Despite the available fertility preservation guidelines, barriers still exist that hinder the implementation of oncofertility practice. This article reports an oncofertility framework that defines the key components of oncofertility care, including a model for service development and the role of health care professionals across specialities.
Keywords: Fertility preservation, Oncofertility, Models of care, Competency, Training, Cancer
Abstract
Background.
Despite international evidence about fertility preservation (FP), several barriers still prevent the implementation of equitable FP practice. Currently, oncofertility competencies do not exist. The aim of this study was to develop an oncofertility competency framework that defines the key components of oncofertility care, develops a model for prioritizing service development, and defines the roles that health care professionals (HCPs) play.
Materials and Method.
A quantitative modified Delphi methodology was used to conduct two rounds of an electronic survey, querying and synthesizing opinions about statements regarding oncofertility care with HCPs and patient and family advocacy groups (PFAs) from 16 countries (12 high and 4 middle income). Statements included the roles of HCPs and priorities for service development care across ten domains (communication, oncofertility decision aids, age‐appropriate care, referral pathways, documentation, oncofertility training, reproductive survivorship care and fertility‐related psychosocial support, supportive care, and ethical frameworks) that represent 33 different elements of care.
Results.
The first questionnaire was completed by 457 participants (332 HCPs and 125 PFAs). One hundred and thirty‐eight participants completed the second questionnaire (122 HCPs and 16 PFAs). Consensus was agreed on 108 oncofertility competencies and the roles HCPs should play in oncofertility care. A three‐tier service development model is proposed, with gradual implementation of different components of care. A total of 92.8% of the 108 agreed competencies also had agreement between high and middle income participants.
Conclusion.
FP guidelines establish best practice but do not consider the skills and requirements to implement these guidelines. The competency framework gives HCPs and services a structure for the training of HCPs and implementation of care, as well as defining a model for prioritizing oncofertility service development.
Implications for Practice.
Despite international evidence about fertility preservation (FP), several barriers still prevent the implementation of equitable FP practice. The competency framework gives 108 competencies that will allow health care professionals (HCPs) and services a structure for the development of oncofertility care, as well as define the role HCPs play to provide care and support. The framework also proposes a three‐tier oncofertility service development model which prioritizes the development of components of oncofertility care into essential, enhanced, and expert services, giving clear recommendations for service development. The competency framework will enhance the implementation of FP guidelines, improving the equitable access to medical and psychological oncofertility care.
Introduction
Despite numerous fertility preservation guidelines [1], [2], [3], [4], there are several barriers that continue to hinder the implementation of oncofertility practice [4], [5], [6], [7], [8]. Currently, oncofertility competencies do not exist. Availability would allow health care professionals (HCPs) to define how oncofertility should be developed and outline the specific competency for each component of care [6]. Their availability would help to address the knowledge, skills, and processes needed to deliver services of a high standard [9], [10] and hence improve uptake and use in a number of ways: (a) recognize the best way for HCPs across specialties to collaborate to develop oncofertility services; (b) identify strategies and principles that will improve patient‐centered oncofertility care; (c) prioritize the key components of oncofertility services that will help services define the order in which they implement changes; (d) improve the ways in which HCPs communicate about oncofertility care and support decision making; (e) define the roles that HCPs play in delivering oncofertility care, ensuring care is routine and reliable in every center; (f) have a clearer method for oncofertility professional development and training; and (g) apply strategies that allow for oncofertility quality improvements that can be clearly evaluated.
A competency framework clearly defines and describes the individual competencies required by services and HCPs to be fully effective [11]. Competencies should be both observable and measurable so that they can be evaluated [10], [11].
The aim of this study was to seek knowledge from international HCPs and patient family and advocacy groups (PFA) on the key components of oncofertility clinical care services, priorities for service development, and roles of HCPs. This would allow knowledge translation [12] in the form of oncofertility competencies and a competency framework that will inform the implementation of oncofertility health services.
Materials and Methods
Delphi Methodology
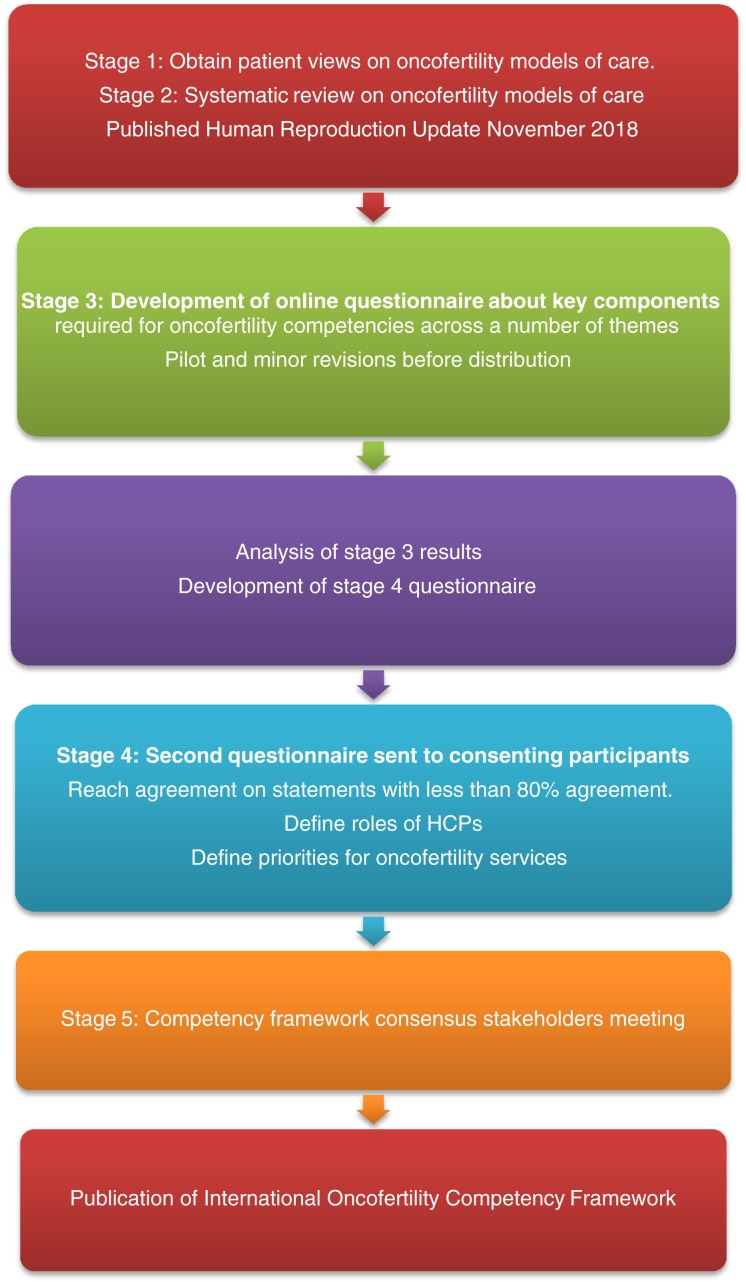
A two‐round quantitative modified Delphi methodology [13] was administered using online questionnaires to conduct a structured feedback process (Fig. 1).
