The use of human tissue samples for biomedical research is under debate, especially as related to questions of permissible uses of stored tissue for future research. Ethical obligations to research participants are considered in this article, which reports on the expectations of cancer patients for the use of archived biospecimens when the prior informed consent is inadequate to the situation and reconsent is not possible.
Keywords: Bioethics, Tissues, Tissue banks, Informed consent
Abstract
Background.
As scientific techniques evolve, historical informed consent forms may inadequately address modern research proposals, leading to ethical questions regarding research with archived biospecimens.
Subjects, Materials, and Methods.
We conducted focus groups among patients with cancer recruited from Massachusetts General Hospital to explore views on medical research, biobanking, and scenarios based on real biospecimen research dilemmas. Our multidisciplinary team developed a structured focus group guide, and all groups were recorded and transcribed. Transcripts were coded for themes by two independent investigators using NVivo software.
Results.
Across five focus groups with 21 participants, we found that most participants were supportive of biobanks and use of their own tissue to advance scientific knowledge. Many favor allowing research beyond the scope of the original consent to proceed if recontact is impossible. However, participants were not comfortable speaking for other patients who may oppose research beyond the original consent. This was viewed as a potential violation of participants’ rights or interests. Participants were also concerned with a “slippery slope” and potential scientific abuse if research were permitted without adherence to original consent. There was strong support for recontact and reconsent when possible and for the concept of broad consent at the time of tissue collection.
Conclusion.
Our participants support use of their tissue to advance research and generally support any productive scientific approach. However, in the absence of broad initial consent, when recontact is impossible, a case‐by‐case decision must be made regarding a proposal's potential benefits and harms. Many participants support broad use of their tissue, but a substantial minority object to use beyond the original consent.
Implications for Practice.
For prospective studies collecting tissue for future research, investigators should consider seeking broad consent, to allow for evolution of research questions and methods. For studies using previously collected tissues, researchers should attempt recontact and reconsent for research aims or methods beyond the scope of the original consent. When reconsent is not possible, a case‐by‐case decision must be made, weighing the scientific value of the biobank, potential benefits of the proposed research, and the likelihood and nature of risks to participants and their welfare interests. This study's data suggest that many participants support broad use of their tissue and prefer science to move forward.
Introduction
Over the past decade, there has been a tremendous amount of debate over the use of human tissue samples in biomedical research and questions related to the permissible uses of stored tissue for future research (e.g., [1], [2], [3], [4], [5]). As scientific techniques evolve and become more powerful, what can be done with archived tissues changes, as do the risks of research, such as those associated with privacy of genetic information. The further the research is removed from the time of sample collection, the less likely it is that the original informed consent document (ICD) will include language that adequately describes the modern research proposal or that meets current standards for informed consent. This continuous evolution of scientific techniques and standards for informed consent gives rise to ethical dilemmas regarding the permissibility of research with archived biospecimens that otherwise represent a highly valuable scientific resource. Researchers and those charged with research oversight must confront these questions, weighing ethical obligations to research participants and the potential benefits of the research to society.
Participants in cancer research often provide biospecimens (typically blood and/or biopsied tissue) for future research [6] as part of clinical trials in which they are enrolled. In addition, many patients receiving routine cancer care provide consent to have blood or extra tissue from surgery stored in a biobank for use in future research. The ICD associated with these donations vary from study to study and institution to institution with regard to the descriptions of the goals, scientific techniques, risks, purpose, and scope of future research [7]. Often, institutional review boards (IRBs) are tasked with making the decision about whether a secondary study can go forward with archived samples, given the consent under which the samples were received. Such decisions are often made on a case‐by‐case basis with little formal guidance from the empirical bioethics literature [2], [8]. Finally, although IRBs include community representatives, deliberations over specific proposals currently take place without a clear sense of what those who provided the research samples would want or expect. It is unclear whether and under what circumstances research participants would expect research to proceed in the hope of scientific advances versus when the conduct of research might be perceived as a violation of the research agreement outlined in the original ICD, a violation that could impact future trust and participation in research.
