This study evaluated and compared the clinical impact of 18F‐FES and 18F‐FDG on the sensitivity of lesion detection, correct staging and individual management plans in patients with newly diagnosed ER positive breast cancer.
Keywords: Newly diagnosed breast cancer, 18F‐FES, 18F‐FDG, Management changes
Abstract
Purpose.
We compared the clinical value of 16a‐18F‐fluoro‐17b‐estradiol (18F‐FES) positron emission tomography (PET)/computed tomography (CT) and 18F‐fluoro‐2‐deoxy‐D‐glucose (18F‐FDG) PET/CT and investigated whether and how 18F‐FES PET/CT affects the implemented management of newly diagnosed estrogen receptor positive breast cancer patients.
Materials and Methods.
We retrospectively analyzed 19 female patients newly diagnosed with immunohistochemistry‐confirmed estrogen receptor (ER)‐positive breast cancer who underwent 18F‐FES and 18F‐FDG PET/CT within 1 week in our center. The sensitivity of 18F‐FES and 18F‐FDG in diagnosed lesions were compared. To investigate the definite clinical impact of 18F‐FES on managing patients with newly diagnosed ER positive breast cancer, we designed two kinds of questionnaires. Referring physicians completed the first questionnaire based on the 18F‐FDG report to propose the treatment regime, and the second was completed immediately after reviewing the imaging report of 18F‐FES to indicate intended management changes.
Results.
In total, 238 lesions were analyzed in 19 patients with newly diagnosed ER‐positive breast cancer. Lesion detection was achieved in 216 sites with 18F‐FES PET and in 197 sites with 18F‐FDG PET/CT. These results corresponded to sensitivities of 90.8% for 18F‐FES versus 82.8% for 18F‐FDG PET/CT in diagnosed lesions. Thirty‐five physicians were given the questionnaires referring to the treatment strategy, with 27 of them completing both questionnaires. The application of 18F‐FES in addition to 18F‐FDG PET/CT changed the management in 26.3% of the 19 patients with newly diagnosed ER‐positive breast cancer.
Conclusion.
Performing 18F‐FES PET/CT in newly diagnosed ER‐positive breast cancer patients increases the value of diagnosis equivocal lesions and treatment management compared with 18F‐FDG PET/CT.
Implications for Practice.
This study investigated whether 16a‐18F‐fluoro‐17b‐estradiol (18F‐FES) positron emission tomography (PET)/computed tomography (CT) affects the clinical management of patients with newly diagnosed estrogen receptor (ER)‐positive breast cancer. Physicians completing two questionnaires comparing the clinical impact of 18F‐FES and 18F‐FDG on individual management plans in patients with newly diagnosed ER‐positive breast cancer confirmed that 18F‐FES scans led to change in management in 26.3% of the 19 patients with newly diagnosed ER positive breast cancer. This retrospective study indicates the potential impact of 18F‐FES PET/CT on intended management of patients with newly diagnosed estrogen receptor positive breast cancer in comparison to 18F‐fluoro‐2‐deoxy‐D‐glucose PET/CT.
摘要
目的。我们比较了 16a‐18F‐氟代‐17b‐雌二醇(18F‐FES) 正电子发射断层扫描(PET)/计算机断层扫描(CT)和 18F‐氟代‐2‐脱氧‐D‐葡萄糖(18F‐FDG)PET/CT 的临床价值,并研究了 18F‐FES PET/CT 是否会以及如何影响新诊断雌激素受体阳性乳腺癌患者的治疗实施。
材料和方法。我们回顾性分析了 19 例新诊断且免疫组化确认雌激素受体(ER)阳性乳腺癌的女性患者,她们在 1 周内于我们的中心接受了18F‐FES 和 18F‐FDG PET/CT 检查。比较了18F‐FES 和 18F‐FDG 对诊断病变的敏感性。为研究18F‐FES 对新诊断 ER 阳性乳腺癌患者治疗的确切临床影响,我们设计了两种问卷。咨询医生根据 18F‐FDG 报告完成了第一份问卷,提出治疗方案,第二份问卷在审查了18F‐FES 的影像学报告后立即完成,以表明预期的治疗变化。
结果。对 19 例新诊断 ER 阳性乳腺癌患者的共 238 处病变进行了分析。采用 18F‐FES PET 检测了 216 个部位的病变,采用 18F‐FDG PET/CT 检测了 197 个部位的病变。在诊断病变中,这些结果对应于 18F‐FES 的敏感度为 90.8%,而 18F‐FDG PET/CT 的敏感度为 82.8%。35 名医生接受了涉及治疗策略的问卷调查,其中 27 名医生完成了两份问卷调查。在 19 例新诊断 ER 阳性乳腺癌患者中,18F‐FES 连同 18F‐FDG PET/CT 的应用改变了 26.3% 的患者的治疗方法。
结论。与 18F‐FDG PET/CT 相比,对新诊断 ER 阳性乳腺癌患者执行 18F‐FES PET/CT 可提高可疑病变的诊断价值和治疗管理。
实践意义:本研究探讨了 16a‐18F‐氟代‐17b‐雌二醇 (18F‐FES) 正电子发射断层扫描 (PET)/计算机断层扫描 (CT) 是否会影响新诊断雌激素受体 (ER) 阳性乳腺癌患者的临床治疗。完成两份比较 18F‐FES 和 18F‐FDG 对新诊断 ER 阳性乳腺癌患者个体治疗计划临床影响调查问卷的医生证实,在 19 名新诊断 ER 阳性乳腺癌患者中,18F‐FES 扫描导致了 26.3% 的患者的治疗方案发生变化。此项回顾性研究表明,与 18F‐氟代‐2‐脱氧‐D‐葡萄糖 PET/CT 相比,18F‐FES PET/CT 对新诊断雌激素受体阳性乳腺癌患者预期治疗会造成潜在影响。
Introduction
Annual estimates suggest that 1.68 million new cases of breast cancer are diagnosed worldwide and result in approximately 400,000 deaths, making breast cancer the major cause of cancer‐related mortality in women [1]. According to cancer statistics from China, breast cancer was estimated to account for 15% of newly diagnosed cancers in 2015 [2]. The diagnosis and staging of breast cancer are predominantly based on physical examination, pathological examination, and imaging [3]. Cancer imaging has evolved from morphological imaging to molecular imaging. Increasing evidence in the literature suggests that positron emission tomography (PET)/computed tomography (CT) has a higher sensitivity and specificity in the staging of many cancers compared with other imaging methods [4]. 18F‐fluoro‐2‐deoxy‐D‐glucose (18F‐FDG) is the most commonly used PET tracer in routine clinical practice for diagnosis and monitoring responses to therapy in oncology [5], [6], [7]. In breast cancer, 18F‐FDG PET‐CT is commonly required for metastatic examination, management response, and suspected recurrence of locally advanced cancer [8], [9], [10]; however, 18F‐FDG is not a cancer‐specific tracer, and benign diseases related to infection or inflammation can also show false‐positive intense 18F‐FDG uptake, which causes difficulty in distinguishing benign disorders from malignant diseases [11], [12].