Figure 1.
Development of an oncofertility competency framework.
Using early evidence identified from the ten domains of oncofertility care (communication, oncofertility decision aids, age‐appropriate care, referral pathways, documentation, oncofertility training, reproductive survivorship care and fertility‐related psychosocial support, supportive care, and ethical frameworks) [14], [15], which covered 33 areas of care, we developed a consensus forecast in the form of a questionnaire.
Selection of Experts
An international expert panel representing cancer, fertility, endocrinology, surgery, and general practice HCPs (counsellors, doctors, nurses, psychologists, social workers, and surgeons) from each specialty was identified in each country by the chief investigators leading this study. Informed experience and knowledge was sought from PFA. The patients did not need to have undergone fertility preservation (FP) to participate but had to be older than 15 years of age as per local ethics board guidelines. Parents of patients up to 25 years of age were included as well as partners of a patient with cancer eligible for the study.
Study Privacy, Anonymity, and Confidentiality
Participants completed the questionnaire independently and were not aware of other participants’ responses. The participant details were kept confidential and were not shared with study participants or researchers.
Consent
Participants provided consent online, which allowed them to proceed to the first question.
Study Ethics
Ethics approval from the South Eastern Sydney Local Health District Human Research Ethics Committee was obtained prior to study commencement (LNR/17/POWH/492 and LNRSSA/17/SCHN/437).
Initial Questionnaire
Questionnaires were developed for HCPs and PFAs. These questionnaires were reviewed by PFA to ensure consensus on appropriate terminology and language and breadth of information captured. The HCPs initial questionnaire included 106 statements for rating on a 5‐point scale (strongly disagree, disagree, undecided, agree, or strongly agree) across 13 sections (supplemental online Table 2). The PFA initial questionnaire consisted of 83 statements. Twenty‐three questions were removed, as PFA representatives felt that specialist knowledge was needed to answer them. Throughout the survey, definitions were given to help the participants understand the statements (supplement online Table 1).
The study information and questionnaires were translated into 9 languages (Arabic, French, German, Hindi, Japanese, Mandarin, and European and Latin American Portuguese and Spanish) by a Department of Health‐certified translator service (Ethnolink). The study information was available on the Future Fertility Web site (www.futurefertility.com) for access by participants.
HCPs were asked to state their current role with oncofertility care and the available oncofertility service provisions in their unit. No personal details were collected from participants except for an e‐mail address from those who wished to take part in the second questionnaire.
Statistical Analysis
The agreement level was set at 80% prior to the start of the study, based on standard Delphi methodology [8]. The mean percentage agreement (agree/strongly agree) was calculated for each statement for all the participants, then separately for the HCPs and PFAs (supplemental online Table 2). Finally, we calculated mean agreement by individual HCP specialties (supplemental online Table 2) and between the 12 high income countries and 4 middle income countries (supplemental online Table 3).
Second Questionnaire
The researchers reviewed the results and comments following the first questionnaire and, for the 9 statements that did not reach the 80% consensus, 14 new statements were developed (supplemental online Table 2).
Questionnaire 2 sought to define the role that individual HCPs should play in oncofertility care and the skills required for these roles. Participants were asked identical questions about each HCP's role and were asked to rank each statement using a 5‐point Likert scale (strongly agree, agree, undecided, disagree, strongly disagree).
Questionnaire 2 also sought to define the priorities for implementing oncofertility care by developing a three‐tier oncofertility framework that allows for progressive service development. This model aimed to allow service development by ranking each provision of service delivery into either essential, enhanced, or expert.
