This review outlines causes of delays in diagnosis and barriers to early diagnosis of cancer in patients in low‐ and middle‐income countries.
Keywords: Cancer, Low‐ and middle‐income country, Early diagnosis, Delay interval
Abstract
Background.
Advanced stage presentation of patients with is common in low‐ and middle‐income countries (LMICs). A comprehensive analysis of existing delays and barriers in LMICs has not been previously reported. We conducted a systematic literature review to comprehensively outline delays and barriers to identify targets for future interventions and provide recommendations for future research in this field.
Materials and Methods.
Multiple electronic databases were searched using a standardized search strategy. Eligible articles were of any language, from LMICs, and published between January 1, 2002, and November 27, 2017. Included studies reported cancer care intervals or barriers encountered. Intervals and associated barriers were summarized by cancer type and geographical region.
Results.
This review included 316 study populations from 57 LMICs: 142 (44.9%) studies addressed time intervals, whereas 214 (67.7%) studies described barriers to cancer diagnosis. The median intervals were similar in the following three stages of early diagnosis: (a) access (1.2 months), (b) diagnostic (0.9 months), and (c) treatment (0.8 months). Studies from low‐income countries had significantly longer access intervals (median, 6.5 months) compared with other country income groups. Patients with breast cancer had longer delay intervals than patients with childhood cancer. No significant variation existed between geographic regions. Low health literacy was reported most frequently in studies describing barriers to cancer diagnosis and was associated with lower education level, no formal employment, lower income, and rural residence.
Conclusion.
Early diagnosis strategies should address barriers during all three intervals contributing to late presentation in LMICs. Standardization in studying and reporting delay intervals in LMICs is needed to monitor progress and facilitate comparisons across settings.
Implications for Practice.
This review draws the attention of cancer implementation scientists globally. The findings highlight the significant delays that occur throughout the cancer care continuum in low‐ and middle‐income countries and describe common barriers that cause them. This review will help shape the global research agenda by proposing metrics and implementation studies. By demonstrating the importance of standardized reporting metrics, this report sets forth additional research and evidence needed to inform cancer control policies.
摘要
背景。在中低收入国家 (LMIC),晚期患者十分常见。以前,人们从未报告有关LMIC癌症治疗延误和障碍现状的综合分析。我们开展了一项系统的文献回顾工作,旨在全面地列述各种延误和障碍,以确定未来干预措施的目标并为该领域中的未来研究提供建议。
材料和方法。采用标准化检索策略在多个电子数据库中进行检索。符合条件的文章为在 2002 年 1 月 1 日至 2017 年 11 月 27 日期间以任何语言在LMIC发表的文章。纳入的研究报告了癌症治疗的时间间隔或遇到的障碍。按照癌症类型和地理区域,我们对时间间隔和相关的障碍进行了总结。
结果。本次回顾工作包含来自 57 个LMIC的 316 项研究人群:142 (44.9%) 项研究提到了时间间隔,而 214 (67.7%) 项研究描述了癌症诊断的障碍。在早期诊断的以下三个阶段中,中位时间间隔相似:(a) 就诊(1.2 个月),(b) 诊断(0.9 个月)以及 (c) 治疗(0.8 个月)。与其他国家收入群体相比,低收入国家的就诊时间间隔明显更长(中位6.5 个月)。与儿童期癌症患者相比,乳腺癌患者的延误时间间隔更长。地理区域之间没有显著差异。在描述癌症诊断障碍的研究中,最常报告的是健康素养低,这一障碍与教育水平较低、无正式职业、收入较低以及在农村居住相关。
结论。早期诊断策略应该解决所有三个时间间隔中延误LMIC患者的诊断和治疗的障碍。对于LMIC的延误时间间隔,人们需要实现研究和报告的标准化,以监控进展情况并促进各种环境之间的对比。
实践意义:本次回顾工作引起了全球癌症一线科学家的关注。研究结果重点指出了在中低收入国家癌症连续治疗期间发生的重大延误问题并描述了导致此类问题的常见障碍。通过提出指标和实践研究,本次回顾工作将帮助制定全球研究议程。通过证实标准化报告指标的重要性,本报告提出了传达癌症控制政策所需的其他研究和证据。
Introduction
Worldwide, it is estimated that 9.6 million people die from cancer annually, with 70% of these deaths occurring in low‐ and middle‐income countries (LMICs) [1]. Cancer is now responsible for approximately 1 in 6 deaths globally, and the number of deaths continues to increase, particularly in LMICs. The higher proportion of patients with cancer in LMICs who have advanced stage disease at the time of diagnosis has led to a greater case fatality rate in LMICs compared with high‐income countries (HICs) [2].