Approximately 70%–80% of breast cancers are hormone receptor (HR)‐positive (estrogen and/or progesterone receptor positive), which makes endocrine therapy an important therapeutic option [13]. Estrogen receptor (ER) plays a key role in the treatment regimen and prognosis [14]. 16a‐18F‐fluoro‐17b‐estradiol (18F‐FES) has been demonstrated to be a noninvasive, molecular imaging technique to observe and quantify in vivo ER expression [15], [16]. Previous studies have shown that the uptake of 18F‐FES could detect the ER‐positive lesions and also highly corresponded to the degree of immunohistochemical (IHC) staining for ER on tumor biopsies [17], [18], [19]. In current clinical studies, however, 18F‐FES PET‐CT is used either to reveal the existence of heterogeneity in the tumors or as a predictor of response to endocrine therapy in patients with advanced or metastatic ER‐positive breast cancer [19], [20], [21], [22].
Although the value of 18F‐FES and 18F‐FDG PET has been extensively studied in metastatic breast cancer [20], [21], the study of 18F‐FES application in cases of newly diagnosed breast cancer is extremely limited. The aims of this study were to evaluate and compare the clinical impact of 18F‐FES and 18F‐FDG on the sensitivity of lesion detection, correct staging, and individual management plans in patients with newly diagnosed ER‐positive breast cancer.
Materials and Methods
Patients and Procedures
Patients with newly diagnosed breast cancer who underwent both 18F‐FES PET/CT and 18F‐FDG PET/CT in Fudan University Shanghai Cancer Center within 1 week, from August 2010 and June 2018, were retrospectively identified from an electronic database. Nineteen treatment‐naive patients with newly diagnosed immunohistochemical confirmed ER‐positive breast cancer were included in our study. All the patients were enrolled from these purposes: predicting response to fulvestrant 500 mg treatment, a phase II study (NCT03507088, n = 14), evaluating ambiguous lesions in routine workup (such as CT, magnetic resonance imaging [MRI], 18F‐FDG; n = 5). The study has been approved by the Fudan University Shanghai Cancer Center Ethic Committee and institutional review boards for clinical investigation. Informed written consent was obtained from all of these enrolled patients.
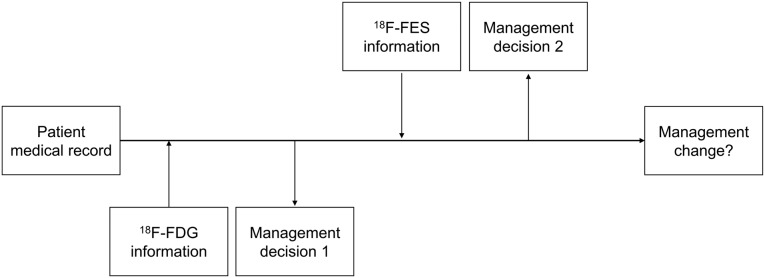
The questionnaire design is depicted in Figure 1. Referring physicians completed the first questionnaire, including the full medical history and 18F‐FDG scan reports to indicate the treatment plan without 18F‐FES PET/CT information, and the second questionnaire, with the addition of an 18F‐FES scan to denote intended management changes.
Figure 1.
The questionnaire process.
Abbreviations: 18F‐FDG, 18F‐fluoro‐2‐deoxy‐D‐glucose; 18F‐FES, 16a‐18F‐fluoro‐17b‐estradiol.
To minimize individual differences, a definitive change in management was considered when over two‐thirds of physicians chose to change the treatment strategy after the FES scan. A change in management was defined as a difference between the pre‐18F‐FES treatment strategy and post‐18F‐FES treatment strategy. Three categories of change in management were defined: (a) change in treatment objective (e.g., from curative to palliative and vice versa); (b) change in surgical management (e.g., surgery carried out or cancelled); and (c) change in systemic treatment (e.g., from endocrine therapy to chemotherapy).
Synthesis of 18F‐FES, 18F‐FDG, and Quality Control
The MMSE precursor and the authentic 19F‐FES were purchased from Jiangsu Huayi Chemical Co, Ltd. (Suzhou, Jiangsu, China). 18F‐FES was prepared according to published methods [23] and modified as reported in our previous study [19]. The total preparation time was approximately 100 min, and the corrected radiochemical yield was approximately 40% at the end of synthesis. 18F‐FDG was produced routinely and automatically by cyclotron (RDS Eclipse ST; Siemens, Knoxville, TN) using an Explora FDG4 module in our center. The 18F‐FES and 18F‐FDG radiochemical purity was greater than 99% and 95%, respectively.