Results
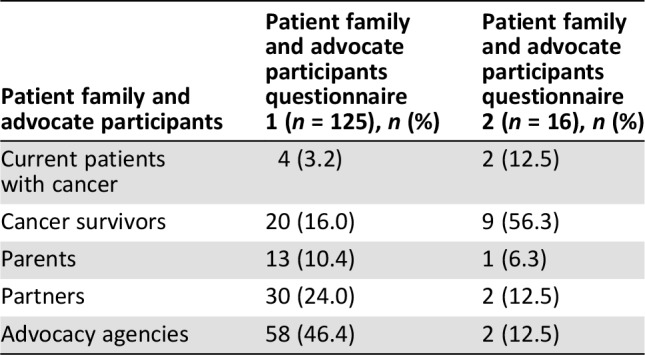
A total of 457 questionnaires were initiated by 332 HCPs (Table 1) and 125 PFAs (Table 2). Seventy‐five percent of the questionnaires were totally completed. The questionnaire was completed in all nine translated languages by participants in 16 countries (Australia, Brazil, Canada, China, Egypt, France, Germany, Greece, India, Italy, Japan, New Zealand, Portugal, South Africa, U.K., and U.S.).
Table 1. Health care professional participation for questionnaire 1 and 2.
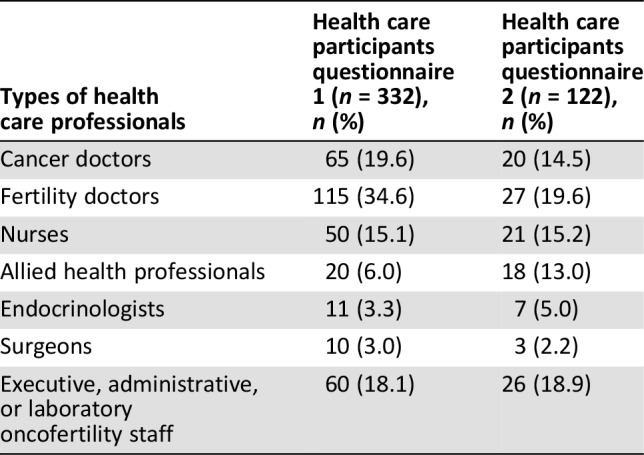
Table 2. Patient family and advocate participation for questionnaire 1 and 2.
Three hundred and six of the 332 HCPs completed information on the types of oncofertility services to which they had access; 138 (45.1%) had a well‐established service, 100 (32.7%) had a developing service, 45 (14.7%) had limited service provision, and 23 (7.5%) had no service.
One hundred and sixty‐nine of the 457 participants who completed questionnaire 1 agreed to take part in questionnaire 2. One hundred and thirty‐eight participants completed questionnaire 2 in stage 4 (81.6% response rate). Of the completed surveys, 122 (88.4%) were completed by HCPs (Table 1) and 16 (11.6%) were completed by PFAs (Table 2).
Of the participants who completed questionnaire 1, 91.0% agreed or strongly agreed with the statements. In questionnaire 2, consensus was reached on 78.6% of the revised statements.
There was agreement that equitable services should be delivered to all patients irrespective of age, gender, religion, culture, and financial abilities. A total of 82.7% of the entire group agreed that oncofertility services needed to be standard practice. The need for interdisciplinary collaboration (95.9%), identifiable services with a clear referral pathway (93.7%), and HCPs knowledgeable about oncofertility guidelines (91.9%) were supported by both HCPs and PFA participants. In questionnaire 1, the statement “oncofertility services need to be provided equitably to all cancer patients irrespective of age” had 74.5% agreement. The question was reworded into three questions for the second questionnaire, all of which reached agreement of 95.7% or higher.
There was agreement between PFAs and HCPs that age‐appropriate oncofertility should be incorporated into oncofertility care across cancer and fertility services. All patients with cancer should receive age‐appropriate information about fertility risks and fertility options (92.9%), HCPs should seek agreement from patients about who should attend the fertility consultation (88.0%), HCPs should include pediatric patients in age‐appropriate discussions (89.15%), and age‐appropriate consultation and waiting spaces should be provided for pediatric and adolescent and young adult (AYA) patients (91.5%).
The role of HCPs was critical for both patients and parents, with a number of participants adding comments about the value of collaboration, inclusion, and the need for further definition of the oncofertility roles of multidisciplinary team staff. An additional statement was added to questionnaire 2 based on comments from participants that staff in different services had different roles and some services do not have access to all types of HCPs: “The role HCPs play in providing oncofertility care should be clearly defined in each cancer or fertility centre” (97.1%). The role of the general practitioner (GP) did not reach agreement in the first questionnaire, but in questionnaire 2, the revised statements reached a consensus.
Having a clearly defined referral pathway (96.1%), process for referral (96.0%), and priorities for urgent cancer cases (93.7%) was agreed upon by both PFAs and HCPs. A total of 85.4% of PFAs agreed that fertility referral should be within two working days, but only 71.8% of HCPs agreed initially. This statement was reworded in the second questionnaire to “patients referred to fertility providers should be seen quickly so that the start of their cancer treatment is not delayed” and reached an agreement of 98.5%. There was agreement that the referral process should be clear, and provide information on past reproductive medical history and fertility status (95%), among other factors.
PFAs and HCPs agreed that cancer centers need to have systems to identify patients with cancer at risk (88.3%), and patients not at risk should also be told they had “no risk of infertility” (86%). Cancer clinicians need to discuss patients’ gonadotoxic risk based on medical history and intended treatment (88.5%) and give patients information about contraception during cancer treatment (95.1%). Patients with cancer should be given an opportunity to discuss oncofertility care with a fertility provider (92.9%), but this should not occur until the cancer physician has discussed treatment (79.6%). Cancer and fertility nurses or navigators should have the expertise to provide patients with cancer with information about reproductive risk and fertility options as well as providing reproductive support (93.5%). The oncofertility consultation should be sensitive to the individual religious or cultural needs of patients (87.5%) and involve HCPs who check patients’ reproductive understanding before having oncofertility consultations (88.2%) and who change consultations to meet the reproductive health literacy needs of patients (91.5%).
