Considering the current impetus to deliver molecularly targeted treatments for cancer, a better understanding of the therapeutic potential of ERBB2 mutations is needed. This article reviews the distribution of ERBB2 mutations in different tumor types, focusing on potential as a novel biomarker.
Keywords: HER2, Gastrointestinal cancer, Tyrosine kinase, ERBB2 mutation, Non‐small cell lung cancer
Abstract
The oncogenic role ERBB2 amplification is well established in breast and gastric cancers. This has led to the development of a well‐known portfolio of monoclonal antibodies and kinase inhibitors targeting the ERBB2 kinase. More recently, activating mutations in the ERBB2 gene have been increasingly reported in multiple solid cancers and were shown to play an oncogenic role similar to that of ERBB2 amplification. Thus, ERBB2 mutations define a distinct molecular subtype of solid tumors and serve as actionable targets. However, efforts to target ERBB2 mutation has met with limited clinical success, possibly because of their low frequency, inadequate understanding of the biological activity of these mutations, and difficulty in separating the drivers from the passenger mutations. Given the current impetus to deliver molecularly targeted treatments for cancer, there is an important need to understand the therapeutic potential of ERBB2 mutations. Here we review the distribution of ERBB2 mutations in different tumor types, their potential as a novel biomarker that defines new subsets in many cancers, and current data on preclinical and clinical efforts to target these mutations.
Implications for Practice.
A current trend in oncology is to identify novel genomic drivers of solid tumors and developing precision treatments that target them. ERBB2 amplification is an established therapeutic target in breast and gastric cancers, but efforts to translate this finding to other solid tumors with ERBB2 amplification have not been effective. Recently the focus has turned to targeting activating ERBB2 mutations. The year 2018 marked an important milestone in establishing ERBB2 mutation as an important actionable target in multiple cancer types. There have been several recent preclinical and clinical studies evaluating ERBB2 mutation as a therapeutic target with varying success. With increasing access to next‐generation sequencing technologies in the clinic, oncologists are frequently identifying activating ERBB2 mutations in patients with cancer. There is a significant need both from the clinician and bench scientist perspectives to understand the current state of affairs for ERBB2 mutations.
Introduction
ERBB2 is a receptor tyrosine kinase that belongs to the EGFR/ERBB/HER family of kinases. Of the four ERBB receptors, ERBB2/HER2 lacks a known ligand, and ERBB3/HER3 lacks kinase activity [1]. Importantly, ERBB3 is the preferred dimerization partner of ERBB2, and together they form the strongest signaling unit among ERBB complexes [1]. The members of ERBB family of kinases were shown to form both homodimers and heterodimers with each other, resulting in the activation of crucial cell signaling pathways, including the PI3K‐AKT pathway [1]. ERBB3 (but not ERBB2) interacts directly with PI3K, and thus the PI3K‐AKT pathway is activated indirectly by ERBB2 through its interaction with ERBB3 [1]. Thus, it is not surprising that the activation of ERBB receptors, because of either protein overexpression or mutation, are implicated in multiple cancers. Mutations in the ERBB2 kinase have been reported in several solid cancers [2], prompting us to evaluate its role in tumorigenesis. Interestingly, ERBB2 amplification and ERBB2 mutation are mostly (in nearly 80%–90% of cases) mutually exclusive and thus represent independent driver events in tumorigenesis [2], [3], [4], [5]. Together, ERBB2 gene amplification and mutation form a sizeable molecular subtype in several cancers and are potential actionable events. Because several ERBB2 kinase inhibitors are already clinically available, it is now important to comprehensively review the available evidence regarding the functional role of ERBB2 mutations in tumorigenicity and drug sensitivity.
Frequency of Mutations and Co‐Occurring Alterations
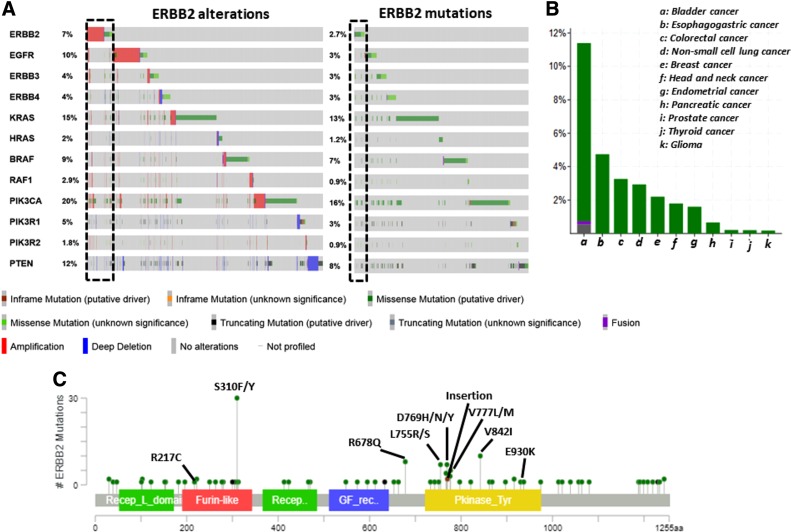
Mutations in the ERBB2 receptor were reported in the extracellular domain (ECD), transmembrane domain (TMD), juxtamembrane domain (JMD), and the intracellular kinase domain (KD). Whereas missense substitutions contribute to approximately 70% of total ERBB2 mutations present in the COSMIC database, insertions and deletions account for nearly 19% and 1.5%, respectively. The frequency of ERBB2 mutation in clinical samples ranges between 0.2% (glioma) to 12.6% (bladder cancer) depending on the cancer type (Table 1). An overall ERBB2 mutation frequency of 3% was reported in a pancancer study (MSK‐IMPACT) involving more than 10,000 tumor samples [6]. An incidence of ERBB2 mutations at a frequency of 3.5% across 400 cancer types was recently reported in a large‐scale targeted exome analysis of 111,176 tumors [7]. A similar ERBB2 mutation frequency of 3.1% was reported in a study that analyzed more than 900 cancer cell lines [8]. Notably, a cumulative somatic mutation frequency of approximately 2.6% was derived from 5,151 samples of multiple cancer types belonging to 14 studies [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22] from cBioPortal (Fig. 1A) [23], [24]. Overall, there is considerable variability in the frequency of ERBB2 mutations in different tumor types (Fig. 1B); most of the common ERBB2 mutations were expressed in tumor samples (Fig. 1C) indicating that they are oncogenic drivers in multiple cancer types.
Table 1. ERBB2 mutation frequencies in solid cancers.

Frequencies from studies that involved highest number of samples for a particular cancer (studies with less than 100 samples were excluded) were collected and shown along with references. Data collected from cBioPortal.
Abbreviations: Broad, Broad Institute of Harvard and Massachustts Institute of Technology; DFCI, Dana‐Farber Cancer Institute; Inserm, Institut national de la santé et de la recherche médicale; MSK, Memorial Sloan Kettering; MSKCC, Memorial Sloan Kettering Cancer Center; QCMG, Queensland Centre for Medical Genomics; TCGA, The Cancer Genome Atlas.
Figure 1.
Frequency of ERBB2 mutations from select studies. (A): Oncoplot of mutations plus copy number alterations of key genes involved in the ERBB2 pathway (left) and mutations only oncoplot of the same gene list (right) in tumor samples across cancer types for which both DNA and RNA sequencing was available in cBioPortal. Frequency of ERBB2 mutations and other key ERBB2 pathway mutations are schematically illustrated. (B): ERBB2 mutation frequency in pooled data of different tumor types from 14 next‐generation sequencing studies is shown. (C): Frequency of ERBB2 mutations whose expression was confirmed from RNAseq is schematically represented.