PET/CT Imaging
For the 18F‐FDG PET/CT imaging, all patients were instructed to fast for at least 6 hours. At the time of the tracer injection, the patients presented blood glucose levels less than 10 mmol/L. Patients with medical comorbidities, such as diabetes, a chronic infection, or chronic inflammatory conditions, were not enrolled to prevent the sensitivity and specificity of 18F‐FDG PET/CT imaging. Before and after injecting 7.4 MBq/kg body weight of 18F‐FDG intravenously, the patients were kept lying comfortably in a quiet, dimly lit room and were administered 1 L of plain water orally before the PET/CT scanning. The scanning consisted of a whole‐body PET/CT examination (2–3 minutes per table position) initiated 1 hour after administration of the tracer using a Siemens Biograph 16 HR PET/CT scanner.
Because all the patients were newly diagnosed and treatment naive, a washout period of the ER antagonist was not required [24]. Approximately 222 MBq (6 mCi) of 18F‐FES was injected intravenously over 1 to 2 minutes. The scanning was initiated 1 hour after administration of the tracer on the same PET/CT scanner as the 18F‐FDG.
Image Interpretation
Lesions identified via 18F‐FDG or 18F‐FES PET were corroborated by CT and/or other imaging. PET images were processed as for a typical clinical scan, corrected for radioactive decay of the tracer, and normalized to the injected dose (ID) and body weight (BW). This processing results in regional standardized uptake values (SUV): SUV = A / (ID / BW), where A is the tissue tracer uptake in microcuries per gram for the hottest pixel in the tumor (SUVmax), ID is the injected dose in millicuries, and BW is the body weight in kilograms. 18F‐FES SUVmax was used to quantify the ER expression. The cutoff value of 18F‐FES positivity and negativity was set at 1.8 based on our previous study [19]. In terms of the 18F‐FDG PET/CT scan, the lesions showing significant uptake (visually higher compared with the surrounding tissues) were defined as positive by two board‐certified nuclear medicine physicians with over 5 years of experience. In patients with uncountable and widespread bone metastases, an arbitration count of up to 10 lesions of the largest 18F‐FES PET or 18F‐FDG PET intensity lesions were taken for the calculation.
Statistical Analysis
The number of lesions either 18F‐FES or 18F‐FDG positive was calculated as the total number and excluded if both were negative. The differences in tracer uptake between different sensitivities were calculated and compared. Because of this high physiological uptake of 18F‐FES in liver tissues, liver lesions were excluded from the analyses. Statistical analyses were conducted using IBM SPSS Statistics 20 (IBM Corporation, Armonk, NY). For intended management, changes were analyzed using the ratio statistics and expressed as 95% confidence interval (CI).
Results
Patient Population
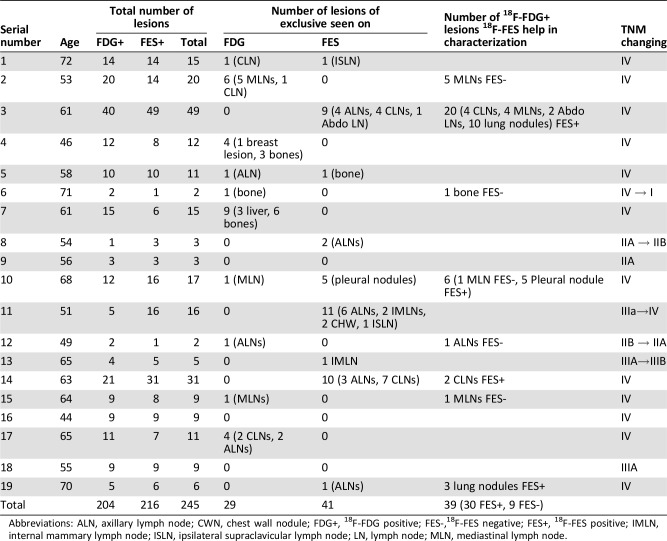
We analyzed the data of 19 patients with newly diagnosed ER positive breast cancer who underwent both 18F‐FES PET/CT and 18F‐FDG PET/CT within 1 week in our center. At the initial diagnosis, the patients were aged between 44 and 72 years (median, 61). The patient data are summarized in Table 1.
Table 1. Changes in TNM stage by 18F‐FES versus 18F‐FDG based on the study population (no. 19).
Abbreviations: ALN, axillary lymph node; CWN, chest wall nodule; FDG+, 18F‐FDG positive; FES‐,18F‐FES negative; FES+, 18F‐FES positive; IMLN, internal mammary lymph node; ISLN, ipsilateral supraclavicular lymph node; LN, lymph node; MLN, mediastinal lymph node.
18F‐FES and 18F‐FDG PET/CT Data Analysis
In total, 245 lesions were identified in 19 patients with newly diagnosed ER‐positive breast cancer. These lesions were identified as 18F‐FDG positive, 18F‐FES positive, or both. In 245 lesions, seven mediastinal lymph nodes (MLNs) were 18F‐FDG positive but 18F‐FES negative. During treatment follow‐up, all the other lesions were responsive in subsequent 18F‐FDG PET/CT and/or other imaging, but the seven MLNs did not change following treatment based on the character of the CT image. Therefore, we defined this MLN lesion as false positive of 18F‐FDG (2.9%). We will discuss these remaining 238 lesions in the following sections [25].