The promotion of early diagnosis and access to treatment of cancer is a pillar of any country's comprehensive cancer control strategy. For example, patients with breast cancer who spend more than 3 months between symptom development and treatment have a 12% increased 5‐year mortality rate compared with patients who waited less than 3 months [3]. Although questions remain on whether increased time to diagnosis and treatment of symptomatic cancer is consistently associated with poorer outcome, there is general acceptance in the value of early or timely cancer diagnosis. A large systematic literature review examined this question and concluded that there is evidence to support earlier diagnosis being associated with earlier stage diagnosis and improved survival for breast, colorectal, head and neck, testicular cancers, melanoma and, to a lesser extent, for pancreatic, prostate, and bladder cancers [4].
In 2017, the World Health Organization (WHO) published the WHO Guide to Cancer Early Diagnosis to support the strengthening of early diagnosis programs around the world [5]. The document outlined cancer early diagnosis into three sequential steps: access to care, evaluation of disease, and access to subsequent treatment [5]. Each of these steps corresponds to an interval: presentation, diagnosis, and treatment, respectively. This provides a clear framework for cancer control programs to systematically address barriers that may impede timely cancer care at each step (supplemental online Fig. 1).
Prior to designing effective interventions that promote early diagnosis and access to treatment, it is necessary to understand current delays and barriers to timely care. The extent of varied time intervals in cancer diagnosis for different cancers and settings in LMICs is not well established. Furthermore, studies to date in LMICs have reported variable indicators that has limited comparison across sites [6]. Barriers to early diagnosis have been predominantly studied in HICs, where barriers such as poor health literacy have been associated with delays in presentation and diagnosis of symptomatic cancer (supplemental online Fig. 2) [7], [8]. Currently, it is unknown if these findings are translatable to LMICs.
Although research on delays in cancer care and their barriers have been reported in select LMICs, such information has not been collated and synthesized in a standardized cancer early diagnosis framework. A thorough review of data from LMICs allows for analysis of the overall landscape of delays in cancer diagnosis and access to treatment. Comparisons among LMICs by cancer types also allows for the identification of deficient research fields that exist. Previous literature reviews have been limited to single cancer types or specific geographical regions [9], [10].
In this review, we conducted a systematic literature review aiming to (a) provide a comprehensive overview of existing cancer care intervals and barriers reported in current literature to identify targets for future interventions and (b) critique current reporting methods in literature from LMICs while providing recommendations for reporting of delays and barriers in future research. A baseline of cancer presentation, diagnosis, and treatment intervals and delays by cancer type studied will be described, as well as the common barriers that result in delays in cancer diagnosis. These findings will ultimately inform future research in cancer early diagnosis by identifying limitations in current literature and targets for intervention.
Materials and Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guideline. This systematic review has been registered on the PROSPERO database (CRD42017083868).
Inclusion
We included any full text article that addressed delays in early diagnosis of any cancer, describing studies conducted in low‐, lower‐middle‐, or upper‐middle‐income countries (collectively designated as LMICs), as defined by the World Bank based on per capita gross national income in 2016 [11]. Studies were required to contain either (a) defined or reported delay intervals in the diagnosis of any symptomatic cancer or (b) reported predictive factors or barriers that delayed early diagnosis of any symptomatic cancer.
