Tumor treating fields, a noninvasive cancer treatment using low intensity alternating electric fields, offers clinical opportunities with unique challenges. This review focuses on the mechanism of action of this treatment, the known pre‐clinical and clinical experience, and the practical issues surrounding its use in the multidisciplinary management of patients with solid malignancies.
Keywords: Tumor‐treating fields, Optune, Glioblastoma, Alternating electric fields, Solid malignancy
Abstract
Tumor‐treating fields (TTFields) are a noninvasive antimitotic cancer treatment consisting of low‐intensity alternating electric fields delivered to the tumor or tumor bed via externally applied transducer arrays. In multiple in vitro and in vivo cancer cell lines, TTFields therapy inhibits cell proliferation, disrupts cell division, interferes with cell migration and invasion, and reduces DNA repair. Human trials in patients with primary glioblastoma showed an improvement in overall survival, and trials in patients with unresectable malignant pleural mesothelioma showed favorable outcomes compared with historical control. This led to U.S. Food and Drug Administration approval in both clinical situations, paving the way for development of trials investigating TTFields in other malignancies. Although these trials are ongoing, the existing evidence suggests that TTFields have activity outside of neuro‐oncology, and further study into the mechanism of action and clinical activity is required. In addition, because TTFields are a previously unrecognized antimitotic therapy with a unique mode of delivery, the oncological community must address obstacles to widespread patient and provider acceptance. TTFields will likely join surgery, systemic therapy, and radiation therapy as a component of multimodality management of patients with solid malignancies.
Implications for Practice.
Tumor‐treating fields (TTFields) exhibit a broad range of antitumor activities. Clinically, they improve overall survival for patients with newly diagnosed glioblastoma. The emergence of TTFields has changed the treatment regimen for glioblastoma. Clinicians need to understand the practical issues surrounding its use in the multidisciplinary management of patients with glioblastoma. With ongoing clinical trials, TTFields likely will become another treatment modality for solid malignancies.
Introduction
Despite decades of investigation, there has been only modest improvement in outcomes for patients with glioblastoma (GBM). In the 1970s, surgical resection and whole brain radiation therapy, the standard of care at the time, resulted in a median overall survival of 9 months [1]. By the early 2000s, radiotherapy field sizes had decreased, and survival improved with the addition of temozolomide (TMZ) chemotherapy, but the median overall survival was still only 15 months [2].
Stupp and colleagues then initiated a phase III randomized trial of 695 patients, known as EF‐14, comparing TMZ chemotherapy with external beam radiation followed by monthly TMZ versus the same treatment with the addition of a novel cancer treatment modality called tumor‐treating fields (TTFields) [3]. TTFields (marketed as Optune therapy; Novocure, St. Helier, Jersey) are low intensity (1–3 V/cm), intermediate frequency (100–300 kHz) electric fields delivered via an array of electrodes applied to the scalp. Although the frequency of these TTFields is too fast for nerve stimulation and too slow for the creation of heat or ionization of cell structures, preclinical and clinical data suggest that they act much like other cytotoxic modalities. Specifically, although taxanes inhibit microtubule formation, micrographic study suggests that TTFields also interfere with microtubule formation, and as certain chemotherapies inhibit specific cell cycle phases, studies have shown that TTFields interfere primarily with the M phase of cell division [4], [5]. Lastly, although ionizing radiation of approximately 1020 Hz causes single‐ and double‐strand DNA breaks that lead to cellular apoptosis, TTFields of approximately 105 Hz interfere with mitosis, resulting in membrane rupture and cellular destruction [6].
The results of EF‐14, reported in 2015 by Stupp and colleagues in the Journal of the American Medical Association, demonstrated an improved progression‐free survival, overall survival (15.6 vs. 20.5 months), and quality of life, without any ill effects on cognitive function [3]. Based on the results of this large, randomized, controlled phase III clinical trial. The National Comprehensive Cancer Network (NCCN) guidelines for central nervous system cancers now include TTFields therapy in combination with TMZ following standard brain radiation therapy with concurrent TMZ as a recommended postoperative adjuvant treatment option for patients with newly diagnosed supratentorial glioblastoma.
Long‐term follow‐up of EF‐14 demonstrated that the 5‐year overall survival for patients with GBM treated with TTFields plus TMZ was more than double that of patients treated by TMZ alone (13% vs. 5%; p = .0037) [7]. Several phase II trials of TTFields in other cancers, including recurrent ovarian cancer, advanced pancreatic cancer, and non‐small cell lung cancer (NSCLC), have been reported, and the launch of several phase III studies for these same cancers has been announced.
In light of these results, we are witnessing the development of a new cancer treatment modality that complements existing surgical, systemic, and radiation therapy techniques. As with any new modality, TTFields bring basic science and clinical opportunities but also bring unique and practical challenges. In this review, we focus on TTFields’ mechanism of action, review existing preclinical and clinical experience, and address some of the practical issues surrounding TTFields’ use in the multidisciplinary management of patients with solid malignancies.
Materials and Methods
PubMed was searched for English‐language articles published before February 28, 2019, using the search terms “tumor treating fields,” “Optune,” “solid malignancy,” “glioblastoma,” and “alternating electrical fields.” Abstracts were searched from recent congresses, including those of the American Society of Clinical Oncology, American Association of Cancer Research, Society of Neuro‐Oncology, and European Society for Medical Oncology. Ongoing clinical trial were compiled through a search of active trials on ClinicalTrials.gov and Novocure.com.
Mechanism of Action
It has long been appreciated that electric currents of 1 kHz or less can stimulate nerves, leading to membrane depolarization and muscle contraction and that externally generated electric fields (e.g., pacemakers) can be used to manipulate the body's existing electrical system. Higher energies, on the order of 900 MHz or more, are sufficiently energetic to produce heat and serve as the primary component of thermal microwave ablation. So, although the use of electric fields in medicine is not new, TTFields are unique in that they are of intermediate frequency (100 to 300 kHz) and therefore too low to generate heat while too high to cause membrane depolarization.
