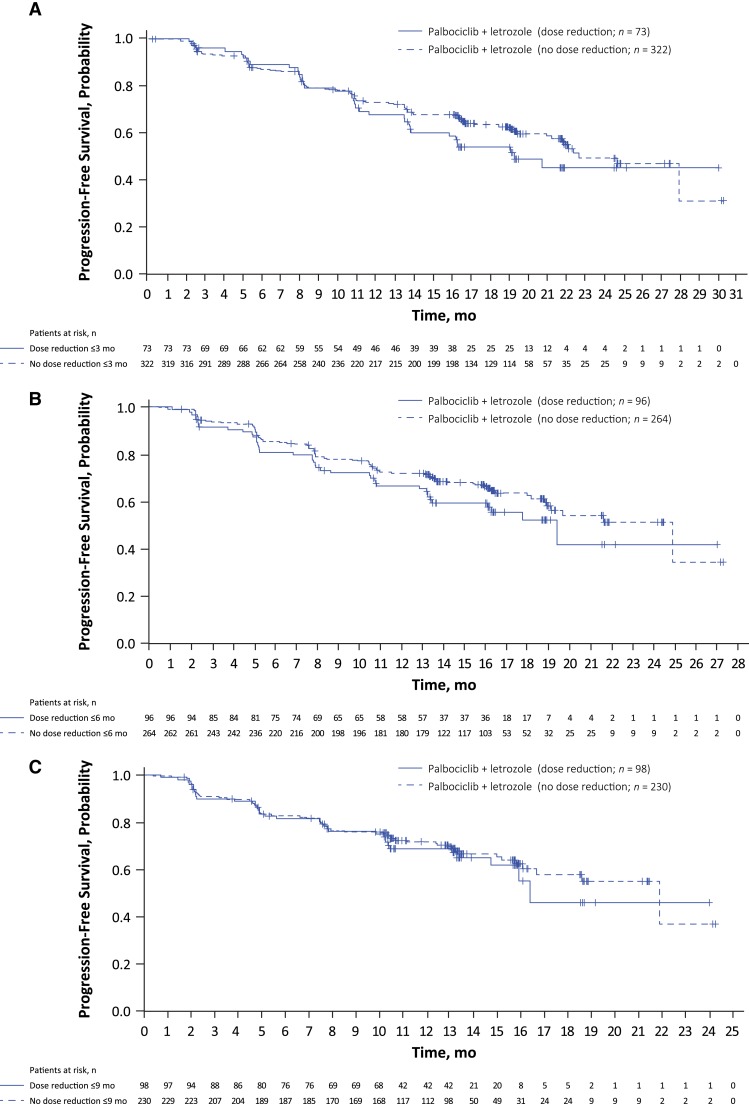
A landmark analysis using three different landmarks (3, 6, and 9 months) suggested that in patients receiving palbociclib plus letrozole, PFS was similar among those who experienced dose reduction and those who did not (Fig. 4A–C).
Figure 4.
Progression‐free survival landmark analysis for patients experiencing palbociclib dose reduction versus no dose reduction. Landmark set at (A) 3 months, (B) 6 months, and (C) 9 months. Patients with a progression‐free survival time ≤ landmarks were excluded from the corresponding analysis.
A second landmark analysis assessed PFS for patients who developed palbociclib‐associated grade 3/4 neutropenia at 3, 6, and 9 months (supplemental online Fig. 4A–C). These results suggest that the occurrence of grade 3/4 neutropenia does not appear to compromise efficacy (i.e., PFS benefit).
Tumor Response: Assessment of Laboratory Data
The investigator‐assessed confirmed objective response rate (ORR) was similar among patients receiving palbociclib, irrespective of the severity of neutropenia (supplemental online Table 5). The ORR (95% CI) was 41.6% (32.9%–50.8%) for patients with maximum grade 1/2 neutropenia, 44.6% (38.9%–50.5%) for maximum grade 3/4 neutropenia, and 43.7% (38.9%–48.6%) for any‐grade neutropenia. Investigator‐assessed confirmed clinical benefit response rates (95% CI) were also similar, irrespective of neutropenia severity, although slightly higher for patients with maximum grade 3/4 neutropenia (87.6% [83.3%–91.1%]) versus maximum grade 1/2 neutropenia (82.4% [74.6%–88.6%]; supplemental online Table 5).