Interestingly, there is also variability in the distribution of the types of ERBB2 mutation (missense, nonsense, or indels) depending on the specific cancer type. Based on the distribution, ERBB2 mutations can be referred to as either “common” or “uncommon” within a cancer type. According to the type of mutation, common ERBB2 mutations (Fig. 1C) can be broadly grouped into three classes: class I, point mutations in the ECD (R217C and S310F/Y), TMD, and JMD (R677Q); class II, insertion mutation in the KD (A775_G776insYVMA); and class III, point mutations in the KD (Fig. 2A). The distribution of different mutation classes varies across tumor types (Fig. 2A). Analysis of the COSMIC database revealed that class I mutations are predominant in liver, pancreatic, cervix, urinary tract, and skin cancers; class II mutations in lung and ovarian cancers; and class III mutations in multiple cancers (Fig. 2A). Among class III ERBB2 mutations, D769Y is frequent in esophageal and salivary gland cancers (Fig. 2A). L755S was reported mainly in breast cancers and cancers of small intestine (Fig. 2A). V777L was predominant in breast, pancreas, salivary gland, and liver cancers (Fig. 2A). V842I was reported in cancers of salivary gland, stomach, pancreas, small intestine, large intestine, ovary, and endometrium (Fig. 2A). The remaining class III mutations were specific to a single cancer type: H878Y (liver), E930D (prostate), and F1030C (liver; Fig. 2A). In addition to ECD and JMD mutations, class I mutations also include certain uncommon mutations that span the TMD such as V659E and G660D [25].
Figure 2.
Schematic representation of the frequency of mutation type among ERBB2‐mutated cancers. (A): Bubble size represents the frequency of each mutation (shown on the x‐axis along with the exon) within each cancer type (y‐axis). The approximate percentage of each ERBB2 mutation type among the total ERBB2‐mutated samples within a cancer type is shown inside the bubble. Smaller bubble sizes indicate less than 5% frequency. The bubbles were color‐coded according to their drug sensitivity based on the MANO study [36]. Green, sensitive to five inhibitors lapatinib, sapitinib, afatinib, neratinib, and osimertinib; red, resistant to all the five inhibitors; blue, lapatinib resistance; gray, sapitinib resistance; black, data not available. The classification of ERBB2 mutations based on the location of ERBB2 receptor and the type of mutation is shown below (blue). (B): The preclinical activity (drug sensitivity) of different mutations toward ERBB2 inhibitors is shown. The table is color‐coded according to the drug sensitivity based on the MANO study [36]: green, sensitive; yellow, intermediate resistance; red, highly resistant.
Abbreviations: ECD, extracellular domain; ins, insertion; JMD, juxtamembrane domain; KD, kinase domain; TMD, transmembrane domain.
Large‐scale comprehensive next‐generation sequencing studies also allows for the identification of co‐occurring and mutually exclusive alterations with ERBB2 mutations. Based on 14 studies that reported both next‐generation DNA and RNA sequencing data from a variety of solid tumors (n = 5,151) [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22] on cBioPortal [23], [24], ERBB2 alterations were found to display significant (p < .001) co‐occurrence with ERBB3, RAF1, PIK3CA, and PIK3R2 alterations but mutual exclusivity with KRAS alterations (Fig. 1A). These findings indicate that both the MAPK and PI3K pathways play a significant role in ERBB2 mutation‐mediated cell transformation and tumorigenesis. Co‐occurrence of ERBB3 alterations also highlights its cooperative role with ERBB2 mutations [1].
In Vitro Activity of ERBB2 Mutations
Overexpression of wild‐type ERBB2 kinase was shown to cause transformation of multiple cell types [26], [27], [28] and induce tumors in mouse models of cancer [29], [30]. Thus, ERBB2 amplification in breast cancer was considered an important oncogenic event leading to the classification of ERBB2‐positive breast cancers as a distinct molecular subtype. Following the identification of ERBB2 mutations in multiple solid cancers, the question, however, remained over their oncogenic nature akin to ERBB2 amplification. The first evidence was presented for the lung cancer‐specific exon 20 insertion mutation, which displayed constitutive kinase activity and tumorigenicity [31], [32]. Subsequent reports demonstrated the oncogenicity of several ERBB2 kinase mutants in murine Ba/F3 and NMuMg cell lines [33], [34]. Interestingly, variability in transformation potential was observed among ERBB2 point mutants of the kinase domain [34], [35], [36]. Notably, the lapatinib‐resistant point mutants were shown to stabilize the active conformation of kinase and displayed enhanced transformation ability compared with that of the wild‐type kinase [34]. However, certain less frequent ERBB2 mutations failed to confer transformation ability in cell lines, suggesting that not all mutants are activating in nature and may represent passenger mutations [35]. These studies, together with the fact that the ERBB2 amplification and mutation are mutually exclusive, indicate that a majority of ERBB2 mutations that are reported in solid cancers are oncogenic in nature, thus making them an actionable target. Targeting the activity of ERBB2 kinase is possible by treatment either with monoclonal antibodies or with kinase inhibitors. Anti‐ERBB2 antibodies such as trastuzumab or pertuzumab bind to the extracellular region of the protein, thereby blocking receptor dimerization and enzymatic activation of the kinase. Small molecule inhibitors bind the intracellular kinase domain of the ERBB2 receptor, thus blocking oncogenic activity and downstream signaling.
Preclinical Activity of Anti‐ERBB2 Monoclonal Antibodies
Receptor dimerization was shown to be important both for ERBB2 autokinase activity [37] and activation of the kinase‐defective ERBB3 protein [38]. Treatment with monoclonal antibodies blocks receptor dimerization, leading to the inhibition of downstream signaling and proliferation in cells that overexpress wild‐type ERBB2 kinase. It is thus important to know if mutant ERBB2 kinases also respond similarly to trastuzumab. A recent preclinical study reported varied responses to trastuzumab among common ERBB2 mutants [36]. Class I mutants are sensitive, whereas class II mutants and a subset of class III mutants (L755S, D769Y, V777L, and V842I) displayed trastuzumab resistance (Fig. 2B). In this study, Ba/F3 cells that overexpressed ERBB2 mutants were treated with trastuzumab [36]. In light of these observations, the efficacy of trastuzumab can thus be predicted for a small subset of patients with combined ERBB2 amplification and mutation. In agreement with this prediction, trastuzumab treatment was reported to cause good clinical response in ERBB2‐mutant tumors that also displayed ERBB2 amplification [39], [40], [41]. However, the question that remained unanswered is about the efficacy of trastuzumab toward cells that express ERBB2 mutants at endogenous levels analogous to that observed in the majority of tumor samples.
It is also important to note that the tumor cells may acquire ERBB2 amplification during the course of disease progression, and if the ERBB2 mutant allele is amplified, this may result in the acquired susceptibility or resistance to trastuzumab treatment based on the type of ERBB2 mutation.
It is also important to note that the tumor cells may acquire ERBB2 amplification during the course of disease progression [42], [43], and if the ERBB2 mutant allele is amplified, this may result in the acquired susceptibility or resistance to trastuzumab treatment based on the type of ERBB2 mutation. In addition to cell‐intrinsic factors such as ERBB2 amplification, the efficacy of trastuzumab was also attributed to antibody‐mediated cell‐mediated cytotoxicity (ADCC) as observed in cells that expressed low levels of ERBB2 protein [44]. This is an encouraging observation as it raises the possibility of treating patients who carry an ERBB2 mutation that was shown to be trastuzumab resistant in preclinical assays. Thus, combining trastuzumab (that may activate ADCC) with an ERBB2 kinase inhibitor may result in a superior treatment outcome. Consistent with this hypothesis, a combination of lapatinib and trastuzumab was shown to successfully treat a patient with non‐small cell lung cancer (NSCLC) with a type II insertion ERBB2 mutation [45]. Similarly, trastuzumab was effective in treating ERBB2 nonamplified breast cancer that harbored the class I S310F mutation [46].
