A proper classification system for gastric cancer has long been needed. This study aimed to develop and validate a molecular classification system based on simplified methods, such as immunohistochemistry, and to assess the association between the classification system and HER2 status.
Keywords: Gastric cancer, Molecular classification, Prognosis, Microsatellite instability, Epithelial‐mesenchymal transition
Abstract
Background.
Gastric cancer (GC) is a heterogeneous disease, and substantial efforts have been made to develop a molecular biology‐based classification system for GC. Analysis of the genomic signature is not always feasible, and thus, we aimed to (i) develop and validate a practical immunohistochemistry (IHC)‐ and polymerase chain reaction (PCR)‐based molecular classification of GC and (ii) to assess HER2 status according to this classification.
Materials and Methods.
A total of 894 consecutive patients with GC from two individual cohorts (training, n = 507; validation, n = 387) were classified using Epstein‐Barr virus (EBV) in situ hybridization, microsatellite instability (MSI) testing, and IHC for E‐cadherin and p53.
Results.
We were able to classify patients into five groups in the training cohort: group 1 (MSI+), group 2 (EBV−, MSI−, non‐epithelial‐mesenchymal transition [non‐EMT]‐like, p53−), group 3 (EBV+), group 4 (EBV−, MSI−, non‐EMT‐like, p53+), and group 5 (EBV−, MSI−, EMT‐like). In the training cohort, each group showed different overall survival (OS) after gastrectomy (p < .001); group 1 had the best prognosis, and group 5 showed the worst survival outcome. The significant impact of the classification system on OS was also verified in the validation cohort (p = .004). HER2 positivity was observed in 6.5% of total population, and most of HER2‐positive cases (93.1%) were included in groups 2 and 4.
Conclusion.
We developed and validated a modified IHC‐ and PCR‐based molecular classification system in GC, which showed significant impact on survival, irrespective of stage or other clinical variables. We also found close association between HER2 status and non‐EMT phenotype in our classification system.
Implications for Practice.
Molecular classification of gastric cancer suggested by previous studies mostly relies on extensive genomic data analysis, which is not always available in daily practice. The authors developed a simplified immunohistochemistry‐ and polymerase chain reaction‐based molecular classification of gastric cancer and proved the prognostic significance of this classification, as well as the close association between HER2 status and certain groups of the classification, in the largest consecutive cohort of gastric cancer. Results of this study suggest that this scheme is a cost‐effective, easy‐to‐implement, and feasible way of classifying gastric cancer in daily clinical practice, also serving as a practical tool for aiding therapeutic decisions and predicting prognosis.
摘要
背景。胃癌 (GC) 是一种异质性疾病,人们已付出大量努力来开发基于分子生物学的GC分类系统。基因组标签分析并非总是可行,因此,我们旨在 (i) 开发和验证一种基于免疫组织化学 (IHC) 和聚合酶链反应 (PCR) 的实用型GC分子分类并 (ii) 根据此项分类来评估 HER2 状态。
材料和方法。利用 EB 病毒 (EBV) 原位杂交、微卫星不稳定性 (MSI) 检测以及 E 钙粘蛋白和 p53 IHC ,我们对来自两个独立队列(训练,n = 507;验证,n = 387)的共计 894 名连续的GC患者进行分类。
结果。我们可以将训练队列中的患者划分为 5 个小组:第 1 组(MSI+)、第 2 组 [EBV−,MSI−,非上皮间质转化非 (EMT) 样,p53−]、第 3 组(EBV+)、第 4 组(EBV−,MSI−,非 EMT 样,p53+)以及第 5 组(EBV−,MSI−,EMT 样)。在训练队列中,每个小组中的患者在胃切除术后显示出不同的总生存期 (OS) (p < 0.001);第 1 组预后最好,第 5 组预后最差。此外,我们也在验证队列中验证了分类系统对OS的显著影响 (p = 0.004)。就总体而言,我们在 6.5% 的患者中观察到了 HER2 阳性,大多数 HER2 阳性的病例 (93.1%) 出现在第 2 组和第 4 组。
结论。我们开发并验证了一个基于IHC和PCR的改良GC分子分类系统,在不考虑疾病分期或其他临床变量的情况下,该系统显示了对生存期的显著影响。我们还在分类系统中发现了 HER2 状态与非 EMT 表型之间的密切联系。
实践意义:既往研究提出的胃癌分子分类主要依赖于大量的基因组数据分析,而此类分析在日常实践中并非总是可及。作者开发了一种基于免疫组织化学和聚合酶链反应的简化版胃癌分子分类,并在最大的癌症连续队列中证实了这种分类的预后意义以及 HER2 状态与特定分类小组之间的密切联系。此项研究结果表明,该方案是一种在日常临床实践中对胃癌进行分类的具有成本效益且易于实施的可行方式,也可以作为一种协助制定医疗决策和判断预后的实用工具。
Introduction
Despite recent advances in treatment strategies for gastric cancer (GC) during the last decade [1], [2], GC remains the third most common cause of cancer‐related deaths worldwide according to 2018 global cancer statistics [3]. GC is also recognized for its highly heterogeneous clinicopathologic features [4], [5], [6]. Therefore, the need for proper classification of GC offering prognostic and therapeutic guidance has long been recognized.
The molecular classification suggested by The Cancer Genome Atlas (TCGA) was a landmark study [7]. It classified GC into four types: Epstein‐Barr virus (EBV)‐positive type, microsatellite instability (MSI) type, genomically stable (GS) type, and chromosomal instability (CIN) type. Similarly, the Asian Cancer Research Group (ACRG) proposed a classification based on MSI status, epithelial‐mesenchymal transition (EMT) signatures by gene expression, and TP53 mutation signatures [8]. Although enormous amounts of data gathered by extensive molecular genetic experiments were integrated into these classification models, they are barely used in daily clinical practice because of high cost and the time‐consuming nature of genomic tests.