Discussion
This detailed safety analysis of PALOMA‐2 showed that the combination of palbociclib plus letrozole was generally well tolerated in postmenopausal women with ER+/HER2− ABC who had not received prior systemic anticancer therapies for their advanced disease. AEs, including treatment‐related hematologic AEs, are consistent with the known safety profile for palbociclib plus letrozole. Treatment‐related hematologic toxicities, including a high incidence of neutropenia, were manageable by dosing interruption, dose reduction, cycle delay, and/or standard medical therapy. The clinical patterns of neutropenia and other hematologic toxicities with palbociclib were consistent with observations in PALOMA‐1 and PALOMA‐3 [13], [14], [16] and rarely led to permanent treatment discontinuation in PALOMA‐2; although neutropenia occurred at a higher frequency than other AEs, the incidence of febrile neutropenia remained low (1.8%), and this toxicity did not result in any permanent treatment discontinuations. Anemia and thrombocytopenia also occurred more frequently in patients treated with palbociclib plus letrozole, albeit with a substantially lower incidence than neutropenia, and those hematologic toxicities were mostly grade 1/2 in severity.
Neutropenia (any grade) occurred at a higher frequency in patients receiving palbociclib plus letrozole compared with placebo plus letrozole (95.3% vs. 18.9%). However, this significantly higher frequency of neutropenia did not compromise the duration of treatment, which was longer for patients receiving palbociclib plus letrozole compared with patients receiving placebo plus letrozole (19.8 vs. 13.6 months, respectively). The median time to onset of any‐grade neutropenia and grade ≥ 3 neutropenia was 15 days and 28 days, respectively, for patients receiving palbociclib plus letrozole. In this study, the median duration of grade ≥ 3 neutropenia (by episode) was 7 days. Although approximately two‐thirds of palbociclib dose reductions were attributed to neutropenia, dose reductions did not appear to compromise PFS, suggesting that effective therapeutic levels of palbociclib were maintained. A potential explanation for this observation is that patients who required dose reductions may have experienced fewer dose delays, and, consequently, the exposure over time may have been similar to those patients who did not require dose reductions. A recent exposure‐response analysis of PALOMA‐2 showed that PFS was similar between Asian and non‐Asian patients, even though Asian patients had more frequent dose reductions [21]. This finding was likely the result of the average palbociclib exposure being higher in Asian patients because of lower clearance versus non‐Asians. Thus, in both of these incidences, dose reductions may not have necessarily resulted in lower exposure over time.
Findings in the present study showed that clinical characteristics, such as Asian ethnicity and low baseline absolute neutrophil counts, may increase the risk of developing grade 3/4 neutropenia. Additionally, an analysis of the Asian subgroup in PALOMA‐2 revealed that Asian patients had lower mean and median baseline absolute neutrophil counts compared with non‐Asians [22]. These observations highlight the importance of closely monitoring these patients and intervening with dose adjustments when indicated. However, because hematologic AE severity appears to vary by patient, this recommendation to closely monitor patients for neutropenia should apply to all patients receiving palbociclib as a means to reduce the incidence or recurrence of more severe grades of neutropenia.
Approximately 11% of patients in the palbociclib plus letrozole arm received G‐CSF to manage neutropenia during the study. A similar percentage was reported in the PALOMA‐3 study, which was performed in women with HR+/HER2− ABC with prior progression on endocrine therapy and in which only a minority of patients (<11%) in the palbociclib plus fulvestrant arm received G‐CSF to manage neutropenia [20]. It should be noted that G‐CSF was administered based on the investigator's judgment, as its use was not a protocol recommendation for the management of neutropenia. Considering the low incidence of febrile neutropenia and rapid neutrophil recovery reported in patients administered palbociclib [13], [14], [20], there is no recommendation for prophylactic use of G‐CSF or antibiotics.
Neutropenia associated with cytotoxic chemotherapeutic agents is typically the result of DNA damage and apoptotic cell death of bone marrow mononuclear cells [15], resulting in acute onset of neutropenia and a prolonged suppression of neutrophils [23]. However, neutropenia observed with palbociclib therapy is typically transient, reflecting a cytostatic effect on neutrophil precursors in the bone marrow, with a reinitiation of the cell cycle during the 1 week treatment‐free period [13], [15]. Hu et al. demonstrated in tumor cells that chemotherapeutic agents and palbociclib both inhibited cell proliferation and induced cellular senescence without cellular apoptosis, whereas in bone marrow cells, apoptosis was observed with chemotherapeutic agents but not with palbociclib (i.e., cytotoxic vs. cytostatic) [15]. Consequently, the palbociclib‐free week between dosing cycles helps restore neutrophil count, whereas dose modification or short interruptions of therapy can be used to prevent higher grade neutropenia episodes without affecting anticancer efficacy in tumor cells [15]. The recommended dosing schedule for palbociclib (3 weeks on, 1 week off) provides a safe way to manage neutropenia without compromising efficacy.