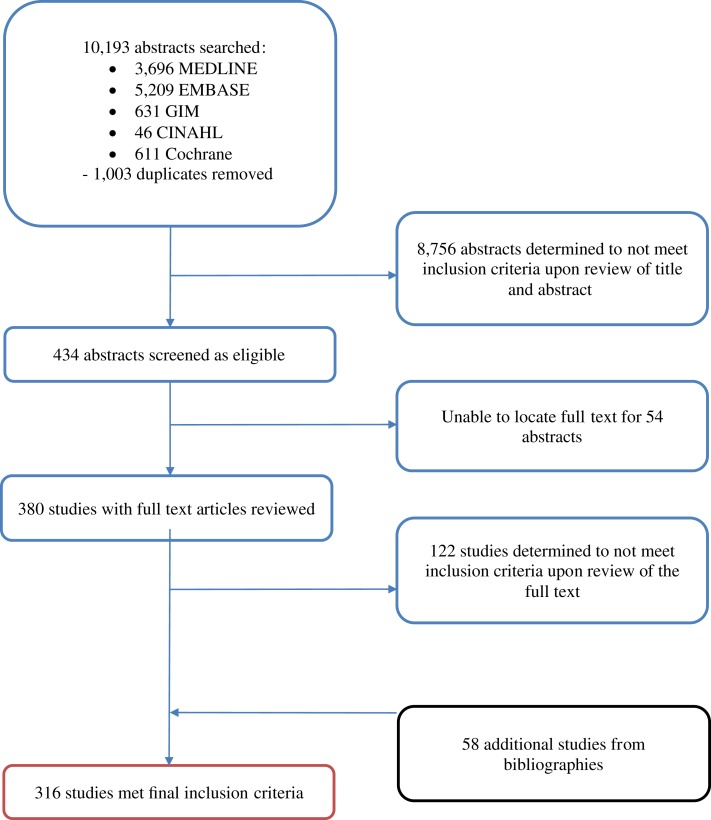
Search for published reports was conducted in English, with no language restriction on published reports. Any article in a language other than English was translated using a web‐based translation tool [12]. Searches were limited to studies conducted in LMICs. A search filter for LMICs was adapted from the Cochrane Effective Practice and Organization of Care, limiting each search to relevant studies. Date of publication was restricted to a recent 15‐year period (January 1, 2002, to November 27, 2017) to emphasize the most relevant data available in this field that reflect the current situation in countries. Studies that evaluated cancer screening or the diagnosis of cancer in an asymptomatic population were excluded to distinguish this review's focus, early diagnosis, from screening. Only published original studies were included for analysis (Fig. 1). A full list of inclusion and exclusion criteria is presented in supplemental online Table 1a.
Figure 1.
Study selection flowchart. The original search returned 10,193 abstracts in total. Only 316 studies met final inclusion criteria and were analyzed in this study.
Abbreviation: GIM, Global Index Medicus.
Search
The search was performed on Nov 27, 2017, and initiated on Ovid MEDLINE with keywords including “cancer,” “interval,” “barrier,” “downstaging,” “early diagnosis,” and “delayed diagnosis” (supplemental online Table 1B). We refined our search by adapting search terms from previous relevant literature and through consultation with WHO librarians. The search was adapted to Ovid EMBASE, Global Index Medicus, CINAHL EBSCO, and the Cochrane Library (consisting of Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Health Technology Assessment Database, and the National Health Service Economic Evaluation Database). A manual search was performed by examining bibliographies of included articles to identify relevant articles not captured in our database searches, using a “snowballing” approach [13].
Data Collection
The initial returned results were collated onto a spreadsheet. Two independent reviewers, N.B. and L.Q., screened abstracts for eligible studies. Each reviewer independently recorded eligible studies on the spreadsheet. Any disagreement regarding eligibility was resolved through discussion between the two reviewers and a third reviewer, A.I. After obtaining the full text of eligible studies, N.B. and L.Q. collectively inspected full texts for studies suitable for final inclusion. Relevant study data were abstracted onto a separate spreadsheet, with both N.B. and L.Q. reviewing all entered data for accuracy.
Information extracted on studies included details on country and WHO region in which the study was conducted, country income‐level, study aim, methods, study population characteristics, study design (cross‐sectional, qualitative, controlled intervention, cohort study, case series, pre‐ and post studies, or case control studies), tumor anatomical site, and results describing interval duration, predictive factors of delay, or barriers to cancer diagnosis. The WHO regions include African region (AFR), American region (AMR), East Mediterranean region (EMR), European region (EUR), Southeast Asian region (SEAR), and Western Pacific region (WPR). Details of the reported interval delays and barriers by type were recorded and compared with the framework described by the WHO Guide to Cancer Early Diagnosis [5] (supplemental online Figs. 1 and 2).
The types of intervals studied were summarized with descriptive statistics, according to time interval reported and measurement unit of delay interval used. These data were subanalyzed by type of cancer and country income‐level. These intervals were compared by interval type, country income‐level, and cancer type, using statistical t tests.
The types of barriers reported were summarized and presented as a frequency table. The barriers were compared according to cancer type, country income‐level, and study design (qualitative or quantitative).
Interval Definitions
Because of their commonly reported frequency, the main types of intervals analyzed were (a) time from symptom(s) onset to presentation to the health system or health professional (first contact point; presentation interval), (b) time from presentation to confirmed diagnosis (diagnostic interval), and (c) time from confirmed diagnosis to commencing treatment (treatment interval). As we expected different time definitions of delay intervals across the included studies and different cancer types, we did not define delays as greater than a certain number of days.