Understanding the exact mechanism of action of TTFields on cells requires a brief review of electromagnetic theory. Simply described, an electric field is the vectoral force that surrounds a positively charged source as it acts upon a negatively charged test particle. In a situation in which there are two parallel electrodes, the magnitude of the electric field is expressed as the difference in voltage between the two electrodes divided by their distance, that is, V/cm. If a charged particle is placed within a constant and uniform electric field, it will move toward the oppositely charged electrode, whereas dipolar particles (those with a positive and negative charge) will rotate and align with the oppositely charged electrodes. This is a process known as dipole alignment. In a situation in which the polarity alternates from one electrode to the other, charged particles will move back and forth, whereas dipoles will rotate in synchrony with the alternating charge.
One last principle of electric fields, referred to as dielectrophoresis, is of particular importance when charged particles are placed within a nonuniform converging electric field where field intensity is higher toward one of the electrodes. In this situation, dipolar particles will not only rotate and align with the oppositely charged electrodes; they will also move toward the area of higher field intensity. These two concepts, dipole alignment and dielectrophoresis, have particular bearing on the cellular effects of TTFields.
In Vitro Cellular Effects
Large biological molecules, such as certain proteins, are composed of both positive and negative charges separated spatially such that they have large dipole moments. When exposed to an electric field, these large molecules are influenced and rotate depending upon the field's orientation. In any biological process governed by precise spatial alignment, such as mitosis, externally applied electric fields should theoretically disrupt this process. This hypothesis has been tested in numerous human cancer cell lines and the cellular effects partially elucidated (Table 1) [4], [5], [6], [8], [9], [10], [11].
Table 1. Summary of in vitro evidence of tumor‐treating fields.
Abbreviation: N/A, not applicable.
Inhibition of Cell Proliferation.
In 2004, Kirson et al. first reported that low‐intensity (1–3 V/cm), intermediate‐frequency (100–300 kHz), alternating electric fields had a profound inhibitory effect on a variety of tumor cell lines [4]. Application of TTFields on human ovarian cancer cells, for example, leads to a significant reduction in cell counts as compared with untreated cells, and the inhibitory effect are nonthermal [11]. Using XTT cell proliferation and clonogenic assays, investigators from Wayne State University demonstrated that TTFields at 200 kHz markedly reduced cell proliferation and clonogenicity in two patient‐derived glioblastoma and gliosarcoma cell lines [12].
Cell Cycle‐Specific Effects.
Alternating electric fields selectively affect specific portions of the cell cycle. During metaphase, TTFields interfere with mitotic spindle formation and the tubulin polymerization process [4]. TTFields decrease the ratio of polymerized tubulin to total tubulin, leading to abnormal chromosome segregation and caspase‐dependent apoptosis of daughter cells [6]. During anaphase, septin protein complexes normally migrate toward the cell midline in preparation for cell division, as they normally stabilize the contractile apparatus; however, in the presence of TTFields, these septin protein complexes misalign, resulting in aberrant mitotic exit [13]. Lastly, during telophase, dielectrophoresis results in a higher‐intensity electric field at the cleavage furrow. This disrupts the separation of daughter cells, leading to mitotic exit and cell death [4].
Reduction of Cancer Cell Migration and Invasion.
Other antitumor effects of TTFields include inhibition of cell migration and invasion [8], [10], [14]. Application of TTFields in vitro significantly reduced human glioma cell migration and invasion as compared with untreated cells using wound healing and Boyden chamber assays [15]. When applied perpendicularly to the course of migration, TTFields’ effects on cell migration were more profound than when applied in two directions or in a parallel direction. Although the mechanism of action is unclear, TTFields do affect cell motility [14].
Inhibition of Angiogenesis.
Kim et al. demonstrated that TTFields suppressed angiogenesis by downregulating VEGF, HIF1α, and matrix metalloproteinases 2 and 9, using U87 and U373 glioblastoma cell lines [10]. Chen et al. also showed that vessel numbers and level of VEGF were all decreased by application of nonionizing, nanosecond pulsed electric fields [16].
Reduction of DNA Repair.
In NSCLC cell lines, TTFields significantly downregulate DNA damage repair pathway genes such as BRCA1, and irradiating cells in the presence of TTFields results in an increased number of double‐strand breaks, chromatid aberrations, and radical oxygen species [16]. Investigations suggest that PARP1 inhibition could complement BRCA1 inhibition, as PARP1 is important in single‐strand DNA repair, whereas BRCA1 is important in double‐strand break repair [17]. Cells that attempt cell division in the presence of unrepaired single‐strand breaks would propagate those breaks into double‐strand breaks, leading to cell death.
Synergistic Effects with Other Therapies.
Several studies have shown that TTFields exhibit additive and synergistic effects with chemotherapeutic agents [4], [5], [11], [18]. Kirson et al. combined TTFields with paclitaxel and cyclophosphamide in breast carcinoma as well as TMZ in glioma cell lines. They concluded that the efficacy of these chemotherapeutic agents in vitro was increased by one to three orders of magnitude by the addition of TTFields [4]. More recently, the combination of cisplatin and TTFields in an NSCLC mouse model resulted in the smallest tumors when compared with cisplatin or TTFields alone [18].
TTFields have also demonstrated synergistic effects with ionizing radiation [19]. When the GBM cell lines U373 and U87 were treated with TTFields for 24 hours followed by 137Cs γ‐ray radiation (dose rate, 3.8 Gy/min; total dose, 2–6 Gy), the combined treatments significantly increased DNA damage and mitotic abnormalities compared with either alone. In addition, application of TTFields to irradiated glioma cells impaired repair of irradiation‐induced or chemically induced DNA damage, possibly by blocking homologous recombination repair [20].
Immunologic Effects.
TTFields also influence immunogenic cell death. In both murine Lewis lung carcinoma and ovarian surface epithelial cell cultures, TTFields resulted in elevated cell surface expression of calreticulin, decreased intracellular ATP levels, and promoted HMGB1 secretion, all markers of immunogenic cell death [21].
TTFields treatment also has synergistic effects with immunotherapeutic agents. Combining TTFields therapy with an immune checkpoint inhibitor (anti‐PD‐1) in vivo significantly decreased tumor volume compared with either therapy alone [21].