There was strong agreement from both HCPs and PFAs that decision aids should be used to support patients with oncofertility understanding and decision making (90.3%), and the decision aids should be integrated into cancer and fertility services as standard practice (90.4%). This includes use in pediatric and AYA patients (92.6%).
Agreement was reached about the need for HCPs to be aware of medical and surgical complications of FP and clearly disclose risks of FP to patients (97.0%) while assessing a patient's suitability to have FP procedures (95.4%). In the first questionnaire the statement “FP procedures should be organized to be done at the same time as other procedures reducing time delays and anaesthetic procedures” did not get consensus (78.5%), and this statement was modified in questionnaire 2 to “If patients are due to have staging procedures under anaesthetic FP procedures should be coordinated with them if possible” (88.4%).
Methods of improving oncofertility care included oncofertility training for all HCPs in cancer and fertility centers (88.5%), cancer and fertility HCPs observing each other's practice so that knowledge and consistency of care was improved (87.5%), cancer and fertility HCPs having access to oncofertility educational training material (82.1%), communication skills training (85.6%), and access to ethics boards for challenging cases (86.3%). The use of online training programs did not reach the criteria for inclusion (68.9%).
Continuity of oncofertility care from diagnosis into survivorship was seen as very important (91.6%), with services needing to respond to the reproductive needs of cancer survivors (94.2%), which may change as patients get older (91.4%). Reproductive follow‐up guidelines need to be implemented into cancer centers (92.1%).
Psychological support should be provided to all patients irrespective of the decision to have FP (90.7%), and this support should be age‐appropriate (95.2%) and available from diagnosis to survivorship (95.6%). Allied health professionals should have expertise to provide fertility‐related emotional support, counseling, or therapeutic interventions (88.8%), but these services should be provided by credentialed staff with the expertise and training (79.5%).
A number of governance issues were identified to streamline service development, which include the development of several standard operating procedures (see supplemental online Table 2).
Agreement was not reached on 3 of the 14 revised statements. These related to how information should be communicated to patients by cancer specialists. A total of 92.6% of the 108 agreed statements had agreement between high and middle income countries based on agree/strongly agree scores. Eight statements did not have agreement with middle income participants, including the role of nurses in communication, advice and support (74.4%), the coordination of fertility preservation under the same anaesthetic (70%), correspondence about oncofertility care (77.8%), and four statements about ethical frameworks (supplemental online Table 3).
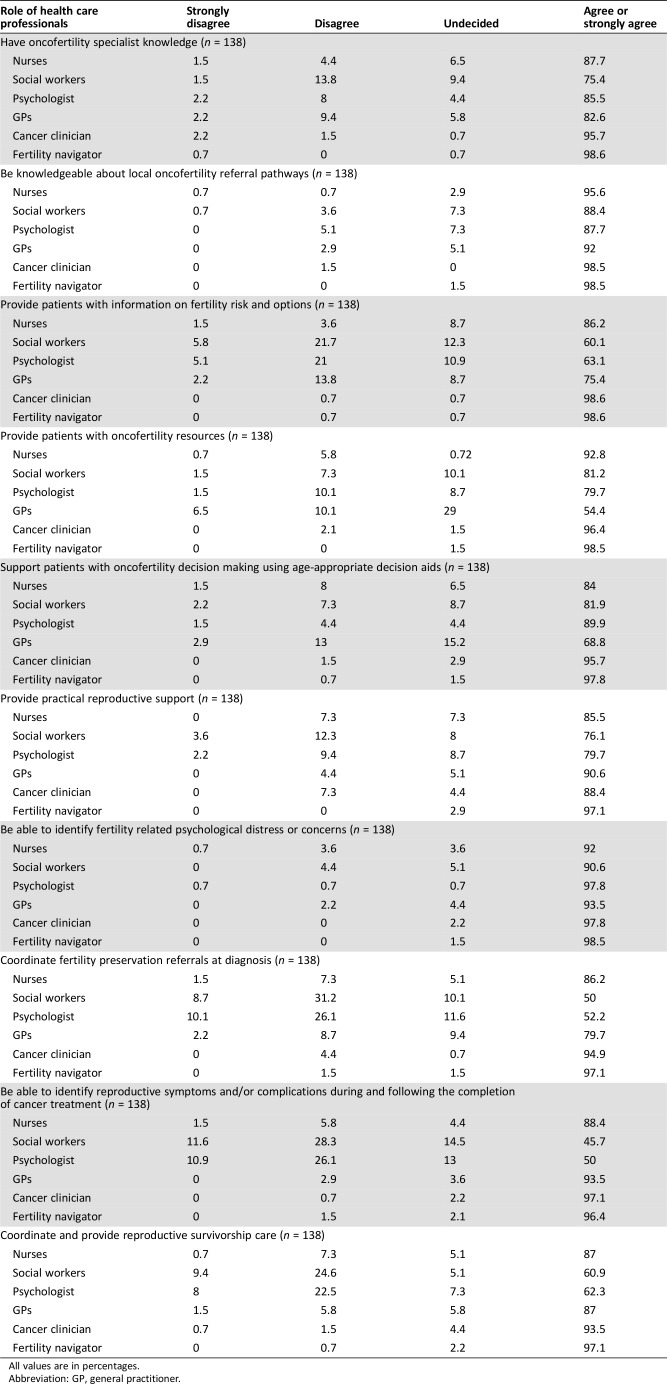
In questionnaire 2, the participants reached agreement on the roles individual HCPs play in providing oncofertility care (Table 3). There was strong support for all HCPs working with patients with cancer having specialist oncofertility knowledge (cancer doctors 95.7%, fertility navigator 98.6%, GPs 82.6%, nurses 87.7%, social workers 75.4%, psychologists 85.5%), although consensus was not reached for social workers. There was also support for all HCPs to know about local oncofertility referral pathways that have been developed (cancer doctors 98.5%, fertility navigator 98.5%, GPs 92.0%, nurses 95.6%, social workers 88.4%, psychologists 87.7%).