Out of a total of 238 lesions, 197 lesions showed 18F‐FDG uptake and 216 lesions were avid in 18F‐FES PET. Therefore, the sensitivity of 18F‐FDG and 18F‐FES in the diagnosis of ER‐positive lesions in newly diagnosed breast cancer was 82.8% and 90.8%, respectively. Because of the commonly known limitation of FES (high background uptake in the liver), we calculated the sensitivity of 18F‐FES and 18F‐FDG without liver lesions as well. Of the 238 lesions, we excluded 3 liver lesions. Out of 235 lesions without liver metastases, 194 lesions were 18F‐FDG positive, whereas 216 lesions remained avid in 18F‐FES PET, showing a sensitivity of 82.5% and 91.9%.
Of the 245 lesions, we also observed 41 lesions (16.7%) that were exclusively detected by 18F‐FES PET scan yet absent in 18F‐FDG PET scanning, seen at the following sites: lymph nodes (including supraclavicular, neck, axillary, internal mammary, mediastinal, and abdominal), bone, pleural, and chest wall nodule. These lesions were defined as metastases and classified as false negative lesions of 18F‐FDG. Additionally, 39 lesions of 18F‐FDG positive (15.9%) were either uncommon sites of metastatic lesions (n = 15) or common sites of inflammatory changes (n = 24, 13 lung lesions and 11 MLNs). Of these ambiguous lesions, 30 lesions (76.9%) were 18F‐FES positive, confirming the presence of ER‐positive metastases. Hence, 18F‐FES added the value of diagnosis in 71 of 80 equivocal lesions (88.8%) in 19 patients with newly diagnosed ER‐positive breast cancer.
Staging Changed After 18F‐FES PET Scan
Detailed changes in the TNM stage before and after 18F‐FES PET scanning is summarized in Tables 1 and 2. Of the 19 patients with newly diagnosed ER‐positive breast cancer, 5 patients (26.3%) were staged differently by 18F‐FES PET and 18F‐FDG PET. M staging was adjusted in two patients, N stage in four patients, and N as well as M staging in one patient. In terms of the TMN status of these patients, the physicians indicated that 18F‐FES PET imaging led not only to upstaging in three cases due to evidence of suspected metastases but also to downstaging in two cases.
Table 2. Treatment management changes for five patients after 18F‐FES.
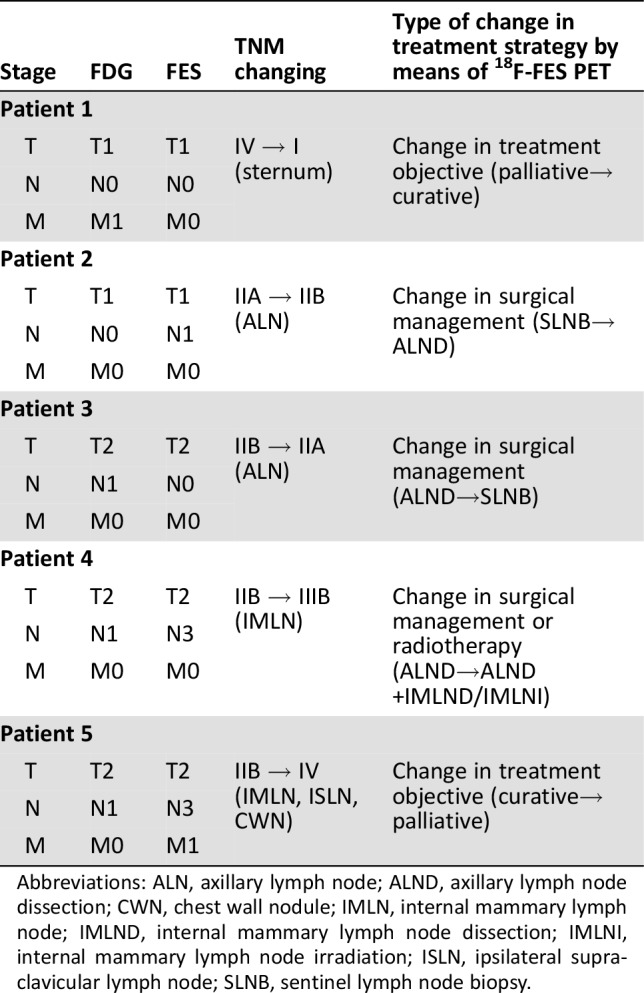
Abbreviations: ALN, axillary lymph node; ALND, axillary lymph node dissection; CWN, chest wall nodule; IMLN, internal mammary lymph node; IMLND, internal mammary lymph node dissection; IMLNI, internal mammary lymph node irradiation; ISLN, ipsilateral supraclavicular lymph node; SLNB, sentinel lymph node biopsy.
Referring Physicians and Questionnaires
Subsequent treatment of these patients was taken from the medical history as follows: (a) five patients received radical surgery, radiotherapy, adjuvant chemotherapy, and endocrine therapy; (b) two patients received surgery, adjuvant chemotherapy, and endocrine therapy; (c) two patients received palliative chemotherapy and endocrine therapy; and 4) nine patients received palliative endocrine therapy only. The management of one patient was not determined because of loss of follow‐up. Of the patients, 36.8% received radical surgery, 26.3% received adjuvant radiotherapy, 36.8% received adjuvant chemotherapy and adjuvant endocrine therapy, and 57.9% received palliative treatment (chemotherapy and/or endocrine therapy).
Thirty‐five different physicians were given the questionnaires referring to the treatment strategy of 19 patients with newly diagnosed ER‐positive breast cancer. Twenty‐seven of the physicians (including 12 surgeons and 15 oncologists) completed both questionnaires. Table 2 summarizes the impact of 18F‐FES PET/CT on intended management. Based on predefined standards, the intended management was changed in five patients (0.263; 95% CI, 0.045–0.481). Most physicians chose to change the therapeutic goal after 18F‐FES PET/CT in two cases (0.158; 95% CI, −0.023 to 0.338) and change surgical management in three cases (0.105; 95% CI, −0.047 to 0.257), as depicted in Table 3.