This challenge emerges frequently in cancer research and other areas of medicine and will continue if not increase because of the evolving nature of science, ethical norms, and governance [1]. Given the rapid growth of biobanking and data sharing, evaluating the views of current and potential research participants on these questions is essential. Although forms of broad consent have been used by some institutions for years, the revised Common Rule formalized this option and therefore may diminish this challenge for future biobanks; guidelines for use of previously collected samples will continue to be important and should be informed by empirical research.
The existing literature demonstrates strong public support across multiple countries for biobanking [9], [10], [11]. Others have demonstrated support for broad consent to unspecified future research, and outlined concerns over privacy, recontact, and anonymization that have shaped national biobanking policies [1], [12], [13]. For example, in a survey of Canadians’ perceptions of biobanking, Joly et al. found that the majority of their 114 participants supported biobanking, 42% favored one‐time broad consent, 12% favored broad consent for future research but restricted to specified diseases, and 29% preferred recontact and consent for each future project [10]. Those surveyed were concerned about biobank governance structure, protection of confidentiality, and control of information. Similarly, McGuire et al. found that focus group participants supported biobanks but favored explicit information about data sharing in informed consent documents for genetic research [14].
Several studies have specifically evaluated cancer patients’ views regarding biobanking. Braun et al. interviewed 30 patients with cancer, finding support for biobanking and preferences for broad consent and confidentiality [15]. In one of the larger studies to date, Bryant et al. found that among 224 patients with cancer, 84% were willing to have leftover tissue used for research, and the vast majority (96%) supported biobanking for future use [16]. The majority supported broad one‐time consent (71%) and linkage to clinical data (62%). Vermeulen et al. studied consent preferences for biobanking among patients with colon or breast cancer in the Netherlands. Among 76 patients, there was broad support for biobanking, and 71% indicated a preference for opt‐out consent, with the majority favoring provision of information about the biobank [4]. Finally, Pentz et al. explored potential racial/ethnic differences in preferences for biobanking among patients with cancer and found that in a diverse sample of 315 patients, 95% supported biobanking, and most favored one‐time broad consent without recontact [5].
Despite the growing literature demonstrating support for broad consent and biobanking in future research, to our knowledge, no prior study has directly addressed the views of patients with cancer regarding the real and pressing problem of what research can be conducted with archived biospecimens obtained prior to modern informed consent practices. The importance of this issue has been highlighted in several conceptual and policy‐oriented papers outlining the ethical dilemma. Petrini describes the potential conflict between the scientific value of archived specimens, the need to protect tissue donors’ confidentiality, the need to respect research participants’ autonomy, and the potential impact on advancement of knowledge and public trust in research if these issues are not resolved successfully [1]. Our group has previously outlined the challenges that can emerge with consideration of data sharing with genetic information derived from archived biospecimens and highlighted key considerations for policy decisions [8]. Similarly, both Helgesson et al. and Bathe and McGuire describe the challenges (such as intellectual property, anonymization, protection from genetic discrimination, and protection of autonomy) inherent in research involving archived biospecimens without modern informed consent and further propose ethical frameworks to help determine if research should be permitted [2], [3].
The current study is designed to provide data on the views and expectations of patients with cancer on the real‐world dilemma of whether to proceed with research using archived biospecimens when the scientific techniques and/or ethical standards have evolved such that the adequacy of prior informed consent is unclear and reconsent is not feasible.
Subjects, Materials, and Methods
Study Design
This qualitative study involved focus groups among patients with cancer discussing their views and expectations regarding use of tissue for cancer research and responses to scenarios involving proposed research with stored tissue. The study was reviewed by the Massachusetts General Hospital/Partners Institutional Review Board and deemed exempt.
Patients and Recruitment
We recruited adult patients with cancer from the oncology clinics at Massachusetts General Hospital to focus groups on the basis of referral from their oncologist. Following referral, patients were contacted by study staff at their clinic visit or by phone. Eligible patients were any English‐speaking adult patients with a history of cancer. Participants were divided among five groups based on their availability and schedule preferences.