The development of practical classification systems for predicting prognosis or treatment response in patients with cancer would be highly useful for clinics. In breast cancer, for example, chemotherapy plans are usually guided by the results of simple ancillary tests, which serve as excellent surrogates representing molecular subtypes of breast cancer: immunohistochemistry (IHC) for hormonal receptors [9] and HER2 protein expression, and additional molecular analysis such as in situ hybridization (ISH) testing if necessary [10].
Based on these lessons from breast cancer, efforts have been made to find reliable ways to replace the original genome‐based molecular classification with more simplified method‐based classification models for patients with GC in routine clinical practice. Setia et al. proposed a classification model based on EBV status, mismatch repair (MMR) protein, and E‐cadherin and p53 expression assessed by IHC and showed that their classification had prognostic significance in 146 patients with GC [11]. A similar model was also suggested by Ahn et al., using MLH1 protein expression alone to represent MSI subtype [12]. However, a consensus concerning these alterative classification methods, especially regarding the best IHC method and interpretation criteria, has not been reached. In addition, although HER2 status remains the only companion diagnostic and treatment predictive factor in GC, the associations between HER2 status and molecular subgroups of GC are not well studied, partially because of the small number and heterogeneity of HER2‐positive tumors [4], [13]. More detailed calibration of classification criteria and additional validation of the model using a large population of patients with GC are required for this protein expression‐based approach to be adopted into clinical practice.
The aims of this study were as follows: (A) to develop and validate a molecular classification system for GC based on simplified methods such as IHC, which has prognostic significance and can be easily used in clinics, and (B) to assess the association between the modified molecular classification system and HER2 status.
Materials and Methods
Study Population
A total of 894 consecutive patients with GC from two institutions were included; 507 patients were treated in Seoul National University Bundang Hospital (Seongnam, Republic of Korea) from 2003 to 2005 (training cohort), and the remaining 387 patients were treated in Seoul National University Hospital (Seoul, Republic of Korea) in 2004 (validation cohort). All patients underwent curative or palliative surgical resection, followed by additional adjuvant or palliative chemotherapy if clinically indicated. Clinicopathologic characteristics including overall survival (OS) were retrieved retrospectively from pathology reports and medical records. OS was defined as the time period between the date of surgery and the date of death due to any cause or censoring.
Formalin‐fixed, paraffin‐embedded tissue blocks from surgically resected samples were obtained from the archives in the Department of Pathology from two institutions. The tissue microarrays (TMAs) were made from the representative 2‐mm cores selected by an experienced gastrointestinal pathologist (H.S.L.), as described previously (SuperBioChips Laboratories, Seoul, Republic of Korea) [14].
This study was approved by the institutional review boards (IRBs) of Seoul National University Bundang Hospital and Seoul National University Hospital (IRB numbers: B‐1407‐260‐305 and 1706‐105‐860).
IHC and EBV ISH
IHC for E‐cadherin (clone 36, mouse monoclonal; BD Biosciences, San Jose, CA), p53 (DO7, mouse monoclonal; Dako; Agilent Technologies, Santa Clara, CA), HER2 (4B5; rabbit monoclonal; predilution; Ventana Medical Systems, Tucson, AZ, USA), and MLH1 (BD Biosciences) were performed on 3‐μm‐thick slides using an automated immunostainer (BenchMark XT; Ventana Medical Systems), following the manufacturer's guidelines. To assess the EBV status of the study population, we performed EBV ISH with the INFORM EBV‐encoded RNA probe (Ventana Medical Systems).
The IHC and EBV ISH were interpreted by two pathologists (J.K. and H.S.L.) who were blinded to the clinical information. The cases showing strong membrane staining of E‐cadherin were interpreted as positive expression, whereas altered expression of E‐cadherin was defined as either complete loss of membrane staining or an aberrant cytoplasmic staining pattern. For p53, strong nuclear staining in more than 10% of the tumor cells was considered p53 positive, and samples with less than 10% positive cells were interpreted as p53 negative; the cases showing weak, scattered, or patchy positivity were regarded as negative [15], [16]. Tumors showing complete loss of nuclear MLH1 expression were interpreted as loss or aberrant expression [12]. The scoring of HER2 IHC was performed according to Dako HercepTest guidelines for GC as follows [17]: 0, no staining or membrane staining in less than 10% of tumor cells; 1+, faint partial membranous staining in at least 10% of tumor cells; 2+, weak‐to‐moderate complete or basolateral membranous staining in at least 10% of tumor cells; 3+, moderate‐to‐strong complete membranous staining in at least 10% of tumor cells (supplemental online Fig. 1A).
MSI Testing
MSI status was determined by a polymerase chain reaction (PCR)‐based method using the allele profiles of five markers (BAT‐26, BAT‐25, D5S346, D17S250, and S2S123) in tumor cells compared with matched normal samples. PCR products were analyzed by a DNA autosequencer (ABI 3731 Genetic Analyzer; Thermo Fisher Scientific, Waltham, MA). The interpretation of MSI status was performed according to the Revised Bethesda Guidelines [18], briefly as follows: tumors with novel bands in at least two markers were classified as MSI high, tumors with additional alleles in one marker were defined as MSI low, and tumors having identical bands in all five markers were designated as microsatellite stable (MSS).
