This article reports a new EHBH‐MVI scoring system, based on preoperative serological and postoperative pathological data, that was developed with the goal of predicting long‐term survival in patients with hepatocellular carcinoma with microvascular tumor invasion and to identify patients who may benefit from adjuvant therapy based on the prediction of poor postoperative prognosis using the new scoring system.
Keywords: Hepatocellular carcinoma, Microvascular invasion, R0 liver resection, Scoring system
Abstract
Background.
Microvascular invasion (MVI) is associated with poor postoperative survival outcomes in patients with hepatocellular carcinoma (HCC). An Eastern Hepatobiliary Surgery Hospital (EHBH) MVI scoring system was established to predict prognosis in patients with HCC with MVI after R0 liver resection (LR) and to supplement the most commonly used classification systems.
Materials and Methods.
Patients with HCC with MVI who underwent R0 LR as an initial therapy were included. The EHBH‐MVI score was developed from a retrospective cohort from 2003 to 2009 to form the training cohort. The variables associated with overall survival (OS) on univariate analysis were subsequently investigated using the log‐rank test, and the EHBH‐MVI score was developed using the Cox regression model. It was validated using an internal prospective cohort from 2011 to 2013 as well as three independent external validation cohorts.
Results.
There were 1,033 patients in the training cohort; 322 patients in the prospective internal validation cohort; and 493, 282, and 149 patients in the three external validation cohorts, respectively. The score was developed using the following factors: α‐fetoprotein level, tumor encapsulation, tumor diameter, hepatitis B e antigen positivity, hepatitis B virus DNA load, tumor number, and gastric fundal/esophageal varicosity. The score differentiated two groups of patients (≤4, >4 points) with distinct long‐term prognoses outcomes (median OS, 55.8 vs. 19.6 months; p < .001). The predictive accuracy of the score was greater than the other four commonly used staging systems for HCC.
Conclusion.
The EHBH‐MVI scoring system was more accurate in predicting prognosis in patients with HCC with MVI after R0 LR than the other four commonly used staging systems. The score can be used to supplement these systems.
Implications for Practice.
Microvascular invasion (MVI) is a major determinant of survival outcomes after curative liver resection for patients with hepatocellular carcinoma (HCC). Currently, there is no scoring system aiming to predict prognosis of patients with HCC and MVI after R0 liver resection (LR). Most of the widely used staging systems for HCC do not use MVI as an independent risk factor, and they cannot be used to predict the prognosis of patients with HCC and MVI after surgery. In this study, a new Eastern Hepatobiliary Surgery Hospital (EHBH) MVI scoring system was established to predict prognosis of patients with HCC and MVI after R0 LR. Based on the results of this study, postoperative adjuvant therapy may be recommended for patients with HCC and MVI with an EHBH‐MVI score >4. This score can be used to supplement the currently used HCC classifications to predict postoperative survival outcomes in patients with HCC and MVI.
Introduction
Hepatocellular carcinoma (HCC) is a common cause of cancer‐related death worldwide [1]. Hepatitis B virus (HBV) infection is associated with 70%–90% of patients with hepatocellular carcinoma (HCC) in the endemic Asia‐Pacific regions [2], [3], [4]. Liver resection (LR) or liver transplantation is still the first‐line curative‐intent therapy for early and intermediate stages of HCC [5], [6], [7]. Unfortunately, the 5‐year recurrence rates after curative liver resection (R0 LR) are as high as 70%–80%, which seriously limits the long‐term survival of patients with HCC [8], [9].
Microvascular tumor invasion (MVI) of HCC is common in HCC and is associated with early tumor recurrence with reduced survival outcomes [10]. MVI occurs in 15%–60% of resected specimens from patients with HCC [11]. Previous studies have confirmed MVI to be a poor prognostic factor of tumor recurrence and long‐term survival after R0 LR [12], [13], [14], [15]. There is still no universally accepted adjuvant therapy after R0 LR for patients with HCC with MVI, although adjuvant therapies have been shown to be beneficial in selected patients [16], [17], [18]. The best way to select these patients for postoperative adjuvant therapy remains to be identified. A scoring system that can predict prognosis of patients with HCC with MVI after R0 LR is needed.
There are many HCC staging systems currently in use [19], [20], [21], [22]. The most commonly used systems are the Barcelona Clinic Liver Cancer (BCLC) staging, the Okuda staging, the Cancer of the Liver Italian Program (CLIP) scoring system, and the tumor‐node‐metastasis (TNM) staging. The BCLC staging has been endorsed as the staging system and treatment algorithm for HCC by the European Association for the Study of Liver Diseases and the American Association for the Study of Liver Diseases [5], [6], [7], [19]. The BCLC system is based on tumor‐related parameters (tumor size, number of nodules, vascular invasion, and extrahepatic spread) and patient characteristics, including the Child‐Pugh liver function class and performance status. The Okuda staging system is based on the tumor area in the liver (> or < 50% of liver area involved) and three parameters that are related to liver function: ascites, serum albumin, and serum bilirubin levels [23]. This was the first staging system to combine liver function status with tumor parameters. The CLIP scoring system combines four tumor‐related features—tumor extent, tumor morphological features, serum α‐fetoprotein levels, and portal vein thrombosis—with a cirrhosis severity index and the Child‐Pugh score to stratify patients with HCC into different groups [24]. The American Joint Committee on Cancer/International Union Against Cancer staging system for HCC uses a TNM classification system to predict survival after resection [25]. The TNM system is based on tumor characteristics and the extent of tumor invasion; it does not take into account the underlying hepatic function. These staging systems do not include MVI as a risk factor; therefore, whether MVI has any impact on prognosis cannot be determined by them.
In this study, a new Eastern Hepatobiliary Surgery Hospital (EHBH) MVI scoring system was established, based on preoperative serological and postoperative pathological data, with the aim to accurately predict postoperative long‐term survival in patients with HCC with MVI after R0 LR as well as to identify a subgroup of patients with HCC with MVI who may benefit from postoperative adjuvant therapy based on the prediction of poor postoperative prognosis by this new scoring system.
Materials and Methods
Patients
Between January 2003 and December 2009, data from consecutive patients who underwent R0 LR for histologically confirmed HCC and MVI were collected at the EHBH. This group formed the training cohort of this study. From January 2011 to December 2013, an independent cohort of consecutive patients with HCC with diagnosed MVI after R0 LR as an initial therapy was prospectively studied in EHBH to form the prospective internal validation cohort. The study was approved by the Institutional Ethics Committee of the EHBH. Informed consent was obtained from all the patients for their data to be used for research. For the three other research centers, the data were collected from the patients at different time periods: for the Affiliated Tumour Hospital of Guangxi Medical University (ATH) from 2013 to 2017, Zhongshan Hospital (ZSH) from 2011 to 2013, and West China Hospital (WCH) from 2014 to 2016. Similar inclusion criteria were used in the four centers. Again, appropriate approvals and consents were obtained in each of these centers.