Table 3. Roles of health care professionals.
All values are in percentages.
Abbreviation: GP, general practitioner.
Nurses should be able to identify fertility‐related medical (88.4%) and psychological symptoms (92.0%) and to provide oncofertility care navigation [provide patient information (86.2%), provide patient resources (92.8%), coordination of FP (86.2%), or survivorship follow‐up (87.0%) and provide decision support (84.0%) and practical support (85.5%)].
Equally, our results showed the importance of allied health professionals providing resources (social workers 81.9%, psychologists 89.9%, and fertility navigators 98.5%), providing support with decision making (social workers 81.2%, psychologists 79.7%, and fertility navigators 97.8%), and practical reproductive support (social workers 76.1%, psychologists 79.7%, and fertility navigators 97.1%; Table 3).
Although GPs were expected to have specialist knowledge about oncofertility care (82.6%) and referral pathways (92.0%), they are not expected to provide information on fertility risk and options (75.4%), provide resources, or support patients with oncofertility decisions (68.8%). The role of GPs was to provide coordination for oncofertility care at diagnosis (79.7%) and survivorship (87.0%) and be able to identify fertility‐related medical (93.5%) or psychological distress or concerns (93.5%; Table 3).
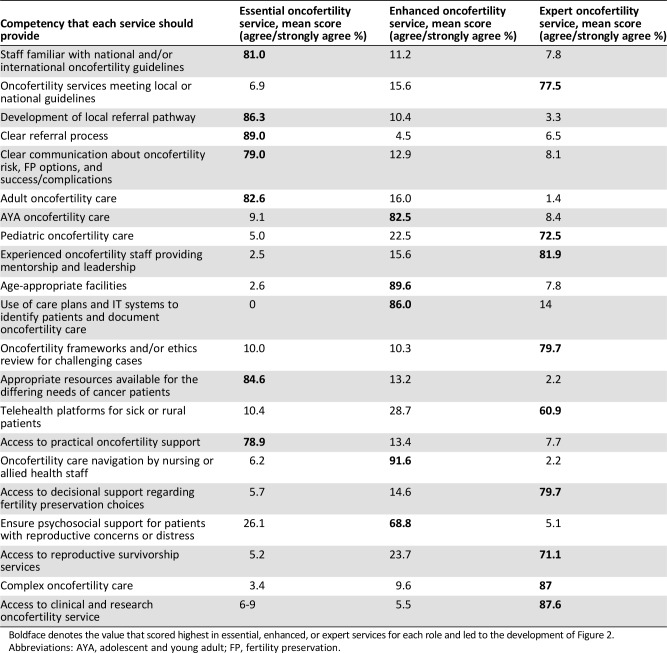
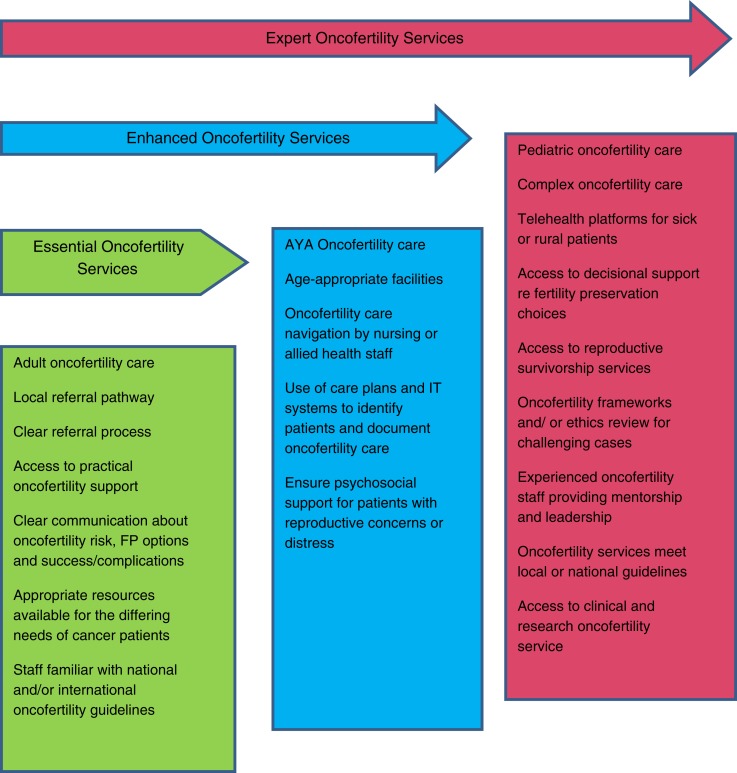
Finally, in questionnaire 2, the participants were able to reach agreement on the priorities for developing oncofertility services (supplemental online Table 3). Taking the highest agree/strongly agree mean results in Table 4, we developed a three‐tier model (Fig. 2). This framework uses the data to show the progressive development of services by concentrating on priorities of oncofertility care.
Table 4. Mean agreement percentages for the implementation of consensus statement priorities (n = 138).