Table 3. The proportion of changes in management after 18F‐FES PET.
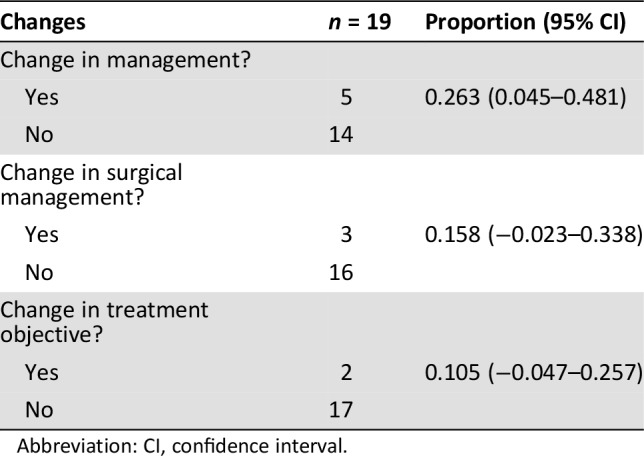
Abbreviation: CI, confidence interval.
As shown before, the implementation of 18F‐FES PET/CT changed the treatment strategy in five cases. The results of the 18F‐FES scans were further confirmed by postoperative pathology in three cases (Figs. 2, 3).
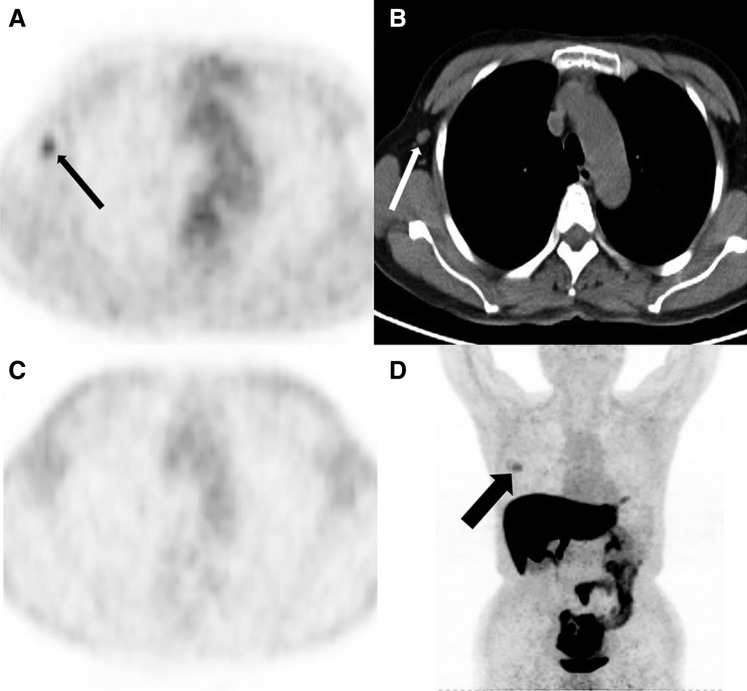
Figure 2.
A 49‐year‐old woman with newly diagnosed estrogen receptor‐positive breast cancer. (A): Axial 18F‐FDG positron emission tomography (PET) shows right axillary focal uptake (black thin arrow). (B): Axial computed tomography (CT) shows the right axillary lymph node on one level (white thin arrow). (C): At the same level, there is no focal uptake in the 18F‐FES PET. (D): 18F‐FES PET MIP showed breast mass uptake (black thick arrow) but no focal uptake in right axillary lymph node. The right axillary lymph node was not a metastasis as proven by pathology after operation.
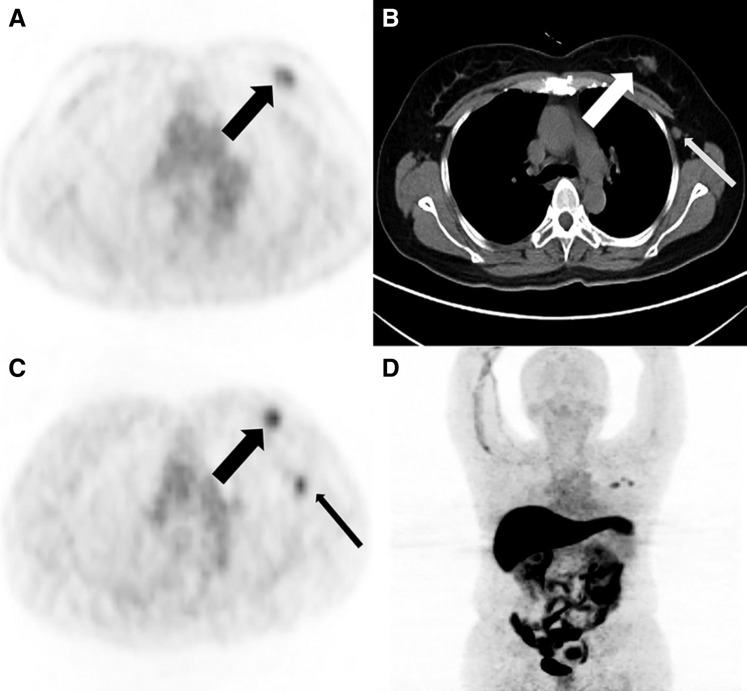
Figure 3.