In Vivo Effects
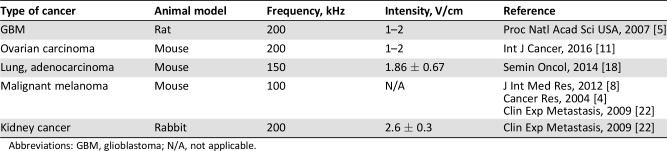
Several animal models have been used to elucidate the in vivo effects of TTFields therapy (Table 2) [4], [5], [8], [11], [18], [22]. TTFields inhibit tumor growth in rat, mouse, and rabbit models for GBM, melanoma, adenocarcinoma of lung, and ovarian and kidney cancer. In these models, TTFields result in a substantial tumor size decrease compared with controls. TTFields reduce the extent of lung metastasis using both a melanoma mouse model and a kidney cancer rabbit model [22].
Table 2. Summary of in vivo evidence of tumor‐treating fields.
Abbreviations: GBM, glioblastoma; N/A, not applicable.
Clinical Safety and Efficacy
Completed clinical trials using TTFields for different solid malignancies are summarized in Table 3.
Table 3. Summary of completed clinical trials on application of TTFields for solid malignancies.
Abbreviations: AACR, American Association for Cancer Research annual meeting; GBM, glioblastoma; IASLC, International Association for the Study of Lung Cancer; NSCLC, non‐small cell lung cancer; TTFields, tumor‐treating fields.
Glioblastoma
Based upon encouraging preclinical evidence, a pilot phase I/II clinical trial was performed in 20 patients with histologically confirmed glioblastoma: 10 with recurrent disease treated with TTFields as monotherapy and 10 with primary disease treated with TTFields and adjuvant TMZ after they completed concurrent radiation and TMZ [5]. No device‐related or increased TMZ‐related adverse effects were observed, and progression‐free and overall survival were improved over historical controls. The long‐term survival outcome for these patients was recently reported, and 4 (two primary and two recurrent) of the original 20 patients are still alive, without relapse, and no longer receiving treatment 12 years after initiating TTFields therapy [23]. Interestingly, two of the four surviving patients exhibited early radiological evidence of progression but continued the TTFields therapy and after a median of 4 months experienced tumor regression.
The encouraging result of the pilot trial prompted a phase III prospective trial for patients with recurrent GBM known as EF‐11 [24]. In this trial, 237 patients were randomized to TTFields as monotherapy (120 patients) or physician's best choice of chemotherapy (117 patients). TTFields monotherapy had similar efficacy as chemotherapy with a median survival of 6.6 versus 6.0 months (p = .27), 1‐year survival rate of 20% versus 20%, and a progression‐free survival at 6 months of 21.4% versus 15.1% (p = .13). Additionally, TTFields treatment had fewer severe adverse events (6% vs. 16%; p = .02), and quality of life favored TTFields, resulting in U.S. Food and Drug Administration (FDA) approval in 2011 for treatment of recurrent GBM [23]. Subsequent analysis showed that patients with TTFields adherence of at least 75% (at least 18 hours/day) had higher median overall survival (7.7 vs. 4.5 months; p = .04) [25].
For patients with primary disease, a second large phase III trial (EF‐14) was published in 2015 after enrolling 695 of a planned 700 patients [3]. All patients completed standard concurrent chemoradiation and were stratified by MGMT methylation and resection status before being randomized to receive maintenance treatment with either TTFields plus TMZ (n = 466) or TMZ alone (n = 229). Treatment with TTFields was delivered continuously (≥18 hours/day) via four transducer arrays placed on the shaved scalp and connected to a portable battery. The primary endpoints were progression‐free survival and overall survival, with a preplanned interim analysis evaluating the outcomes for the first 315 patients with at least 18 months of follow‐up. TTFields plus TMZ significantly improved progression‐free survival (7.1 vs. 4.0 months; p = .001) while adding a 5‐month overall survival benefit (20.5 vs. 15.6 months; p = .004) compared with TMZ alone. As a result, the FDA granted approval for the use of TTFields in primary GBM, and the NCCN guidelines for central nervous system cancers now include TTFields therapy in combination with TMZ following standard brain radiation therapy with concurrent TMZ as a recommended postoperative adjuvant treatment option for patients with newly diagnosed supratentorial glioblastoma.
The updated result of EF‐14 showed that adding TTFields to TMZ resulted in significantly improved 5‐year overall survival compared with TMZ alone (13% vs. 5%; p = .0037), and the improvement in overall survival was seen across all patient subgroups including age, extent of resection, performance status, and MGMT methylation status [7]. As previously reported in EF‐11, the degree of adherence was predictive of outcome, and a secondary analysis showed that higher electric field intensity (≥1.05 V/cm) to the tumor bed was associated with better median survival (25.0 vs. 21.6 months; hazard ratio, 0.76; p = .043) as compared with lower electric field intensity [26]. These results essentially void the argument made by some investigators that the beneficial effects of TTFields may be due to a placebo effect.
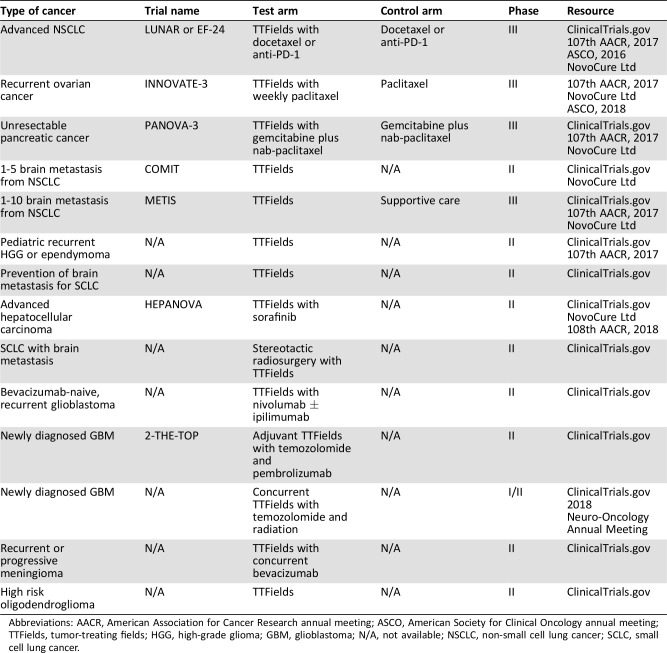
As mentioned before, in vitro study demonstrated there were synergistic effects between TTFields and ionizing radiation [19]. Currently, a phase I/II trial is in progress to test the eligibility and efficacy of concurrent treatment of TTFields with TMZ and radiotherapy for newly diagnosed GBM [27]. In addition, phase II trials are in progress to test the efficacy of concurrent treatment of TTFields with immunotherapeutic agents, including (a) adjuvant TTFields with pembrolizumab and TMZ after definitive TMZ and radiotherapy for newly diagnosed GBM and (b) TTFields and nivolumab with and without ipilimumab for recurrent GBM (Table 4).