Focus Group Methodology
A semistructured focus group discussion guide was developed by a multidisciplinary team with expertise in research oversight, patient advocacy, cancer care, translational research, oncology, genetics, psychology, and bioethics. The guide was based on literature review and cases experienced by the investigators. Three cases were presented that involved ethical questions related to (a) secondary use that included germline genetic testing not described in the original consent; (b) secondary use that included research on a cancer type not described in the original consent; and (c) secondary use that included the sharing of deidentified data with a national database, rather than restricting access to a research consortium, as described in the original consent (Table 1). Interview guide domains included general attitudes toward cancer research, views and concerns regarding tissue collection for research, privacy concerns, attitudes toward genetic research, and views on specific scenarios involving discrepancies between the proposed research and the informed consent form (differences in scientific tests; types of cancer tested; and scope of data shared).
Table 1. Scenarios designed to assess comfort with different kinds of departures from the informed consent document.
At the start of each focus group, participants were asked to complete a brief demographic questionnaire. Participants received a handout including a one‐page glossary of terms and the scenarios. Participants identified themselves by first name only and were asked to respect the confidentiality of others in the group. All focus groups were conducted by the principal investigator (J.P.), while a second research team member took detailed field notes (J.T.R. or K.Q.). Each session lasted approximately 90 minutes and was recorded. Participants were provided with parking vouchers and refreshments but were not otherwise compensated.
Qualitative Analysis
Audio recordings of the focus groups were professionally transcribed, checked by members of the research team, and scrubbed of identifying information. Two team members (J.T.R. and K.Q.) independently coded the transcripts using NVivo 11 (QSR International, Melbourne, Australia), a qualitative research and analysis software package. The independently coded transcripts were merged and coding discrepancies were evaluated using the coding comparison tool. The coders reviewed each coding discrepancy, came to consensus, and finalized the data set. After all codes were reviewed, an overall Kappa coefficient, measuring inter‐rater agreement, was calculated at 0.86.
Results
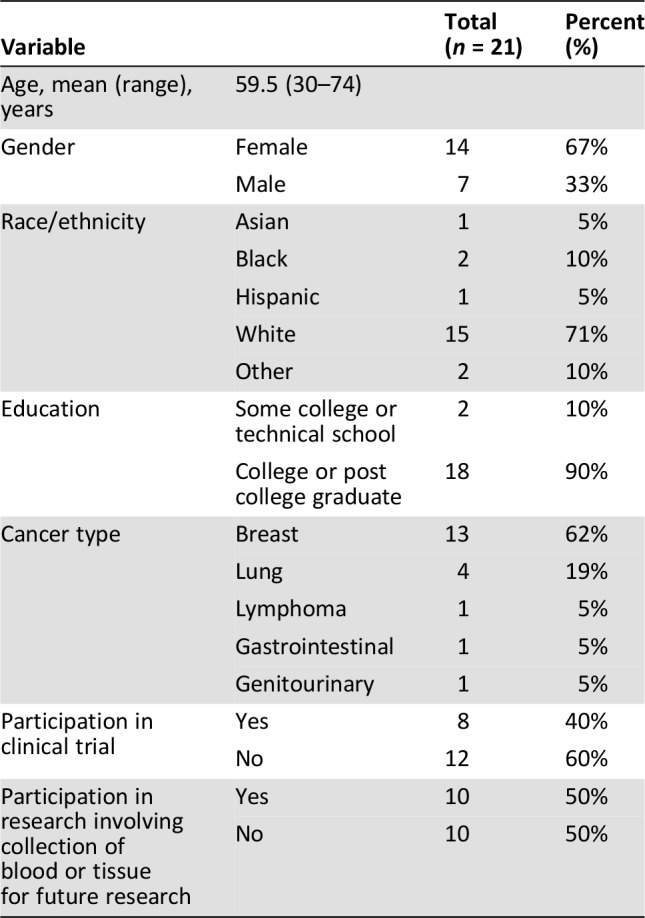
We recruited 21 participants to five focus groups (Table 2). A total of 32 patients were contacted, with 11 declining or unable to schedule a time to participate. Fourteen women and seven men with median age of 59.5 (30–79 years) participated in the focus groups. The majority (71%) of participants identified as white. All had at least some college or technical school, and most (90%) had completed college and/or graduate school. The majority had received a breast cancer diagnosis, but five cancer types were represented, and patients with both early‐stage and advanced disease were included. The sample was split evenly between those who had participated in research involving the storage of tissue for future research and those who had not.