Assessment of Study Quality
The quality of each study included in the review was assessed by N.B., L.Q., and A.I. and focused on participant selection bias, measurement bias, and control for confounding. Assessment of quantitative studies employed a tool developed by the U.S. National Heart, Lung and Blood Institute, whereas assessment of qualitative studies employed a tool from the Critical Appraisal Skills Programme [14], [15]. Study quality was grouped into three categories (high, intermediate, or low). Low quality studies were included in the Figure 2 heat map and supplemental online Table 4 but excluded from other analyses.
Data Analysis
Because of the heterogeneity in the data, smaller subanalyses were conducted using pooled data from studies that presented comparable data. Otherwise, a narrative synthesis was used to summarize the main findings. Descriptive statistics were calculated using Stata SE version 12 (StataCorp, College Station, TX). All studies included in the review were geographically mapped using R‐3.4.0 (https://www.R-project.org/). The Wilcoxon rank sum test or the unpaired t test was used to test for differences in median interval lengths by cancer type and country income‐level. The chi‐square test statistic was used to evaluate differences in proportions of reported barriers by study design. All reported p values are two sided.
Results
The search of six electronic databases yielded 10,193 abstracts, of which 1,003 duplicate abstracts were removed (Fig. 1). Upon review of title and abstract, a total of 8,756 articles were determined ineligible, and an additional 54 were excluded because a published full text could not be found. After review of full texts and the manual addition of 58 bibliographic references, 301 manuscripts were finally included, with 316 study populations described. The primary reasons for article exclusion were (a) study did not assess barriers or intervals of care, (b) study site in HICs; (c) study focused on screening, or (d) study was not primary research. Upon study quality assessment, 189 were determined to be of high quality, 121 of intermediate quality, and 6 of low quality.
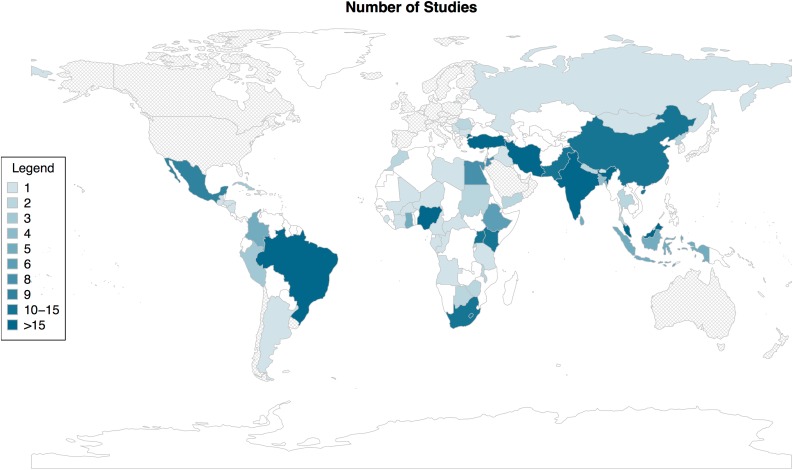
The geographical distribution of included studies revealed a widespread representation of LMICs across all six WHO regions (Fig. 2, supplemental online Table 2). Of the 57 LMICs represented, 14 were low‐income, 20 were lower‐middle income, and 23 were upper‐middle income countries. Among countries that had at least one study included, there was a median of two (range, 1–32) studies published per country. The number and proportion of LMICs within each WHO region that had at least one study included in this review were 21 (44%) in AFR, 10 (40%) in AMR, 9 (43%) in EMR, 5 (26%) in EUR, 8 (73%) in SEAR, and 4 (20%) in WPR.
Figure 2.
Heat map of studies included in systematic review by country in which study was conducted. Colors in map represent the number of studies included in the review by country: countries in gray are high‐income countries (excluded from review); countries in white are low‐, lower‐middle, or upper‐middle income countries with no published study included in the review.
After excluding the six low‐quality publications, the review included 96 studies addressing time intervals, 168 studies addressing barriers to cancer diagnosis, and 46 studies addressing both (Table 1). Subsequent analyses of interval duration included 142 studies and analyses of barriers to cancer diagnosis included 214 studies. Overall, the most frequently reported studies included in our review were cross‐sectional in design (n = 260, 84%), conducted in upper‐middle‐income countries (n = 160, 52%), and focused on breast cancer (n = 127, 41%). Common cancers reported differed by WHO region; for example, AFR contributed 21 cervical cancer studies but only two head and neck cancer studies (supplemental online Table 3).