Table 4. Summary of ongoing clinical trials on application of TTFields for solid malignancies.
Abbreviations: AACR, American Association for Cancer Research annual meeting; ASCO, American Society for Clinical Oncology annual meeting; TTFields, tumor‐treating fields; HGG, high‐grade glioma; GBM, glioblastoma; N/A, not available; NSCLC, non‐small cell lung cancer; SCLC, small cell lung cancer.
Other Solid Malignancies
Although the level I clinical evidence relates to patients with GBM, there is now a growing body of experience revealing TTFields’ activity in other cancers, and there are several planned and active trials in diseases other than primary brain tumors (Table 3).
TTFields in combination with pemetrexed were tested in advanced NSCLC in a phase II study in which 41 patients with inoperable advanced NSCLC who had tumor progression after chemotherapy were enrolled and received pemetrexed with TTFields applied to the chest and upper abdomen until disease progression [28]. TTFields combined with pemetrexed were tolerated; there were no device‐related serious adverse events, and the median overall survival was 13.4 months. Because the 1‐ and 2‐year survival of 57% and 26%, respectively, was better than historical controls, a phase III randomized control study (the LUNAR study or EF‐24) launched and is currently enrolling patients to study the efficacy of TTFields in combination with docetaxel or anti‐PD‐1 for advanced NSCLC [29].
The use of TTFields and concurrent paclitaxel in 31 patients with heavily pretreated, platinum‐resistant, unresectable ovarian cancer resulted in a progression‐free survival of 8.9 months (compared with 3.9 months in paclitaxel‐alone historical controls), with only two patients (6.4%) having severe skin irritation [30]. A phase III trial known as INNOVATE‐3 will now test TTFields (at 200 kHz) concomitantly with weekly paclitaxel in earlier‐stage, recurrent, platinum‐resistant ovarian carcinoma.
Results from a phase II study testing TTFields in combination with gemcitabine for unresectable pancreatic adenocarcinoma demonstrated improved efficacy compared with historical controls, with a median progression‐free survival of 8.3 months and median overall survival of 14.9 months [31]. This same trial enrolled 20 patients to TTFields in combination with gemcitabine plus nab‐paclitaxel and reported no TTFields‐related serious side effects. The median progression‐free survival was 12.7 months, which was longer than published historical results. The median overall survival was not reached, and the 1‐year survival rate was 72% [32]. A phase III trial known as PANOVA‐3 will investigate TTFields (150 kHz) concomitantly with standard chemotherapy for front‐line therapy of unresectable pancreatic adenocarcinoma.
Recently, the result of the STELLAR phase II trial of TTFields for mesothelioma was presented at the International Association for the Study of Lung Cancer's 19th World Conference on Lung Cancer [33]. Patients with mesothelioma who received TTFields with pemetrexed and cisplatin or carboplatin experienced median overall survival of 18.2 months compared with 12.1 months in historical control, with no increase in systemic toxicity [33]. Based upon the final STELLAR data, very recently, the U.S. FDA approved TTFields (the NovoTTF‐100L system; Novocure) to be used in conjunction with a standard two‐drug chemotherapy for unresectable malignant pleural mesothelioma. TTFields are the first treatment in more than 15 years that the FDA has approved for mesothelioma since the approval of pemetrexed in 2004.
In addition, phase II/III trials are in progress to study the efficacy of TTFields for brain metastasis from NSCLC (the METIS trial and COMIT trial), advanced hepatocellular carcinoma (the HEPANOVA trial), recurrent meningioma, high‐risk oligodendroglioma, and pediatric high‐grade glioma and ependymoma (Table 4).
Side Effects and Quality of Life
Based on the results from EF‐14 [5], [7], compared with TMZ alone, the addition of TTFields to TMZ in patients with newly diagnosed glioblastoma was not associated with any significant increase (44% vs. 48%; p = .58) in systemic toxic effects including fatigue; infection; hematological, gastrointestinal vascular, and respiratory disorders; and neurological disorders (headache and seizure). The only notable exception was a higher incidence of localized skin toxicity (medical device site reaction beneath the transducer arrays) in patients who received TTFields. Mild to moderate skin irritation was observed in 43% of patients treated with TTFields and severe skin reaction (grade 3) in 2% of patients.
A secondary analysis of EF‐14 showed that health‐related quality of life did not differ significantly between treatment arms except for more itchy skin with TTFields therapy [34]. Deterioration‐free survival was significantly longer with TTFields for global health, physical and emotional functioning, pain, and leg weakness, likely related to improved progression‐free survival. Time to deterioration, reflecting the influence of treatment, did not differ significantly except for itchy skin (TTFields worse; 8.2 vs. 14.4 months; p < .001) and pain (TTFields improved; 13.4 vs. 12.1 months; p < .01). Role, social, and physical functioning were not affected by TTFields.
Cost‐Effectiveness for TTFields Therapy
Guzauskas et al. analyzed the cost‐effectiveness for TTFields therapy by using the data from EF‐14 [35]. They calculated an undiscounted increase in mean survival of 1.8 life years for TTFields plus TMZ versus TMZ alone. The incremental cost‐effectiveness ratio was $150,452 per life year gained and $197,336 per quality‐adjusted life year gained. Mean lifetime survival and quality‐adjusted survival substantially increased with treatment with TTFields plus TMZ compared with TMZ alone in patients with newly diagnosed GBM. Therefore, treatment with TTFields can be considered cost‐effective within the reported range of willingness‐to‐pay thresholds in the U.S. based on the results of this analysis.