Chemotherapy‐induced neutropenia has been associated with an increased risk of life‐threatening infections in patients with cancer [24], [25], and in this study, overlapping infections (all grades) in patients with neutropenia (all grades) were observed in 51.8% of patients in the palbociclib plus letrozole arm. However, 93.2% of these infections were grade 1/2 in severity, and no patient in the palbociclib arm experienced a fatal infection. Moreover, in patients with grade 3/4 neutropenia, 31.2% of patients had an overlapping infection (all grades), the majority (93.5%) of which were grade 1/2.
Palbociclib appeared to be well tolerated in this study, resulting in a permanent discontinuation rate due to AEs of 9.7%. However, the observed increased incidence of AEs could potentially have a negative impact on patient health and quality of life. Consequently, a recent analysis of PALOMA‐2 was performed using the Functional Assessment of Cancer Therapy–Breast and EuroQoL–5 Dimension questionnaires to assess patient‐reported health‐related quality of life [26]. Patient responses demonstrated that adding palbociclib to letrozole therapy maintained health‐related quality of life and was associated with improved pain scores in treatment‐naive postmenopausal women with ER+/HER2− ABC compared with those treated with letrozole alone. In particular, no statistically significant difference was observed for either index score in patients with or without neutropenia [26].
Although the CDK 4/6 inhibitors currently approved for the treatment of HR+/HER2− advanced or metastatic breast cancer share similar overall AE profiles, there are several important differences with respect to hematologic cytopenic effects [6], [7], [27], [28], [29]. For example, whereas both palbociclib (PALOMA‐2) [14] and ribociclib (phase III MONALEESA‐2) [30] had similarly high incidences of any‐grade and grade 3/4 neutropenia (80% and 66%, respectively, for palbociclib vs. 74% and 59% for ribociclib), the phase III MONARCH 3 study showed a relatively lower rate of neutropenia with abemaciclib (41% and 21%), which may potentially be due to the greater potency of abemaciclib against CDK4 versus CDK6 [29]. However, the incidence of diarrhea was much lower for palbociclib [14] and ribociclib [30] compared with abemaciclib [29] (26% and 1% for palbociclib and 35% and 1% for ribociclib vs. 81% and 10% for abemaciclib). Palbociclib also demonstrated a lower incidence of thrombocytopenia compared with abemaciclib (16% and 2% vs. 36% and 2%) [14], [29]. Because all three of these CDK 4/6 inhibitors have shown clinical benefit in the treatment of HR+/HER2− ABC [27], the choice of an appropriate CDK 4/6 agent in clinical practice will likely be driven by the PFS benefit and safety considerations given the clinical characteristics of each individual patient.
Conclusion
In PALOMA‐2, palbociclib in combination with letrozole was well tolerated, with a safety profile similar to that observed in previous clinical studies. Neutropenia was the most common AE associated with palbociclib treatment; however, it rarely resulted in permanent discontinuation from study treatment, and the risk of developing febrile neutropenia was low. Consistent with its mechanism of action, palbociclib‐induced neutropenia was generally transient and readily managed through dose and treatment schedule adjustments, without the need for G‐CSF support, while appearing to maintain PFS benefits in most women with ER+/HER2− ABC.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This study was sponsored by Pfizer Inc. Pfizer provided financial support for this study and would like to extend their appreciation to all patients who participated in the study. Editorial and medical writing support was provided by Alan J. Klopp, Ph.D., C.M.P.P., and Anny Wu, Pharm.D., of Complete Healthcare Communications, LLC (North Wales, PA), a CHC Group company, and was funded by Pfizer. Full responsibility for the final manuscript rested solely with the authors.