Boldface denotes the value that scored highest in essential, enhanced, or expert services for each role and led to the development of Figure 2.
Abbreviations: AYA, adolescent and young adult; FP, fertility preservation.
Figure 2.
Three‐tiered oncofertility service development model.
Abbreviations: AYA, adolescent and young adult; FP, fertility preservation; IT, information technology.
Discussion
This is the first consensus framework giving guidance about the core competencies of oncofertility care and implementation strategies. One hundred and eight statements gained international agreement that will enhance the implementation of FP guidelines [1], [2], [3]. These guidelines [1], [2], [3] establish best practice and give recommendations for FP uptake and use; however, they do not consider the skills required by HCPs or the oncofertility services to effectively implement these guidelines. This is especially important in view of particular oncofertility requirements for pediatric patients, complex patients, patients in rural and regional centers, and patients with additional health care needs. The development of this competency framework gives HCPs and services a structure for the components of care required as part of a service, the roles of HCPs, and how service development can be prioritized. The value of these additional elements cannot be overstated.
This study has a strong foundation, building on previous patient advocacy work [16] and comprehensive oncofertility literature reviews [6], [14], [15], [16], ensuring the competency statements were evidence based. The PFA engagement and participation in this study strengthened the development and review of the Delphi questionnaires, giving equal weight to HCP and PFA responses and ensuring that the outcomes are patient focused. The Delphi method relies on the key assumption that consensus forecasts from a group are generally more accurate than those from individuals. This study had the advantage of a large international group of HCP experts representing multidisciplinary roles in a number of different specialties and PFAs who provide the patient's perspective.
A diverse range of countries were included in this study with diversity in clinical practices around the world and different availability of resources in caring for patients’ oncofertility needs [5], [17]. Despite these differences, participants from middle income and higher income countries had agreement on consensus views, which is consistent with the literature showing how important reproductive needs and concerns are to cancer patients around the world [18].
The competency framework consensus statements cover ten components of oncofertility care (supplemental online Table 2). Elements of each of the 10 components of care overlap, but a number of key themes are required for themes to be developed: understanding about local patient groups who require the service, identification of HCP champions who are willing to collaborate on service development, collaboration between multiple HCPs across specialties, training of HCPs involved in service delivery, training of HCPs working in cancer and fertility services, coordination of services, and development of governance structure and care navigation.
The three‐tiered framework for oncofertility service development allows a method to prioritize the gradual implementation of different components of care. The framework progression requires an increase in the level of oncofertility training and expertise HCPs have, increase in staffing, and increase in the complexity of cases that are seen. Over time, a number of additional tiers may appear with smaller expert services that do not undertake some components of the framework, such as pediatric care. Although research was seen as a priority for expert services in our model over time, essential and enhanced services should also be involved in oncofertility research.
Traditionally, cancer services have had access to nursing and allied HCPs providing care navigation and practical and psychological support but despite these opportunities, nursing and allied HCPs have felt unable to provide this level of support because of a fear of upsetting patients [19], time constraints [20], HCPs being unsure what their role is [21], and lack of oncofertility training or expertise in this area [22], [23]. Our results will provide clearer guidance on the roles of key HCPs. Further research is required to look at the strategy for upskilling a large number of HCPs and for prioritizing which HCPs should receive training first.
There are a number of initiatives being developed to improve education for HCPs and a priority for cancer services should be to ensure that staff working in the clinical areas should be provided with training [24], [25], [26]. Depending on the size of the service, available resources, or availability of staffing, HCPs may have different roles which overlap the ones which we have described in our framework. Identification of the service needs based on the local population and proposed oncofertility framework will allow services to identify staff who can provide the same service.
Collaboration across services will ensure that some of these competencies are reached more quickly. Collaborating to develop new resources [27], [28], [29], [30], [31] or adapting resources that have already been developed, implementing successful oncofertility training programs [19], [25], [32], and using templates for standard operating procedures should help to decrease the burden for staff, reduce the time for implementation, and reduce the costs of implementing models of care.
Strengths
This collaboration brings together the views of PFAs and HCPs from 16 countries who responded independently of other participants. The electronic format and translation allowed for wide international engagement and was cost‐effective, enabling greater participant involvement.
Limitations
The development of the competency framework only had limited primary physician involvement and, in order to implement primary care changes, more engagement will be required. Complex medical needs may require additional supports that have not been addressed. The oncofertility services across the world currently have enormous variability in cultural and religious views, availability of oncofertility services, financial support, and access to appropriate resources.
Future Directions
Although the International Oncofertility Competency Framework is evidenced, informed, and promising, its implementation needs to be tested for feasibility, acceptability, uptake, and sustainment [33] and evaluated in terms of challenging organizational context and differences in resources and staffing in services around the world. Formative evaluation will be used to provide critical information about the implementation of “change management” and enable our international team to study the complexity of integrating this competency framework into standard practice across a number of different health care settings.
Conclusion
The implementation of an oncofertility competency framework will assist services to define their model of care and referral pathways and deliver oncofertility services of a consistent, high standard as per internationally available guidelines.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
The G‐FORCE research group acknowledge the work that the Australasian Oncofertility Consortium Group and international patient representatives provided in determining the pillars for the models of care and their work with the research team to develop the questionnaires. We are grateful to all the patient representatives and health care professionals who shared their experience in order for us to develop this work.
This work is supported by Kids Cancer Alliance (KCA), a CINSW Translational Cancer Research Centre (15/TRC/1‐04) and funding and support from a Churchill Fellowship from the Churchill Foundation (Australia) in 2015. AA position is supported by the KCA and CanTeen funding from the Federal Health Department.