The inclusion criteria were patients with HCC with (a) no macrovascular invasion or extrahepatic metastasis; (b) good liver function with a Child‐Pugh A score or B7 (score ≤ 7); (c) preoperative serological examination and contrast‐enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen; (d) R0 LR (no gross residual tumor, negative microscopic margins) with ≥1 cm resection margins; (e) a histopathological diagnosis of MVI in the resected specimens as determined by two experienced pathologists [10], [17]; and (f) complete pathological data. Patients who underwent preoperative anticancer treatments, had a history of other cancers, had incomplete clinical data, or had undergone palliative tumor resection, portal vein embolization, or associating liver partition and portal vein ligation for staged hepatectomy procedures were excluded. Eligible patients who underwent R0 LR between 2005 and 2009 at EHBH were retrospectively included in the training cohort for the development of the EHBH‐MVI score. Another cohort of patients who were operated between 2011 and 2013 at the same center were prospectively included as the internal validation cohort. Patients who underwent R0 LR from the ATH, ZSH, and WCH during the study period were retrospectively entered to form the three external validation cohorts.
Preoperative and Postoperative Examinations
Routine preoperative examinations included imaging and serological examinations. All patients underwent a standard liver imaging protocol [26], which included abdominal ultrasonography, contrast‐enhanced MRI and/or computed tomographic scan of the abdomen, and plain radiography or noncontrast computed tomographic scan of the chest. All the radiological data were reviewed by two different and independent radiologists in their respective hospitals based on uniform diagnostic standards. Routine preoperative laboratory examinations included routine blood tests, liver and renal function tests, hepatitis B and C serology, HBV DNA load, and serum α‐fetoprotein (AFP) level. Gastric/esophageal varices was diagnosed based on esophagogastroduodenoscopy and/or contrast‐enhanced CT and MRI.
Routine postoperative examinations included histopathology and immunohistochemical examinations. All the resected specimens were cut into slices of approximately 3–5 mm thick and fixed in 1% formalin. The liver slices, which contained tumor tissues and noncancerous adjacent nontumorous tissues, were embedded in paraffin, cut into 4‐μm sections, and stained with hematoxylin and eosin. At least one slice of nontumorous liver parenchyma 1 cm away from the tumor edge was examined [27]. Currently, the diagnosis of MVI is only determined by histologic examination of the resected surgical specimens. MVI was defined as invasion of tumor cells in a portal vein, hepatic vein, or a large capsular vessel of the surrounding hepatic tissues, partially or totally lined by endothelial cells visible only by microscopy [10], [28]. Other pathological indexes used in this study included tumor diameter (maximum diameter), number of tumors, tumor encapsulation, and cirrhosis. All the histopathological evaluations were performed by two independent and experienced pathologists who were blinded to the clinical data in their respective hospitals based on uniform diagnostic standards.
Liver Resection
R0 LR was performed using techniques that have been described previously [17], [29]. Intraoperative ultrasonography was performed routinely to assess the number and size of the lesions and the relationship of the tumors to the vascular structures. Pringle’s maneuver was routinely used with a clamp/unclamp time of 10 minutes/5 minutes. R0 anatomical resection was our preferred surgical method for our patients. Anatomical resection was preferred for a single tumor or for multiple tumor nodules within one liver segment or neighboring segments. For multiple bilobar tumor nodules, anatomical resection was used for the main tumor, whereas satellite nodules were resected nonanatomically with an adequate resection margin [30]. In patients with an inadequate liver remnant volume, nonanatomical resection was used to achieve a negative resection margin. A negative resection margin was defined as a lack of visible tumor cells left in the remnant liver at all the resection margins.
Follow‐Up
Follow‐up examinations for all the patients were conducted using laboratory tests, which included serum AFP, liver function, and abdominal ultrasonography, as well as contrast‐enhanced CT once every 2–3 months for the first year and then once every 6 months after treatment until death or dropout from the follow‐up program. When tumor recurrence was diagnosed, patients underwent further investigations. Appropriate treatments were given, which included percutaneous ethanol injection, radiofrequency ablation, transarterial chemoembolization, or liver re‐resection, depending on the general condition of the patient, the liver functional reserve, the pattern of tumor recurrence, the patient’s wish, and the recommended treatment by the multidisciplinary team.
Statistical Analysis
The endpoints of this study were overall survival (OS) and recurrence‐free survival (RFS). OS was measured from the date of R0 LR to the date of patient’s death or the date of last follow‐up visit. RFS was calculated from the date of R0 LR to the date when tumor recurrence was diagnosed. Survival curves were calculated using the Kaplan‐Meier method and compared using the log‐rank test. Median survival times and their 95% confidence intervals (CIs) were reported. Continuous variables were expressed as mean ± SD and compared using the unpaired, two‐tailed t test or Mann‐Whitney U test. Categorical variables were compared using the chi‐square test or the Fisher’s exact test. Continuous variables, such as AFP and size (diameter) of the tumor, were transformed into categorical variables. Each of these continuous variables was divided into two or three leveled categorical data by setting one or two break point(s), respectively, which were then represented by one or two binary variable(s) [30]; p values were calculated for each set of break points with univariate or multivariate Cox proportional hazards regression, and the set of break points with the lowest p value was retained if the value reached significance. Univariate analysis of OS time was performed on the estimation set. The log‐rank test was performed to detect significant parameters on univariate analysis. Parameters with a p value (log‐rank) of <.05 in the univariate analyses were entered into multivariate analysis. Multivariate Cox regression analysis with a stepwise selection was performed to detect independent predictors of OS (the entry criteria for selection into the final multivariate model was p < .05). The regression coefficients (β) of the Cox regression model were divided by the median of the regression coefficients (β) of all the parameters in the model and approximated to the nearest unit (1.00 units) to obtain simple point numbers to facilitate bedside calculation of the EHBH‐MVI score [30].