Table 1. Basic information on the 310 studies that were high or medium quality.
Head and neck cancers include oral, laryngeal, and esophageal.
Cancer type and number of studies listed in supplemental online Table 5.
Abbreviation: AFR, African region; AMR, American region; EMR, East Mediterranean region; EUR, European region; IQR, interquartile range; SEAR, Southeast Asian region; WPR, Western Pacific region.
Time Intervals to Cancer Diagnosis
Altogether, 142 studies assessed one or more interval along the patient pathway to access cancer diagnosis and treatment (Table 2). The presentation interval (time from symptom onset to presentation to a health care professional) was most frequently reported among all cancer types (n = 113, 80%). Fewer studies reported on intervals within the health care system after patient presentation, with 45 (32%) reporting on the diagnostic interval (time from first presentation to a health care professional to confirmed diagnosis) and 28 (20%) studies reporting on the treatment interval (time from confirmed diagnosis to commencement of treatment). This was especially apparent among studies of breast cancer and those conducted in low‐income countries (LICs) (Table 2).
Table 2. Number of studies by type of interval, cancer type, and income‐level of country in which study was conducted.
Many studies assessed more than one interval in the cancer care continuum.
Complete list of other cancers in supplemental online Table 5.
The included studies used different metrics to measure delay intervals. The median interval in months was the most common metric reported (supplemental Table 4). However, 80 (33%) of the 246 intervals described in all studies that examined presentation, diagnostic, and treatment intervals reported a mean interval, despite its susceptibility to influence by outliers. This susceptibility was highlighted by the 18 studies that reported both median and mean presentation intervals. Among these studies, the mean interval was significantly greater than the median (5.45 vs. 2.05 months, p = .0002).
Median interval duration was analyzed by cancer type and country income‐level (Table 3). Studies of childhood cancers reported substantially shorter median presentation interval (0.5 months) compared with studies of breast cancer (2.2; p = .0244) and to all other adult cancers combined (2.0; p = .0097; Table 3). The diagnostic and treatment intervals were also shorter for childhood cancers compared with those of breast and other cancers combined, but these differences were not statistically significant.
Table 3. Number of studies and median delay in months (IQR) by type of interval, cancer type, and country income‐level.
Other cancer types were examined as a combined group due to the limited number of studies of individual types of cancer. Complete list is located in supplemental online Table 5.
p value comparing difference in median delay using Wilcoxon rank sum test.
p value comparing difference in median delay using unpaired t test.
Abbreviations: BC, breast cancer; CC, childhood cancer; IQR, interquartile range; L, lower; LM, lower‐middle; UM, upper‐middle.
Upon analysis of the median interval length by country income‐level (Table 3, supplemental online Fig. 3), LICs had significantly longer presentation intervals (6.5 months) compared with either lower‐middle income (1.4, p = .0269) or upper‐middle‐income countries (1.0, p = .0056). Neither the diagnostic nor treatment interval were significantly different between LICs and lower‐middle‐income or upper‐middle‐income countries.
Barriers to Cancer Early Diagnosis
In total, 214 studies reported on nine categories of barriers to early diagnosis of cancer (Table 4). Studies examined health literacy for either the general community or health care professionals and reported knowledge of signs and symptoms, risk factors, and navigating health care services. Cancer stigma consisted of examining fear, shame, and embarrassment of cancer diagnoses from the patient to their family or community. Access to primary care included inability to obtain appointments with a health care professional and lack of transportation. This resonated with access to diagnostics and geographical limitations to treatment. Inaccurate diagnoses were often described as false reassurance given by health care professional, delaying eventual treatment. Poor coordination of care consisted of patients who were lost to follow‐up after diagnosis or were not promptly referred for ongoing treatment. Financial barriers consisted of lacking health insurance and inability to afford imaging or subsequent treatment. Finally, sociocultural barriers included obstructive behavior from dominant family members, as well as opposition to opposite‐gender examinations (i.e., male physicians performing breast examinations).
Table 4. Number of studies and proportion that reported on barriers to care by cancer and country income‐level.
Many studies assessed more than one barrier.
Complete list of other cancers in supplemental online Table 5.
Abbreviations: LM, lower‐middle; UM, upper‐middle.
