Infrequent occurrence of certain types of tumors makes treatment decisions more difficult because of the limited clinical experience. This article reports on incidence trends and survival outcomes for patients diagnosed with small cell carcinoma of the head and neck, an infrequent neuroendocrine neoplasm with an unfavorable prognosis.
Keywords: Small cell carcinoma, Squamous cell carcinoma, Incidence and survival of head and neck cancer
Abstract
Background.
Small cell carcinomas of the head and neck (SmCCHNs) are rare neoplasms with an unfavorable prognosis. Population‐based data describing survival and prognostic factors for SmCCHN are limited.
Methods.
Data were obtained from the U.S. National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database for 1973–2013. Patient and tumor‐related characteristics for SmCCHN were compared with those for squamous cell carcinoma of the head and neck (SCCHN). Survival was compared by constructing Kaplan‐Meier curves and Cox proportional hazard models with and without propensity score matching.
Results.
The data set included 609 SmCCHN and 227,943 SCCHN cases. Both histological subtypes were more common in men than women and more common in white patients. SmCCHN was most likely to originate in the larynx, glottis and hypopharynx, or salivary glands and to present with more advanced stage and grade. SCCHN was most likely to originate in the oral cavity and was found infrequently in the salivary glands. Overall 5‐ and 10‐year survival estimates were 27% and 18% for SmCCHN and 46% and 31% for SCCHN, respectively. In multivariable survival analyses adjusting for age, sex, race, marital status, year of diagnosis, stage, grade, and receipt of radiation, the hazard ratio (HR) comparing SmCCHN with SCCHN was 1.53 with a 95% confidence interval (CI) from 1.39 to 1.68. Average 5‐year survival varied widely between the histologic types when comparing tumor sites: 14.5% for SmCCHN versus 48.9% for SCCHN in the oropharynx. In propensity score matched analyses, the corresponding HR was 1.27 (95% CI, 1.15–1.40).
Conclusion.
Compared with SCCHN, SmCCHN carries a worse survival and is more likely to present with more advanced stage.
Implications for Practice.
Small cell carcinoma of the head and neck (SmCCHN) is a rare subtype of head and neck cancer. In this Surveillance, Epidemiology, and End Results (SEER) data analysis, the characteristics and survival of SmCCHN are compared with those of the common squamous cell carcinoma of the head and neck. Results show that SmCCHN carries a worse prognosis and tends to present at a more advanced stage; SmCCHN also is ten times more likely to originate from the salivary glands. These findings may have implications for clinical practice, as location of the tumor may strongly associate with the pathologic diagnosis. If a SmCCHN is diagnosed, a disseminated disease is likely; hence vigilance in staging procedures is indicated.
Introduction
Small cell carcinoma of the head and neck (SmCCHN) is an infrequently encountered neuroendocrine neoplasm [1] that carries an unfavorable prognosis [2]. Because of the rarity of the disease, no randomized controlled trials have been conducted to guide therapy in these rare cancers. Until recently, the limited literature consisted of case studies or small case series [3], [4], [5], [6]. To our knowledge, only one long‐term analysis of population‐based data on SmCCHN has been performed; however, our series is significantly larger [7].
SmCCHN occurs in nearly all structures of the head and neck and most frequently originates in the larynx [7], [8]. Accepted therapeutic modalities include surgical resection, radiation therapy, or chemotherapy, although a recent study showed that there is no therapeutic advantage to treating with surgery in addition to chemotherapy and radiation in locally advanced disease [9]. The infrequent occurrence of this tumor in the head and neck makes it difficult for investigators to gain clinical experience treating these tumors or report more than limited personal experience or case studies. SmCCHN seems to be rarely associated with human papilloma virus (HPV) [10], [11] but usually shows positive staining for p16 because of dysregulation of the Rb pathway, potentially complicating diagnosis [12]. The most common histological type of malignancy found in the head and neck is squamous cell carcinoma [13]. Squamous cell carcinoma of the head and neck (SCCHN) accounts for 3.3% of malignancies in the U.S., with 11,260 deaths expected in 2009 [14]. Smoking is a well‐characterized risk factor, and HPV has emerged as an important factor in the rise of oropharyngeal cancer in developed countries [15] as well as in developing countries [16]. In contrast to SmCCHN, SCCHN is well described with clearly established treatment guidelines [17]. SCCHN is also increasingly characterized with respect to molecular targets, leading to more specific targeted therapeutic options [17].