Preclinical Activity of Anti‐ERBB2 Small Molecule Kinase Inhibitors
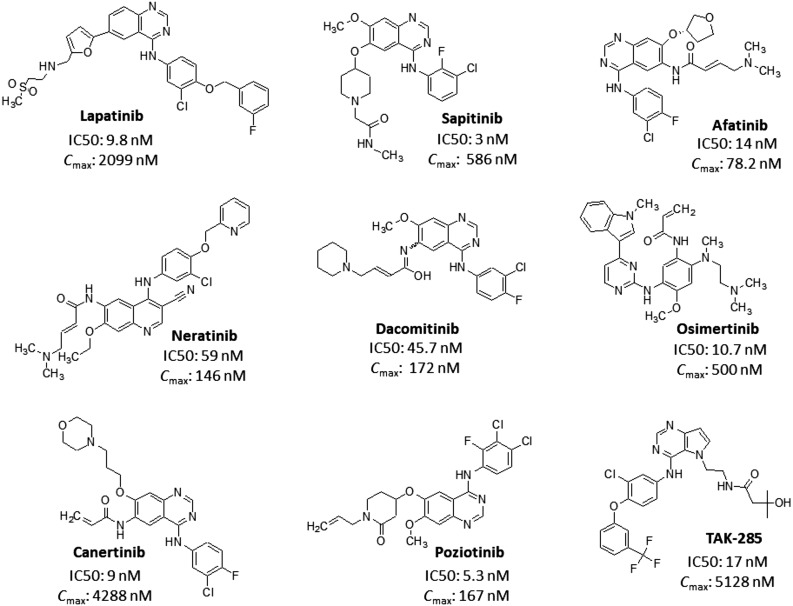
Several ERBB2 inhibitors (Fig. 3) were developed and reported to have activity against wild‐type and mutant ERBB2 kinase [36]. Almost all the inhibitors displayed ERBB2 inhibitory activity (IC50) toward wild‐type ERBB2 kinase well within the maximum achievable serum concentrations (Fig. 3) [47], [48], [49], [50], [51], [52], [53], [54], [55]. Lapatinib (Tykerb) is a dual EGFR/ERBB2 inhibitor (ERBB2 IC50, 9.8 nM; EGFR IC50, 10.2 nM) [56], approved for the treatment of metastatic breast cancer. Sapitinib is a reversible pan‐ERBB kinase inhibitor (EGFR IC50, 4 nM; ERBB2 IC50, 3 nM; and ERBB3 IC50, 4 nM) [57], which is undergoing clinical trials for metastatic breast cancer, NSCLC, and colorectal cancer. Afatinib (Gilotrif) is a second‐generation irreversible inhibitor of EGFR (IC50, 0.5 nM) and ERBB2 (IC50, 14 nM) kinases [58] that was approved for the treatment of NSCLC. Neratinib (Nerlynx), also a second‐generation irreversible inhibitor of EGFR (IC50, 92 nM) and ERBB2 (IC50, 59 nM) kinases [59], was approved for the treatment of ERBB2‐positive breast cancer. Dacomitinib is a second‐generation pan‐ERBB irreversible inhibitor (EGFR IC50, 6 nM; ERBB2 IC50, 45.7 nM; and ERBB4 IC50, 73.7 nM) [60] and an investigational drug in the treatment of NSCLC. Osimertinib (Tagrisso/Tagrix) is a third‐generation kinase inhibitor approved for the treatment of EGFR TK mutation‐positive NSCLC. Canertinib is a second‐generation, experimental, irreversible inhibitor of EGFR (IC50, 1.5 nM) and ERBB2 (IC50, 9 nM) kinases with potent activity against esophageal cancer [61]. Poziotinib is a third‐generation, irreversible, pan‐ERBB inhibitor (EGFR IC50, 3.2 nM; ERBB2 IC50, 5.3 nM; and ERBB4 IC50, 23.5 nM) that is being tested in various clinical trials [62]. TAK‐285 is an investigational irreversible pan‐ERBB inhibitor (EGFR IC50, 23 nM; ERBB2 IC50, 17 nM; and ERBB4 IC50, 260 nM) [63].
Figure 3.
ERBB2 inhibitors. Schematic representation of selective ERBB2 as well as pan‐ERBB inhibitors along with the IC50 value for ERBB2 kinase and maximum achievable serum concentration.
Abbreviations: Cmax, maximum achievable serum concentration; IC50, inhibitory activity.
Initial in vitro studies reported differential lapatinib sensitivity among ERBB2 mutations; L755S/P (exon 19) [34] and A775_G776insYVMA (exon 20) [35] were reported to cause lapatinib resistance. Both L755S and A775_G776insYVMA were also shown to be cross‐resistant to irreversible inhibitors neratinib [64], [65] and afatinib [65], [66]. A recent in vitro study demonstrated that the class I ECD mutations are sensitive to all the tested inhibitors but that the class II insertion mutation displayed a variable degree of resistance toward ERBB2 inhibitors (Fig. 2B) [36]. Importantly, osimertinib displayed activity toward all class I and class III mutants, including L755S, but remained ineffective toward the class II exon‐20 insertion mutant (Fig. 2B) [36]. However, it is interesting to note that the second‐generation EGFR inhibitor poziotinib displayed significant activity against the class II insertion mutation with several‐fold greater potency than afatinib and osimertinib [67]. Thus, it is now possible to inhibit the activity of all the common ERBB2 mutants that were reported in solid cancers. In spite of the promising activity of ERBB2 inhibitors, it is still possible that drug‐resistant mutations may arise following treatment, as has been predicted using in vitro screening systems [34], [68]. But the current availability of activity profiles for a large panel of ERBB2 mutants toward a battery of kinase inhibitors will be very useful for a physician to choose alternate inhibitors that may overcome possible drug resistance.
Resistance to ERBB2 Inhibitors
In addition to the primary ERBB2 mutation, several other factors may influence the clinical response to kinase inhibitors (Fig. 4). Multiple resistance mechanisms were reported in preclinical models that expressed wild‐type ERBB2 kinase. Emergence of secondary mutations in the kinase domain that abrogate inhibitor binding to the ERBB2 kinase has already been reported. For example, ERBB2‐L755S emerged as drug‐resistant mutation after ERBB2‐targeted therapy [69]. Other mechanisms include overexpression of receptors that are dimeric partners of ERBB2 kinase (EGFR and ERBB3) [70], [71] and activation of downstream signaling pathways because of mutations [72]. ERBB2‐ERBB3 dimers are the strongest signaling complexes that lead to the activation of PI3K‐AKT pathway (Fig. 4). Interestingly, disruption of heterodimers alone by using trastuzumab was not sufficient to exert antiproliferative properties if the PI3K‐AKT pathway was active because of the mutant PI3K kinase; a combination of trastuzumab with either PI3K or mTOR inhibitors proved to be effective [73], [74], [75]. In preclinical studies, mutant PI3K was shown to confer an aggressive phenotype and therapeutic resistance in breast cancer models with ERBB2 amplification [76]. Clinically also, loss of PTEN or mutations in PI3K is strongly associated with resistance to ERBB2 blockers [77], [78], [79]. Coamplification of additional genes such as Brk tyrosine kinase [80] and RAR‐α [81], induction of Bim together with the downregulation of survivin [82], enhanced production of heregulin [83], and activation of the MET kinase [84] were some of the mechanisms reported to cause lapatinib resistance in cells that expressed wild‐type ERBB2 kinase. Thus, it is important to monitor if the above‐mentioned ERBB2‐independent mechanisms also emerge in ERBB2‐mutant cancer models following the treatment with ERBB2 inhibitors.
Figure 4.
ERBB2 signaling depicting actionable targets. Molecular mechanisms underlying resistance toward ERBB2 kinase inhibitors are schematically shown. Homo‐ and heterodimers involving ERBB2 are shown along with ERBB2 blockers (antibodies and inhibitors in green). Other actionable drug targets whose activity can be blocked to overcome drug resistance along with the examples of their available inhibitors are also shown. The PI3K‐AKT pathway, which is prominent in resistance toward ERBB2 blockers, is highlighted in red.
Several strategies were tested to overcome trastuzumab or lapatinib resistance. Dacomitinib was shown to overcome de novo resistance toward trastuzumab as well as acquired resistance to lapatinib [85]. Osimertinib was shown to overcome inhibitor cross‐resistance displayed by the L755S mutation [36]. Importantly, wild‐type ERBB2 kinase is a client protein for HSP90 chaperone, and the activity of HSP90 is essential for ERBB2 stability and activity [86]. Inhibition of HSP90 leads to the degradation of ERBB2 kinase and consequently results in tumor cell death. HSP90 inhibition was shown to overcome trastuzumab‐resistant breast and gastric cancer cells with ERBB2 amplification [87].