Qualitative studies examining barriers generally consisted of smaller sample sizes and involved either randomly or purposely selected individuals undergoing focus group interview sessions used open‐ended questioning. Qualitative studies tended to identify more barriers than quantitative studies, using predesigned questionnaires developed by the authors. A total of 97 cross‐sectional studies quantitatively examined health literacy alone. These included sample sizes ranging from 35 to 122,058; convenient samples were often obtained for smaller sizes, and cluster sampling was used for larger sample populations. These questionnaires that often examined knowledge, awareness, risk factors, symptoms, investigations, and management were also employed for studies of health care professionals.
Health literacy was the most frequently reported barrier (n = 186, 87%), regardless of cancer type. The least frequently reported barrier was limited access to diagnostics [22] (10%). With the exception of health literacy, all other barriers were more frequently reported by studies of childhood cancers compared with other cancers. There was no obvious difference in barriers reported by country income‐level (Table 4).
Of the 186 studies that reported health literacy, 64 presented factors significantly associated with cancer knowledge using univariate or multivariate linear regression (Table 5). All studies reported significance using a cutoff value of p < .05. Factors positively associated with greater health literacy were more years of formal education, having formal employment, increased income, urban residence, and a personal or family history of cancer. Age, sex, and marital status were reported as being not significantly associated with increased level of health literacy (Table 5).
Table 5. Number of countries that reported on factors and their association (negative association, no association, positive association) with cancer knowledge.
Statistical significance determined by studies that report a p value of ≤. 05.
Discussion
This systematic review describes the current landscape of delays in presentation, diagnostic, and treatment, and the barriers to cancer care in LMICs. It presents the largest sample of published studies to date, investigating cancer care intervals and barriers in LMICs, with the largest number of cancer types and range of countries. The review described the published literature by cancer type, geographic region, country income‐level, and detailed subgroup analyses of delay duration by type of interval, barriers to care, and factors associated with cancer knowledge.
This review demonstrates that delays are common during every step of the cancer care continuum, across cancer types and country income‐levels. Although a fixed cutoff value for defining delays was not employed within our study, our findings feature values that may be compared among cancer types and country income‐levels. This decision was made in accordance to recommendations to encourage analysis of continuous time interval data rather than dichotomized groups [6]. It may be noted that a target of 30 days per interval has generally been used in cancer program planning [16].
Our review illustrates that, in LMICs, although more studies report on the presentation interval, the duration of the presentation, diagnostic, and treatment intervals are not substantially different from one another, highlighting the need to address delays at all steps in the patient pathway. This has significant policy implications: focusing on one strategy, such as increasing awareness, without addressing others may not significantly reduce overall delays. As a comparator with HICs, reported intervals for cancers amenable to early diagnosis interventions in Denmark have been recently described as median 9 days for presentation and 34 days for the diagnostic interval, for 13,921 and 20,195 patients analyzed, respectively [17]. This demonstrates a substantially reduced presentation interval compared with LMICs but a comparable diagnostic interval. In essence, presentation intervals may potentially be influenced more by country income‐level.
Patient presentation intervals are significantly longer in LICs. However, our analysis is limited by the small number of studies from LICs (n = 3 for diagnostic interval and n = 0 for treatment interval). These findings suggest that studies focusing on diagnostic and treatment delays in LICs are profoundly needed, and targeted programmes that address health literacy, cancer stigma, and access to primary care should be prioritized in LICs. Similarly, comparisons across cancer types may also be useful to highlight where interventions are needed for certain cancers and where lessons can be learned from studies of other cancers. For example, there are longer time durations observed in all intervals for breast cancers compared with childhood cancers. Although the number of studies is limited, there may be opportunities to learn about successful interventions that decrease diagnostic and treatment intervals in pediatric care settings.
We found considerable heterogeneity in the metrics used by studies to describe the duration of intervals in the cancer care pathway. Although overall the most commonly reported interval is the median, a substantial number of studies report intervals using means. Our analysis of the subset of studies reporting median interval durations, although limited by the number of observations, is justified and informative based on allowing for high‐level comparisons across studies, cancer types, and country income‐level. Interpreting mean intervals, however, should be attempted cautiously, owing to their susceptibility to be skewed by outliers. This is highlighted in the comparison of the 18 studies that reported both a median and mean presentation interval, demonstrating the reported mean intervals were consistently longer than the median interval, where a few patients with long presentation interval affected the overall statistic. This suggests that vulnerable populations are subjected to prolonged intervals in accessing treatment. Furthermore, although the median of the median intervals for LMICs was approximately 1 month per interval, the interquartile ranges demonstrates that there is significant variability with some studies reporting median intervals of more than 4 months. Better understanding the differences between subpopulations in one study and populations between studies is an important research priority for targeted strategies.