The abilities of the different systems, such as the Okuda staging system, CLIP staging, TNM stage, and BCLC staging, to differentiate prognosis were compared using survival analysis and the area under the receiver operating characteristic (ROC) curve (AUC) for each score. To perform this test, patients censored before 1, 3, and 5 years were excluded from the analysis. To further validate the discriminative ability of the EHBH‐MVI scoring system, the new EHBH‐MVI score was analyzed as a survival predictor in each subgroup of the commonly used staging systems by the log‐rank test methodology. To avoid overoptimistic results due to model development and evaluation using the same data set, the prognostic performance of the EHBH‐MVI scoring system was assessed in one independent prospective internal validation cohort and three independent external validation cohorts (ATH, ZSH, and WCH) to guarantee stability of the model. All the reported p values were two‐sided. A significance level of .05 was applied throughout. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Clinical Characteristics of the Patients
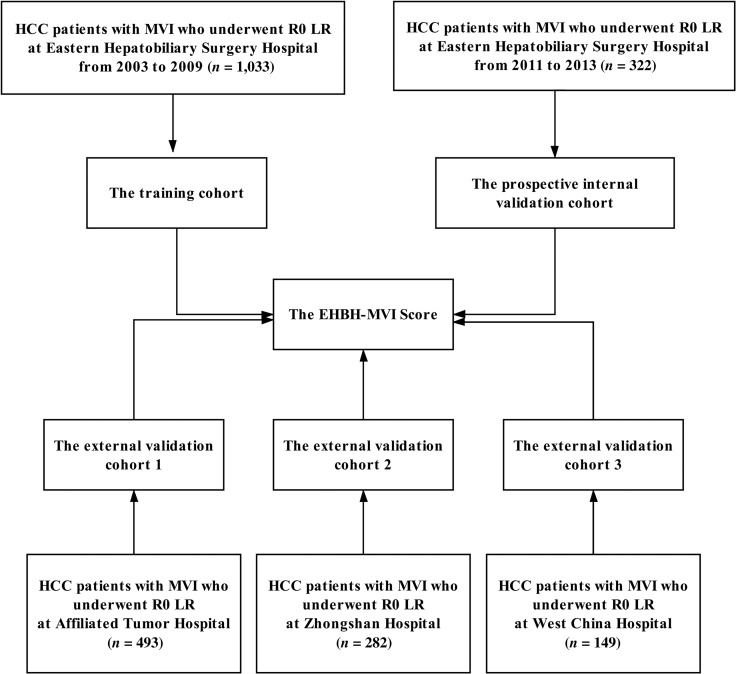
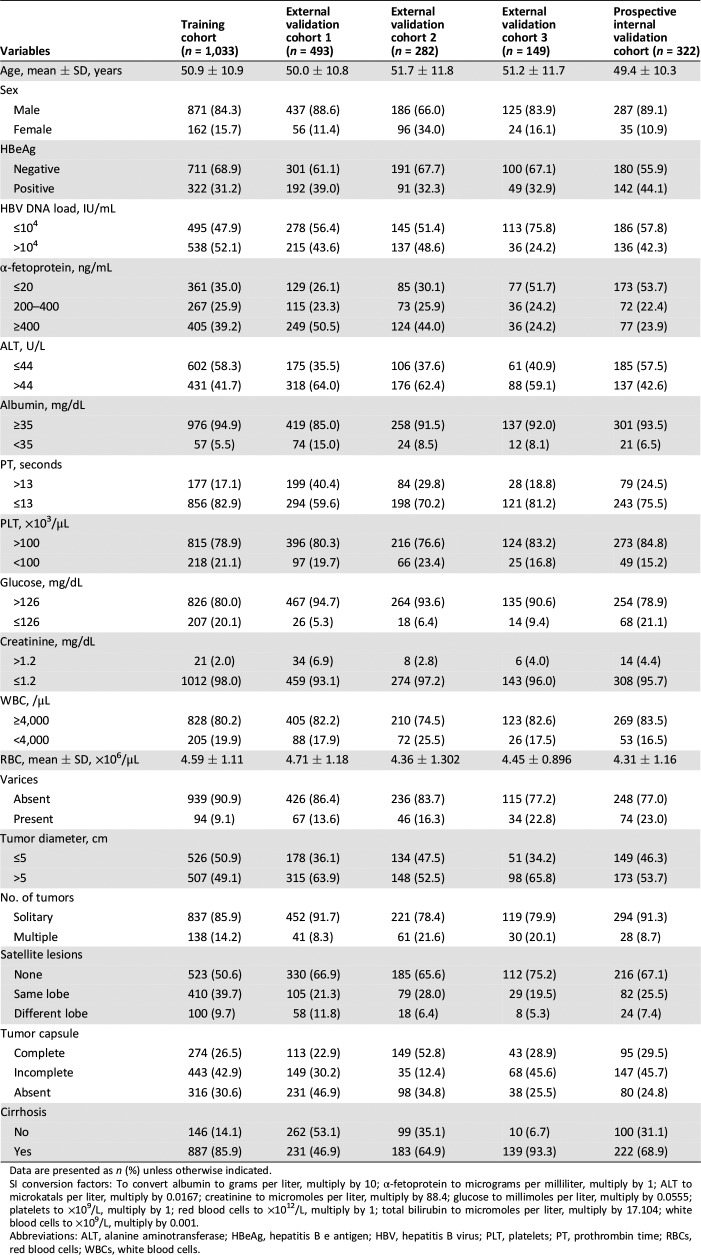
In Figure 1, 1,033 patients with HCC with MVI who underwent R0 LR at EHBH between 2003 and 2009 met the inclusion criteria in the training cohort. In the internal prospective validation cohort, 322 patients operated at EHBH between 2011 and 2013 were included. For the three external validation cohorts, 493 patients who were operated at ATH formed the validation cohort 1, 282 patients at ZSH formed the validation cohort 2, and 149 patients at WCH formed the validation cohort 3. The baseline characteristics of the training cohort and the four validation cohorts are provided in Table 1. Some clinicopathologic data were slightly different among the training and the validation cohorts.
Figure 1.
Flow chart showing the selection process of patients with HCC with MVI who underwent R0 LR in the training cohort (n = 1,033), the prospective internal validation cohort (n = 322), and the three external validation cohorts (n = 493, 282, and 149, respectively).
Abbreviations: EHBH, Eastern Hepatobiliary Surgery Hospital; HCC, hepatocellular carcinoma; LR, liver resection; MVI, microvascular invasion.
Table 1. The clinicopathological characteristics in patients with hepatocellular carcinoma with microvascular invasion in the different cohorts.
Data are presented as n (%) unless otherwise indicated.
SI conversion factors: To convert albumin to grams per liter, multiply by 10; α‐fetoprotein to micrograms per milliliter, multiply by 1; ALT to microkatals per liter, multiply by 0.0167; creatinine to micromoles per liter, multiply by 88.4; glucose to millimoles per liter, multiply by 0.0555; platelets to ×109/L, multiply by 1; red blood cells to ×1012/L, multiply by 1; total bilirubin to micromoles per liter, multiply by 17.104; white blood cells to ×109/L, multiply by 0.001.