There has been little information describing the incidence and survival of SmCCHN over time, and to our knowledge, there have been no randomized trials to guide therapy [18]. In this study, we analyzed the incidence trends and survival outcomes for patients diagnosed with SmCCHN in the Surveillance, Epidemiology, and End Results (SEER) database in various demographic groups. The data on SmCCHN were compared with the similar data on SCCHN to ensure that treating oncologists are aware of the distinct features of the tumor and inform patients appropriately.
Materials and Methods
Data were obtained from the U.S. National Cancer Institute's SEER program, which provides data on newly diagnosed cancer cases and reports summary statistics on cancer prevalence, incidence, survival, and mortality in the U.S.
Using the SEER database, cases with a histological category of small cell neoplasms (International Classification of Diseases in Oncology, third edition [ICD‐O‐3] codes 8002, malignant tumor, small cell type; 8041/3, small cell carcinoma not otherwise specified [NOS]; 8042/3, oat cell carcinoma; 8043/3, small cell carcinoma, fusiform cell; 8044/3, small cell carcinoma, intermediate cell) were selected. The comparison group included only malignancies classified as squamous cell carcinomas (ICD‐O‐3 codes 8050/3 through 8084/3).
The International Classification of Diseases in Oncology, third edition, was used to select cases reported to SEER from 1973 to 2013 that originated from the head and neck region. Seventy sites within the head and neck region were analyzed. Because of the low incidence of small cell carcinoma in the head and neck since 1973, the sites were separated into groups based on anatomical location for comparison purposes. The same groups were used to organize the squamous cell carcinomas in the analysis.
The following anatomical groups were included in the analysis. Oral cavity included malignant neoplasms from C01.9 (base of tongue) to C06.9 (mouth, NOS) and C14.8 (overlapping lesion of lip, oral cavity, and pharynx). Salivary glands included C07.9 (parotid gland) to C08.9 (major salivary gland, NOS). Oropharynx included C09.0 (tonsillar fossa) to C09.9 (tonsil, NOS), C10.0 (vallecula), C10.2 (lateral wall of oropharynx) to C10.9 (oropharynx NOS), and C14.0 (pharynx, NOS) to C14.2 (Waldeyer's ring). Nasopharynx included C11.0 (superior wall of nasopharynx) to C11.9 (nasopharynx, NOS). Nasal cavity and sinuses included C30.0 (nasal cavity) to C31.9 (accessory sinus, NOS). Larynx, glottis, and hypopharynx included C10.1 (anterior surface of epiglottis), C12.9 (pyriform sinus) to C13.9 (hypopharynx NOS), and C32.0 (glottis) to C32.9 (larynx, NOS).
All patients in the SEER database from 1973 to 2013 were characterized by sex, race, age at diagnosis, and disease stage. Because of the numerous changes in cancer staging over the last four decades, “SEER historic stage A” variable was used. This classification scheme covered the entire study period, classifying cancer cases as localized, regional, distant, or unstaged [19].
Survival outcomes were also collected from the SEER database for both small cell and squamous cell carcinomas of the head and neck. Patients with missing survival information were excluded from the analysis. Overall survival was defined as months from diagnosis to death or last follow‐up if alive.
Statistical Analysis
Statistical analysis was conducted using SAS Version 9.4 (SAS Institute, Cary, NC) and SAS macros or software developed at the Biostatistics and Bioinformatics Shared Resource at Winship Cancer Institute. The significance level was set at a two‐sided α error of .05. Descriptive statistics for each variable were reported. The distributions of covariates across the two study cohorts (SmCCHN vs. SCCHN) were compared using the chi‐square test for categorical variables and analysis of variance for continuous variables. The unadjusted survival analyses for each independent variable of interest were conducted using Cox proportional hazards models and log‐rank tests. A multivariable Cox proportional hazard model was fit using a backward variable selection method applying an α = .05 removal criteria. Kaplan‐Meier survival plots were produced to compare the survival curves in patients with SmCCHN and SCCHN across all other variables of interest.