To that end, our analysis of barriers to cancer care identifies particularly vulnerable groups at risk of delays, including patients who report lack of formal education, lack of formal employment, and rural residency. These findings emphasize the role of addressing social determinants which are essential to increasing cancer knowledge, improving cancer outcomes, and strengthening engagement with the health sector [18].
Setting a Research Agenda
From our review of all eligible peer‐reviewed journal articles that describe delays in cancer care in LMICs and barriers that cause them, we have identified gaps in current knowledge and the need for further research. We propose three recommendations to guide the research agenda in LMICs.
Firstly, the recently published WHO Guide to Cancer Early Diagnosis outlines a framework for the reporting of cancer delay intervals [5]. The delay findings presented here indicate that cancer systems should emphasize the reporting of medians as they are less subject to the effect of outliers. Our review is the first to demonstrate its utility across cancer types and specifically in LMICs [19]. This review advises standardization of study reporting to allow for better data interpretation across studies [5].
Secondly, our review demonstrates no significant differences in reported duration among the different intervals across cancer types in LMICs. However, there is a significant inequality in the number of studies that focused on barriers to cancer diagnosis or treatment, compared with studies of barriers to patient presentation. The lack of studies on factors associated with delays after patients present to the health care system should drive research examining health system barriers to access treatment. Future studies of barriers may also consider inclusion of qualitative or mixed methods to capture a wider range of barriers identified by patients with cancer and health care providers. As described in our review, qualitative study designs using in‐depth interviews were able to obtain greater specific details regarding barriers through their use of open‐ended questioning. However, the broad use of cross‐sectional studies that employed a wide range of study methodology may benefit from standardizing survey designs, as well as sampling techniques. Future research may seek to adopt conceptual frameworks more widely such as the Theoretical Domains Framework and examine the role of validated surveys to streamline the collection of data relating to health system‐related barriers [20].
Finally, our findings on the delay intervals in the cancer care pathway should be interpreted with global cancer incidence and mortality in sight. Although it is reassuring that high burden cancers in LMICs, such as cervical and breast cancers, were well represented in this review, there is a lack of other common cancers studied such as prostate, lung, colorectal, and stomach cancers [1]. Studies should address these underrepresented cancers to increase our understanding of high burden cancers that disproportionately affect LMICs.
Limitations
Our findings summarize published studies that reported heterogeneous data. Best efforts were made to summarize data with descriptive statistics and pooled analyses in preference over narrative syntheses. The published studies included were of different study designs, quality, and varying evidence level—ranging from qualitative interviews to prospective cohort studies. Although a wide range of barriers was explored within our study, the commonly reported questionnaires used for causes of delays were not externally validated. This likely led to potential barriers not explored or missed. In addition, only 10% of the included studies were from LICs, despite LICs making up over 20% of LMICs. Both of these factors may affect the generalizability of our findings. Nevertheless, this review represents the largest collection to date of studies of delay intervals and barriers to cancer care indicators by cancer types and other study characteristics in LMICs.
Future Directions
Interventions that improve early detection of cancer will be able to address other noncommunicable diseases (NCDs) as well. Fragmented models of service delivery can be integrated into an NCD early diagnosis and treatment program. The analysis of these interventions should include the study of outcomes for other NCDs. Focusing on strong primary care services in LMICs can improve outcomes while reducing costs to individuals, communities, and the health care system [21].
We emphasize the importance of standardizing the reporting of cancer care intervals and barriers in all cancers and across countries, to improve translation of research findings. Our review highlights gaps in LMICs data availability, as well as certain cancers that constitute significant burden of disease yet represent a small proportion of the published literature on these topics. Finally, more data are needed to examine how cancer early diagnosis programs can be integrated into primary care service delivery models and NCD prevention and control initiatives and to capture individual‐level patient outcomes [22], [23].
Conclusion
Although some countries and some cancer types have been represented, more studies are needed in LICs and on all high burden cancers, and greater efforts are needed to standardize the measurement and reporting of delay intervals. Nevertheless, we have presented the differences across cancer types and country income‐levels. Future studies should identify effective interventions that address the barriers that may be responsible for causing delays in cancer diagnosis to improve patient outcomes.
See http://www.TheOncologist.com for supplemental material available online.
Contributed equally.