Table 2. Demographics of focus group participants.
Broad but Not Unanimous Support
Although participants had generally favorable views of science and of clinical trials, this did not translate to uniform support for the secondary use of tissues beyond the initial consent. Across the groups, there was a willingness to allow the research to proceed (both generally and for specific scenarios), primarily motivated by an interest in advancing research and clinical care for future patients and families, and including a sense of duty to give back to a research enterprise from which they had benefited. Participants expressed a sense that researchers should “just use it” as long as the research was legitimate and focused on the common good.
“Well, clinical research is what keeps us alive. We have the knowledge today, but when we get sick, and we have something newer come, especially if you have something which is incurable—so‐called, incurable for today—maybe tomorrow, will be. Maybe in 20 years. That's why giving samples, for example, is an absolute necessity. It can save all of us, sick or not.” FG3/S5
“…I was benefiting from so many people who had done that before me. And I just felt like it was also moral obligations. I thought there was no question that I was benefiting and fortunate to be here, and I needed to be part of whatever was going forward.” FG4/S5
Participants understood that research and knowledge evolves and that the research proposed in the scenarios was part of this natural trajectory. Several participants also expressed hope that current research might lead to direct benefits for current patients. However, this often‐enthusiastic support came with several caveats, discussed below.
Concerns About Secondary Use
Even people who were comfortable with unconsented secondary uses of their own tissues voiced concerns on behalf of others who might have reservations about such uses. Participants recognized that there would likely be a diversity of views among research participants.
“…I think if this question were posed to me, I would say yes on the original form of consent. I think probably the vast majority of people would have said [that] also, but I think maybe the question is, do you know that 100% people would? And is it worth going against that minority of people who would not want that to be done for the majority of people who would? So I think it's a difficult question.” FG4/S3
There was also heightened concern about the third scenario, involving the sharing of genomic data with a national database:
“My initial reaction was okay [to pursue the proposed research], but…I flipped, and I think in my case it's because it's genetic code. If it was not genetic code, but something else, who cares. But because genetic code…can identify you, who knows what they can do with that five, ten, three years from now. That makes me nervous.” FG3/S2
Four of the five focus groups included one or more participants who were consistently against unconsented secondary uses. Those who were against unconsented secondary uses voiced concerns regarding scientific abuse and doubts about the research process in general. Further, the case of Henrietta Lacks was mentioned spontaneously in three of the five groups, despite this work taking place in Boston, MA, not Baltimore, MD, and there being no mention of this case in the study guide or by the moderator. Overall, about one third of our participants voiced serious reservations or opposition to unconsented secondary uses.
“So imagine that I participated. I let you use my tissue for somatic mutations. And 10 years later—I'm reading a study that took place at MGH on lymphoma, and I'm thinking, ‘Wow, you know, I gave tissue to that.’ And we discovered germline mutations. ‘Oh my God.’ And that's just one person. I would be furious because you violated my trust.” FG2/S9
Participants’ concerns included scientific abuse of the sort Mrs. Lacks and her family suffered, tissue uses that they found morally objectionable (e.g., immortalization, commercialization, cloning), legal ramifications of violating the consent “contract,” the notion of a slippery slope (i.e., if these unconsented uses are permitted, nothing will be prohibited), and violations of patient trust. Without prompting, our participants hit on most of the historical and contemporary “hot button” bioethics concerns, including return of results and its relationship to deidentification, privacy/confidentiality, insurance and employment discrimination, the right not to know, human genetic modification, enhancement, and tissue and data ownership.