Propensity score matching method was also implemented. A logistic regression model predicting small cell carcinomas versus squamous cell carcinomas was carried out to estimate the propensity score by all variables of interest. Patients with SmCCHN were matched to SCCHN referents at a ratio of 1:3 based on the propensity score using a greedy algorithm [20]. After matching, the balance of covariates between two cohorts was evaluated by the standardized differences, and a value of <0.1 was considered as negligible imbalance [21]. The effects were estimated in the matched sample by a Cox model with a robust variance estimator for overall survival [22].
Results
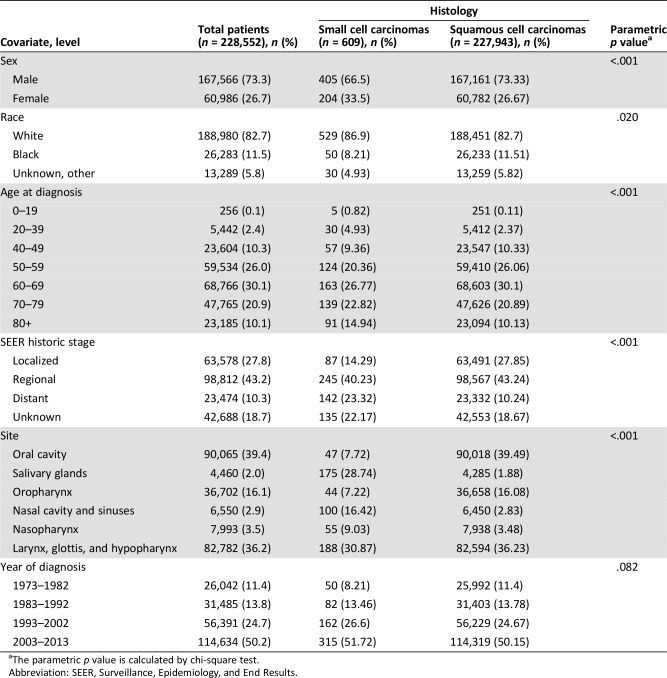
The complete data set included 228,552 patients identified in the SEER database. The survival analysis data set excluded patients without survival data, totaling 609 SmCCHN cases and 227,943 cases of SCCHN. The demographics of the patients are presented in Table 1, and patients were stratified by sex, race, age, stage, site in the head and neck, and year of diagnosis.
Table 1. Epidemiological data for small cell and squamous cell carcinoma of the head and neck.
The parametric p value is calculated by chi‐square test.
Abbreviation: SEER, Surveillance, Epidemiology, and End Results.
Both SmCCHN (87%) and SCCHN (83) histologic types were more common in white patients than in other racial groups (p = .02). Compared with the SCCHN cohort, patients with SmCCHN included lower proportions of males (73% vs. 67%). The two malignancies tend to occur in older patients, with only 15% of SmCCHN and 13% of SCCHN occurring in patients diagnosed under the age of 50 years (p < .001).
Patients with SmCCHN in the SEER database presented with distant metastases in 23.3% of cases compared with only 10% of SCCHN cases (p < .001). SmCCHN was most commonly reported in the larynx, glottis, and hypopharynx (31%) followed by the salivary glands 29%. This distribution of primary tumor sites was different from that observed in SCCHN, in which the oral cavity was the most common location (39%) followed by the larynx, glottis, and hypopharynx (36%). Only 2% of SCCHN cases occurred in the salivary glands, the least common location for this histologic type in the head and neck region but a common site for SmCCHN.
In both groups, an increasing number of patients was included in the database with each decade. The relative percentage of SmCCHN diagnosis relative to SCCHN diagnosis remained stable regardless of the decade.
The overall 5‐year and 10‐year survival estimates for SmCCHN were 26% and 18%, respectively (Fig. 1). The corresponding values for SCCHN were 46% and 30%. Table 2 shows the survival in patients with SmCCHN and SCCHN stratified by sex, race, age at diagnosis, SEER historic stage, site, and year of diagnosis.
Figure 1.
Kaplan‐Meier plot for survival in small cell carcinoma and squamous cell carcinoma of the head and neck. This figure compares the 10‐year survival of all patients in the Surveillance, Epidemiology, and End Results (SEER) database with small cell and squamous cell carcinoma. The number of surviving patients is seen at the bottom of the graph. Median 5‐year and 10‐year survival for the two histologic subtypes is depicted below the graph.