A 54‐year‐old woman with newly diagnosed ER‐positive breast cancer. (A): Axial 18F‐FDG positron emission tomography (PET) show left breast mass uptake (black thick arrow). (B): Axial computed tomography (CT) shows a mass at the site of 18F‐FDG PET uptake, which is primary breast cancer (white thick arrow) and axillary lymph node (black thin arrow). (C): At the same level, 18F‐FES PET shows the mass (black thick arrow) and axillary lymph node (black thin arrow), considered to be estrogen receptor‐positive lesions. (D): 18F‐FES PET MIP demonstrates 18F‐FES avid focus in left breast mass and axillary lymph node. The left axillary lymph node contained metastases as proven by pathology after operation.
Among the 27 physicians, 15 (55.6%) found that the implementation of 18F‐FES PET/CT in addition to 18F‐FDG PET/CT added value to the decision making of the treatment strategy in patients with newly diagnosed ER positive breast cancer.
Discussion
To our knowledge, this is the first explorative study conducted to systematically evaluate the clinical value of 18F‐FES PET/CT and 18F‐FDG PET/CT and investigate whether and how 18F‐FES affects the implemented management of patients with newly diagnosed estrogen receptor‐positive breast cancer. Previous studies have successfully demonstrated that 18F‐FES PET/CT is a sensitive method to monitor regional estrogen binding in advanced and metastatic ER‐positive breast cancer [26] and validated that 18F‐FES uptake quantitation correlates well with ER expression measured by IHC [16], [18], [27]. Our previous study has confirmed this favorable statement as well [19].
Our retrospective review of 19 patients with newly diagnosed ER‐positive breast cancer demonstrates that 18F‐FES PET and 18F‐FDG PET scanning both showed high sensitivity in the detection of suspected lesions. 18F‐FES PET showed a higher sensitivity in the diagnosis of metastatic lesions than 18F‐FDG PET (90.8% vs. 82.8%, respectively). The most critical shortcoming of 18F‐FES PET/CT is that it cannot be reliably measured in liver metastases because of high background 18F‐FES uptake [21]. Considering this, we recalculated the sensitivity of both tracers without liver metastatic sites. 18F‐FES was slightly higher in the sensitivity of lesion diagnosis (90.8% to 91.9%). In the study by Gupta et al., different results were observed in 10 treatment‐naive patients with ER‐positive breast cancer, where higher sensitivities in 18F‐FDG PET compared with 18F‐FES PET were reported (92.21% vs. 75.32%, respectively) [25]. Notably, some lesions of ER‐positive characteristics were converted to ER‐negative phenotypes after treatment, and the heterogeneity of 18F‐FES uptake was higher in patients with recurrent or metastatic breast cancer than untreated patients [19], [28]. Because of the transformation of some lesions from ER positive to ER negative, 18F‐FES PET sensitivity was lower than 18F‐FDG PET in lesions diagnosis.
Distinguishing inflammatory lesions from malignant disease is notoriously difficult via 18F‐FDG PET/CT [29] because the lungs and MLNs are one of the main sites of inflammatory lesions and can give rise to 18F‐FDG false positive results. 18F‐FES PET/CT, with higher specificity for the recognition of ER positive lesions, can play a significant role in the identification of 18F‐FDG equivocal lesions [21]. In this study, seven lesions (MLNs) were found to be 18F‐FDG positive but 18F‐FES negative. Under the outcome of subsequent treatment and the character of CT image, we can conclude that these seven MLN lesions were 18F‐FDG false positive for disease. Confirming the presence of ER‐positive lesions, 71 out of 80 equivocal metastatic lesions on 18F‐FDG scans were shown to uptake 18F‐FES. Hence, 18F‐FES PET/CT showed a significant potential as a reference in some equivocal lesions detected via 18F‐FDG PET/CT.
Our results showed for the first time that 18F‐FES PET/CT could impact the management of newly diagnosed breast cancer. In the current study, the addition of 18F‐FES PET/CT changed the treatment strategy in 23.6% of the patients. A series of studies have demonstrated that 18F‐FDG PET/CT can correct the initial clinical stage of breast cancer [30], [31]. Ulaner et al. conducted a retrospective review of 238 patients with ER‐positive/HER2‐negative breast cancer and found that correct staging with 18F‐FDG PET/CT led to the detection of 32 patients (13.4%) with unforeseen distant metastases, mainly in initial clinical stage IIB and stage III patients [32]. Notably, our study was based on the results of 18F‐FDG PET/CT imaging to further analyze the potential role of 18F‐FES PET/CT in correct staging and management of patients with newly diagnosed breast cancer. In the current study, 18F‐FES PET/CT led to intended treatment management changes in 5 of 19 patients (0.263, 95% CI 0.045–0.481), which is a considerable probability of changing treatment decisions. We believe that the role of 18F‐FES PET/CT in changing staging and treatment management will be more prominent compared with other traditional imaging methods in newly diagnosed ER‐positive breast cancer. Further studies are needed to validate this point of view.