When in Doubt, Ask
Even among the majority of participants who were comfortable with unconsented secondary use of their tissues, there was general agreement that when proposed secondary research would conflict with the original consent, investigators should attempt to reconsent those whose tissues are to be used. The need for reconsent had one or more ardent supporters in all five focus groups and was the general consensus in one group. Both legal and moral arguments were raised by our participants. Some people viewed consent documents as contracts between researchers and participants, and others wanted to be viewed as partners in the research.
“So setting the legalities aside, I think you have an ethical obligation to update donors if the terms of the relationship have changed. And it's no different from any other retailer today where you constantly get e‐mails like, ‘We've updated the terms and conditions of our privacy policy. Click here to accept, or you can no longer use our site,’ or whatever. It shouldn't be that hard for you guys to reach out to people with an update. And if they opt out of it, then you can't use their stuff.” FG2/S8
“To be quite honest, and this may or may not sound rational, but I feel that it's my tissue, it's mine. I have the right to know what's going to happen with it.” FG5/S5
Some participants had less stringent thresholds for requiring reconsent. The feeling was that if the risks and benefits of the proposed secondary research are the same as the original project, the research could go forward, but if the risks have changed or increased, investigators should reconsent or recruit new tissue donors.
In cases in which reconsent is impossible or impracticable, our focus group participants had a range of views about whether and how to move forward. But again, many of our participants were happy to have their tissues used for many or all types of secondary research, and even suggested consent approaches that would facilitate this, including the use of broad consent language. Support for broad consent language was spontaneously voiced in four out of five focus groups.
“We ought to expand [consent forms] and say, ‘Look guys, we don't know what's around the road in 10 years. The doors are open. You give it to us. We're going to go there.’ And then you got to accept the fact that there's good police. We will do good with it.” FG5/S4
One participant suggested liberal use of archived samples after a period of time had passed:
“…someone just makes a blanket rule. Anything that's over 15 years or more, we can use for anything.” FG4/S1
Overall, the sense was that researchers should plan ahead for future research needs and interests. In cases in which samples were collected under more restrictive consents and reconsent is not possible or practicable, there was no consensus on how to proceed. Our participants’ views ranged from a simple prohibition on secondary research to the view that if the majority would be okay with it, then researchers can proceed, to simply allowing researchers to proceed, on the belief that the research could help people.
Two important observations were documented in the field notes from these focus groups. First, participants with a history of cancer but no specific knowledge of or training in molecular biology or research appeared able to grasp the issues and delve into detailed discussions of the ethics of unconsented secondary research, genetics research, deidentification, and data sharing. Virtually all participants voiced opinions on these questions, and many raised issues that have been raised previously in the bioethics literature. Second, within the focus groups, participants were able to influence the opinions of others, and both the moderator and research assistants noted shifts in the discussion both toward and away from permissibility of the research based on fellow group members’ arguments.
Discussion
Secondary use of clinical and research tissue is an increasing focus of debate as technology and research capabilities evolve. Particularly in oncology, where interventions are developed targeting molecular changes that may be seen across cancer types, consent forms restricted to specific cancers or techniques have become outdated. At the same time, the value of molecular analysis of archived cancer tissue has increased. Further, to enable replication of published results and to maximize the value of the initial investment required to collect human tissues, researchers have been urged, if not required, to share research samples and data. These modern scientific techniques and practices may conflict with the research and risks described in older ICD provided to research participants years or decades earlier.
Our study solicited views of a patient population likely to have clinical or research samples already stored at a large academic medical center or hospital. Our participants were supportive of research generally and were particularly interested in research that could produce benefits for patients like them, the community at large, and for the next generation. Many of our participants said they were happy to have their tissues used in research, even if that research was not in line with the original informed consent document. However, this generally pro‐research group of patients with cancer was nonetheless sensitive to how others might feel about such research and were wary of allowing secondary uses that violate the original consent. Indeed, a sizable minority (approximately one third) of our research participants found secondary uses of tissues not in compliance with original consent to be ethically problematic. Even those in favor of broad use of their own tissue frequently raised concerns about how failure to adhere to consent could lead to a loss of trust and lack of societal support for research. Further research is needed to determine if our data bear out in larger, more diverse populations and if there are differences between, for example, those with early‐ and late‐stage disease regarding their views of the permissibility of secondary uses of their samples.