Author Contributions
Conception/design: Véronique Diéras, Nadia Harbeck, Anil Abraham Joy, Eric Gauthier, Patrick Schnell, Ave Mori, Hope S. Rugo
Financial support: Eric Gauthier
Administrative support: Eric Gauthier
Provision of study material or patients: Véronique Diéras, Nadia Harbeck, Anil Abraham Joy, Johannes Ettl, Sunil Verma, Eric Gauthier, Hope S. Rugo
Collection and/or assembly of data: Véronique Diéras, Nadia Harbeck, Anil Abraham Joy, Johannes Ettl, Eric Gauthier, Hope S. Rugo
Data analysis and interpretation: Véronique Diéras, Nadia Harbeck, Anil Abraham Joy, Johannes Ettl, Sunil Verma, Dongrui R. Lu, Eric Gauthier, Patrick Schnell, Ave Mori, Hope S. Rugo
Manuscript writing: Véronique Diéras, Nadia Harbeck, Anil Abraham Joy, Karen Gelmon, Johannes Ettl, Sunil Verma, Dongrui R. Lu, Eric Gauthier, Hope S. Rugo, Patrick Schnell, Ave Mori, Richard S. Finn
Final approval of manuscript: Véronique Diéras, Nadia Harbeck, Anil Abraham Joy, Karen Gelmon, Johannes Ettl, Sunil Verma, Dongrui R. Lu, Eric Gauthier, Patrick Schnell, Ave Mori, Hope S. Rugo, Richard S. Finn
Disclosures
Véronique Diéras: Roche, Genentech, Pfizer, Novartis, Eli Lilly & Co, Abbvie, Odonate Therapeutics, Seattle Genetics, Daiichi Sankyo (C/A, H, SAB); Nadia Harbeck: Eli Lilly & Co, Novartis, Pfizer (C/A, H); Anil Abraham Joy: Pfizer, AstraZeneca, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Novartis, Puma, Roche (C/A); Karen Gelmon: Novartis, A&Z Pharmaceutical, Pfizer, Mylan, Merck, Nanostring, Genomic Health, Roche (C/A); Johannes Ettl: Pfizer, Novartis, Eli Lilly & Co, Roche (C/A), Celgene (RF), Pfizer, Novartis, Eli Lilly & Co, Roche, Celgene (H), Pfizer, Celgene (other—Travel Support); Sunil Verma: Amgen, AstraZeneca, Bayer, Daiichi Sankyo, Pfizer, Novartis, Roche (C/A); Dongrui R. Lu: Pfizer (E, OI), Pfizer (Spouse‐E, OI); Eric Gauthier: Pfizer (E, OI); Patrick Schnell: Pfizer (E, OI); Ave Mori: Pfizer (E, OI); Hope S. Rugo: Pfizer, Merck, Novartis, Eli Lilly & Co, Genentech, OBI Pharma, Plexxikon, Odonate Therapeutics, Daiichi Sankyo, Eisai, Macrogenics (RF), Celltrion (H), Eli Lilly & Co, Mylan, Pfizer, Daiichi Sankyo, Amgen, Merck, Puma, AstraZeneca (other—travel support); Richard S. Finn: Astra Zeneca, Bayer, Bristol‐Myers Squibb, Eisai, Eli Lilly & Co, Novartis, Pfizer, Merck, Roche/Genentech (C/A).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Rugo HS, Rumble RB, Macrae E et al. Endocrine therapy for hormone receptor‐positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 2016;34:3069–3103. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso F, Costa A, Senkus E et al. 3rd ESO‐ESMO international consensus guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol 2017;28:16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network . Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer. Version 1. 2018. Available at https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed April 9, 2018.
- 4.Hayes EL, Lewis‐Wambi JS. Mechanisms of endocrine resistance in breast cancer: An overview of the proposed roles of noncoding RNA. Breast Cancer Res 2015;17:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afinitor (everolimus). Full Prescribing Information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2018.