Abbreviation: CI, confidence interval.
Table 2. 5‐year survival for small cell and squamous histology.

Abbreviations: CI, confidence interval; SEER, Surveillance, Epidemiology, and End Results.
In SmCCHN, the 5‐year survival rate in female patients was 22% and in male patients was 30%. The 5‐year survival in male and female patients with SCCHN was similar (47% and 46%, respectively). The average 5‐year survival in SmCCHN for white and black patients was 26% and 25%, respectively, but the difference between these patients and their SCCHN counterparts was more dramatic, with the average 5‐year survival for SCCHN in white patients being 48% and in black patients 35%.
The age at diagnosis was a prognostic indicator more for SCCHN than for SmCCHN. In SCCHN, the average 5‐year survival was best for patients aged 0–19 years (71%) and worst for patients who were aged more than 80 years (22%). SmCCHN occurring in patients aged more than 80 years also carried a poor average 5‐year prognosis of 17%. None of the five patients with SmCCHN aged 0–19 years survived beyond 5 years.
The average 5‐year prognosis varied widely between the two histologic type groups when survival was compared within each anatomic site. In the oropharynx, SmCCHN carried the worst average 5‐year survival (15%), whereas SCCHN in this site carried the best 5‐year survival (49%). SmCCHN occurring in the salivary glands had a 36% average 5‐year survival, whereas SCCHN in the same site had a similar prognosis, with 35% surviving at 5‐years after diagnosis. SmCCHN originating in the nasal cavity paranasal sinuses also had a favorable prognosis of 35%.
Over the last four decades, survival for both types of head and neck cancer has improved. In the case of SmCCHN, average 5‐year survival was 14% in the decade 1983–1992, which improved to 35% in the most recent decade. Similarly, the most recent decade showed the best average 5‐year survival for SCCHN, of 50%.
In the multivariable analyses adjusting for age, sex, race, marital status, year of diagnosis, stage, grade, and receipt of radiation, the hazard ratio (HR) comparing SmCCHN with SCCHN was 1.53 with a 95% confidence interval (CI) from 1.39 to 1.68 (Table 3). For all types of head and neck cancer, elderly, male, black, and not married persons had worse prognosis compared with their respective SCCHN groups. Other factors independently associated with lower survival included more advanced stage and tumor grade and earlier decades of diagnosis.
Table 3. Univariate and multivariable association with survival.
Bold indicates significant value.
Abbreviations: —, reference group; CI, confidence interval; HR, hazard ratio; SEER, Surveillance, Epidemiology, and End Results.
The propensity score matched analysis included 608 cases of SmCCHN and 1,720 cases of SCCHN. The HR for histology confirmed the overall population HR comparing SmCCHN and SCCHN cases and was 1.27 (95% CI, 1.15–1.40; p < .001) (Fig. 2).
Figure 2.
Kaplan‐Meier plot for matched small cell and squamous cell histology cases. This figure depicts the matched plot for small cell and squamous cell histology. Patients with squamous cell carcinoma of the head and neck were matched with demographically similar patients with small cell carcinoma of the head and neck to control for variation in group and isolate the effect of histology on survival.
Abbreviation: CI, confidence interval.
Discussion
To our knowledge, only one large‐scale SEER analysis has been conducted to define trends in the incidence and survival of SmCCHN [7]. In a recent SEER analysis of SmCCHN, Kuan et al. focused on sinonasal primary disease and reported an improvement in outcome compared with other sites of SmCCHN. Our analysis confirms this finding but adds that when SmCCHN of the salivary glands are considered, they actually portend the most favorable prognosis for all head and neck small cell carcinomas. Our analysis also assessed nearly three times as many patients as the Kuan cohort with SmCCHN because of a broader definition of head and neck cancer, which includes the oral cavity, oropharynx, and salivary glands. This allowed us to provide information about a more robust cohort of patients with SmCCHN. This analysis additionally contrasts the presentation and clinical behavior of SmCCHN with common SCCHN histology. This analysis clarifies important prognostic information for patients with SmCCHN and is useful to patients and providers. Importantly, the data gathered from this matched analysis document that SmCCHN, and specifically certain subsets, has a significantly worse prognosis compared with SCCHN after controlling for other epidemiological factors.