Our participants suggested two main solutions to the divergence of opinion among those who have enrolled in trials, which have been much discussed in the literature: reconsent for existing samples and broad consent for prospective samples. Today, existing samples are stripped of identifiers, defining any subsequent use as not human subjects research, although some institutions do require oversight of secondary uses of tissue, even when deidentified. Our research suggests that some people would find this practice problematic for secondary uses in conflict with the original consent.
For research going forward, our participants’ views were aligned both with those represented in the literature and with the recent revision to the Common Rule. The revised Rule stipulates that the consent process for any research involving the collection of identifiable information or biospecimens includes either “(i) A statement that identifiers might be removed from the identifiable private information or identifiable biospecimens and that, after such removal, the information or biospecimens could be used for future research studies or distributed to another investigator for future research studies without additional informed consent from the subject or the legally authorized representative, if this might be a possibility; or (ii) A statement that the subject's information or biospecimens collected as part of the research, even if identifiers are removed, will not be used or distributed for future research studies” [17]. The revised Rule also requires the inclusion of additional statements, where applicable, regarding the possibility of whole genome sequencing of tissues, potential commercial uses, and the return of individual research results. Finally, the revised Rule permits broad consent in some contexts, including, “for the storage, maintenance, and secondary research use of identifiable private information or identifiable biospecimens (collected for either research studies other than the proposed research or non‐research purposes)…” Our research suggests that these modifications may be welcomed by many participants, as they could lead to increased clarity for both research participants and researchers interested in secondary use of stored tissues. Furthermore, the fact that our participants were able to grasp both the pragmatic and ethical issues associated with the secondary use of stored tissues and have nuanced discussions of the same suggests that patients could be a valuable resource when determining the governance of such resources.
It is important to note that our participants, although representative of the patient population at Massachusetts General Hospital, are not representative of the broader U.S. population. We spoke with a small sample of largely white, highly educated patients. Furthermore, people who are willing to participate in focus group research may be more likely to have higher levels of trust and more positive views of scientific research. In a small qualitative study, we are unable to assess the frequency of views on these issues in the broader population. Because of the focus group format, we cannot know whether opposition to unconsented secondary research was associated with prior participation in research, or nonparticipation, or with any other characteristic. Finally, although we did spend some time explaining the content and meaning of genetic data, the people we spoke with may have nonetheless had misconceptions about the potential benefits and risks of this research that could have influenced their views. These focus group findings, including remaining uncertainties, have formed the basis for a population‐based survey to further identify and begin to quantify views on these issues among patients with cancer.
Conclusion
These data suggest that for prospective studies, researchers should consider seeking broad consent to allow for some evolution of research questions and methods. For studies using previously collected tissues, researchers should attempt recontact and reconsent for research aims or methods beyond the scope of the original consent. When reconsent is not possible and the proposed secondary use falls into the gray area between what is clearly within the scope of the original consent and clearly outside that scope, a case‐by‐case decision must be made, and our data suggest that many (although not all) participants support broad use of their tissue and just want the science to move forward. There is a strong interest among many research participants in seeing their blood or tissue used to maximal scientific benefit. However, a substantial minority are uncomfortable with unconsented secondary use and a violation of the original consent risks diminishing trust in science for some patients. Finally, even among those who are comfortable with unconsented secondary use of their own tissue, there is reluctance to speak for other patients and a recognition that violating the wishes of even a minority of research participants is problematic. The interests of both those who wish to see broad and productive use of their tissue and those who oppose any secondary use beyond the scope of the original consent must be taken into account as these cases arise.
Acknowledgments
We are grateful to the patients who participated in this research during what is always a trying process of treatment for cancer. We are also grateful to the Greenwall Foundation for supporting this work through their Making A Difference in Real‐World Bioethics Dilemmas funding program.