Author Contributions
Conception/design: Antoinette Anazodo, Paula Laws, Shanna Logan, Carla Saunders, Jo Travaglia, Brigitte Gerstl, Natalie Bradford, Richard Cohn, Mary Birdsall, Ronald Barr, Nao Suzuki, Seido Takae, Ricardo Marinho, Shuo Xiao, Qiong‐Hua Chen, Nailini Mahajan, Madhuri Patil, Devika Gunasheela, Kristen Smith, Leonard Sender, Cláudia Melo, Teresa Almeida‐Santos, Mahmoud Salama, Leslie Appiah, Irene Su, Sheila Lane, Teresa K. Woodruff, Allan Pacey, Richard A. Anderson, Francoise Shenfield, Elizabeth Sullivan, William Ledger
Provision of study material or patients: Antoinette Anazodo, Paula Laws, Shanna Logan, Carla Saunders, Jo Travaglia, Brigitte Gerstl, Natalie Bradford, Richard Cohn, Mary Birdsall, Ronald Barr, Nao Suzuki, Seido Takae, Ricardo Marinho, Shuo Xiao, Qiong‐Hua Chen, Nailini Mahajan, Madhuri Patil, Devika Gunasheela, Kristen Smith, Leonard Sender, Cláudia Melo, Teresa Almeida‐Santos, Mahmoud Salama, Leslie Appiah, Irene Su, Sheila Lane, Teresa K. Woodruff, Allan Pacey, Richard A. Anderson, Francoise Shenfield, Elizabeth Sullivan, William Ledger
Collection and/or assembly of data: Antoinette Anazodo, Paula Laws, Brigitte Gerstl
Data analysis and interpretation: Antoinette Anazodo, Paula Laws, Shanna Logan, Carla Saunders, Jo Travaglia, Brigitte Gerstl, Natalie Bradford, Richard Cohn, Mary Birdsall, Ronald Barr, Nao Suzuki, Seido Takae, Ricardo Marinho, Shuo Xiao, Qiong‐Hua Chen, Nailini Mahajan, Madhuri Patil, Devika Gunasheela, Kristen Smith, Leonard Sender, Cláudia Melo, Teresa Almeida‐Santos, Mahmoud Salama, Leslie Appiah, Irene Su, Sheila Lane, Teresa K. Woodruff, Allan Pacey, Richard A. Anderson, Francoise Shenfield, Elizabeth Sullivan, William Ledger
Manuscript writing: Antoinette Anazodo, Paula Laws, Shanna Logan, Carla Saunders, Jo Travaglia, Brigitte Gerstl, Natalie Bradford, Richard Cohn, Mary Birdsall, Ronald Barr, Nao Suzuki, Seido Takae, Ricardo Marinho, Shuo Xiao, Qiong‐Hua Chen, Nailini Mahajan, Madhuri Patil, Devika Gunasheela, Kristen Smith, Leonard Sender, Cláudia Melo, Teresa Almeida‐Santos, Mahmoud Salama, Leslie Appiah, Irene Su, Sheila Lane, Teresa K. Woodruff, Allan Pacey, Richard A. Anderson, Francoise Shenfield, Elizabeth Sullivan, William Ledger
Final approval of manuscript: Antoinette Anazodo, Paula Laws, Shanna Logan, Carla Saunders, Jo Travaglia, Brigitte Gerstl, Natalie Bradford, Richard Cohn, Mary Birdsall, Ronald Barr, Nao Suzuki, Seido Takae, Ricardo Marinho, Shuo Xiao, Qiong‐Hua Chen, Nailini Mahajan, Madhuri Patil, Devika Gunasheela, Kristen Smith, Leonard Sender, Cláudia Melo, Teresa Almeida‐Santos, Mahmoud Salama, Leslie Appiah, Irene Su, Sheila Lane, Teresa K. Woodruff, Allan Pacey, Richard A. Anderson, Francoise Shenfield, Elizabeth Sullivan, William Ledger
Disclosures
The authors indicated no financial relationships.