The major findings of this analysis reveal significant differences in patients with SmCCHN versus SCCHN. Although all types of head and neck carcinomas are more common in men, SmCCHN is somewhat more likely than SCCHN to occur in female and in white patients. Both histological types are more likely to occur in older patients. The most striking difference is in the sites of the tumors within the head and neck. Salivary gland disease is second only to laryngeal disease in SmCCHN. In SCCHN, the salivary glands are the least common site of involvement.
The constant relative percentage of SmCCHN diagnosis compared with SCCHN is surprising. Both histological subtypes reported more cases in each successive decade, but the proportion of cases diagnosed as SmCCHN remained stable. This may be explained by a lack of widespread community knowledge about both subtypes, leading to a lack of preventative measures, or a lack of environmental influence on this disease, in addition to the HPV‐positive disease that significantly affects SCCHN incidence.
The comparison of 5‐year survival outcomes showed several interesting findings between these two tumor types. The 5‐year survival outcomes for women with SmCCHN were poorer compared with those of men, despite similar survival for both sexes in SCCHN. Interestingly, black and white patients had a similar 5‐year survival rate in SmCCHN, whereas SCCHN carried a worse prognosis among black patients. This finding may suggest that SmCCHN is itself a poor prognostic indicator that impacts prognosis to a greater degree and supersedes the influence on survival of factors influenced by racial background, such as social or access to care factors.
The sites of tumor involvement and 5‐year survival showed that the prognosis varies dramatically with histologic type in the different sites within the head and neck. Of note is the best prognosis for SmCCHN in the salivary glands. This was a reassuring finding, as it was also one of the most common sites for SmCCHN.
Higher stage at presentation was independently associated with a worse survival outcome. Patients with SmCCHN were much more likely than patients with SCCHN to present with distant metastases, contributing to a poorer prognosis. This is not surprising because of the aggressive nature of the disease and the fact that SmCCHN is less likely to occur at visually obvious locations such as the oral cavity. In light of the poor prognosis, inexperienced physicians should consider whole‐body computed tomography to evaluate for distant metastasis at diagnosis.
It is encouraging that there is an improvement in 5‐year survival in later decades in both histologic subtypes. In the last three decades, the average 5‐year survival rate has improved more dramatically for SmCCHN than for SCCHN. This trend is promising and indicates that treatments for both histologic types are improving, a hopeful sign for decades to come.
Our findings should be interpreted with a thorough understanding of the strengths and limitations of the publicly available SEER data. The database has a large sample size that affords sufficient power to detect moderate associations and allows for a variety of multivariable analyses. Population‐based identification of patients, as opposed to institution‐based studies, allows for greater external validity. Significant limitations exist in our analysis related to the lack of important clinical and demographic variables available in the SEER database. Notably, the publicly available SEER data do not include information pertaining to chemotherapy, facility of diagnosis or long‐term insurance information that play a role in prognosis. Additionally, for many cases, staging data were not available, which limited the ability for robust analysis of stage at presentation. Furthermore, the relatively small sample size of SmCCHN available within the SEER database made it difficult to appreciate a significant trend in prognosis with age as was clearly visible in patients with SCCHN. For the above reasons, both cancer registry‐based and institution‐based studies provide useful, nonoverlapping information that contributes to the evidence despite their strengths and limitations [23].
Conclusion
SmCCHN is a rare clinical entity that confers a worse survival than SCCHN. Fortunately, there has been improvement in prognosis for both types of cancers in recent years. Our study was limited by the small sample size of SmCCHN available within the SEER database.
Acknowledgments
The authors thank Anthea Hammond for editorial support.