Bright‐Field Dual‐Color HER2 Silver ISH
HER2 silver ISH (SISH) was performed using INFORM HER2 DNA and INFORM Chromosome 17 (CEP17) probes (Ventana Medical Systems) on an automatic SISH staining device (BenchMark XT, Ventana Medical Systems) according to the manufacturer's guidelines and interpreted as described previously [19]. Briefly, SISH signals were counted in 20 nonoverlapping tumor cell nuclei within the area of hot spot using ×60 objectives by three pathologists (J.K., A.N.S., and H.S.L.) who were blinded to the results of HER2 IHC. HER2 amplification was defined as an HER2/CEP17 ratio of ≥2.0 in 20 tumor nuclei (supplemental online Fig. 1B). For equivocal cases with HER2/CEP17 ratio from 0.8 to 2.2, signals from 20 additional tumor nuclei were counted, and HER2/CEP17 ratio was recalculated.
The following criteria were applied for determination of the overall HER2 status: (A) cases showing HER2 IHC score of 3+ and (B) cases with HER2 IHC score 2+ and HER2 amplification by SISH were regarded as HER2 positive.
IHC‐ and PCR‐Based Molecular Classification of GC
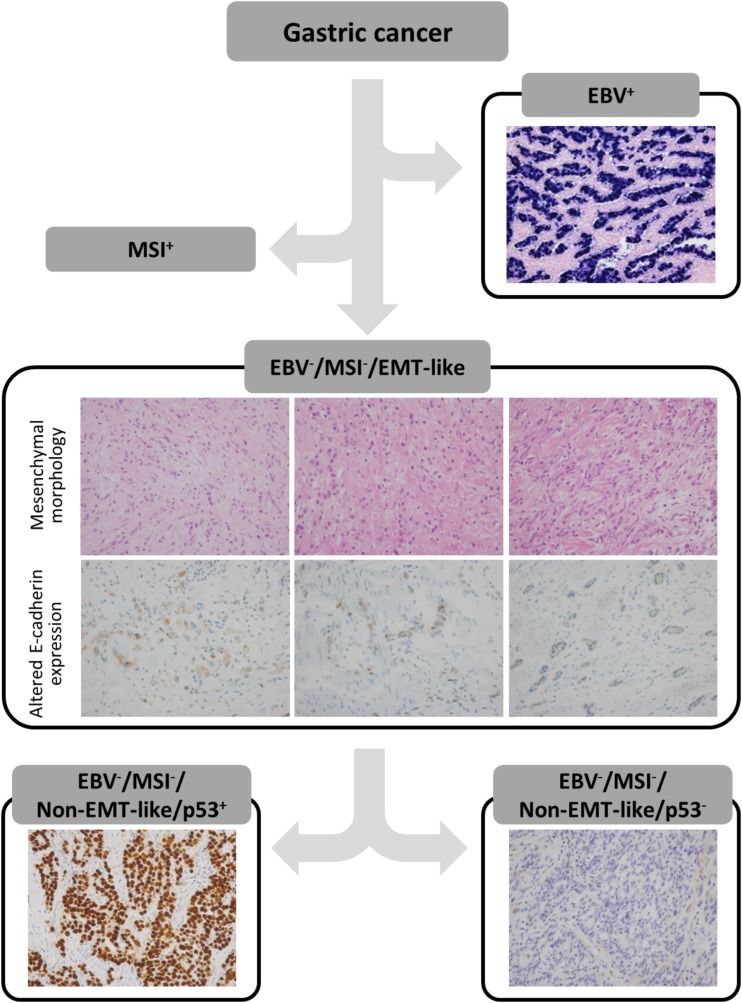
We subdivided the study population into five categories of molecular classification of GC, according to the process adapted from previously reported methods [11], [12]. As shown in Figure 1, EBV+ GCs were initially sorted out, and MSI‐high GCs were classified as the MSI+ group. Among the rest of the MSS or MSI‐low GCs, cases showing mesenchymal‐like morphologic features or altered E‐cadherin expression were classified into the EBV−, MSI−, EMT‐like group. Specifically, mesenchymal‐like morphologic features included (A) tumor cells infiltrating into stromal tissue as single cells or (B) elongated tumor cells resembling histiocytes or stromal cells such as myofibroblasts. Determination of mesenchymal‐like morphologic features was performed independently by two pathologists (J.K. and H.S.L.). The remaining cases were subdivided according to p53 IHC results; those with positive p53 IHC results were assigned to the EBV−, MSI−, non‐EMT‐like, p53+ group, and the rest were assigned to the EBV−, MSI−, non‐EMT‐like, p53− group. Finally, we named each group as follows: group 1 (MSI+), group 2 (EBV−, MSI−, non‐EMT‐like, p53−), group 3 (EBV+), group 4 (EBV−, MSI−, non‐EMT‐like, p53+), and group 5 (EBV−, MSI−, EMT‐like).
Figure 1.
Schematic flow of immunohistochemistry‐ and polymerase chain reaction‐based molecular classification. After EBV+ and MSI+ gastric cancers (GCs) were sorted out, EMT‐like group was determined by morphology and E‐cadherin expression by tumor cells. The remaining cases were further classified according to p53 immunohistochemistry results.
Abbreviations: EBV, Epstein‐Barr virus; EMT, epithelial‐mesenchymal transition; MSI, microsatellite instability.
Statistical Analysis
Clinicopathologic characteristics in two cohorts were analyzed by chi square, Fisher's exact, linear‐by‐linear, and Kruskal‐Wallis tests, as appropriate. Kaplan‐Meier analyses of OS according to molecular classification were performed using log‐rank tests. Univariable and multivariable analyses were performed by log‐rank test and Cox proportional hazard model, respectively. A p value less than .05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics 22.0 (IBM, Armonk, NY) and R statistical package 3.4.2 (http://www.r-project.org).