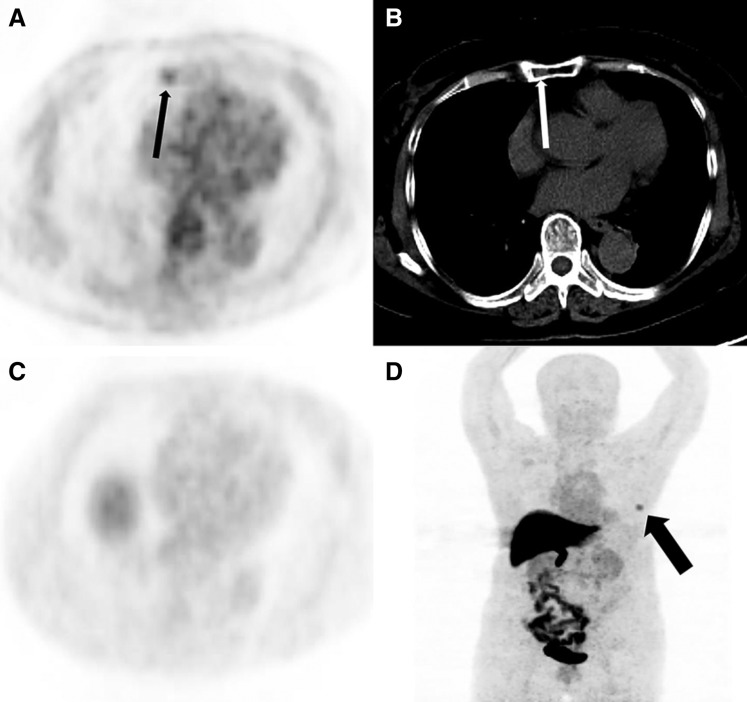
18F‐FES PET/CT that changed the course of operation was seen in almost half of the cases in operated patients. In one case, 18F‐FDG PET/CT showed one extra lesion not seen on 18F‐FES PET/CT (one solitary bone lesion in the sternum). Thus, 18F‐FES PET/CT downstaging a patient from stage IV to stage I lead to a management change from operation contraindications to operation indications and palliative to curative (Fig. 4). This change in therapeutic strategy was crucial to the patient's prognosis; however, this case has not been confirmed by pathology and is simply considered to be a hemangioma in accordance with the recent follow‐up imaging techniques including MRI and CT.
Figure 4.
A 71‐year‐old woman with newly diagnosed estrogen receptor‐positive breast cancer. (A): Axial 18F‐FDG positron emission tomography (PET) shows right manubrium focal uptake (black thin arrow). (B): Axial computed tomography (CT) show an inhomogeneous density of partial manubrium on one level (white thin arrow). (C and D): 18F‐FES PET has no uptake in the manubrium (black thick arrow shows breast mass uptake). The manubrium lesion was a considered to be a hemangioma during a recent follow‐up.
The value of FES is recognized by more than half of referring physicians based on the questionnaire of the present study (55.6%). The study has several limitations. First, the sample size was relatively modest because the population we studied had newly diagnosed breast cancer, whereas other studies of 18F‐FES mainly focused on recurrent or metastatic breast cancer. In addition, because of the resolution limitations of PET, small lesions may not show 18F‐FDG or 18F‐FES uptake, which could lead to an underestimation of the total number of lesions. Furthermore, the major drawback of 18F‐FES is its high liver physiological uptake, which makes it unable to detect and diagnose liver lesions. Finally, we did not have access to serial tumor biopsies, which could have contributed to a comprehensive comparison with PET/CT imaging.
Conclusion
18F‐FES PET/CT scanning can be helpful in the diagnosis and treatment management of newly diagnosed ER‐positive breast cancer, especially in patients with equivocal lesions on 18F‐FDG PET/CT scanning. The proper application of 18F‐FES PET/CT could optimize the individual treatment strategy by avoiding ineffective and excessive management.
Acknowledgments
The authors are grateful to questionnaire survey supports from Department of Breast Surgery and Department of Medical Oncology from Fudan University Shanghai Cancer Center. Additionally, we would like to thank data analysis supports from biostatistician Miao Mo at Department of Cancer Prevention, Fudan University Shanghai Cancer Center.
This research is supported by the Shanghai Committee of Science and Technology Fund (No. 19ZR1411300), the Shanghai Engineering Research Center of Molecular Imaging Probes Program (No. 19DZ2282200) and the National Natural Science Foundation of China (No. 81874114). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Contributed equally.
Contributor Information
Biyun Wang, Email: pro_wangbiyun@163.com.
Zhongyi Yang, Email: yangzhongyi21@163.com.
Author Contributions
Conception/design: Cheng Liu, Chengcheng Gong, Biyun Wang Zhongyi Yang,
Provision of study material or patients: Yingjian Zhang, Biyun Wang, Zhongyi Yang
Collection and/or assembly of data: Cheng Liu, Chengcheng Gong, Shuai Liu, Yingjian Zhang, Yongping Zhang, Xiaoping Xu, Huiyu Yuan
Data analysis and interpretation: Cheng Liu, Chengcheng Gong
Manuscript writing: Cheng Liu, Chengcheng Gong, Biyun Wang, Zhongyi Yang
Final approval of manuscript: Cheng Liu, Chengcheng Gong, Shuai Liu, Yingjian Zhang, Yongping Zhang, Xiaoping Xu, Huiyu Yuan, Biyun Wang, Zhongyi Yang
Disclosures
The authors indicated no financial relationships.