Clinical Activity of Anti‐ERBB2 Agents Against ERBB2 Mutant Cancers
ERBB2 amplification is a well‐established therapeutic target in the treatment of breast and gastroesophageal junction cancers. Monoclonal antibodies are the primary agents for targeting ERBB2 amplification in these tumors. Small molecule tyrosine kinase inhibitors have also shown activity in this tumor population. Early efforts to target ERBB2‐amplified other solid tumors using chemotherapy plus trastuzumab have not been effective [88], [89]. More recently the focus is shifting toward targeting activating ERBB2 mutations in other solid tumors.
Monoclonal Antibodies.
Monoclonal antibodies in combination with chemotherapy have shown activity in ERBB2‐mutated NSCLC [39], [90]. The EUHER2 study is a retrospective analysis of anti‐ERBB2 directed therapies in ERBB2 exon 20 mutation‐positive NSCLC (Table 2). The study enrolled 101 patients, and 58 received monoclonal antibodies targeting ERBB2 kinase. All of them received trastuzumab in combination with cytotoxic chemotherapy except for three patients receiving single agent trastuzumab, and one patient received ado‐trastuzumab emtansine or T‐DM1. The overall response rate was 51%, and a progression‐free survival of 4.8 months was observed in this group [91]. At present there are no ongoing trials evaluating trastuzumab in combination with chemotherapy in patients with advanced NSCLC. However, the combination of trastuzumab and pertuzumab is being evaluated in ERBB2‐overexpressed, ‐amplified, or ‐mutated tumors in the My Pathway basket trial [92]. Preliminary data have shown activity and safety of this combination in heavily pretreated colorectal (n = 34) and biliary cancers (n = 8), with overall response rates of 37% and 50%, respectively [93], [94]. Margetuximab is also a monoclonal antibody targeting ERBB2 that is engineered for increased Fc‐domain binding affinity to both low affinity variants of the activating Fcγ receptor, CD16 [95]. It has shown activity in solid tumors, but at present it is primarily being evaluated in advanced breast and gastric carcinomas.
Table 2. Studies reporting therapeutic outcome with ERBB2 kinase inhibitors.
One patient received T‐DM1.
Includes exon 20 insertions and point mutations in all major domains of ERBB2.
Includes lung, endometrial, salivary, biliary, ovarian, bladder, colorectal, and other cancers.
ERBB2 exon 20 mutations.
Abbreviations: mAb, monoclonal antibody; NSCLC, non‐small cell lung cancer; TKI, tyrosine kinase inhibitor.
Antibody Drug Conjugates.
Preliminary data from another ongoing trial with 18 patients have shown clinical activity with T‐DM1 in ERBB2‐mutated NSCLC with an overall response rate of 44% and median progression‐free survival of 5 months (Table 2) [96]. In this study ERBB2 expression by immunohistochemistry (IHC) did not predict for response to T‐DM1 [97]. ERBB3 overexpression was detected in seven (39%) patients, and five of those patients had a partial response to T‐DM1. These findings suggest that ERBB3 expression may predict for response to T‐DM1 in this population, although further confirmation is required. Newer ERBB2‐directed monoclonal antibodies undergoing evaluation include trastuzumab‐deruxtecan (DS‐8201a), an ERBB2 targeted antibody drug conjugate with a novel topoisomerase I inhibitor [98]. In a phase I trial, DS‐8201a showed activity in a cohort of ERBB2‐expressing (IHC ≥1+) solid tumors with a reported response rate of 36.4% (8 of 22 patients). Subsequent expansion of the NSCLC cohort (n = 18) reported confirmed response rate of 58.8% (10 of 17 patients). The median duration of response was 9.9 months, and grade 3 or higher toxicities were reported in 25% of patients with one patient death because of interstitial lung disease (Table 2) [99]. The response rate in patients with confirmed ERBB2 mutations was 72.7% (8 of 11 patients), and median duration of response was 11.9 months.
In both studies ERBB2 overexpression by IHC did not predict for treatment response. Tumor response to T‐DM1 and DS‐8201a was seen across different ERBB2 mutation subtypes, including exon 20 insertion and missense mutations. The presence of ERBB2 mutations was not necessarily associated with ERBB2 overexpression and/or amplification. These preliminary findings raise an interesting question of how antibody drug conjugates target ERBB2 mutated tumors. Further studies are required to answer this question, and the ongoing phase II expansion trial of DS‐8201a may provide some answers.
Tyrosine Kinase Inhibitors.
The EUHER2 cohort included 29 patients who were treated with one of the tyrosine kinase inhibitors: neratinib, lapatinib, or afatinib. The overall response rate was 7.4% with median progression‐free survival of 3.4 months (Table 2) [91]. In another cohort of 27 patients with advanced ERBB2‐mutant lung cancers, treatment with afatinib was associated with a response rate of 13% (Table 2) [100]. Of the 27 patients, 16 had the exon 20 YVMA in‐frame insertion, five other exon 20 in‐frame insertions, three missense mutations in exons 8 and 17, and one single nucleotide polymorphism. All three responders had in‐frame insertion in exon 20, and two of the three had the YVMA insertion. Durable responses with afatinib treatment were reported in isolated cases of lung cancer with exon 20 insertion mutations [65], [101], [102], [103], [104]. An objective response rate of 19% was achieved with afatinib in a cohort of 28 heavily pretreated ERBB2 mutation‐positive patients [105]. The median time to treatment failure was 2.9 months for the entire cohort; however, patients with exon 20 p.A775_G776insYVMA mutations (n = 10) had a response rate of 33% and median time to treatment failure of 9.6 months.
Dacomitinib is an irreversible ERBB1, ERBB2, and ERBB4 inhibitor that was evaluated in a phase II trial against advanced ERBB2 mutant (n = 26) or ERBB2 amplified (n = 4) NSCLC (Table 2) [106]. The ERBB2 mutations were primarily exon 20 insertion mutations (n = 25). Partial responses were reported in 3 out of 26 patients (12%) with ERBB2 exon 20 mutations, and no responses were reported in ERBB2‐amplified patients. The median progression‐free survival for the mutant cohort was 3 months, and the median overall survival was 9 months. Poziotinib is another tyrosine kinase inhibitor with a similar target profile. In a cohort of 13 patients with ERBB2 exon 20 insertion or point mutation‐positive NSCLC, treatment with poziotinib was associated with a response rate of 50% and disease control rate of 83%. The drug was associated with significant toxicity, including grade 3 or greater toxicity in more than 50% of patients and one death because of pneumonitis (Table 2) [67].
The SUMMIT trial evaluated neratinib against a variety of advanced stage solid tumors with ERBB2 or ERBB3 mutations [2]. Only the breast cancer cohort (n = 25) met the prespecified endpoint of response rate of at least 30%. However, responses to neratinib were reported in ERBB2 mutant lung, salivary, cervical, and biliary cancers. No responses were reported in the colorectal and bladder cancer cohorts. Despite the limited benefit from neratinib, the SUMMIT trial provided interesting insights on the sensitivities of different ERBB2 mutations to neratinib. Neratinib showed activity across all mutation types in patients with breast cancer. In other solid tumor cohorts, neratinib showed activity against S310 mutation only in cervical and biliary tract tumors. In the case of V777 mutations, activity was seen primarily in endometrial cancers. Interestingly, no ERBB2 exon 20 insertion‐mutant NSCLC showed objective tumor response to treatment with neratinib.
In the phase II PUMA‐NER‐4201, patients with ERBB2‐mutated NSCLC were randomized to receive either single agent neratinib (n = 17) or neratinib plus temsirolimus (n = 43) (Table 2) [107]. Single agent neratinib failed to show any partial response, but six (35%) patients exhibited stable disease. Eight patients (19%) in the combination arm had partial response, and 14 (32.5%) patients had stable disease. TAK‐788 is a dual EGFR and ERBB2 inhibitor being evaluated in patients with relapsed or refractory NSCLC and who have tested positive for activating EGFR tyrosine kinase mutations and ERBB2 mutations [108]. Of the 13 patients with ERBB2 mutations, one patient had an unconfirmed partial response. Further updates are expected from the phase II expansion of this study.