Results
Clinicopathologic Characteristics
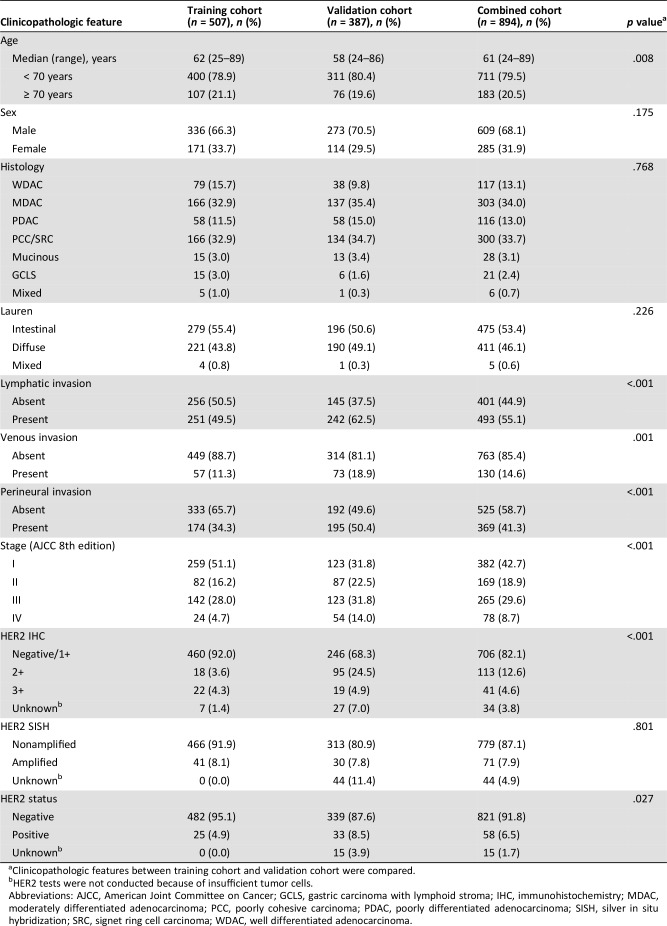
Clinicopathologic characteristics of the two cohorts are summarized in Table 1. Patients in the validation cohort were relatively younger in terms of age than those in the training cohort (p = .008), and there were no significant gender differences between cohorts. The overall distributions of GC subtypes according to World Health Organization (WHO) and Lauren classification were similar between the two cohorts. However, GCs in the validation cohort showed more aggressive pathologic features, including lymphatic invasion (62.5% vs. 49.5%; p < .001), venous invasion (18.9% vs. 11.3%; p = .001), perineural invasion (50.4% vs. 34.3%; p < .001), and advanced stage according to American Joint Committee on Cancer (AJCC) 8th edition (p < .001). The validation cohort also showed higher HER2 IHC scores (p < .001) and overall HER2 positivity (8.5% vs. 4.9%; p = .027).
Table 1. Clinicopathologic features of the study population.
Clinicopathologic features between training cohort and validation cohort were compared.
HER2 tests were not conducted because of insufficient tumor cells.
Abbreviations: AJCC, American Joint Committee on Cancer; GCLS, gastric carcinoma with lymphoid stroma; IHC, immunohistochemistry; MDAC, moderately differentiated adenocarcinoma; PCC, poorly cohesive carcinoma; PDAC, poorly differentiated adenocarcinoma; SISH, silver in situ hybridization; SRC, signet ring cell carcinoma; WDAC, well differentiated adenocarcinoma.
Results of Molecular Classification
We applied the modified molecular classification to the two cohorts separately. Results are summarized in Table 2. In the training cohort, 45 cases out of 507 (8.9%) were in group 1 (MSI+), 191 (37.7%) in group 2 (EBV−, MSI−, non‐EMT‐like, p53−), 51 (10.1%) in group 3 (EBV+), 144 (28.4%) in group 4 (EBV−, MSI−, non‐EMT‐like, p53+), and 76 (15.0%) in group 5 (EBV−, MSI−, EMT‐like). In the validation cohort, the overall distribution of five classes showed no statistically significant differences compared with training cohort (p = .071): group 1 (8.3%; 32/387), group 2 (46.0%; 178/387), group 3 (7.2%; 28/387), group 4 (27.6%; 107/387), and group 5 (10.9%; 42/387).
Table 2. The results of molecular classification in training and validation cohorts.
Abbreviation: EBV, Epstein‐Barr virus; EMT, epithelial‐mesenchymal transition; MSI+, microsatellite instability high.
We compared the proportions of different molecular groups categorized by our classification system among different stages (AJCC 8th edition) as shown in supplemental online Table 1 (combined cohort), and significant differences were observed (p < .001). The proportion of group 1 cases within stage IV was only 2.6%, which was notably lower compared with other stages (6.8% in stage I, 17.2% in stage II, and 7.5% in stage III). Group 2 showed a predilection toward stage I (54.7%) when compared with other stages (37.3% in stage II, 27.2% in stage III, and 32.1% in stage IV). The proportions of group 3 within each stage were relatively consistent: 9.2% (stage I), 7.7% (stage II), 9.1% (stage III), and 9.0% (stage IV). The distribution of group 4 among the four stages was also relatively even, ranging from 26.0% to 32.1%. However, group 5 showed marked preponderance toward advanced stage: 25.7% (88/343) of patients with stage III/IV versus 5.4% (30/551) of patients with stage I/II.
Comparison of Methods for Determination of MSI Status
In order to test whether IHC for MMR protein could serve as the appropriate alternative to PCR‐based assessment of MSI status, we performed MLH1 IHC in all available cases (supplemental online Fig. 2). Loss of MLH1 was found in 61 (7.0%) cases out of 874 tested samples. Discordant results between MLH1 IHC and PCR‐based study were found in 20 cases (2.3%): 5 cases found to be MSI− by PCR analysis showed loss of nuclear MLH1, and 15 cases classified as MSI+ by PCR showed retained expression of MLH1 (supplemental online Table 2). When focusing on cases with MSI+ results by PCR, we found that 21.1% (15/71) of these samples showed retained MLH1 expression.