Author Contributions
Conception/design: Nathan R. Brand, Liang G. Qu, André M. Ilbawi
Provision of study material or patients: Nathan R. Brand, Liang G. Qu
Collection and/or assembly of data: Nathan R. Brand, Liang G. Qu
Data analysis and interpretation: Nathan R. Brand, Liang G. Qu, Ann Chao
Manuscript writing: Nathan R. Brand, Liang G. Qu
Final approval of manuscript: Nathan R. Brand, Liang G. Qu, Ann Chao, André M. Ilbawi
Disclosures
The authors indicated no financial relationships.
References
- 1.Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Unger‐Saldaña K. Challenges to the early diagnosis and treatment of breast cancer in developing countries. World J Clin Oncol 2014;5:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards MA, Westcombe AM, Love SB et al. Influence of delay on survival in patients with breast cancer: A systematic review. Lancet 1999;353:1119–1126. [DOI] [PubMed] [Google Scholar]
- 4.Neal RD, Tharmanathan P, France B et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 2015;112(suppl 1)S92–S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . Guide To Cancer Early Diagnosis. Geneva, Switzerland: World Health Organization, 2017. Available from: http://apps.who.int/iris/bitstream/10665/254500/1/9789241511940‐eng.pdf?ua=1. Accessed January 10, 2017. [Google Scholar]
- 6.Weller D, Vedsted P, Rubin G et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br J Cancer 2012;106:1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones CEL, Maben J, Jack RH et al. A systematic review of barriers to early presentation and diagnosis with breast cancer among black women. BMJ Open 2014;4:e004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forbes LJ, Warburton F, Richards MA et al. Risk factors for delay in symptomatic presentation: A survey of cancer patients. Br J Cancer 2014;111:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma K, Costas A, Shulman LN et al. A systematic review of barriers to breast cancer care in developing countries resulting in delayed patient presentation. J Oncol 2012;2012:121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espina C, McKenzie F, os‐Santos‐Silva I. Delayed presentation and diagnosis of breast cancer in African women: A systematic review. Ann Epidemiol 2017;27:659–671.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fantom, NJ, Serajuddin U. The World Bank's classification of countries by income (English). Policy Research working paper; no. WPS 7528. Washington DC: The World Bank; 2016. [Google Scholar]
- 12.Doc translator. Available from: https://www.onlinedoctranslator.com/. Accessed December 23, 2017.
- 13.Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: Audit of primary sources. BMJ 2005;331:1064–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Study Quality Assessment Tools. Available from: https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools. Accessed January 7, 2017.
- 15.Critical Appraisal Skills Programme Qualitative Research Checklist. Available from: http://docs.wixstatic.com/ugd/dded87_25658615020e427da194a325e7773d42.pdf. Accessed January 10, 2017.
- 16.World Health Organization . Cancer control: Knowledge into Action: WHO Guide for Effective Programmes: Early Detection. Geneva, Switzerland: World Health Organization, 2007. [Google Scholar]
- 17.Flytkjær Virgilsen L, Møller H, Vedsted P. Cancer diagnostic delays and travel distance to health services: A nationwide cohort study in Denmark. Cancer Epidemiol 2019;59:115–122. [DOI] [PubMed] [Google Scholar]
- 18.Marmot M, Friel S, Bell R et al. Closing the gap in a generation: Health equity through action on the social determinants of health. Lancet 2008;372:1661–1669. [DOI] [PubMed] [Google Scholar]
- 19.Brasme JF, Morfouace M, Grill J et al. Delays in diagnosis of paediatric cancers: A systematic review and comparison with expert testimony in lawsuits. Lancet Oncol 2012;13:e445–e459. [DOI] [PubMed] [Google Scholar]
- 20.Geerligs L, Rankin NM, Shepherd HL et al. Hospital‐based interventions: A systematic review of staff‐reported barriers and facilitators to implementation processes. Implement Sci 2018;13:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Package of Essential Noncommunicable (PEN) Disease Interventions for Primary Health Care in Low‐Resource Settings. Geneva, Switzerland: World Health Organization, 2010. [Google Scholar]
- 22.Rubin G, Berendsen A, Crawford SM et al. The expanding role of primary care in cancer control. Lancet Oncol 2015;16:1231–1272. [DOI] [PubMed] [Google Scholar]
- 23.Kruk ME, Nigenda G, Knaul FM. Redesigning primary care to tackle the global epidemic of noncommunicable disease. Am J Public Health 2015;105:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]