References
- 1.Oktay K, Harvey BE, Loren AW. Fertility preservation in patients with cancer: ASCO clinical practice guideline update summary. J Oncol Pract 2018;14:381–385. [DOI] [PubMed] [Google Scholar]
- 2.Peccatori FA, Azim HA Jr, Orecchia R et al. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2013;24(suppl 6):vi160–vi170. [DOI] [PubMed] [Google Scholar]
- 3.Yasmin E, Balachandren N, Davies MC et al. Fertility preservation for medical reasons in girls and women: British fertility society policy and practice guideline. Hum Fertil (Camb) 2018;21:3–26. [DOI] [PubMed] [Google Scholar]
- 4.Anazodo A, Ataman‐Millhouse L, Jayasinghe Y et al. Oncofertility‐An emerging discipline rather than a special consideration. Ped Blood Cancer 2018;65:e27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rashedi AS, de Roo SF, Ataman LM et al. Survey of fertility preservation options available to patients with cancer around the globe. J Glob Oncol 2018:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anazodo A, Laws P, Logan S et al. How can we improve oncofertility care for patients? A systematic scoping review of current international practice and models of care. Human reproduction update, 2019;25:159–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambertini M, Fontana V, Massarotti C et al. Prospective study to optimize care and improve knowledge on ovarian function and/or fertility preservation in young breast cancer patients: Results of the pilot phase of the PREgnancy and FERtility (PREFER) study. Breast 2018;41:51–56. [DOI] [PubMed] [Google Scholar]
- 8.Biglia N, Torrisi R, D'Alonzo M et al. Attitudes on fertility issues in breast cancer patients: An Italian survey. Gynecol Endocrinol 2015;31:458–464. [DOI] [PubMed] [Google Scholar]
- 9.Roach, M.S. The Human Act of Caring: A Blueprint for the Health Professions. Ottowa, Ontario: The CanadianHospital Association, 1987. [Google Scholar]
- 10.Dolan, G. Assessing student nurse clinical competency: Will we ever get it right? J Clin Nurs 2003;12:132–141. [DOI] [PubMed] [Google Scholar]
- 11.Royal College of Nursing . An integrated career and competence framework for nurses working in the field of long‐term follow‐up and late effects care of children and young people after cancer. London, U.K.:Royal College of Nursing, 2011:1–20. [Google Scholar]
- 12.Grimshaw JM, Eccles MP, Lavis JN et al. Knowledge translation of research findings. Implement Sci 2012;7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okoli C, Pawlowski SD. The Delphi method as a research tool: An example, design considerations and applications. Information & Management 2004;42:15–29. [Google Scholar]
- 14.Logan S, Perz J, Ussher J et al. Clinician provision of oncofertility support in cancer patients of a reproductive age: A systematic review. Psychooncology 2018;27:748–756. [DOI] [PubMed] [Google Scholar]
- 15.Logan S, Perz J, Ussher J et al. A systematic review of patient oncofertility support needs in reproductive cancer patients aged 14 to 45 years of age. Psychooncology 2018;27:401–409. [DOI] [PubMed] [Google Scholar]
- 16.Anazodo AC, Gerstl B, Stern CJ et al. Utilizing the experience of consumers in consultation to develop the Australasian Oncofertility Consortium Charter. J Adolesc Young Adult Oncol 2016;5:232–239. [DOI] [PubMed] [Google Scholar]
- 17.Salama M, Ataman L, Taha T et al. Building oncofertility core competency in developing countries: Experience From Egypt, Tunisia, Brazil, Peru, and Panama. J Glob Oncol 2018;4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melan K, Amant F, Veronique‐Baudin J et al. Fertility preservation healthcare circuit and networks in cancer patients worldwide: What are the issues? BMC Cancer 2018;18:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vadaparampil ST, Clayton H, Quinn GP et al. Pediatric oncology nurses’ attitudes related to discussing fertility preservation with pediatric cancer patients and their families. J Pediatr Oncol Nurs 2007;24:255–263. [DOI] [PubMed] [Google Scholar]
- 20.Ussher JM, Cummings J Dryden A et al. Talking about fertility in the context of cancer: health care professional perspectives. Eur J Cancer Care (Engl) 2016;25:99–111. [DOI] [PubMed] [Google Scholar]
- 21.King L, Quinn GP, Vadaparampil ST et al. Oncology social workers’ perceptions of barriers to discussing fertility preservation with cancer patients. Soc Work Health Care 2008;47:479–501. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs A, Kashanian JA, Clayman ML et al. Pediatric oncology providers’ attitudes and practice patterns regarding fertility preservation in adolescent male cancer patients. J Pediatr Hematol Oncol 2016;38:118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinn GP, Vadaparampil ST, Lee JH et al. Physician referral for fertility preservation in oncology patients: A national study of practice behaviors. J Clin Onco 2009;27:5952–5957. [DOI] [PubMed] [Google Scholar]
- 24.Vadaparampil ST, Gwede CK, Meade C et al. ENRICH: A promising oncology nurse training program to implement ASCO clinical practice guidelines on fertility for AYA cancer patients. Patient Educ Couns 2016;99:1907–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinn GP, Woodruff TK, Knapp CA et al. Expanding the oncofertility workforce: Training allied health professionals to improve health outcomes for adolescents and young adults. J Adolesc Young Adult Oncol 2016;5:292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forman EJ, Anders CK, Behera MA. Pilot survey of oncologists regarding treatment‐related infertility and fertility preservation in female cancer patients. J Reprod Med. 2009;54:203–207. [PMC free article] [PubMed] [Google Scholar]
- 27.de Man AM, Rashedi A, Nelen W et al. Female fertility in the cancer setting: Availability and quality of online health information. Hum Fertil (Camb) 2018:1–9. [DOI] [PubMed] [Google Scholar]
- 28.Quinn GP, Vadaparampil ST, Malo T et al. Oncologists’ use of patient educational materials about cancer and fertility preservation. Psychooncology, 2012;21:1244–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy D, Sawczyn KK, Quinn JP. Using a patient‐centered approach to develop a fertility preservation brochure for pediatric oncology patients: A pilot study. J Pediatr Adolesc Gynecol 2012;25:114–121. [DOI] [PubMed] [Google Scholar]
- 30.Murphy D, Kashal P, Quinn GP et al. Development of a Spanish language fertility educational brochure for pediatric oncology families. J Pediatr Adolesc Gynecol 2014;27:202–209. [DOI] [PubMed] [Google Scholar]
- 31.Merrick H, Wright E, Pacey AA et al. Finding out about sperm banking: What information is available online for men diagnosed with cancer? Hum Fertil (Camb) 2012;15:121–128. [DOI] [PubMed] [Google Scholar]
- 32.Vadaparampil ST, Hutchins NM, Quinn GP. Reproductive health in the adolescent and young adult cancer patient: An innovative training program for oncology nurses. J Cancer Educ 2013;28:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proctor EK, Landsverk J, Aarons G et al. Implementation research in mental health services: An emerging science with conceptual, methodological, and training challenges. Adm Policy Ment Health 2009;36:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]