Pyrotinib is a pan‐ERBB tyrosine kinase inhibitor that has been conditionally approved in combination with capecitabine for the treatment of ERBB2‐positive advanced breast cancer in China [109], [110]. Pyrotinib showed activity in xenografts and organoids established from a patient tumor sample with ERBB2 exon 20 insertion mutation‐positive lung adenocarcinoma [111]. The study included a phase II cohort of 15 patients with ERBB2‐mutant NSCLC treated with pyrotinib 400 mg daily. The objective response rate was 53.3%, and median overall survival was 12.9 months. Pyrotinib was overall well tolerated; 60% of patients developed drug‐related adverse effects, but these were all grade 1–2 events not requiring dose reduction or discontinuation. These findings hold considerable promise in the effort to target ERBB2 exon 20 insertion mutation.
Targeting ERBB2 mutation in solid tumors continues to be an ongoing challenge. The low prevalence of ERBB2 mutation, differentiating drivers from the passenger alterations, and identifying the appropriate treatment (monoclonal antibody vs. tyrosine kinase inhibitors) are significant challenges in effectively targeting ERBB2 mutations. Interpreting the data from the clinical trials is further hampered by the low sample sizes and significant variability in the efficacy of the agents to target these mutations. A case in point is neratinib in the SUMMIT trial: it failed to elicit objective tumor response in any patients with NSCLC with ERBB2 exon 20 insertion mutation, whereas afatinib, pyrotinib, poziotinib, and dacomitinib have shown activity against this mutation. Also, there is variability in response to tyrosine kinase inhibitor treatment depending on the type of ERBB2 mutation; the best responses have been primarily reported in patients with exon 20 insertion mutations. Afatinib, pyrotinib, and poziotinib have shown activity in patients with ERBB2 exon 20 insertion mutation‐positive NSCLC even though many of them had been heavily pretreated. Both pyrotinib and poziotinib were associated with higher response rate than afatinib, but these studies are not comparable. Moreover, the samples sizes are small, and poziotinib was also associated with significant toxicity.
The low prevalence of ERBB2 mutation, differentiating drivers from the passenger alterations, and identifying the appropriate treatment (monoclonal antibody vs. tyrosine kinase inhibitors) are significant challenges in effectively targeting ERBB2 mutations.
Similarly, dual ERBB2 inhibition with trastuzumab plus pertuzumab in the MyPathway trial appear to be promising in patients with ERBB2 expressing, amplified, or mutated biliary and colorectal cancers. Novel agents such as DS‐8201a may also represent the way forward in treating ERBB2 expressing, amplified, or mutated solid tumors, although further confirmation in larger studies is needed.
Conclusion
Recent studies have clearly established the role of ERBB2 mutation in tumorigenesis and as an actionable target in several solid cancers. In vitro studies have shown that the common ERBB2 mutations are vulnerable to at least one available clinically approved ERBB2 kinase inhibitor. The limited clinical activity of the single agent kinase inhibitors may be the result of additional factors such as the role played by type of ERBB2 mutation, co‐occurring mutations and alterations, rapid development of resistance mechanisms, and other alterations in the tumor environment. The clinical benefit from ERBB2 tyrosine kinase inhibitors varied depending on the mutation type as well as the anatomic site of cancer. In NSCLC, ERBB2 tyrosine kinase inhibitors have primarily shown activity against exon 20 insertion mutations and not so much with the other mutation types, including missense mutations in the kinase domain, whereas in biliary tract, cervical, and endometrial cancers, neratinib showed activity against missense mutations at residues S310 and V777. Further studies that focus on specific ERBB2 mutations in each tumor type are needed to establish the role of ERBB2 kinase inhibitors in the clinic. Until such studies are completed, the focus should be on enrolling all patients with activating ERBB2 mutation‐positive solid tumors in clinical trials. Interestingly, ERBB2‐directed monoclonal antibodies and antibody drug conjugates have shown considerable promise in this population and merit further exploration. It appears that they may have activity against both the ERBB2‐mutated as well as the ‐overexpressed and/or ‐amplified tumors. A viable strategy for the future may be in using monoclonal antibodies and antibody drug conjugates in the broader population of ERBB2 activated (including mutated, overexpressed, or amplified) solid tumors, whereas a highly selective approach that considers the tumor type and specific mutation may be needed for ERBB2 tyrosine kinase inhibitors.
ERBB2 mutation may also serve as independent biomarker for treatment response to chemotherapy in some solid tumors. ERBB2 mutation‐positive lung [112] and bladder cancers [113] had better treatment outcomes with chemotherapy. Recently, ERBB2 mutation was also shown to cause resistance toward antiestrogen treatment in estrogen receptor‐positive breast cancer cells, which could be reversed by the neratinib treatment [114], [115]. In addition, co‐occurrence of ERBB2 mutation and amplification was associated with poor response toward trastuzumab or lapatinib treatment but displayed significant response toward neratinib treatment [116]. Overall, ERBB2 mutation, either alone or together with ERBB2 amplification, defines a new molecular subtype in solid cancers and could offer patients additional options in their fight against cancer.
Acknowledgments
R.K.K. received funding from the Government of India's University Grants Commission (UGC)‐Faculty Recharge Programme (FRP) scheme, grant F.4‐5(136‐FRP)/2014(BSR). D.R.V. received funding from the UGC Universities with Potential for Excellence (UPE) scheme, grant F‐14‐5/2012(NS/PE).
Contributor Information
Janakiraman Subramanian, Email: jsubramanian@saint-lukes.org.
Rama Krishna Kancha, Email: ickrishna@gmail.com.
Author Contributions
Conception/design: Janakiraman Subramanian, Ashiq Masood, Rama Krishna Kancha
Collection and/or assembly of data: Janakiraman Subramanian, Archana Katta, Ashiq Masood, Rama Krishna Kancha
Data analysis and interpretation: Janakiraman Subramanian, Archana Katta, Dashavantha Reddy Vudem, Rama Krishna Kancha
Manuscript writing: Janakiraman Subramanian, Archana Katta, Dashavantha Reddy Vudem, Rama Krishna Kancha
Final approval of manuscript: Janakiraman Subramanian, Rama Krishna Kancha
Disclosures
Janakiraman Subramanian: Biocept, Paradigm (RF), Aleixion, AstraZeneca, Bristol‐Myers Squibb, Boehringer‐Ingelheim, Pfizer (SAB), AstraZeneca, Boehringer‐Ingelheim, Eli Lilly & Co. (H); Ashiq Masood: Bristol‐Myers Squibb, Boehringer‐Ingelheim (C/A), Biocept (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Yarden Y and Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127–137. [DOI] [PubMed] [Google Scholar]
- 2.Hyman DM, Piha‐Paul SA, Won H et al. HER kinase inhibition in patients with HER2‐ and HER3‐mutant cancers. Nature 2018;554:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arcila ME, Chaft JE, Nafa K et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 2012;18:4910–4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chmielecki J, Ross JS, Wang K et al. Oncogenic alterations in ERBB2/HER2 represent potential therapeutic targets across tumors from diverse anatomic sites of origin. The Oncologist 2015;20:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li BT, Ross DS, Aisner DL et al. HER2 amplification and HER2 mutation are distinct molecular targets in lung cancers. J Thorac Oncol 2016;11:414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zehir A, Benayed R, Shah RH et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017;23:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pahuja KB, Nguyen TT, Jaiswal BS et al. Actionable activating oncogenic ERBB2/HER2 transmembrane and juxtamembrane domain mutations. Cancer Cell 2018;34:792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barretina J, Caponigro G, Stransky N et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012;483:603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Network . Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research Network . Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research Network . Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013;499:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network ; Kandoth C, Schultz N, Cherniack AD et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Research Network . Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014;507:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research Network . Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas Research Network . Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014;159:676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciriello G, Gatza ML, Beck AH et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 2015;163:506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Network . Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015;517:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson D, Van Allen EM, Wu YM et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cancer Genome Atlas Research Network . The molecular taxonomy of primary prostate cancer. Cell 2015;163:1011–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey P, Chang DK, Nones K et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47–52. [DOI] [PubMed] [Google Scholar]
- 22.Robertson AG, Kim J, Al‐Ahmadie H et al. Comprehensive molecular characterization of muscle‐invasive bladder cancer. Cell 2017;171:540–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerami E, Gao J, Dogrusoz U et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao J, Aksoy BA, Dogrusoz U et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto H, Toyooka S, Ninomiya T et al. Therapeutic potential of afatinib for cancers with ErbB2 (HER2) transmembrane domain mutations G660D and V659E. The Oncologist 2018;23:150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Fiore PP, Pierce JH, Kraus MH et al. erbB‐2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science 1987;237:178–182. [DOI] [PubMed] [Google Scholar]
- 27.Yu D, Wolf JK, Scanlon M et al. Enhanced c‐erbB‐2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res 1993;53:891–898. [PubMed] [Google Scholar]
- 28.Noguchi M, Murakami M, Bennett W et al. Biological consequences of overexpression of a transfected c‐erbB‐2 gene in immortalized human bronchial epithelial cells. Cancer Res 1993;53:2035–2043. [PubMed] [Google Scholar]
- 29.Guy CT, Webster MA, Schaller M et al. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA 1992;89:10578–10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Creedon H, Balderstone LA, Muir M et al. Use of a genetically engineered mouse model as a preclinical tool for HER2 breast cancer. Dis Model Mech 2016;9:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang SE, Narasanna A, Perez‐Torres M et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 2006;10:25–38. [DOI] [PubMed] [Google Scholar]
- 32.Perera SA, Li D, Shimamura T et al. HER2YVMA drives rapid development of adenosquamous lung tumors in mice that are sensitive to BIBW2992 and rapamycin combination therapy. Proc Natl Acad Sci USA 2009;106:474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trowe T, Boukouvala S, Calkins K et al. EXEL‐7647 inhibits mutant forms of ErbB2 associated with lapatinib resistance and neoplastic transformation. Clin Cancer Res 2008;14:2465–2475. [DOI] [PubMed] [Google Scholar]
- 34.Kancha RK, von Bubnoff N, Bartosch N et al. Differential sensitivity of ERBB2 kinase domain mutations towards lapatinib. PLoS One 2011;6:e26760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greulich H, Kaplan B, Mertins P et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci USA 2012;109:14476–14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagano M, Kohsaka S, Ueno T et al. High‐throughput functional evaluation of variants of unknown significance in ErbB2. Clin Cancer Res 2018;24:5112–5122. [DOI] [PubMed] [Google Scholar]
- 37.Samanta A, LeVea CM, Dougall WC et al. Ligand and p185c‐neu density govern receptor interactions and tyrosine kinase activation. Proc Natl Acad Sci USA 1994;91:1711–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kancha RK, von Bubnoff N, Duyster J. Asymmetric kinase dimer formation is crucial for the activation of oncogenic EGFRvIII but not for ERBB3 phosphorylation. Cell Commun Signal 2013;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cappuzzo F, Bemis L, Varella‐Garcia M. HER2 mutation and response to trastuzumab therapy in non‐small‐cell lung cancer. N Engl J Med 2006;354:2619–2621. [DOI] [PubMed] [Google Scholar]
- 40.Weiler D, Diebold J, Strobel K et al. Rapid response to trastuzumab emtansine in a patient with HER2‐driven lung cancer. J Thorac Oncol 2015;10:e16–e17. [DOI] [PubMed] [Google Scholar]
- 41.Shih J, Bashir B, Gustafson KS et al. Cancer signature investigation: ERBB2 (HER2)‐activating mutation and amplification‐positive breast carcinoma mimicking lung primary. J Natl Compr Canc Netw 2015;13:947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng S, Tripathy D, Shete S et al. HER‐2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci USA 2004;101:9393–9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fabi A, Di Benedetto A, Metro G et al. HER2 protein and gene variation between primary and metastatic breast cancer: Significance and impact on patient care. Clin Cancer Res 2011;17:2055–2064. [DOI] [PubMed] [Google Scholar]
- 44.Collins DM, O'Donovan N, McGowan PM et al. Trastuzumab induces antibody‐dependent cell‐mediated cytotoxicity (ADCC) in HER‐2‐non‐amplified breast cancer cell lines. Ann Oncol 2012;23:1788–1795. [DOI] [PubMed] [Google Scholar]
- 45.Falchook GS, Janku F, Tsao AS et al. Non‐small‐cell lung cancer with HER2 exon 20 mutation: Regression with dual HER2 inhibition and anti‐VEGF combination treatment. J Thorac Oncol 2013;8:e19–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jasra S, Opyrchal M, Norton L et al. A rare case of S310F somatic ERBB2 mutation in a HER2‐nonamplified breast cancer. Clin Breast Cancer 2017;17:e37–e41. [DOI] [PubMed] [Google Scholar]
- 47.Burris HA, Hurwitz HI, Dees EC et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol 2005;23:5305–5313. [DOI] [PubMed] [Google Scholar]
- 48.Kurata T, Tsurutani J, Fujisaka Y et al. Inhibition of EGFR, HER2 and HER3 signaling with AZD8931 alone and in combination with paclitaxel: Phase I study in Japanese patients with advanced solid malignancies and advanced breast cancer. Invest New Drugs 2014;32:946–954. [DOI] [PubMed] [Google Scholar]
- 49.Wind S, Schmid M, Erhardt J et al. Pharmacokinetics of afatinib, a selective irreversible ErbB family blocker, in patients with advanced solid tumours. Clin Pharmacokinet 2013;52:1101–1109. [DOI] [PubMed] [Google Scholar]
- 50.Ito Y, Suenaga M, Hatake K et al. Safety, efficacy and pharmacokinetics of neratinib (HKI‐272) in Japanese patients with advanced solid tumors: A phase 1 dose‐escalation study. J Clin Oncol 2012;42:278–286. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi T, Boku N, Murakami H et al. Phase I and pharmacokinetic study of dacomitinib (PF‐00299804), an oral irreversible, small molecule inhibitor of human epidermal growth factor receptor‐1, ‐2, and ‐4 tyrosine kinases, in Japanese patients with advanced solid tumors. Invest New Drugs 2012;30:2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J, Salminen A, Yang X et al. Effects of 31 FDA approved small‐molecule kinase inhibitors on isolated rat liver mitochondria. Arch Toxicol 2017;91:2921–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simon GR, Garrett CR, Olson SC et al. Increased bioavailability of intravenous versus oral CI‐1033, a pan erbB tyrosine kinase inhibitor: Results of a phase I pharmacokinetic study. Clin Cancer Res 2006;12:4645–4651. [DOI] [PubMed] [Google Scholar]
- 54.Kim TM, Lee KW, Oh DY et al. Phase 1 Studies of poziotinib, an irreversible pan‐HER tyrosine kinase inhibitor in patients with advanced solid tumors. Cancer Res Treat 2018;50:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doi T, Takiuchi H, Ohtsu A et al. Phase I first‐in‐human study of TAK‐285, a novel investigational dual HER2/EGFR inhibitor, in cancer patients. Br J Cancer 2012;106:666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rusnak DW, Lackey K, Affleck K et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB‐2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor‐derived cell lines in vitro and in vivo. Mol Cancer Ther 2001;1:85–94. [PubMed] [Google Scholar]