- 6.Ibrance (palbociclib). Full Prescribing Information. New York, NY: Pfizer Inc; 2018. [Google Scholar]
- 7.Kisqali (ribociclib). Full Prescribing Information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017. [Google Scholar]
- 8.McCain J. First‐in‐class CDK4/6 inhibitor palbociclib could usher in a new wave of combination therapies for HR+, HER2‐ breast cancer. P T 2015;40:511–520. [PMC free article] [PubMed] [Google Scholar]
- 9.Fry DW, Harvey PJ, Keller PR et al. Specific inhibition of cyclin‐dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004;3:1427–1438. [PubMed] [Google Scholar]
- 10.Marzec M, Kasprzycka M, Lai R et al. Mantle cell lymphoma cells express predominantly cyclin D1a isoform and are highly sensitive to selective inhibition of CDK4 kinase activity. Blood 2006;108:1744–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn RS, Dering J, Conklin D et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor‐positive human breast cancer cell lines in vitro. Breast Cancer Res 2009;11:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrance (palbociclib). Summary of Product Characteristics . Freiburg, Germany: Pfizer Inc; 2016. [Google Scholar]
- 13.Finn RS, Crown JP, Lang I et al. The cyclin‐dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first‐line treatment of oestrogen receptor‐positive, HER2‐negative, advanced breast cancer (PALOMA‐1/TRIO‐18): A randomised phase 2 study. Lancet Oncol 2015;16:25–35. [DOI] [PubMed] [Google Scholar]
- 14.Finn RS, Martin M, Rugo HS et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016;375:1925–1936. [DOI] [PubMed] [Google Scholar]
- 15.Hu W, Sung T, Jessen B et al. Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clin Cancer Res 2016;22:2000–2008. [DOI] [PubMed] [Google Scholar]
- 16.Cristofanilli M, Turner NC, Bondarenko I et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone‐receptor‐positive, HER2‐negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA‐3): Final analysis of the multicentre, double‐blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–439. [DOI] [PubMed] [Google Scholar]
- 17.Zangardi ML, Spring LM, Blouin GC et al. Ribociclib for post‐menopausal women with HR+/HER2‐ advanced or metastatic breast cancer. Expert Rev Clin Pharmacol 2017;10:1169–1176. [DOI] [PubMed] [Google Scholar]
- 18.Polk A, Kolmos IL, Kümler I et al. Specific CDK4/6 inhibition in breast cancer: A systematic review of current clinical evidence. ESMO Open 2016;1:e000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeMichele A, Clark AS, Tan KS et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: Phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res 2015;21:995–1001. [DOI] [PubMed] [Google Scholar]
- 20.Verma S, Bartlett CH, Schnell P et al. Palbociclib in combination with fulvestrant in women with hormone receptor‐positive/HER2‐negative advanced metastatic breast cancer: Detailed safety analysis from a multicenter, randomized, placebo‐controlled, phase 3 study (PALOMA‐3). The Oncologist 2016;21:1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng J, Yu Y, Durairaj C et al. Palbociclib exposure‐response analyses in treatment of hormone‐receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER–) advanced breast cancer (ABC). Presented at: 40th Annual San Antonio Breast Cancer Symposium; December 5‐9, 2017; San Antonio, TX.
- 22.Im SA, Mukai H, Park IH et al. Palbociclib plus letrozole as first‐line therapy in postmenopausal Asian women with ER+/HER2– metastatic breast cancer: Results from the phase 3, randomized PALOMA‐2 study. J Glob Oncol 2019;5:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tofts PS, Chevassut T, Cutajar M et al. Doubts concerning the recently reported human neutrophil lifespan of 5.4 days. Blood 2011;117:6050‐6052;6053–6054. [DOI] [PubMed] [Google Scholar]
- 24.Crawford J, Dale DC, Lyman GH. Chemotherapy‐induced neutropenia: Risks, consequences, and new directions for its management. Cancer 2004;100:228–237. [DOI] [PubMed] [Google Scholar]
- 25.Lyman GH. Chemotherapy dose intensity and quality cancer care. Oncology (Williston Park) 2006;20:16–25. [PubMed] [Google Scholar]
- 26.Rugo HS, Dieras V, Gelmon KA et al. Impact of palbociclib plus letrozole on patient‐reported health‐related quality of life: Results from the PALOMA‐2 trial. Ann Oncol 2018;29:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwata H. Clinical development of CDK4/6 inhibitor for breast cancer. Breast Cancer 2018;25:402–406. [DOI] [PubMed] [Google Scholar]
- 28.Verzenio (abemaciclib). Full Prescribing Information. Indianapolis, IN: Eli Lilly & Co. USA, LLC.; 2018.
- 29.Goetz MP, Toi M, Campone M et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35:3638–3646. [DOI] [PubMed] [Google Scholar]
- 30.Hortobagyi GN, Stemmer SM, Burris HA et al. Ribociclib as first‐line therapy for HR‐positive, advanced breast cancer. N Engl J Med 2016;375:1738–1748. [DOI] [PubMed] [Google Scholar]