TTFields Planning and Delivery
Although it is tempting to draw comparisons between TTFields and ionizing radiation, their mechanisms of action and modes of delivery are unique. The most important and useful distinction between the two is to compare their frequency: TTFields are approximately 105 Hz, resulting in a wavelength of 3 km, whereas ionizing radiation is approximately 1020 Hz, resulting in a wavelength of 0.003 nm. This difference provides considerable insight into why TTFields require a different approach to planning and delivery from that established for ionizing radiation. Another distinction is how we quantify the delivered energy: an ionizing radiation dose is energy deposited in tissue and expressed as joules per kilogram, whereas a TTFields dose is change in energy over time or power and expressed as watts per volume.
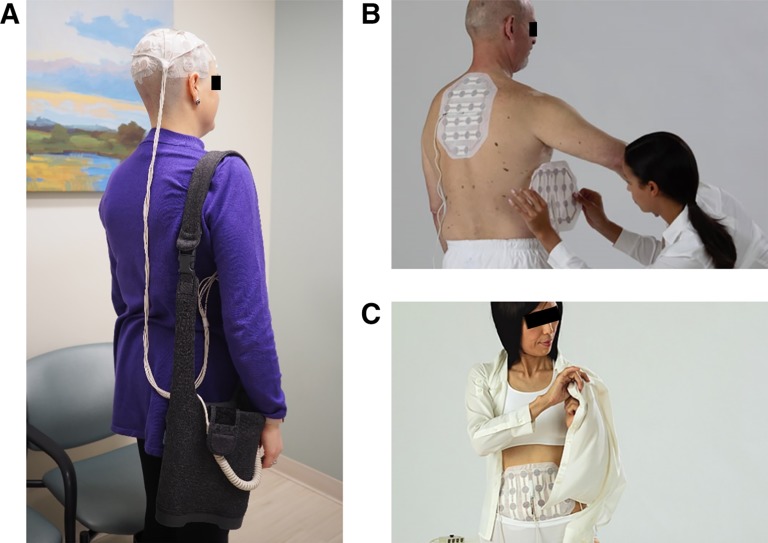
The first FDA‐approved delivery method for TTFields in patients with recurrent or primary GBM is the medical device Optune. Using two paired transducer arrays applied to the shaved scalp and connected to a portable battery via a small electric field generator, the device delivers an alternating electric field. A voltage difference between the anterior and posterior and the left and right lateral transducers creates the electric field within the brain at a frequency of 200 kHz with an intensity of 1 to 3 V/cm. Figure 1A shows an example of a patient with the transducer arrays on shaved scalp and the battery in a shoulder bag. Examples of transducer arrays on body are shown in Figure 1B and C.
Figure 1.
Example diagrams of patients with the tumor‐treating fields device. (A): A patient with the transducer arrays on shaved scalp and the portable battery in a shoulder bag. (B): Transducer arrays are placed on a patient's back for mesothelioma or lung cancer. (C): Transducer arrays are placed on abdomen or pelvis for ovarian cancer. Figure 1B and 1C are reproduced with permission from Novocure. © 2019 Novocure.
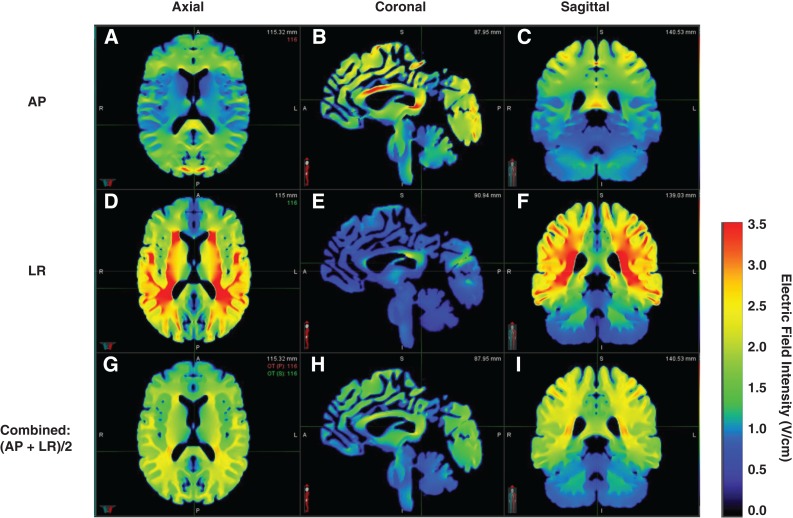
Unlike planning software and the ionization chambers used in radiation oncology to visualize and measure ionizing radiation dose, it is quite challenging to visualize and measure TTFields intensities. Several studies have provided insight into the intensity distribution of TTFields in the human brain using a finite element method [36], [37], [38]. Miranda et al. constructed a realistic head model from magnetic resonance imaging (MRI) images segmented into five different tissue types: scalp, skull, cerebrospinal fluid, gray matter, and white matter [37]. A volume mesh was then generated, and typical values for the conductivity (σ) and relative permittivity (εr) were applied to the segmented tissues. A finite element physics solver then solves Maxwell's equation between mesh interfaces and displays the intensity across the segmented tissues (Fig. 2). The simulation results show that the calculated electric field is nonuniform (ranging between 0.5 and 2.0 V/cm) but is predictable in that the highest intensities occur near the interface of tissues with significantly different conductivities such as the lateral ventricles and outside the necrotic core of tumors [36], [37], [39].
Figure 2.
Example of calculated electric field distribution within the brain. The axial, coronal, and sagittal views of calculated electric field distributions for anterior‐posterior and left‐right transducer arrays as well as combined electric fields are shown, respectively.
Abbreviations: AP, anterior‐posterior; LR, left‐right.
Although routinely calculating patient‐specific TTFields intensity distributions is not yet practical, it is of particular interest because both preclinical and clinical studies have shown that the inhibitory effects of TTFields start at 1.0 V/cm and increase with increasing intensity [4], [5], [26]. To maximize the field intensity to the region where most brain failures occur (2.0 cm around the primary tumor), Wenger et al. compared a personalized array layout with a symmetric one and concluded that adapting the array layout to the tumor location resulted in a 2‐fold increase in the field intensity [40].