Survival Analysis According to Clinicopathologic Features and Molecular Classification
Survival analyses according to various clinicopathologic features were performed using log‐rank tests. EBV status itself was not a significant prognostic indicator (training, p = .788; validation, p = .734; combined, p = .791; supplemental online Fig. 3A), whereas we observed OS differences according to MSI status (supplemental online Fig. 3B), which was statistically significant within combined population (training, p = .226; validation, p = .112; combined, p = .045). There were significant OS differences according to the results of E‐cadherin and p53 IHC results (p < .001 and p < .001, respectively, in combined population; supplemental online Fig. 3C–D). However, no survival difference was observed between HER2‐negative and HER2‐positive subgroups (training, p = .507; validation, p = .437; combined, p = .668; supplemental online Fig. 3E).
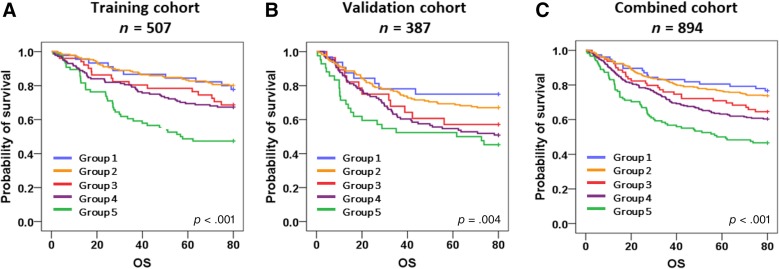
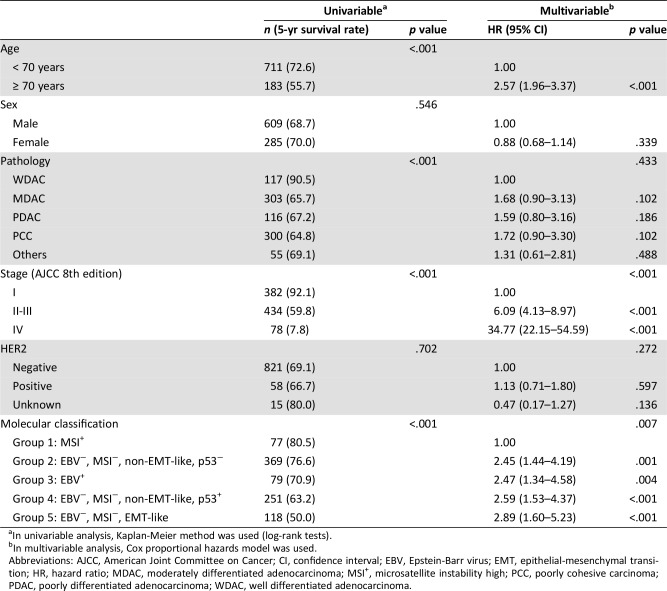
We also performed survival analyses according to the molecular classification, and Kaplan‐Meier survival curves are shown in Figure 2. We found significant OS differences according to the molecular classification in the training cohort (p < .001), the validation cohort (p = .004), and in the combined population (p < .001) as well. In all settings, group 1 (MSI+) and group 2 (EBV−, MSI−, non‐EMT‐like, p53−) showed the best OS outcome, whereas group 5 (EBV−, MSI−, EMT‐like) had the worst OS. When OS between subgroups were compared with each other (supplemental online Table 3), group 5 consistently stood out as the poor prognostic indicator in most of the combinations. Univariable analysis on the combined population showed the significant prognostic impacts of advanced age, pathology, advanced stage, and molecular classification (p < .001). In addition, we confirmed that the modified molecular classification (p = .007) is a statistically significant predictor of OS, independent of other clinical parameters including stage (Table 3).
Figure 2.
Survival analysis according to molecular classification. In the training cohort (A), groups 1 (microsatellite instability [MSI]+) and 2 (Epstein‐Barr virus [EBV]−, MSI−, non‐epithelial‐mesenchymal transition [non‐EMT]‐like, p53−) showed the best survival outcome, groups 3 (EBV+) and 4 (EBV−, MSI−, non‐EMT‐like, p53+) showed intermediate prognosis, and group 5 (EBV−, MSI−, EMT‐like) was noted for poor prognosis (p < .001). Similar patterns of differences in overall survival were recapitulated in the validation cohort (p = .004) (B) and the combined cohort (p < .001) (C).
Abbreviation: OS, overall survival.
Table 3. Univariable and multivariable analysis of overall survival in the combined cohort (n = 894).
In univariable analysis, Kaplan‐Meier method was used (log‐rank tests).
In multivariable analysis, Cox proportional hazards model was used.
Abbreviations: AJCC, American Joint Committee on Cancer; CI, confidence interval; EBV, Epstein‐Barr virus; EMT, epithelial‐mesenchymal transition; HR, hazard ratio; MDAC, moderately differentiated adenocarcinoma; MSI+, microsatellite instability high; PCC, poorly cohesive carcinoma; PDAC, poorly differentiated adenocarcinoma; WDAC, well differentiated adenocarcinoma.