Abbreviations: ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; PLT, platelets; PT, prothrombin time; RBCs, red blood cells; WBCs, white blood cells.
Univariate and Multivariable Cox Regression Analyses in the Training Cohort
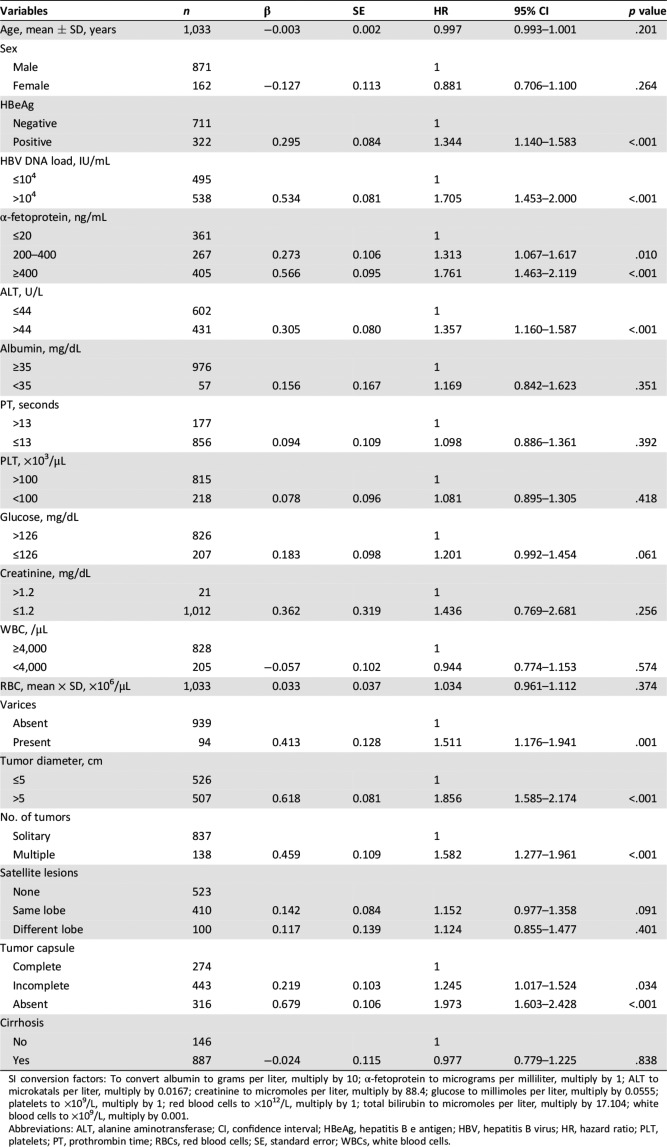
On univariate analyses (Table 2), hepatitis B e antigen (HBeAg) positivity (p < .001), HBV DNA load >104 IU/mL (p < .001), AFP ≥400 ng/mL (p < .001), alanine aminotransferase >44 U/L (p < .001), presence of gastric fundus/esophagus varicosity (p = .001), tumor diameter >5 cm (p < .001), multiple tumors (p = .002), and absence of tumor encapsulation (p < .001) were associated with significantly worse OS in the training cohort.
Table 2. Univariate analysis for overall survival in patients with hepatocellular carcinoma with microvascular invasion in the training cohort (n = 1,033).
SI conversion factors: To convert albumin to grams per liter, multiply by 10; α‐fetoprotein to micrograms per milliliter, multiply by 1; ALT to microkatals per liter, multiply by 0.0167; creatinine to micromoles per liter, multiply by 88.4; glucose to millimoles per liter, multiply by 0.0555; platelets to ×109/L, multiply by 1; red blood cells to ×1012/L, multiply by 1; total bilirubin to micromoles per liter, multiply by 17.104; white blood cells to ×109/L, multiply by 0.001.
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HR, hazard ratio; PLT, platelets; PT, prothrombin time; RBCs, red blood cells; SE, standard error; WBCs, white blood cells.
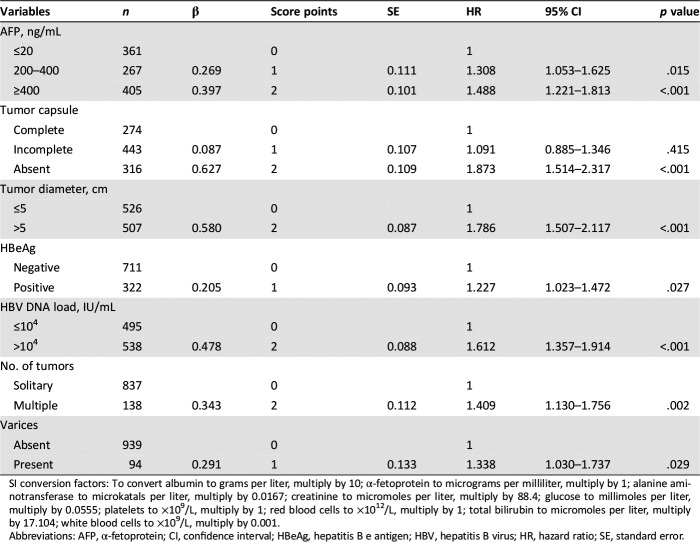
On multivariable Cox regression analyses, AFP (≤20, 200–400, >400 ng/mL), tumor encapsulation (complete, incomplete, absent), tumor diameter (≤5, >5 cm), HBeAg (negative, positive), HBV DNA load (≤104, >104 IU/mL), number of tumors (solitary, multiple), and gastric fundus/esophagus varicosity (absent, present) remained as significant predictors of OS. The calculated regression coefficients (β, B‐values) were multiplied by a factor of 3, and the maximum number of integers was determined to facilitate the EHBH‐MVI score calculation [Up‐rounded integer, such as β of (0 − 0.333) × 3 = 1; (0.334 − 0.666) × 3 = 2] (Table 3). A score of 4 was chosen as a cutoff for prognostication with the EHBH‐MVI score based on ROC analysis. The new EHBH‐MVI score for a patient was calculated using the following equation, by adding the sum of the multiplication of the seven factors by their respective weights:
Table 3. Multivariable Cox regression analysis of overall survival in patients with hepatocellular carcinoma with microvascular invasion in the training cohort (n = 1,033).