Author Contributions
Conception/design: Marta B. Bean, Nabil F. Saba
Collection and/or assembly of data: Marta B. Bean
Data analysis and interpretation: Yuan Liu, Renjain Jiang
Manuscript writing: Marta B. Bean, Nabil F. Saba
Final approval of manuscript: Conor Ernst Steuer, Mihir Patel, Mark William McDonald, Kristin Ann Higgins, Jonathan Jay Beitler, Dong Moon Shin, Nabil F. Saba
Disclosures
Mihir Patel: Intuitive Surgical (H); Kristen Ann Higgins: AstraZeneca, Varian Medical Systems (C/A), Genentech (SAB), RefleXion Medical (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Renner G. Small cell carcinoma of the head and neck: A review. Semin Oncol 2007;34:3–14. [DOI] [PubMed] [Google Scholar]
- 2.De Felice F, Lei M, Guerrero Urbano T. Controversies in small cell carcinoma of the head and neck: Prophylactic cranial irradiation (PCI) after primary complete initial remission. Cancer Treat Rev 2015;41:725–728. [DOI] [PubMed] [Google Scholar]
- 3.Violet Wilmot V, Nixon IJ, Nixon IF. Small cell neuroendocrine carcinoma of tracheostomy site in a patient with a history of juvenile laryngeal papillomatosis. BMJ Case Rep 2016;2016:bcr2016216370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatoum GF, Patton B, Takita C et al. Small cell carcinoma of the head and neck: The University of Miami experience. Int J Radiat Oncol Biol Phys 2009;74:477–481. [DOI] [PubMed] [Google Scholar]
- 5.Babin E, Rouleau V, Vedrine PO et al. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. J Laryngol Otol 2006;120:289–297. [DOI] [PubMed] [Google Scholar]
- 6.Lee SS, Lee JL, Ryu MH et al. Extrapulmonary small cell carcinoma: Single center experience with 61 patients. Acta Oncol 2007;46:846–851. [DOI] [PubMed] [Google Scholar]
- 7.Kuan EC, Alonso JE, Tajudeen BA et al. Small cell carcinoma of the head and neck: A comparative study by primary site based on population data. Laryngoscope 2017;127:1785–1790. [DOI] [PubMed] [Google Scholar]
- 8.Gnepp DR. Small cell neuroendocrine carcinoma of the larynx. A critical review of the literature. J Otorhinolaryngol Relat Spec 1991;53:210–219. [DOI] [PubMed] [Google Scholar]
- 9.Pointer KB, Ko HC, Brower JV et al. Small cell carcinoma of the head and neck: An analysis of the National Cancer Database. Oral Oncol 2017;69:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misawa K, Kawasaki H, Matsuo R et al. Human papillomavirus‐associated small cell carcinoma/neuroendocrine carcinoma of the oropharynx: A report of two cases. Springerplus 2016;5:1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraft S, Faquin WC, Krane JF. HPV‐associated neuroendocrine carcinoma of the oropharynx: A rare new entity with potentially aggressive clinical behavior. Am J Surg Pathol 2012;36:321–330. [DOI] [PubMed] [Google Scholar]
- 12.Alos L, Hakim S, Larque AB et al. p16 overexpression in high‐grade neuroendocrine carcinomas of the head and neck: Potential diagnostic pitfall with HPV‐related carcinomas. Virchows Arch 2016;469:277–284. [DOI] [PubMed] [Google Scholar]
- 13.Sikora AG, Toniolo P, Delacure MD. The changing demographics of head and neck squamous cell carcinoma in the United States. Laryngoscope 2004;114:1915–1923. [DOI] [PubMed] [Google Scholar]
- 14.Jemal A, Siegel R, Ward E et al. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225–249. [DOI] [PubMed] [Google Scholar]
- 15.Ang KK, Harris J, Wheeler R et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murthy V, Swain M, Teni T et al. Human papillomavirus/p16 positive head and neck cancer in India: Prevalence, clinical impact, and influence of tobacco use. Indian J Cancer 2016;53:387–393. [DOI] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Network . Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015;517:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Laan TP, Plaat BE, van der Laan BF et al. Clinical recommendations on the treatment of neuroendocrine carcinoma of the larynx: A meta‐analysis of 436 reported cases. Head Neck 2015;37:707–715. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute . Surveillance, Epidemiology, and End Results Program. Available at http://seer.cancer.gov. Accessed September 20, 2016.
- 20.Parsons LS. Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Poster presented at: SAS Users Group International (SUGI) 26; April 22–25, 2001; Long Beach, CA; 214‐26.
- 21.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: A Monte Carlo study. Stat Med 2007;26:734–753. [DOI] [PubMed] [Google Scholar]
- 22.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc 1989;84:1074–1078. [Google Scholar]
- 23.Ludwig MS, Goodman M, Miller DL et al. Postoperative survival and the number of lymph nodes sampled during resection of node‐negative non‐small cell lung cancer. Chest 2005;128:1545–1550. [DOI] [PubMed] [Google Scholar]