Distinction Between Signet Ring Cell Carcinoma and Poorly Cohesive Carcinoma
According to the 2010 WHO classification, poorly cohesive carcinomas (PCC), including signet ring cell carcinomas (SRCs), account for one of the major histologic patterns of GCs. Although WHO classification does not provide distinction between PCC and pure SRCs, our classification was able to discriminate these two histologic subtypes. As shown in supplemental online Table 4, 47.2% (91/193) of PCCs were classified as group 5 (EBV−, MSI−, EMT‐like), whereas only 8.4% (9/107) of SRCs were in group 3; most of pure SRCs (93/107; 86.9%) fell into group 2 (EBV−, MSI−, non‐EMT‐like, p53−) or group 4 (EBV−, MSI−, non‐EMT‐like, p53+). In addition to the differences in molecular classification, these two histologic features were also associated with significant difference regarding prognosis: SRCs showed significantly better OS compared with PCCs (supplemental online Fig. 4).
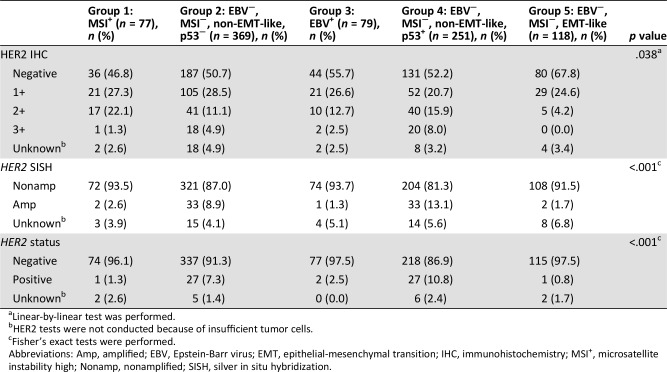
Association Between HER2 Status and Molecular Classification
We assessed HER2 status by performing IHC and SISH, and the results were compared among the groups categorized by our molecular classification system (Table 4). Among 41 patients who were HER2 3+ by IHC, 38 (92.7%) were included in group 2 or 4 (p = .038). Similarly, 93.0% (66/71) of the HER2‐positive patients by SISH were in group 2 or 4 (p < .001). When considering both IHC and SISH results in the determination of HER2 positivity, we noted that a highly significant association exists between HER2 status and molecular classification (p < .001): the majority of HER2‐positive patients (93.1%; 54/58) were included in either group 2 or group 4.
Table 4. Association between HER2 status and molecular classification.
Linear‐by‐linear test was performed.
HER2 tests were not conducted because of insufficient tumor cells.
Fisher's exact tests were performed.
Abbreviations: Amp, amplified; EBV, Epstein‐Barr virus; EMT, epithelial‐mesenchymal transition; IHC, immunohistochemistry; MSI+, microsatellite instability high; Nonamp, nonamplified; SISH, silver in situ hybridization.
Discussion
In this study, we developed and validated a modified IHC‐ and PCR‐based molecular classification system using two independent, large cohorts of patients with GC. We demonstrated the prognostic impact of this simplified molecular classification in patients with GC and also showed close association between HER2 status and certain molecular classification subgroups.
Before the era of TCGA, EBV+ and MSI+ GCs were consistently regarded as the most distinctive subtypes [20], [21], [22], and recent efforts focused on how to classify the remaining cases of GCs other than those two types. In addition to EBV+ and MSI+ GCs, TCGA Research Network proposed GS and CIN subtypes: the former represented by close association with diffuse type GC by Lauren classification with frequent mutations in CDH1 and RHOA genes, whereas the latter is characterized by genomic instability most notably caused by TP53 alterations. Although a number of clinicopathologic characteristics, including tumor location, age, and histologic features, showed significant associations with TCGA's molecular classification, survival differences were not found between the four classes [7]. Subsequently, Cristescu et al. suggested another classification based on MSI status, EMT signature, and TP53 activity, thereby classifying GC into four subtypes, and they found significant differences in survival between the subtypes, which were further validated in additional cohorts of GCs [8].
Despite these improvements with respect to the molecular classification of GC, the impact of this progress on daily diagnostic practice is limited. The most important reason for this discrepancy is that the genome‐based molecular tests are too expensive and labor‐intensive to be used for clinics. The need for more practical and feasible classification systems that can be easily used in routine clinical practice has been increasing. Therefore, studies of IHC‐based molecular classification systems as alternative approaches have been conducted, and some results suggest that the IHC‐based classification would be easily applicable and have prognostic significance [11], [12], [23], [24].
We have proposed a modified version of a previously described IHC‐based molecular classification. First, we adopted PCR‐based determination of MSI status rather than the previously used IHC‐based method, which determined MSI status by confirming loss of MLH1 expression in GC tumor cells compared with adjacent nonneoplastic cells [11], [12]. Numerous studies have suggested the usefulness and noninferiority of MMR protein IHC as a simpler way of screening for MSI status compared with PCR‐based method [25], [26], [27]. Using the tissue samples of current study population, we also performed MLH1 IHC and found that the IHC‐based method showed highly concordant results compared with the PCR‐based assessment. However, it is reported that minority of cases retain intact MLH1 protein expression, although they show obvious MSI phenotype by PCR analyses [28], [29]. Similarly, when confined within MSI+ cases determined by PCR in our analysis, we also found a higher discordance rate, suggesting the limited sensitivity of MLH1 IHC. Considering the marked increase in importance of MSI from a treatment related perspective—that MSI tumors show excellent response to immunotherapeutic agents [30]—we think that determination of MSI status should be done by the most sensitive method available, and we thus implemented this PCR‐based approach into our classification.