SI conversion factors: To convert albumin to grams per liter, multiply by 10; α‐fetoprotein to micrograms per milliliter, multiply by 1; alanine aminotransferase to microkatals per liter, multiply by 0.0167; creatinine to micromoles per liter, multiply by 88.4; glucose to millimoles per liter, multiply by 0.0555; platelets to ×109/L, multiply by 1; red blood cells to ×1012/L, multiply by 1; total bilirubin to micromoles per liter, multiply by 17.104; white blood cells to ×109/L, multiply by 0.001.
Abbreviations: AFP, α‐fetoprotein; CI, confidence interval; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; HR, hazard ratio; SE, standard error.
EHBH‐MVI Score = AFP (≤20 ng/mL = 0; 200–400 ng/mL = 1; >400 ng/mL = 2) + tumor encapsulation (complete = 0, incomplete = 1, absent = 2) + tumor diameter (≤5 cm = 0, >5 = 2) + HBeAg (negative = 0, positive = 1) + HBV DNA load (≤104 = 0, >104 IU/mL = 2) + number of tumors (solitary = 0, multiple = 2) + gastric fundus/esophagus varicosity (absent = 0, present = 1)
The EHBH‐MVI Score Predicted Survival in the Training and Validation Cohorts
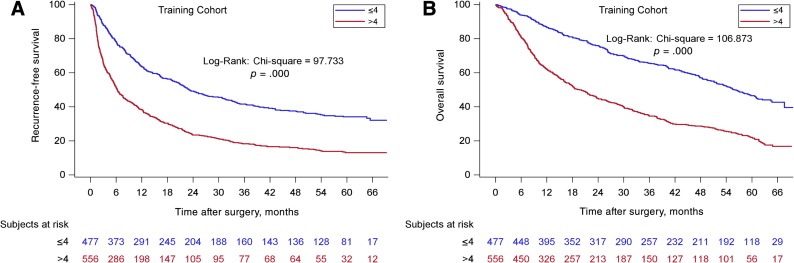
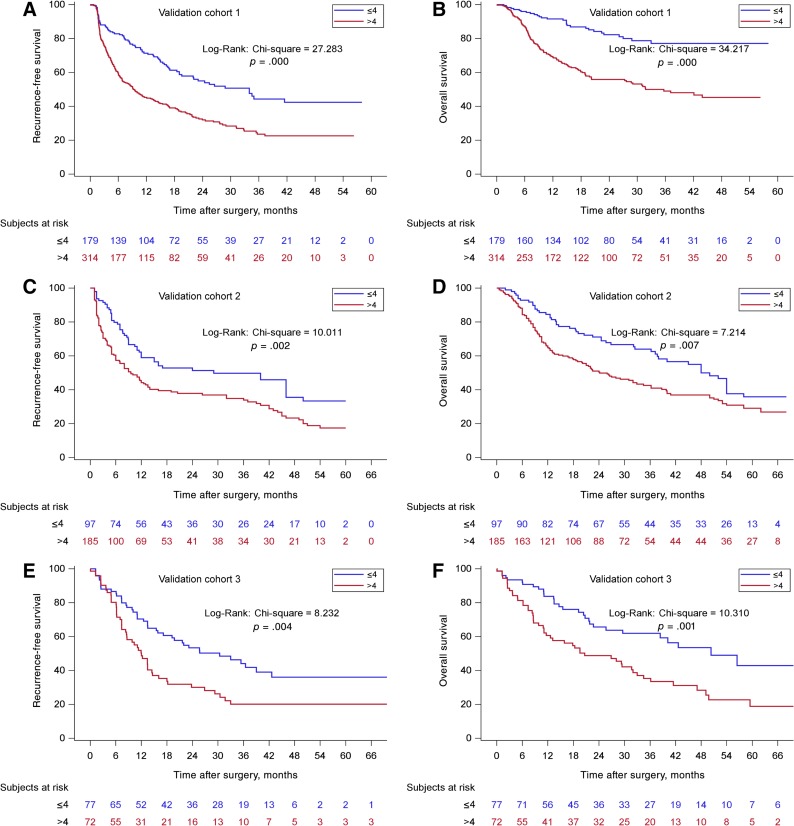
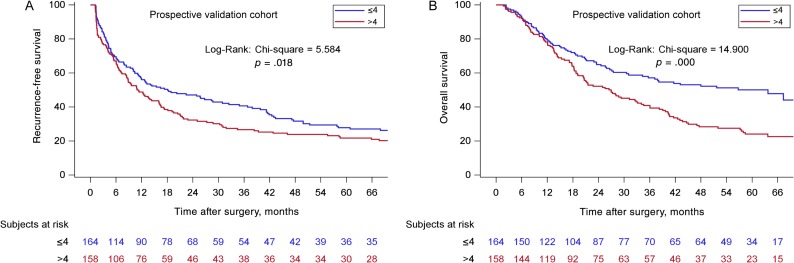
According to the new scoring model, two groups of patients with significantly different OS (p < .001) were identified. The supplemental online Table 1 shows the details of the analyses. For the training cohort from EHBH (Fig. 2), the median OS for the ≤4 group was 55.8 (95% CI: 51.1–61.1) months, and the 1‐, 3‐, and 5‐year OS were 86.5%, 66.6%, and 46.6%, respectively (n = 477). The median OS for the >4 groups was 19.6 (95% CI: 16.4–23.5) months, and the 1‐, 3‐, and 5‐year OS were 62.0%, 34.5%, and 22.0%, respectively (n = 556, p < .001). The EHBH‐MVI scoring performed equally well in the validation cohorts. For the three external validation cohorts (Fig. 3), the median OS of the cohorts from ATH, ZSH, and WCH for the ≤4 groups were significantly longer than the median OS for the >4 groups (p < .001, p = .007, p = .001, respectively). For the prospective internal validation cohort (Fig. 4), the median OS for the ≤4 group was 63.7 (95% CI: 34.3–82.5) months, and the 1‐, 3‐, and 5‐year OS were 79.5%, 57.1%, and 50.1%, respectively (n = 164); the median OS for the >4 group was 27.0 (95% CI: 20.4–34.1) months, and the 1‐, 3‐, and 5‐year OS were 78.2%, 40.8%, and 24.1%, respectively (n = 158, p ≤ .001). For the three external validation cohorts, the median OS of the cohorts from ATH, ZSH, and WCH for the ≤4 groups were significantly longer than the median OS for the >4 groups (p < .001, p = .007, p = .001, respectively).
Figure 2.
Kaplan‐Meier curves in estimating recurrence‐free survival (RFS) and overall survival (OS) by the Eastern Hepatobiliary Surgery Hospital (EHBH) microvascular invasion (MVI) score in the training cohort. (A): The prognostic significance of the two EHBH‐MVI score subgroups (≤4, n = 477/>4 points, n = 556) for RFS (p < .001). (B): The prognostic significance of the two EHBH‐MVI score subgroups (≤4, n = 477/>4 points, n = 556) for OS (p < .001).