- 57.Hickinson DM, Klinowska T, Speake G et al. AZD8931, an equipotent, reversible inhibitor of signaling by epidermal growth factor receptor, ERBB2 (HER2), and ERBB3: A unique agent for simultaneous ERBB receptor blockade in cancer. Clin Cancer Res 2010;16:1159–1169. [DOI] [PubMed] [Google Scholar]
- 58.Li D, Ambrogio L, Shimamura T et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27:4702–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rabindran SK, Discafani CM, Rosfjord EC et al. Antitumor activity of HKI‐272, an orally active, irreversible inhibitor of the HER‐2 tyrosine kinase. Cancer Res 2004;64:3958–3965. [DOI] [PubMed] [Google Scholar]
- 60.Engelman JA, Zejnullahu K, Gale CM et al. PF00299804, an irreversible pan‐ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 2007;67:11924–11932. [DOI] [PubMed] [Google Scholar]
- 61.Smaill JB, Rewcastle GW, Loo JA et al. Tyrosine kinase inhibitors. 17. Irreversible inhibitors of the epidermal growth factor receptor: 4‐(phenylamino)quinazoline‐ and 4‐(phenylamino)pyrido[3,2‐d]pyrimidine‐6‐acrylamides bearing additional solubilizing functions. J Med Chem 2000;43:1380–1397. [DOI] [PubMed] [Google Scholar]
- 62.Nam HJ, Kim HP, Yoon YK et al. Antitumor activity of HM781‐36B, an irreversible pan‐HER inhibitor, alone or in combination with cytotoxic chemotherapeutic agents in gastric cancer. Cancer Lett 2011;302:155–165. [DOI] [PubMed] [Google Scholar]
- 63.Ishikawa T, Seto M, Banno H et al. Design and synthesis of novel human epidermal growth factor receptor 2 (HER2)/epidermal growth factor receptor (EGFR) dual inhibitors bearing a pyrrolo[3,2‐d]pyrimidine scaffold. J Med Chem 2011;54:8030–8050. [DOI] [PubMed] [Google Scholar]
- 64.Bose R, Kavuri SM, Searleman AC et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov 2013;3:224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kosaka T, Tanizaki J, Paranal RM et al. Response heterogeneity of EGFR and HER2 exon 20 insertions to covalent EGFR and HER2 inhibitors. Cancer Res 2017;77:2712–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kavuri SM, Jain N, Galimi F et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov 2015;5:832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robichaux JP, Elamin YY, Tan Z et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20‐selective kinase inhibitor in non‐small cell lung cancer. Nat Med 2018;24:638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu X, De Angelis C, Burke KA et al. HER2 reactivation through acquisition of the HER2 L755S mutation as a mechanism of acquired resistance to HER2‐targeted therapy in HER2. Clin Cancer Res 2017;23:5123–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zuo WJ, Jiang YZ, Wang YJ et al. Dual characteristics of novel HER2 kinase domain mutations in response to HER2‐targeted therapies in human breast cancer. Clin Cancer Res 2016;22:4859–4869. [DOI] [PubMed] [Google Scholar]
- 70.Ritter CA, Perez‐Torres M, Rinehart C et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res 2007;13:4909–4919. [DOI] [PubMed] [Google Scholar]
- 71.Rexer BN, Ghosh R, Narasanna A et al. Human breast cancer cells harboring a gatekeeper T798M mutation in HER2 overexpress EGFR ligands and are sensitive to dual inhibition of EGFR and HER2. Clin Cancer Res 2013;19:5390–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi W, Jiang T, Nuciforo P et al. Pathway level alterations rather than mutations in single genes predict response to HER2‐targeted therapies in the neo‐ALTTO trial. Ann Oncol 2017;28:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Junttila TT, Akita RW, Parsons K et al. Ligand‐independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC‐0941. Cancer Cell 2009;15:429–440. [DOI] [PubMed] [Google Scholar]
- 74.Kataoka Y, Mukohara T, Shimada H et al. Association between gain‐of‐function mutations in PIK3CA and resistance to HER2‐targeted agents in HER2‐amplified breast cancer cell lines. Ann Oncol 2010;21:255–262. [DOI] [PubMed] [Google Scholar]
- 75.O'Brien NA, McDonald K, Tong L et al. Targeting PI3K/mTOR overcomes resistance to HER2‐targeted therapy independent of feedback activation of AKT. Clin Cancer Res 2014;20:3507–3520. [DOI] [PubMed] [Google Scholar]
- 76.Hanker AB, Pfefferle AD, Balko JM et al. Mutant PIK3CA accelerates HER2‐driven transgenic mammary tumors and induces resistance to combinations of anti‐HER2 therapies. Proc Natl Acad Sci USA 2013;110:14372–14377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chandarlapaty S, Sakr RA, Giri D et al. Frequent mutational activation of the PI3K‐AKT pathway in trastuzumab‐resistant breast cancer. Clin Cancer Res 2012;18:6784–6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loibl S, von Minckwitz G, Schneeweiss A et al. PIK3CA mutations are associated with lower rates of pathologic complete response to anti‐human epidermal growth factor receptor 2 (HER2) therapy in primary HER2‐overexpressing breast cancer. J Clin Oncol 2014;32:3212–3220. [DOI] [PubMed] [Google Scholar]
- 79.Majewski IJ, Nuciforo P, Mittempergher L et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2‐targeted therapies in breast cancer. J Clin Oncol 2015;33:1334–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiang B, Chatti K, Qiu H et al. Brk is coamplified with ErbB2 to promote proliferation in breast cancer. Proc Natl Acad Sci USA 2008;105:12463–12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paroni G, Fratelli M, Gardini G et al. ynergistic antitumor activity of lapatinib and retinoids on a novel subtype of breast cancer with coamplification of ERBB2 and RARA. Oncogene 2012;31:3431–3443. [DOI] [PubMed] [Google Scholar]
- 82.Tanizaki J, Okamoto I, Fumita S et al. Roles of BIM induction and survivin downregulation in lapatinib‐induced apoptosis in breast cancer cells with HER2 amplification. Oncogene 2011;30:4097–4106. [DOI] [PubMed] [Google Scholar]
- 83.Chakrabarty A, Rexer BN, Wang SE et al. H1047R phosphatidylinositol 3‐kinase mutant enhances HER2‐mediated transformation by heregulin production and activation of HER3. Oncogene 2010;29:5193–5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen CT, Kim H, Liska D et al. MET activation mediates resistance to lapatinib inhibition of HER2‐amplified gastric cancer cells. Mol Cancer Ther 2012;11:660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kalous O, Conklin D, Desai AJ et al. Dacomitinib (PF‐00299804), an irreversible pan‐HER inhibitor, inhibits proliferation of HER2‐amplified breast cancer cell lines resistant to trastuzumab and lapatinib. Mol Cancer Ther 2012;11:1978–1987. [DOI] [PubMed] [Google Scholar]
- 86.Kancha RK, Bartosch N, Duyster J. Analysis of conformational determinants underlying HSP90‐kinase interaction. PLoS One 2013;8:e68394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wainberg ZA, Anghel A, Rogers AM et al. Inhibition of HSP90 with AUY922 induces synergy in HER2‐amplified trastuzumab‐resistant breast and gastric cancer. Mol Cancer Ther 2013;12:509–519. [DOI] [PubMed] [Google Scholar]
- 88.Gatzemeier U, Groth G, Butts C et al. Randomized phase II trial of gemcitabine‐cisplatin with or without trastuzumab in HER2‐positive non‐small‐cell lung cancer. Ann Oncol 2004;15:19‐27. [DOI] [PubMed] [Google Scholar]
- 89.Lara PN Jr, Laptalo L, Longmate J et al. Trastuzumab plus docetaxel in HER2/neu‐positive non‐small‐cell lung cancer: a California Cancer Consortium screening and phase II trial. Clin Lung Cancer 2004;5:231–246. [DOI] [PubMed] [Google Scholar]
- 90.Goss GD, Felip E, Cobo M et al. Association of ERBB mutations with clinical outcomes of afatinib‐ or erlotinib‐treated patients with lung squamous cell carcinoma: Secondary analysis of the LUX‐Lung 8 randomized clinical trial. JAMA Oncol 2018;4:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mazieres J, Barlesi F, Filleron T et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2‐targeted drugs: Results from the European EUHER2 cohort. Ann Oncol 2016;27:281–286. [DOI] [PubMed] [Google Scholar]
- 92.Genentech, Inc. My Pathway: A study evaluating herceptin/perjeta, tarceva, zelboraf/cotellic, erivedge, alecensa, and tecentriq treatment targeted against certain molecular alterations in participants with advanced solid tumors. ClinicalTrials.gov Web site. https://clinicaltrials.gov/ct2/show/NCT02091141. Published March 19, 2014. Accessed March 11, 2019.