Another key modification of our method is the implementation of histologic characteristics in defining group 5, the EBV−, MSI−, EMT‐like subtype. Previous studies used E‐cadherin IHC as the alternative way to define the group that corresponds to the GS subtype in TCGA's classification [7] or to the MSS/EMT group in the ACRG classification [8]. This idea is largely based on the notion that the E‐cadherin protein, coded by the CDH1 gene, is the key mediator of the EMT process. However, at least in GCs, the correlation between E‐cadherin IHC results and CDH1 gene alteration is not clear [31]; therefore, we thought that defining the EMT feature solely based on E‐cadherin IHC would be insufficient. Instead, we suggested adding the histologic criteria of “mesenchymal morphology” into the definition of the EBV−, MSI−, EMT‐like subtype and showed the consistently poor OS of this group compared with others, supporting the clinical usefulness of this approach. Furthermore, the combination of histologic features and immunophenotypic characteristics enabled us to distinguish between pure SRCs and PCC. In addition to proving that these two types with similar but distinct histology were classified differently, we were also able to show significant survival differences between these two.
Most importantly, we succeeded in validating the prognostic significance of this classification system in an independent cohort of patients with GC. To our knowledge, this is the first study in which a molecular classification system for GC using simplified methods that are feasible in real clinical practice setting was validated in an independent large cohort of patients with GC. In addition, we found that this modified molecular classification system is the independent prognostic factor in consecutive GC population by multivariable analysis, irrespective of other clinical factors including stage, further strengthening the prognostic significance of this classification.
In addition to prognostic significance, the major purpose of developing a classification system is for the proper guidance of the therapeutic approaches. Recent evidence suggests that groups 1 (MSI+) and 3 (EBV+) may be the ideal candidates for immunotherapy, considering their high expression of PD‐L1 protein, numerous numbers of tumor infiltrating immune cells, and higher mutational burdens [32], [33]. Therefore, we suggest that these two groups should be classified by routine tests of EBV and MSI status. The poor prognosis of group 5 patients implies that finding actionable targets in this population is critical. A recent study proposed that the IGF‐receptor 1 (IGF1R) inhibitor molecule, linsitinib, showed promising efficacy against mesenchymal phenotype (MP) GCs using cell line‐derived xenograft mouse models [34]. Further clarification of the association between MP subtype GCs and group 5 GCs by our classification system should be conducted to set up adequate therapeutic strategies for group 5 GCs. We observed a close association between non‐EMT phenotype groups 2 and 4 and HER2 positivity, as reported before [35], and it is well known that HER2‐positive cases are the ideal targets for trastuzumab‐based therapeutic approach. Still, a more concrete distinction between p53 IHC results and actual TP53 genetic status would be required for better understanding of molecular biological mechanisms of group 2 and 4 GCs.
Our study has the limitations of being a retrospective study on Asian patients with GC from two different institutions; therefore, for this classification to be more generally accepted for use in clinical practice, additional validation on study populations composed of diverse ethnic backgrounds would be ideal. There is also the possibility that the perception of mesenchymal histology may be different among pathologists; therefore, efforts need to be made to achieve precise definitions and consensus. Construction of TMAs enabled us to validate our classification in the largest cohorts of GC. When constructing TMAs, an experienced pathologist (H.S.L.) reviewed the whole slides of each patient and cautiously selected the most representative tumor areas, and the histologic types of tumors were assigned according the major histologic patterns. During these processes, the nature of mixed carcinoma category of GCs might have been under‐represented, and comprehensive characterization of tumor heterogeneity was not ideally performed; how tumor heterogeneity could be reasonably incorporated into the molecular classification should be the next target of interest in future research.
In conclusion, we have developed a simplified IHC‐ and PCR‐based molecular classification of GC and validated its prognostic implications in the largest consecutive GC cohort ever conducted. We suggest that this classification is an easy‐to‐implement, practical, prognostically significant method for daily practice as well as a possible guidance tool for making therapeutic decisions.
Conclusion
The idea of molecular classification of GC was first introduced by TCGA study; however, the utilization of the classification scheme in daily practice was hindered by the feasibility issue of high‐burden molecular genetic studies. We developed a IHC‐, ISH‐, and PCR‐based molecular classification to overcome this feasibility problem, and we showed that this classification has prognostic significance in the largest consecutive GC cohort. We also suggest that this classification can aid making therapeutic decisions and further understanding GC biology.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF‐2016R1D1A1B03931744).
Contributed equally.
Author Contributions
Conception/design: Jiwon Koh, Keun‐Wook Lee, Hye Seung Lee
Provision of study material or patients: Keun‐Wook Lee, Ji‐Won Kim, Jin Won Kim, Do Joong Park, Hyung‐Ho Kim, Woo Ho Kim
Collection and/or assembly of data: Jiwon Koh, Soo Kyung Nam, An Na Seo
Data analysis and interpretation: Jiwon Koh, Keun‐Wook Lee, Soo Kyung Nam, An Na Seo, Hye Seung Lee
Manuscript writing: Jiwon Koh, Keun‐Wook Lee, Hye Seung Lee
Final approval of manuscript: Jiwon Koh, Keun‐Wook Lee, Soo Kyung Nam, An Na Seo, Ji‐Won Kim, Jin Won Kim, Do Joong Park, Hyung‐Ho Kim, Woo Ho Kim, Hye Seung Lee
Disclosures
The authors indicated no financial relationships.