Figure 3.
Kaplan‐Meier curves in estimating recurrence‐free survival (RFS) and overall survival (OS) by the Eastern Hepatobiliary Surgery Hospital (EHBH) microvascular invasion (MVI) score in the three external validation cohorts. (A): The prognostic significance of the two EHBH‐MVI score subgroups (≤4, n = 179/>4 points, n = 314) for RFS in the external validation cohort 1 (p < .001). (B): The prognostic significance of the two EHBH‐MVI score subgroups (≤4, n = 179/>4 points, n = 314) for OS in the external validation cohort 1 (p < .001). (C): The prognostic significance of the two EHBH‐MVI score subgroups (≤4, n = 97/>4 points, n = 185) for RFS in the external validation cohort 2 (p < .005). (D): The prognostic significance of the two EHBH‐MVI score subgroups (≤4, n = 97/>4 points, n = 185) for OS in the external validation cohort 2 (p < .05). (E): The prognostic significance of the two EHBH‐MVI score subgroups (≤4, n = 77/>4 points, n = 72) for RFS in the external validation cohort 3 (p < .005). (F): The prognostic significance of the two EHBH‐MVI score subgroups (≤4, n = 77/>4 points, n = 72) for OS in the external validation cohort 3 (p < .005).
Figure 4.
Kaplan‐Meier curves in estimating recurrence‐free survival (RFS) and overall survival (OS) by the Eastern Hepatobiliary Surgery Hospital (EHBH) microvascular invasion (MVI) score in the prospective internal validation cohort. (A): The prognostic significance of the two EHBH‐MVI score subgroups (≤4, n = 164/>4 points, n = 158) for RFS (p < .05). (B): The prognostic significance of the two EHBH‐MVI score subgroups (≤4, n = 164/>4 points, n = 158) for OS (p < .001).
In addition, the EHBH‐MVI score had good performance in RFS prediction, as shown in supplemental online Table 2, with two significantly different prognostic subgroups in the training cohort (23.03 vs. 6.27 months, p < .001), the three validation cohorts (33.96 vs. 9.07 months in ATH, p < .001; 29.00 vs. 9.53 months in ZSH, p = .002; 30.39 vs. 12.02 months in WCH, p = .004), and the prospective internal validation cohort (18.11 vs. 11.13 months in EHBH from 2011 to 2013, p = .018).
Comparison of the EHBH‐MVI Score and the Current Commonly Used Staging Systems
Kaplan‐Meier curves were generated for the BCLC classification, TNM 7th, Okuda, and CLIP stages, as shown in supplemental online Tables 3 and 4 and supplemental online Figures 1–4. The four commonly used international classification systems showed clear prognostic strata (p < .001, p < .001, p = .009, and p < .001 for OS, respectively; p < .001, p < .001, p = .013, and p < .001 for RFS, respectively). The EHBH‐MVI scoring system showed similar prognostic accuracy to the international standards (p < .001 for OS and RFS). The staging systems in predicting survival outcomes were then compared using the ROC curve area analysis. In the training cohort, the AUCs of the EHBH‐MVI scoring system were greater than the four commonly used international staging systems for HCC (supplemental online Figs. 9, 10). The difference is that these international staging systems were used to predict prognosis in all patients with HCC with or without MVI, but the EHBH‐MVI scoring system focused on predicting OS and RFS in patients with HCC with MVI.
Combining the Subgroups of Patients with Scores of ≤4 and >4 in the New Scoring System Predicted OS and RFS into the Commonly Used International Staging Systems
By combining the subgroups of patients with scores of ≤4 and >4 in the new EHBH‐MVI scoring system into the four commonly used international staging systems for HCC (supplemental online Tables 5 and 6 and supplemental online Figs. 5–8), the two groups of patients with HCC with MVI were shown to have significantly different OS and RFS in the Okuda I and II stages, CLIP early and mid‐term stages, BCLC 0 and A stages, and TNM II stage. Patients in the BCLC B stage and TNM IIIa stage showed no significant difference probably because of the very small sample sizes. These results suggested that this EHBH‐MVI score is a good tool for patients with HCC with MVI.
Discussion
Presence of MVI is associated with worse surgical outcomes in patients with HCC after LR. However, the commonly used international staging systems for HCC were not designed to focus on predicting outcomes of patients with HCC with MVI after R0 LR. In addition, the role of postoperative adjuvant therapy for patients with HCC after LR remains controversial. Failure to demonstrate effectiveness in the use of adjuvant therapy can well be related to our previous inability to include a high‐risk group of patients to develop early HCC recurrence (<2 years) in these studies, and MVI is a known risk factor of early HCC recurrence after LR. This novel prognostic staging system to predict prognosis in patients with HCC and MVI after surgery is important for clinicians and patients in deciding on whether to provide postoperative adjuvant therapy, in advising patients on strategies of postoperative follow‐up, and in designing studies on adjuvant therapies for patients with a predicted higher risk of early HCC recurrence. This study is the first to report and validate on a new scoring system, based on multicenter data in predicting survival outcomes in patients with HCC with MVI treated with R0 LR. A low score (≤4) predicted patients with MVI to have good survival outcomes, and the results were similar to those of patients with HCC without MVI. In contrast, a high score (>4) reliably predicted patients to have worse survival outcomes.
In previous studies, MVI, which was also known as microvascular tumor thrombus, was diagnosed only under microscopy. Its presence has been repeatedly and definitively shown to be a significant poor prognostic factor of HCC recurrence and overall survival after liver resection. Even for patients with small HCCs or for those who were treated with transplantations, presence of MVI in the resected surgical specimens was still a poor prognostic indicator of long‐term survival outcomes [31], [32], [33]. Recent studies have focused on preoperative prediction of MVI. By using a preoperative serological index, a nomogram was reported that could preoperatively predict the presence of MVI in patients with HBV‐related HCC but could not be used to predict survival outcomes [11]. In another study, a radiomic nomogram based on preoperative imaging indicators was reported to show a favorable predictive accuracy on the MVI status in patients with HCC [34]. However, preoperative imaging indicators or serological indicators are less accurate than the gold standard of histopathological diagnosis of MVI, and they can only be used in prediction. In this study, postoperative histopathological indexes combined with preoperative serological indexes were used to establish a new scoring system to predict long‐term survival outcomes in patients with HCC with MVI after R0 LR. Thus, the prediction focused on the long‐term survival outcomes in patients with HCC who had already been confirmed to have MVI on histopathological studies.