Lastly, TTFields have been shown to have synergistic interaction with radiation. Straube et al. investigated the dosimetric impact of TTFields transducer arrays onto radiation treatment plans for GBM [41]. There was a small but clinically insignificant interaction between the TTFields transducer arrays and radiation treatment plan. Therefore, daily replacement of TTFields transducer arrays may not be necessary; however, concomitant use of TTFields with radiotherapy is still considered investigational [27].
The NovoTAL System
A currently available and FDA‐approved software program known as NovoTAL (Novocure) allows for optimization of array layouts based upon specific tumor location [42]. Instead of calculating actual TTFields intensity on patient‐specific MRI anatomy, however, the NovoTAL system stores multiple precalculated array layouts and chooses the layout most applicable to the current patient. This requires the physician to enter 20 head and tumor measurements taken from the T1 postcontrast MRI images (Fig. 3). Some investigators have advocated for planning from the T2‐weighted or fluid‐attenuated inverse recovery MRI images because peritumoral edema often contains tumor, but in our experience this is more likely to result in a symmetric layout and may actually decrease the field intensity to the tumor bed. For this reason, we continue to recommend mapping to the T1 postcontrast enhanced images [43].
Figure 3.
NovoTAL treatment planning using standard T1‐weighted postcontrast magnetic resonance imaging (MRI) image. The process is as follows. (A): Head size measurements 1–3 are obtained using the axial MRI slice directly above the superior edge of the orbit. Reference frame was drawn around the head at the outer margin of the scalp. (B): Head size measurements 4–6 are obtained using the coronal MRI slice at the level of external auditory canal. A reference frame was drawn with the bottom line at the level of inferior margin of the temporal lobe. (C): Tumor location measurements 7–13 are obtained using the axial MRI image showing the maximum diameters of enhancing tumor. (D): Tumor location measurements 14–20 are obtained using the coronal MRI image showing the maximum diameters of enhancing tumor.
Despite being a first‐generation platform for TTFields planning, the NovoTAL system allows for (a) increasing TTFields intensity at the primary tumor site, (b) early correlation between intensity and tumor recurrence, and (c) remapping and adapting the array layout to account for disease status changes. Importantly, having physicians perform the mapping procedure using the NovoTAL system is reliable and reproducible [42].
Role of the Multidisciplinary Team
Before initiating any treatment, multidisciplinary discussion develops consensus among providers and generates a comprehensive multidisciplinary care plan. Just as the multidisciplinary discussion focuses on the inclusion, intensity, and timing of surgery, systemic therapy, and radiation therapy, we have found that the early integration and discussion of TTFields therapy significantly enhances the likelihood of patient and provider acceptance and adherence. In our early experience, if the discussion of TTFields was delayed until the last day of chemoradiation therapy, our patients were less likely to accept them as a treatment option. We also determined that the discussion around the delivery of TTFields was more successful when TTFields were offered in the same manner that we offer other therapies. For example, when offered chemoradiation followed by adjuvant chemotherapy with TTFields as the standard of care, patients were more likely to accept them than when offered TTFields as an option after completing all the other therapies. This is not to say that TTFields are mandatory but rather to include TTFields in our standard approach and make them just as mandatory (or optional) as surgery, systemic therapy, and radiation therapy.
Currently eligible patients for treatment with TTFields include those with histologically confirmed, primary or recurrent, supratentorial GBM. Patients should have a Karnofsky performance status score of 70% or higher and adequate bone marrow, liver, and renal function [3]. TTFields are contraindicated for patients aged 18 years or younger; who are pregnant; who have active implanted medical devices (deep brain stimulators, spinal cord stimulators, vagus nerve stimulators, pacemakers, defibrillators, and programmable shunts); or who have a skull defect (such as missing bone with no replacement), shunt, or bullet fragments.
According to the design of EF‐14, TTFields treatment starts 4 to 7 weeks following completion of chemoradiation for newly diagnosed GBM and is used in conjunction with high‐dose adjuvant TMZ. For those patients treated on the EF‐14 protocol, randomization occurred only after a postchemoradiation MRI of the brain revealed no progression of disease. As discussed above, however, the subgroup analysis showed that all patients, regardless of resection status (biopsy only vs. subtotal vs. total resection), benefitted from TTFields use. For this reason, we have opted to eliminate this interval scan, as the results do not significantly change our decision to initiate therapy.
Once the patient initiates TTFields treatment, monthly visits assess both patient adherence and skin tolerance. Of the patients enrolled on the EF‐14 trial, 44% had mild or moderate skin reactions underneath the transducer arrays; however, only 1% to 2% of skin reactions were grade 3, requiring any medical management other than shifting the placement of the transducer arrays.
Magnetic resonance scans performed every 2 to 3 months assess disease status, but if radiographic progression is detected, TTFields treatment is continued with or without additional local or systemic therapy as long as the patient continues to adhere to and tolerate the treatment [44]. If the progression is an increase in size of at least 25% or if new lesions appear distal to the original tumor bed, we recommend replanning using the NovoTAL system to account for these changes. In the setting of significant clinical deterioration and functional decline, we then consider discontinuation of TTFields therapy on a case‐by‐case basis.
Controversies and Future Directions
Despite the evidence outlined above, considerable controversy surrounds the acceptance of TTFields, particularly among the neuro‐oncology community [45], [46]. Some cite the challenge of understanding the mechanism of action or argue that the clinical benefit is too small [45], [46]. Others reference the regulatory burden of allowing patients in clinical trials to use a device that increases overall survival and potentially masks the effect of the investigational therapy in question. The lack of a sham device in the control arm to rule out a placebo effect has also been criticized [47], as has the fact that the designers of EF‐14 recommended continuation of the therapy despite disease progression, whereas previous investigators have traditionally discontinued therapy in this setting. Lastly, there is the inability to convince patients of the importance of device adherence or to accept therapy in the first place despite the therapy having considerably fewer side effects than surgery, systemic therapy, or radiation therapy.