References
- 1.Ajani JA, D'Amico TA, Almhanna K et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286–1312. [DOI] [PubMed] [Google Scholar]
- 2.Bang YJ, Van Cutsem E, Feyereislova A et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): A phase 3, open‐label, randomised controlled trial. Lancet 2010;376:687–697. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 4.Lee HE, Park KU, Yoo SB et al. Clinical significance of intratumoral HER2 heterogeneity in gastric cancer. Eur J Cancer 2013;49:1448–1457. [DOI] [PubMed] [Google Scholar]
- 5.Shah MA, Ajani JA. Gastric cancer–an enigmatic and heterogeneous disease. JAMA 2010;303:1753–1754. [DOI] [PubMed] [Google Scholar]
- 6.Eom BW, Jung KW, Won YJ et al. Trends in gastric cancer incidence according to the clinicopathological characteristics in Korea, 1999‐2014. Cancer Res Treat 2018;50:1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cristescu R, Lee J, Nebozhyn M et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449–456. [DOI] [PubMed] [Google Scholar]
- 9.Hammond MEH, Hayes DF, Dowsett M et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 2010;134:e48–e72. [DOI] [PubMed] [Google Scholar]
- 10.Lim TH, Lim AST, Thike AA et al. Implications of the Updated 2013 American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations on Human Epidermal Growth Factor Receptor 2 Gene Testing Using Immunohistochemistry and Fluorescence In Situ Hybridization for Breast Cancer. Arch Pathol Lab Med 2016;140:140–147. [DOI] [PubMed] [Google Scholar]
- 11.Setia N, Agoston AT, Han HS et al. A protein and mRNA expression‐based classification of gastric cancer. Mod Pathol 2016;29:772–784. [DOI] [PubMed] [Google Scholar]
- 12.Ahn S, Lee SJ, Kim Y et al. High‐throughput protein and mRNA expression‐based classification of gastric cancers can identify clinically distinct subtypes, concordant with recent molecular classifications. Am J Surg Pathol 2017;41:106–115. [DOI] [PubMed] [Google Scholar]
- 13.Kim JW, Im SA, Kim M et al. The prognostic significance of HER2 positivity for advanced gastric cancer patients undergoing first‐line modified FOLFOX‐6 regimen. Anticancer Res 2012;32:1547–1553. [PubMed] [Google Scholar]
- 14.Lee KS, Nam SK, Koh J et al. Stromal expression of MicroRNA‐21 in advanced colorectal cancer patients with distant metastases. J Pathol Transl Med 2016;50:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HS, Lee HK, Kim HS et al. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol 2003;200:39–46. [DOI] [PubMed] [Google Scholar]
- 16.Chang MS, Lee JH, Kim JP et al. Microsatellite instability and Epstein‐Barr virus infection in gastric remnant cancers. Pathology International 2000;50:486–492. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann M, Stoss O, Shi D et al. Assessment of a HER2 scoring system for gastric cancer: Results from a validation study. Histopathology 2008;52:797–805. [DOI] [PubMed] [Google Scholar]
- 18.Umar A, Boland CR, Terdiman JP et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo AN, Kwak Y, Kim DW et al. HER2 status in colorectal cancer: Its clinical significance and the relationship between HER2 gene amplification and expression. PLoS One 2014;9:e98528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Beek J. EBV‐positive gastric adenocarcinomas: A distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol 2004;22:664–670. [DOI] [PubMed] [Google Scholar]
- 21.Lee HS, Choi SI, Lee HK et al. Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod Pathol 2002;15:632–640. [DOI] [PubMed] [Google Scholar]
- 22.Lee HS, Chang MS, Yang HK et al. Epstein‐Barr virus‐positive gastric carcinoma has a distinct protein expression profile in comparison with Epstein‐Barr virus‐negative carcinoma. Clin Cancer Res 2004;10:1698–1705. [DOI] [PubMed] [Google Scholar]
- 23.Kim HS, Shin SJ, Beom SH et al. Comprehensive expression profiles of gastric cancer molecular subtypes by immunohistochemistry: Implications for individualized therapy. Oncotarget 2016;7:44608–44620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park CK, Park JS, Kim HS et al. Receptor tyrosine kinase amplified gastric cancer: Clinicopathologic characteristics and proposed screening algorithm. Oncotarget 2016;7:72099–72112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindor NM, Burgart LJ, Leontovich O et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol 2002;20:1043–1048. [DOI] [PubMed] [Google Scholar]
- 26.Piñol V, Castells A, Andreu M et al. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA 2005;293:1986–1994. [DOI] [PubMed] [Google Scholar]
- 27.Lee HS, Kim WH, Kwak Y et al. Molecular testing for gastrointestinal cancer. J Pathol Transl Med 2017;51:103–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiaravalli AM, Furlan D, Facco C et al. Immunohistochemical pattern of hMSH2/hMLH1 in familial and sporadic colorectal, gastric, endometrial and ovarian carcinomas with instability in microsatellite sequences. Virchows Arch 2001;438:39–48. [DOI] [PubMed] [Google Scholar]
- 29.Mizoshita T, Tsukamoto T, Cao X et al. Microsatellite instability is linked to loss of hMLH1 expression in advanced gastric cancers: Lack of a relationship with the histological type and phenotype. Gastric Cancer 2005;8:164–172. [DOI] [PubMed] [Google Scholar]
- 30.Le DT, Uram JN, Wang H et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corso G, Carvalho J, Marrelli D et al. Somatic mutations and deletions of the E‐cadherin gene predict poor survival of patients with gastric cancer. J Clin Oncol 2013;31:868–875. [DOI] [PubMed] [Google Scholar]
- 32.Kim ST, Cristescu R, Bass AJ et al. Comprehensive molecular characterization of clinical responses to PD‐1 inhibition in metastatic gastric cancer. Nat Med 2018;24:1449–1458. [DOI] [PubMed] [Google Scholar]
- 33.Koh J, Ock CY, Kim JW et al. Clinicopathologic implications of immune classification by PD‐L1 expression and CD8‐positive tumor‐infiltrating lymphocytes in stage II and III gastric cancer patients. Oncotarget 2017;8:26356–26367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh SC, Sohn BH, Cheong JH et al. Clinical and genomic landscape of gastric cancer with a mesenchymal phenotype. Nat Commun 2018;9:1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kataoka Y, Okabe H, Yoshizawa A et al. HER2 expression and its clinicopathological features in resectable gastric cancer. Gastric Cancer 2013;16:84–93. [DOI] [PubMed] [Google Scholar]