There was a striking sex difference in the incidence of hepatocellular carcinoma, with a strong predominance for men [35]. It is necessary to define an individually planning surveillance strategy with various follow‐up intervals based on patient sex and other risk factors for patients after R0 LR. In many of the MVI risk estimation models, multifocal lesions, large tumor size, incomplete tumor encapsulation, and high serum levels of AFP have been reported to increase the possibility of vascular invasion in HCC [36], [37], [38]. Our study showed these factors to also be significantly associated with the OS and RFS rates in patients with HCC with MVI after R0 LR. These results suggested that these factors were associated with the incidence of MVI, which may further worsen survival outcomes in patients with MVI. In addition, HBeAg (negative/positive), HBV DNA load (≤104/>104), and gastric fundus/esophagus varicosity (absent/present) were also shown in this study to be associated with long‐term survival outcomes. Our research team has reported previously that HBV infection and active HBV replication were associated with the development of vascular invasion in patients with HCC [39], and an MVI risk estimation nomogram showed a high serum HBV DNA load (>104 IU/mL) to be associated with an increased risk of MVI in early T‐stage HCC [11]. These studies supported the findings in the current study that viral load was an important factor associated with prognosis of patients with HCC with MVI after LR. Gastric fundus/esophagus varicosity, an indication of the degree of cirrhosis, was also found to be an independent factor of OS in this study. In addition, we used similar methods to develop an EHBH portal vein tumor thrombus (PVTT) scoring system as an aid to decision making on hepatectomy for patients with HCC with PVTT, which included four factors—total bilirubin, α‐fetoprotein, tumor diameter, and satellite lesions [40]. However, in this EHBH‐MVI scoring system, we input the other factor of liver function, albumin, into univariate analysis (p = .351), which indicated that the liver function may not be associated with prognosis in patients with MVI, probably because patients with HCC with MVI have relatively better liver function than patients with PVTT.
The long‐term survival outcomes of patients with HCC with MVI can vary greatly after R0 LR [41], [42], [43]. Whether postoperative adjuvant therapy should be performed for all these patients is controversial. Currently, our research team showed that postoperative adjuvant transarterial chemoembolization (TACE) after R0 hepatectomy improved survival outcomes in patients with HCC and MVI [17], and the results have been supported by another study using adjuvant radiotherapy [16]. Our new scoring system distinguished different subgroups of patients with HCC with MVI to have a high score (>4) and a low score (≤4). In future postoperative adjuvant TACE or radiotherapy studies, the impact of adjuvant therapy on long‐term survival outcomes in patients with HCC with MVI with predicted poor long‐term survival outcomes (the high‐score subgroup) should be analyzed separately from the low‐score subgroup.
Although our study showed that the four commonly used international staging systems can stratify patients with HCC with MVI after R0 LR into distinct risk categories, the ability of these systems to predict long‐term survival of these patients was suboptimal when compared with the EHBH‐MVI scoring system using ROC analysis, probably because the EHBH‐MVI scoring system focused on the prognosis of patients with MVI. Our novel prognostic staging system, which is specific for patients with HCC and MVI after R0 LR, can improve these international systems; for example, for the BCLC B staging system, patients with HCC with MVI can be subdivided into two groups with different prognoses (≤4/>4) using our score. Thus, this score can become an appropriate supplement to the existing staging systems.
This study has several limitations. First, this is a retrospective study with its inherent defects. However, the analysis on the internal validation cohort was based on prospectively collected data. Furthermore, the results were validated in four validation cohorts to increase reliability. Second, the aim of this study was to identify a suitable subgroup of patients with HCC with MVI for postoperative adjuvant therapy. For patients with an EHBH‐MVI score >4 who are predicted to have poor RFS and OS outcomes, postoperative adjuvant TACE may potentially be beneficial to these patients. However, these conclusions need more high‐level evidence to support. Third, standards in diagnosis and in surgical techniques for patients with HCC with MVI may vary in the different centers. Finally, because this study was conducted in China, most patients had a background of HBV infection. These data require validation from study groups with hepatitis C virus infection or alcoholism being the prevailing etiologies of HCC.
Conclusion
A new EHBH‐MVI scoring system was used to predict prognosis in patients with HCC with MVI after R0 LR. For patients with MVI, postoperative adjuvant therapy may be recommended for those with an EHBH‐MVI score >4, as these patients are predicted to have poor long‐term survival outcomes. The score can be used to supplement the most commonly used international classification systems in distinguishing subgroups of patients with different long‐term survival outcomes among patients with HCC with MVI.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This work was supported by the Key Project of Natural Science Foundation of China (81730097); the grants of the Science Fund for Creative Research Groups (81521091); the National Key Basic Research Program “973 project” (2015CB554000); and the National Natural Science Foundation of China (81602523 and 81702335).
Contributed equally.
Author Contributions
Conception/design: Xiu‐Ping Zhang, Kang Wang, Xu‐Biao Wei, Meng‐Chao Wu, Wan Yee Lau, Shu‐Qun Cheng
Provision of study material or patients: Le‐Qun Li, Hui‐Chuan Sun, Tian‐Fu Wen, Zong‐Tao Chai, Zhen‐Hua Chen, Jie Shi, Wei‐Xing Guo, Dong Xie, Wen‐Ming Cong
Collection and/or assembly of data: Xiu‐Ping Zhang, Kang Wang, Xu‐Biao Wei
Data analysis and interpretation: Xiu‐Ping Zhang, Kang Wang, Xu‐Biao Wei
Manuscript writing: Xiu‐Ping Zhang
Final approval of manuscript: Xiu‐Ping Zhang, Kang Wang, Xu‐Biao Wei, Le‐Qun Li, Hui‐Chuan Sun, Tian‐Fu Wen, Zong‐Tao Chai, Zhen‐Hua Chen, Jie Shi, Wei‐Xing Guo, Dong Xie, Wen‐Ming Cong, Meng‐Chao Wu, Wan Yee Lau, Shu‐Qun Cheng
Disclosures
The authors indicated no financial relationships.