Author Contributions
Conception/design: Debra J.H. Mathews, Eric Campbell, Deborah Collyar, Fay J. Hlubocky, Steven Isakoff, Jeffrey Peppercorn
Provision of study material or patients: Steven Isakoff, Jeffrey Peppercorn
Collection and/or assembly of data: Julia T. Rabin, Katharine Quain, Jeffrey Peppercorn
Data analysis and interpretation: Debra J.H. Mathews, Julia T. Rabin, Katharine Quain
Manuscript writing: Debra J.H. Mathews, Julia T. Rabin, Katharine Quain
Final approval of manuscript: Debra J.H. Mathews, Julia T. Rabin, Katharine Quain, Eric Campbell, Deborah Collyar, Fay J. Hlubocky, Steven Isakoff, Jeffrey Peppercorn
Disclosures
The authors indicated no financial relationships.
References
- 1.Petrini C. "Broad" consent, exceptions to consent and the question of using biological samples for research purposes different from the initial collection purpose. Soc Sci Med 2010;70:217–220. [DOI] [PubMed] [Google Scholar]
- 2.Helgesson G, Dillner J, Carlson J et al. Ethical framework for previously collected biobank samples. Nat Biotechnol 2007;25:973–976. [DOI] [PubMed] [Google Scholar]
- 3.Bathe OF, McGuire AL. The ethical use of existing samples for genome research. Genet Med 2009;11:712–715. [DOI] [PubMed] [Google Scholar]
- 4.Vermeulen E, Schmidt MK, Aaronson NK et al. Opt‐out plus, the patients' choice: Preferences of cancer patients concerning information and consent regimen for future research with biological samples archived in the context of treatment. J Clin Pathol 2009;62:275–278. [DOI] [PubMed] [Google Scholar]
- 5.Pentz RD, Billot L, Wendler D. Research on stored biological samples: Views of African American and White American cancer patients. Am J Med Genet A 2006;140:733–739. [DOI] [PubMed] [Google Scholar]
- 6.Peppercorn J. Toward improved understanding of the ethical and clinical issues surrounding mandatory research biopsies. J Clin Oncol 2013;31:1–2. [DOI] [PubMed] [Google Scholar]
- 7.Beskow LM, Friedman JY, Hardy NC et al. Developing a simplified consent form for biobanking. PLoS One 2010;5:e13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peppercorn J, Shapira I, Deshields T et al. Ethical aspects of participation in the database of genotypes and phenotypes of the National Center for Biotechnology Information: The Cancer and Leukemia Group B Experience. Cancer 2012;118:5060–5068. [DOI] [PubMed] [Google Scholar]
- 9.Ahram M, Othman A, Shahrouri M. Public support and consent preference for biomedical research and biobanking in Jordan. Eur J Hum Genet 2013;21:567–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joly Y, Dalpe G, So D et al. Fair shares and sharing fairly: A survey of public views on open science, informed consent and participatory research in biobanking. PLoS One 2015;10:e0129893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy J, Scott J, Kaufman D et al. Public perspectives on informed consent for biobanking. Am J Public Health 2009;99:2128–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasgupta I, Bollinger J, Mathews DJ et al. Patients' attitudes toward the donation of biological materials for the derivation of induced pluripotent stem cells. Cell Stem Cell 2014;14:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Doherty KC, Burgess MM, Edwards K et al. From consent to institutions: Designing adaptive governance for genomic biobanks. Soc Sci Med 2011;73:367–374. [DOI] [PubMed] [Google Scholar]
- 14.McGuire AL, Hamilton JA, Lunstroth R et al. DNA data sharing: Research participants' perspectives. Genet Med 2008;10:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braun KL, Tsark JU, Powers A et al. Cancer patient perceptions about biobanking and preferred timing of consent. Biopreserv Biobank 2014;12:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryant J, Sanson‐Fisher R, Fradgley E et al. Oncology patients overwhelmingly support tissue banking. BMC Cancer 2015;15:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Office of Human Research Protections . Federal policy for the protection of human subjects. Fed Regist 2017;82:7149–7274. [PubMed] [Google Scholar]