References
- 1.Torre LA, Islami F, Siegel RL et al. Global cancer in women: Burden and trends. Cancer Epidemiol Biomarkers Prev 2017;26:444–457. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 3.Kim I, Choi HJ, Ryu JM et al. Prognostic validation of the American Joint Committee on Cancer 8th staging system in 24,014 Korean patients with breast cancer. J Breast Cancer 2018;21:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czernin J, Allen‐Auerbach M, Nathanson D et al. PET/CT in oncology: Current status and perspectives. Curr Radiol Rep 2013;1:177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avril S, Muzic RF, Jr., Plecha D et al. 18F‐FDG PET/CT for monitoring of treatment response in breast cancer. J Nucl Med 2016;57(suppl 1):34S‐39S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riedl CC, Pinker K, Ulaner GA et al. Comparison of FDG‐PET/CT and contrast‐enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur J Nucl Med Mol Imaging 2017;44:1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitajima K, Miyoshi Y. Present and future role of FDG‐PET/CT imaging in the management of breast cancer. Jpn J Radiol 2016;34:167–180. [DOI] [PubMed] [Google Scholar]
- 8.Segaert I, Mottaghy F, Ceyssens S et al. Additional value of PET‐CT in staging of clinical stage IIB and III breast cancer. Breast J 2010;16:617–624. [DOI] [PubMed] [Google Scholar]
- 9.Nakai T, Okuyama C, Kubota T et al. Pitfalls of FDG‐PET for the diagnosis of osteoblastic bone metastases in patients with breast cancer. Eur J Nucl Med Mol Imaging 2005;32:1253–1258. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z, Yi YL, Liu Y et al. Comparison of whole‐body PET/PET‐CT and conventional imaging procedures for distant metastasis staging in patients with breast cancer: A meta‐analysis. Eur J Gynaecol Oncol 2015;36:672–676. [PubMed] [Google Scholar]
- 11.Ugurluer G, Kibar M, Yavuz S et al. False positive 18F‐FDG uptake in mediastinal lymph nodes detected with positron emission tomography in breast cancer: A case report. Case Rep Med 2013;2013:459753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ataergin S, Arslan N, Ozet A et al. Abnormal 18F‐FDG uptake detected with positron emission tomography in a patient with breast cancer: A case of sarcoidosis and review of the literature. Case Rep Med 2009;2009:785047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rugo HS, Rumble RB, Macrae E et al. Endocrine therapy for hormone receptor‐positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol 2016;34:3069–3103. [DOI] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) ; Davies C, Godwin J, Gray R et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient‐level meta‐analysis of randomised trials. Lancet 2011;378:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hospers GA, Helmond FA, de Vries EG et al. PET imaging of steroid receptor expression in breast and prostate cancer. Curr Pharm Des 2008;14:3020–3032. [DOI] [PubMed] [Google Scholar]
- 16.Peterson LM, Mankoff DA, Lawton T et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F‐fluoroestradiol. J Nucl Med 2008;49:367–374. [DOI] [PubMed] [Google Scholar]
- 17.Dehdashti F, Mortimer JE, Siegel BA et al. Positron tomographic assessment of estrogen receptors in breast cancer: Comparison with FDG‐PET and in vitro receptor assays. J Nucl Med 1995;36:1766–1774. [PubMed] [Google Scholar]
- 18.Gemignani ML, Patil S, Seshan VE et al. Feasibility and predictability of perioperative pet and estrogen receptor ligand in patients with invasive breast cancer. J Nucl Med 2013;54:1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z, Sun Y, Xu X et al. The assessment of estrogen receptor status and its intratumoral heterogeneity in patients with breast cancer by using 18F‐fluoroestradiol PET/CT. Clin Nucl Med 2017;42:421–427. [DOI] [PubMed] [Google Scholar]
- 20.Kurland BF, Peterson LM, Lee JH et al. Estrogen receptor binding (18F‐FES PET) and glycolytic activity (18F‐FDG PET) predict progression‐free survival on endocrine therapy in patients with ER+ breast cancer. Clin Cancer Res 2017;23:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao GJ, Clark AS, Schubert EK et al. 18F‐fluoroestradiol PET: Current status and potential future clinical applications. J Nucl Med 2016;57:1269–1275. [DOI] [PubMed] [Google Scholar]
- 22.van Kruchten M, de Vries EG, Glaudemans AW et al. Measuring residual estrogen receptor availability during fulvestrant therapy in patients with metastatic breast cancer. Cancer Discov 2015;5:72‐81. [DOI] [PubMed] [Google Scholar]
- 23.Mori T, Kasamatsu S, Mosdzianowski C et al. Automatic synthesis of 16 alpha‐[(18)F]fluoro‐17beta‐estradiol using a cassette‐type [(18)F]fluorodeoxyglucose synthesizer. Nucl Med Biol 2006;33:281–286. [DOI] [PubMed] [Google Scholar]
- 24.Linden HM, Kurland BF, Peterson LM et al. Fluoroestradiol positron emission tomography reveals differences in pharmacodynamics of aromatase inhibitors, tamoxifen, and fulvestrant in patients with metastatic breast cancer. Clin Cancer Res 2011;17:4799–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta M, Datta A, Choudhury PS et al. Can (18)F‐fluoroestradiol positron emission tomography become a new imaging standard in the estrogen receptor‐positive breast cancer patient: A prospective comparative study with (18)F‐fluorodeoxyglucose positron emission tomography? World J Nucl Med 2017;16:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Kruchten M, de Vries EGE, Brown M et al. PET imaging of oestrogen receptors in patients with breast cancer. Lancet Oncol 2013;14:e465–e475. [DOI] [PubMed] [Google Scholar]
- 27.Evangelista L, Guarneri V, Conte PF. 18F‐fluoroestradiol positron emission tomography in breast cancer patients: Systematic review of the literature & meta‐analysis. Curr Radiopharm 2016;9:244–257. [DOI] [PubMed] [Google Scholar]
- 28.van Kruchten M, Glaudemans AW, de Vries EF et al. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med 2012;53:182–190. [DOI] [PubMed] [Google Scholar]
- 29.Ergonul AG, Akcam TI, Özdil A et al. Diagnostic value of 18F‐FDG‐PET/CT in benign lung diseases. Kardiochir Torakochirurgia Pol 2018;15:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulaner GA, Castillo R, Goldman DA et al. (18)F‐FDG‐PET/CT for systemic staging of newly diagnosed triple‐negative breast cancer. Eur J Nucl Med Mol Imaging 2016;43:1937–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groheux D, Hindié E, Delord M et al. Prognostic impact of (18)FDG‐PET‐CT findings in clinical stage III and IIB breast cancer. J Natl Cancer Inst 2012;104:1879–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulaner GA, Castillo R, Wills J et al. 18F‐FDG‐PET/CT for systemic staging of patients with newly diagnosed ER‐positive and HER2‐positive breast cancer. Eur J Nucl Med Mol Imaging 2017;44:1420–1427. [DOI] [PubMed] [Google Scholar]