Although a dose of skepticism is essential when evaluating any new modality, it is equally important to remain objective and to manage all forms of conflict of interest [48]. For example, to claim that a benefit is too small does not acknowledge the patient perspective, nor does withholding a therapy because the improved overall survival would interfere with the analysis of some other agent's efficacy. It is equally important to judge therapies consistently; radiotherapy trials do not use sham devices, and medical oncology trials rarely use placebos. Although we acknowledge that the pursuit of new and effective therapies for patients with cancer (and GBM in particular) is necessary, we caution against withholding a therapy with substantial preclinical data and proven clinical benefit. We also urge clinicians to include TTFields as part of the multidisciplinary standard of care discussion and reinforce the importance of adherence throughout the patient's therapy.
Conclusion
We are witnessing the development of a new cancer treatment modality that complements existing surgical, systemic, and radiation therapy techniques. Although controversies abound, data suggest that TTFields have the potential to affect significantly the lives of patients with cancer.
Author Contributions
Conception/design: Yuefeng Wang, Manjari Pandey, Matthew T. Ballo
Provision of study material or patients: Yuefeng Wang, Matthew T. Ballo
Collection and/or assembly of data: Yuefeng Wang, Matthew T. Ballo
Data analysis and interpretation: Yuefeng Wang, Matthew T. Ballo
Manuscript writing: Yuefeng Wang, Manjari Pandey, Matthew T. Ballo
Final approval of manuscript: Yuefeng Wang, Manjari Pandey, Matthew T. Ballo
Disclosures
Manjari Pandey: Novocure (RF); Matthew T. Ballo: Novocure (SAB). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Walker MD, Alexander E Jr, Hunt WE et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg 1978;49:333–343. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Taillibert S, Kanner AA et al. Maintenance therapy with tumor‐treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. JAMA 2015;314:2535–2543. [DOI] [PubMed] [Google Scholar]
- 4.Kirson ED, Gurvich Z, Schneiderman R et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res 2004;64:3288–3295. [DOI] [PubMed] [Google Scholar]
- 5.Kirson ED, Dbalý V, Tovarys F et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci USA 2007;104:10152–101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giladi M, Schneiderman RS, Voloshin T et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep 2015;5:18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stupp R, Taillibert S, Kanner A et al. Effect of tumor‐treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017;318:2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Liu R, Liu J et al. Growth inhibition of malignant melanoma by intermediate frequency alternating electric fields, and the underlying mechanisms. J Int Med Res 2012;40:85–94. [DOI] [PubMed] [Google Scholar]
- 9.Giladi M, Schneiderman RS, Porat Y et al. Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumor treating fields. Pancreatology 2014;14:54–63. [DOI] [PubMed] [Google Scholar]
- 10.Kim EH, Song HS, Yoo SH et al. Tumor treating fields inhibit glioblastoma cell migration, invasion and angiogenesis. Oncotarget 2016;7:65125–65136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voloshin T, Munster M, Blatt R et al. Alternating electric fields (TTFields) in combination with paclitaxel are therapeutically effective against ovarian cancer cells in vitro and in vivo. Int J Cancer 2016;139:2850–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michelhaugh SK, Kiousis S, Wallace‐Povirk A et al. Tumor‐treating fields decrease proliferation and clonogenicity of patient‐derived WHO grade IV glioma cell lines. Abstract presented at: 107th Annual Meeting of the American Association for Cancer Research (AACR); April 1–5, 2017; Washington, D.C.; 3309.
- 13.Gera N, Yang A, Holtzman TS et al. Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLoS One 2015;10:e0125269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silginer M, Weller M, Stupp R et al. Biological activity of tumor‐treating fields in preclinical glioma models. Cell Death Dis 2017;8:e2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia‐Carracedo D, Schneiderman RS, Zeevi E et al. Tumor treating fields (TTFields) affect human glioma cell migration, invasion and adherence properties in vitro. Abstract presented at: 107th Annual Meeting of the AACR; April 1–5, 2017; Washington, D.C.; 900.
- 16.Chen X, Kolb JF, Swanson RJ et al. Apoptosis initiation and angiogenesis inhibition: Melanoma targets for nanosecond pulsed electric fields. Pigment Cell Melanoma Res 2010;23:554–563. [DOI] [PubMed] [Google Scholar]
- 17.Karanam NK, Srinivasan K, Ding L et al. Tumor treating fields elicit a conditional vulnerability to ionizing radiation via the downregulation of BRCA1 signaling and reduced DNA double‐strand break repair capacity in non‐small cell lung cancer cell lines. Cell Death Dis 2017;8:e2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giladi M, Weinberg U, Schneiderman RS et al. Alternating electric fields (tumor‐treating fields therapy) can improve chemotherapy treatment efficacy in non‐small cell lung cancer both in vitro and in vivo. Semin Oncol 2014;41(suppl 6):S35–S41. [DOI] [PubMed] [Google Scholar]
- 19.Kim EH, Kim YH, Song HS et al. Biological effect of an alternating electric field on cell proliferation and synergistic antimitotic effect in combination with ionizing radiation. Oncotarget 2016;7:62267–62279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giladi M, Munster M, Schneiderman RS et al. Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat Oncol 2017;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voloshin T, Tal‐Yitzhaki O, Kaynan N et al. Tumor treating fields (TTFields) plus anti‐PD‐1 therapy induce immunogenic cell death resulting in enhanced antitumor efficacy. Abstract presented at: 107th Annual Meeting of the AACR; April 1–5, 2017; Washington, D.C.; 3665.