- 93.Argiles G, Saro J, Segal NH et al. Novel carcinoembryonic antigen T‐cell bispecific (CEA‐TCB) antibody: Preliminary clinical data as a single agent and in combination with atezolizumab in patients with metastatic colorectal cancer (mCRC). Ann Oncol 2017;28(suppl 3):LBA004A. [Google Scholar]
- 94.Javle MM, Hainsworth JD, Swanton C et al. Pertuzumab + trastuzumab for HER2‐positive metastatic biliary cancer: Preliminary data from MyPathway. J Clin Oncol 2017;35(suppl 4):402A.27893326 [Google Scholar]
- 95.Bang YJ, Giaccone G, Im SA et al. First‐in‐human phase 1 study of margetuximab (MGAH22), an Fc‐modified chimeric monoclonal antibody, in patients with HER 2‐positive advanced solid tumors. Ann Oncol 2017;28:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li BT, Shen R, Buonocore D et al. Ado‐trastuzumab emtansine in patients with HER2 mutant lung cancers: Results from a phase II basket trial. J Clin Oncol 2018;36:2532–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li B, Offin M, Hembrough T et al. Molecular and imaging predictors of response to ado‐trastuzumab emtansine in patients with HER2 Mutant lung cancers: An exploratory phase 2 trial. J Thorac Oncol 2018;13:S599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iwata H, Tamura K, Doi T. Trastuzumab deruxtecan (DS‐8201a) in subjects with HER2‐expressing solid tumors: Long‐term results of a large phase 1 study with multiple expansion cohorts. J Clin Oncol 2018;36(suppl 15):2501A. [Google Scholar]
- 99.Tsurutani J, Park H, Doi S et al. Updated results of phase 1 study of DS‐8201a in HER2‐expressing or ‐mutated advanced non‐small‐cell lung cancer. J Thorac Oncol 2018;13:324. [Google Scholar]
- 100.Lai WV, Lebas L, Barnes TA et al. Afatinib in patients with metastatic HER2‐mutant lung cancers: An international multicenter study. Eur J Cancer 2019;109:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Greve J, Teugels E, Geers C et al. Clinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neu. Lung Cancer 2012;76:123–127. [DOI] [PubMed] [Google Scholar]
- 102.Mazieres J, Peters S, Lepage B et al. Lung cancer that harbors an HER2 mutation: Epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013;31:1997–2003. [DOI] [PubMed] [Google Scholar]
- 103.Li BT, Lee A, O'Toole S et al. HER2 insertion YVMA mutant lung cancer: Long natural history and response to afatinib. Lung Cancer 2015;90:617–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Costa DB, Jorge SE, Moran JP et al. Pulse afatinib for ERBB2 exon 20 insertion‐mutated lung adenocarcinomas. J Thorac Oncol 2016;11:918–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peters S, Curioni‐Fontecedro A, Nechushtan H et al. Activity of afatinib in heavily pretreated patients with ErbB2 mutation‐positive advanced NSCLC: Findings from a global named patient use program. J Thorac Oncol 2018;13:1897–1905. [DOI] [PubMed] [Google Scholar]
- 106.Kris MG, Camidge DR, Giaccone G et al. Targeting HER2 aberrations as actionable drivers in lung cancers: Phase II trial of the pan‐HER tyrosine kinase inhibitor dacomitinib in patients with HER2‐mutant or amplified tumors. Ann Oncol 2015;26:1421–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gandhi L, Besse B, Mazieres J et al. MA04.02. Neratinib ± temsirolimus in HER2‐mutant lung cancers: An international, randomized phase II study. J Thorac Oncol 2017;12(supplement 1):S358–S359. [Google Scholar]
- 108.Doebele RC, Riely GJ, Spira AI et al. First report of safety, PK, and preliminary antitumor activity of the oral EGFR/HER2 exon 20 inhibitor TAK‐788 (AP32788) in non‐small cell lung cancer (NSCLC). J Clin Oncol 2018;36:9015. [Google Scholar]
- 109.Blair HA. Pyrotinib: First global approval. Drugs 2018;78:1751–1755. [DOI] [PubMed] [Google Scholar]
- 110.Ma F, Li Q, Chen S et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan‐ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2‐positive metastatic breast cancer. J Clin Oncol 2017;35:3105–3112. [DOI] [PubMed] [Google Scholar]
- 111.Wang Y, Jiang T, Qin Z et al. HER2 exon 20 insertions in non‐small‐cell lung cancer are sensitive to the irreversible pan‐HER receptor tyrosine kinase inhibitor pyrotinib. Ann Oncol 2019;30:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eng J, Hsu M, Chaft JE et al. Outcomes of chemotherapies and HER2 directed therapies in advanced HER2‐mutant lung cancers. Lung Cancer 2016;99:53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Groenendijk FH, De Jong J, Fransen Van de Putte EE et al. ERBB2 mutations characterize a subgroup of muscle‐invasive bladder cancers with excellent response to neoadjuvant chemotherapy. Eur Urol 2016;69:384–388. [DOI] [PubMed] [Google Scholar]
- 114.Croessmann S, Formisano L, Kinch LN et al. Combined blockade of activating ErbB2 mutations and ER results in synthetic lethality of ER+/HER2 mutant breast cancer. Clin Cancer Res 2019;25:277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nayar U, Cohen O, Kapstad C et al. Acquired HER2 mutations in ER(+) metastatic breast cancer confer resistance to estrogen receptor‐directed therapies. Nat Genet 2019;51:207–216. [DOI] [PubMed] [Google Scholar]
- 116.Cocco E, Javier Carmona F, Razavi P et al. Neratinib is effective in breast tumors bearing both amplification and mutation of ERBB2 (HER2). Sci Signal 2018;11:eaat9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoadley KA, Yau C, Hinoue T et al. Cell‐of‐origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell 2018;173:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ellrott K, Bailey MH, Saksena G et al. Scalable open science approach for mutation calling of tumor exomes using multiple genomic pipelines. Cell Syst 2018;6:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Taylor AM, Shih J, Ha G et al. Genomic and functional approaches to understanding cancer aneuploidy. Cancer Cell 2018;33:676–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao Q, Liang WW, Foltz SM et al. Driver fusions and their implications in the development and treatment of human cancers. Cell Rep 2018;23:227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu J, Lichtenberg T, Hoadley KA et al. An integrated TCGA pan‐cancer clinical data resource to drive high‐quality survival outcome analytics. Cell 2018;173:400–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sanchez‐Vega F, Mina M, Armenia J et al. Oncogenic signaling pathways in The Cancer Genome Atlas. Cell 2018;173:321–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pereira B, Chin SF, Rueda OM et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun 2016;7:11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.The Cancer Genome Atlas . TCGA study identifies genomic features of cervical cancer. News release. National Institutes of Health. January 24, 2017. [Google Scholar]
- 125.Giannakis M, Mu XJ, Shukla SA et al. Genomic correlates of immune‐cell infiltrates in colorectal carcinoma. Cell Rep 2016;15:857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Janjigian YY, Sanchez‐Vega F, Jonsson P et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov 2018;8:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brennan CW, Verhaak RG, McKenna A et al. The somatic genomic landscape of glioblastoma. Cell 2013;155:462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schulze K, Imbeaud S, Letouze E et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet 2015;47:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Campbell JD, Alexandrov A, Kim J et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet 2016;48:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jordan EJ, Kim HR, Arcila ME et al. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov 2017;7:596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cancer Genome Atlas Research Network . Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Armenia J, Wankowicz SAM, Liu D et al. The long tail of oncogenic drivers in prostate cancer. Nat Genet 2018;50:645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hodis E, Watson IR, Kryukov GV et al. A landscape of driver mutations in melanoma. Cell 2012;150:251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stinchcombe T, Stahel RA, Bubendrof L et al. Efficacy, safety, and biomarker results of trastuzumab emtansine (T‐DM1) in patients (pts) with previously treated HER2‐overexpressing locally advanced or metastatic non‐small cell lung cancer (mNSCLC). J Clin Oncol 2017;35(suppl 15):8509A. [Google Scholar]
- 135.Li BT, Makker V, Buonocore DJ et al. A multi‐histology basket trial of ado‐trastuzumab emtansine in patients with HER2 amplified cancers. J Clin Oncol 2018;36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hyman DM, Piha‐Paul SA, Rodon J et al. Neratinib in HER‐2 or HER3‐mutant solid tumors: SUMMIT, a global, multi‐histology, open‐label, phase 2 ‘basket’ study. Cancer Res Treat 2017;77(suppl 13):CT001A. [Google Scholar]