References
- 1.Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301–1314. [DOI] [PubMed] [Google Scholar]
- 3.Chen W. Cancer statistics: Updated cancer burden in China. Chin J Cancer Res 2015;27:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Consensus statements on the prevention and management of hepatitis B and hepatitis C in the Asia‐Pacific region. Core Working Party for Asia‐Pacific Consensus on Hepatitis B and C. J Gastroenterol Hepatol 2000;15:825–841. [DOI] [PubMed] [Google Scholar]
- 5.Marrero JA, Kulik LM, Sirlin C et al. Diagnosis, staging and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver . EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 7.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer . EASL‐EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 2012;56:908–943. [DOI] [PubMed] [Google Scholar]
- 8.Grazi GL, Ercolani G, Pierangeli F et al. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg 2001;234:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim KC, Chow PK, Allen JC et al. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg 2012;99:1622–1629. [DOI] [PubMed] [Google Scholar]
- 10.Cong WM, Bu H, Chen J et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol 2016;22:9279–9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei Z, Li J, Wu D et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus‐related hepatocellular carcinoma within the Milan criteria. JAMA Surg 2016;151:356–363. [DOI] [PubMed] [Google Scholar]
- 12.Mazzaferro V, Llovet JM, Miceli R et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol 2009;10:35–43. [DOI] [PubMed] [Google Scholar]
- 13.Sumie S, Kuromatsu R, Okuda K et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol 2008;15:1375–1382. [DOI] [PubMed] [Google Scholar]
- 14.Huang C, Zhu XD, Ji Y et al. Microvascular invasion has limited clinical values in hepatocellular carcinoma patients at Barcelona Clinic Liver Cancer (BCLC) stages 0 or B. BMC Cancer 2017;17:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan SX, Yang F, Yang Y et al. Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients' poor recurrence‐free survival after hepatectomy. Hepatology 2012;56:2231–2241. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Wang W, Yao X et al. Postoperative adjuvant radiotherapy is associated with improved survival in hepatocellular carcinoma with microvascular invasion. Oncotarget 2017;8:79971–79981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun JJ, Wang K, Zhang CZ et al. Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann Surg Oncol 2016;23:1344‐1351. [DOI] [PubMed] [Google Scholar]
- 18.Ye JZ, Chen JZ, Li ZH et al. Efficacy of postoperative adjuvant transcatheter arterial chemoembolization in hepatocellular carcinoma patients with microvascular invasion. World J Gastroenterol 2017;23:7415–7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruix J, Reig M, Sherman M. Evidence‐based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016;150:835–853. [DOI] [PubMed] [Google Scholar]
- 20.Guy J, Kelley RK, Roberts J et al. Multidisciplinary management of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2012;10:354–362. [DOI] [PubMed] [Google Scholar]
- 21.Soares KC, Cosgrove DC, Herman JM et al. Multidisciplinary clinic in the management of hepatocellular carcinoma. Ann Surg Oncol 2014;21:1059–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roayaie S, Blume IN, Thung SN et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 2009;137:850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okuda K, Ohtsuki T, Obata H et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer 1985;56:918–928. [DOI] [PubMed] [Google Scholar]
- 24.A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients: The Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28:751–755. [DOI] [PubMed] [Google Scholar]
- 25.Vauthey JN, Ribero D, Abdalla EK et al. Outcomes of liver transplantation in 490 patients with hepatocellular carcinoma: Validation of a uniform staging after surgical treatment. J Am Coll Surg 2007;204:1016–1027; discussion 1027–1018. [DOI] [PubMed] [Google Scholar]
- 26.Pomfret EA, Washburn K, Wald C et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl 2010;16:262–278. [DOI] [PubMed] [Google Scholar]
- 27.Zhao H, Chen C, Fu X et al. Prognostic value of a novel risk classification of microvascular invasion in patients with hepatocellular carcinoma after resection. Oncotarget 2017;8:5474–5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evidence‐based practice guidelines for standardized pathological diagnosis of primary liver cancer in China: 2015 [in Chinese]. Zhonghua Gan Zang Bing Za Zhi 2015;23:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi M, Guo RP, Lin XJ et al. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: A prospective randomized trial. Ann Surg 2007;245:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang YF, Zhou J, Wei W et al. Intermediate‐stage hepatocellular carcinoma treated with hepatic resection: The NSP score as an aid to decision‐making. Br J Cancer 2016;115:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng LH, Dong H, Lau WY et al. Novel microvascular invasion‐based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J Cancer Res Clin Oncol 2017;143:293‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg 2011;254:108–113. [DOI] [PubMed] [Google Scholar]
- 33.Jin YJ, Lee JW, Lee OH et al. Transarterial chemoembolization versus surgery/radiofrequency ablation for recurrent hepatocellular carcinoma with or without microvascular invasion. J Gastroenterol Hepatol 2014;29:1056–1064. [DOI] [PubMed] [Google Scholar]
- 34.Peng J, Zhang J, Zhang Q et al. A radiomics nomogram for preoperative prediction of microvascular invasion risk in hepatitis B virus‐related hepatocellular carcinoma. Diagn Interv Radiol 2018;24:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Han J, Xing H et al. Sex difference in recurrence and survival after liver resection for hepatocellular carcinoma: A multicenter study. Surgery 2019;165:516–524. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez‐Peralvarez M, Luong TV, Andreana L et al. A systematic review of microvascular invasion in hepatocellular carcinoma: Diagnostic and prognostic variability. Ann Surg Oncol 2013;20:325–339. [DOI] [PubMed] [Google Scholar]
- 37.Ahn SY, Lee JM, Joo I et al. Prediction of microvascular invasion of hepatocellular carcinoma using gadoxetic acid‐enhanced MR and (18)F‐FDG PET/CT. Abdom Imaging 2015;40:843–851. [DOI] [PubMed] [Google Scholar]
- 38.Banerjee S, Wang DS, Kim HJ et al. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology 2015;62:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei X, Li N, Li S et al. Hepatitis B virus infection and active replication promote the formation of vascular invasion in hepatocellular carcinoma. BMC Cancer 2017;17:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang XP, Gao YZ, Chen ZH et al. An Eastern Hepatobiliary Surgery Hospital/portal vein tumor thrombus scoring system as an aid to decision making on hepatectomy for hepatocellular carcinoma patients with portal vein tumor thrombus: A multicenter study. Hepatology 2019;69:2076–2090. [DOI] [PubMed] [Google Scholar]
- 41.Du M, Chen L, Zhao J et al. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer 2014;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou YF, Wei YG, Yang JY et al. Microvascular invasion patterns affect survival in hepatocellular carcinoma patients after second hepatectomy. J Surg Res 2016;200:82–90. [DOI] [PubMed] [Google Scholar]
- 43.Schlichtemeier SM, Pang TC, Williams NE et al. A pre‐operative clinical model to predict microvascular invasion and long‐term outcome after resection of hepatocellular cancer: The Australian experience. Eur J Surg Oncol 2016;42:1576–1583. [DOI] [PubMed] [Google Scholar]