- 22.Kirson ED, Giladi M, Gurvich Z et al. Alternating electric fields (TTFields) inhibit metastatic spread of solid tumors to the lungs. Clin Exp Metastasis 2009;26:633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rulseh AM, Keller J, Klener J et al. Long‐term survival of patients suffering from glioblastoma multiforme treated with tumor‐treating fields. World J Surg Oncol 2012;10:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stupp R, Wong ET, Kanner AA et al. NovoTTF‐100A versus physician's choice chemotherapy in recurrent glioblastoma: A randomized phase III trial of a novel treatment modality. Eur J Cancer 2012;48:2192–2202. [DOI] [PubMed] [Google Scholar]
- 25.Kanner AA, Wong ET, Villano JL et al.; EF‐11 Investigators. Post hoc analyses of intention‐to‐treat population in phase III comparison of NovoTTF‐100A system versus best physician's choice chemotherapy. Semin Oncol 2014;41(suppl 6):S25–S34. [DOI] [PubMed] [Google Scholar]
- 26.Ballo MT, Urman N, Lavy‐Shahaf G et al. Correlation of tumor treating fields dosimetry to survival outcomes in newly diagnosed glioblastoma: A large‐scale numerical simulation‐based analysis of data from the phase 3 EF‐14 randomized trial. Int J Radiat Oncol Biol Phys 2019;104:1106–1113. [DOI] [PubMed] [Google Scholar]
- 27.Glas M, Scheffler B, Lazaridis L et al. PriCoTTF: A phase I/II trial of tumor treating fields prior and concomitant to radiotherapy in newly diagnosed glioblastoma. Neuro Oncol 2018;20(suppl 6):ACTR‐49A. [Google Scholar]
- 28.Pless M, Betticher DC, Droege C et al. A phase II study of tumor treating fields (TTF) in combination with pemetrexed for advanced non‐small cell lung cancer (NSCLC): Updated survival results. J Clin Oncol 2011;29(suppl 15):e18030A. [Google Scholar]
- 29.Weinberg U, Farber O, Giladi M et al. LUNAR ‐ A phase 3 trial of TTFields in combination with PD‐1 inhibitors or docetaxel for second line treatment of non‐small cell lung cancer (NSCLC). Abstract presented at: 107th Annual Meeting of the AACR; April 1–5, 2017; Washington, D.C.; CT071.
- 30.Vergote I, von Moos R, Manso L et al. Tumor treating fields in combination with paclitaxel in recurrent ovarian carcinoma: Results of the INNOVATE pilot study. Gynecol Oncol 2018;150:471–477. [DOI] [PubMed] [Google Scholar]
- 31.Benavides M, Guillen C, Rivera F et al. PANOVA: A phase II study of TTFields (150kHz) concomitant with standard chemotherapy for front line therapy of advanced pancreatic adenocarcinoma. Abstract presented at: 107th Annual Meeting of the AACR; April 1–5, 2017; Washington, D.C.; CT130.
- 32.Rivera F, Benavides M, Gallego J et al. Tumor treating fields in combination with gemcitabine or gemcitabine plus nab‐paclitaxel in pancreatic cancer: Results of the PANOVA phase 2 study. Pancreatology 2019;19:64–72. [DOI] [PubMed] [Google Scholar]
- 33.Ceresoli G, Aerts J, Madrzak J et al. STELLAR – Final results of a phase 2 trial of TTFields with chemotherapy for first‐line treatment of malignant pleural mesothelioma. Abstract presented at: International Association for the Study of Lung Cancer (IASLC) 19th World Conference on Lung Cancer, September 23–26, 2018; Toronto, Canada; MA12.06.
- 34.Taphoorn MB, Dirven L, Kanner AA et al. Influence of treatment with tumor‐treating fields on health‐related quality of life of patients with newly diagnosed glioblastoma: A secondary analysis of a randomized clinical trial. JAMA Oncol 2018;4:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guzauskas G, Wang BCM, Pollom E et al. The cost effectiveness of tumor treating fields treatment for patients with newly diagnosed glioblastoma based on the EF‐14 trial. Neuro Oncol 2018;20(suppl 6):HOUT‐16A. [Google Scholar]
- 36.Wenger C, Salvador R, Basser PJ et al. The electric field distribution in the brain during TTFields therapy and its dependence on tissue dielectric properties and anatomy: A computational study. Phys Med Biol 2015;60:7339–7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miranda PC, Mekonnen A, Salvador R et al. Predicting the electric field distribution in the brain for the treatment of glioblastoma. Phys Med Biol 2014;59:4137–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lok E, Hua V, Wong RT. Computed modeling of alternating electric fields therapy for recurrent glioblastoma. Cancer Med 2015;4:1697–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wenger C, Salvador R, Basser PJ et al. Modeling tumor treating fields (TTFields) application within a realistic human head model. Conf Proc IEEE Eng Med Biol Soc 2015;2015:2555–2558. [DOI] [PubMed] [Google Scholar]
- 40.Wenger C, Salvador R, Basser PJ et al. Improving tumor treating fields treatment efficacy in patients with glioblastoma using personalized array layouts. Int J Radiat Oncol Biol Phys 2016;94:1137–1143. [DOI] [PubMed] [Google Scholar]
- 41.Straube C, Oechsner M, Kampfer S et al. Dosimetric impact of tumor treating field (TTField) transducer arrays onto treatment plans for glioblastomas ‐ A planning study. Radiat Oncol 2018;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhry A, Benson L, Varshaver M et al. NovoTTF‐100A System (tumor treating fields) transducer array layout planning for glioblastoma: A NovoTAL system user study. World J Surg Oncol 2015;13:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trusheim J, Dunbar E, Battiste J et al. A state‐of‐the‐art review and guidelines for tumor treating fields treatment planning and patient follow‐up in glioblastoma. CNS Oncol 2016;6:29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong ET, Lok E, Swanson KD et al. Response assessment of NovoTTF‐100A versus best physician's choice chemotherapy in recurrent glioblastoma. Cancer Med 2014;3:592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taillibert S, Le Rhun E, Chamberlain MC. Tumor treating fields: A new standard treatment for glioblastoma? Curr Opin Neurol 2015;28:659–664. [DOI] [PubMed] [Google Scholar]
- 46.Cloughesy TF, Lassman AB. NovoTTF: Where to go from here? Neuro Oncol 2017;19:605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampson JH. Alternating electric fields for the treatment of glioblastoma. JAMA 2015;314:2511–2513. [DOI] [PubMed] [Google Scholar]
- 48.Guyatt G, Akl EA, Hirsh J et al. The vexing problem of guidelines and conflict of interest: A potential solution. Ann Intern Med 2010;152:738–774. [DOI] [PubMed] [Google Scholar]