This article evaluates the sensitivity, negative predictive value, and false‐negative rate of sentinel lymph node mapping in detecting lymphatic metastases in low‐risk and high‐risk patients with endometrial cancer.
Keywords: Endometrial cancer, Lymphatic metastases, Lymphadenectomy, Sentinel lymph node biopsy, Indocyanine green
Abstract
Background.
The efficacy of sentinel lymph node (SLN) mapping for high‐risk endometrial cancer remains unclear. This prompted us to evaluate the sensitivity, negative predictive value (NPV), and false‐negative (FN) rate of cervical injection of indocyanine green (ICG) SLN mapping in patients with endometrial cancer.
Materials and Methods.
This prospective interventional study was performed at a single university teaching hospital. Consecutive patients with early‐stage endometrial cancer who underwent laparoscopic surgical staging were included. Cervical injection of ICG and near‐infrared SLN identification and biopsy were performed for all study patients followed by systematic pelvic lymphadenectomy, whereas para‐aortic lymphadenectomy was performed in all patients with high‐risk histologies. SLN detection rates, sensitivity, NPV, and FN rates were calculated.
Results.
Between July 2016 and July 2018, 131 patients were enrolled. The overall SLN detection rate was 93.1%, with a bilateral detection rate of 61.8%. Four positive SLNs were identified in four patients. Lymph node metastasis was observed in four additional patients without positive SLNs. These four patients belonged to a group of patients with a high‐risk subtype. Three of the four patients had isolated para‐aortic node metastases. In low‐risk endometrial cancers, the sensitivity of the SLN technique to identify nodal metastatic disease was 100% (95% confidence interval [CI] 31.0–100), with an NPV and FN rate of 100% (95% CI 95.1–100) and 0%, respectively. In high‐risk endometrial cancers, the sensitivity, NPV, and FN rate were 20% (95% CI 1.0–70.1), 83.3% (95% CI 61.8–94.5), and 80%, respectively.
Conclusion.
Cervical injection of ICG and SLN mapping yielded a low sensitivity and a high FN rate for the identification of node metastasis in endometrial cancer with high‐risk histologies.
Implications for Practice.
The efficacy of sentinel lymph node (SLN) mapping for high‐risk endometrial cancer remains unclear. This study enrolled 131 patients with early‐stage endometrial cancer who underwent cervical injection of indocyanine green SLN mapping followed by systematic pelvic lymphadenectomy and para‐aortic lymphadenectomy. The key result was that SLN mapping yielded a low sensitivity and a high false‐negative rate for the identification of node metastasis in endometrial cancer with high‐risk histologies. The SLN strategy in these patients may increase the risk of missed diagnosis of isolated para‐aortic node metastases and seems to be unacceptable in clinical practice.
Introduction
Approximately 63,230 and 63,400 new cases of endometrial cancer are diagnosed annually in the U.S. and China, respectively [1], [2]. The standard initial treatment for early‐stage disease is surgical staging with lymphadenectomy, which provides prognostic information and identifies high‐risk patients requiring adjuvant therapy [3]. However, there remains controversy regarding this procedure following the reports of no benefit in disease‐free or overall survival in two large randomized studies evaluating the role of lymphadenectomy in endometrial cancer [4], [5]. Moreover, complete pelvic and para‐aortic lymphadenectomy is associated with comorbidities such as lymphoedema, lymphocyst formation, and genitofemoral nerve injury [6]. Sentinel lymph node (SLN) mapping, which informs nodal status while avoiding the surgical morbidity of full regional lymphadenectomy, has emerged as an alternative strategy for lymphatic assessment [7].
Increasing evidence supports the potential of SLN mapping in endometrial cancer. The FIRES trial, the largest multicenter, prospective cohort study to date, investigated 385 patients with stage I endometrial cancer undergoing SLN mapping, reporting a detection rate of 86% and a negative predictive value (NPV) of 99.6% [8]. A systematic review and meta‐analysis of 55 studies reported an overall detection rate of 81% and a 96% sensitivity to detect metastases [9]. Based on the study results, the Society of Gynecologic Oncology recently released a consensus recommendation for SLN mapping in endometrial cancer [10].
However, the majority of studies have included only patients at low risk for lymph node involvement and thus may underestimate the false‐negative (FN) rate. The efficacy of SLN mapping for high‐risk endometrial cancer remains unclear [11]. The 2017 National Comprehensive Cancer Network (NCCN) guidelines for endometrial cancer suggested that “the use of SLN mapping in high‐risk histologies should be undertaken with particular caution” [12]. However, this statement was revised in 2018 based on the results of recent trials indicating that SLN mapping may perform well in high‐risk histologies [13], [14], [15]. However, these trials have not provided definitive accuracy data to surgeons, as they were of retrospective nature, contained small numbers of patients, had no canonical requirement for para‐aortic lymphadenectomy, or used different tracers in a single trial. Thus, a prospective study based on the rational use of tracers and comprehensive surgical staging was required.
We conducted a prospective trial using cervical injection of indocyanine green (ICG) as the tracer for SLN mapping. Systematic pelvic lymphadenectomy and pelvic plus para‐aortic lymphadenectomy to the inferior mesenteric artery were performed for the surgical staging of patients with endometrial cancer with low‐risk and high‐risk histologies, respectively. This trial aimed to estimate the sensitivity, NPV, and FN rate of near‐infrared (NIR)‐ICG SLN mapping in detecting lymphatic metastases in patients with low‐risk and high‐risk endometrial cancer.
Materials and Methods
Patients
Between July 2016 and July 2018, consecutive patients diagnosed with endometrial cancer were evaluated at Shanghai First Maternity and Infant Hospital. The inclusion criteria included age older than 18 years and endometrial cancer with a presumed preoperative stage I or II disease, defined as normal preoperative abdomen and pelvis computed tomography (CT) or magnetic resonance imaging findings and normal pulmonary imaging (chest x‐ray or CT scan) findings. The exclusion criteria included evidence of extrauterine disease; previous treatment for endometrial cancer; or contraindications for receiving the ICG tracer, including a history of hepatic impairment or iodine allergy. All enrolled patients provided written informed consent. The study was approved by the institutional ethics committee. This trial was registered at Chinese Clinical Trial Registry (ChiCTR 1900020483).
Procedures
All procedures were performed by a single surgeon with more than 30 years of postgraduate experience. ICG tracer (Eisai Inc., Liaoning, China) was injected into the cervix before placement of the uterine manipulator. The standardized dose (0.5 mg/mL) was generated by adding 1 mL of the stock solution (2.5 mg/mL) to 4 mL of sterile water. One milliliter (0.5 mg) each of the diluted ICG solution was injected using a spinal needle superficially and deeply into the uterine cervix at 3 and 9 o'clock (total dose: 2 mg). Fluorescence detection in all patients was performed using a PINPOINT endoscopic fluorescence imaging system (Novadaq Technologies, BC, Canada).
All operations were performed laparoscopically. After peritoneal evaluation and washing, NIR imaging was used to visualize ICG in the lymphatics. Successful mapping was defined as the presence of a channel leading directly from the cervix to one or more candidate lymph nodes in at least one hemi‐pelvis. The identified SLNs were retrieved and labeled according to their locations. Complete bilateral lymphadenectomy was then performed in all patients. Patients with high‐risk histologies (grade 3 endometrioid, carcinosarcoma, serous, clear cell, or undifferentiated carcinoma) underwent simultaneous para‐aortic lymphadenectomy to the inferior mesenteric artery and omentectomy. All subsequent surgical staging procedures were performed using a three‐dimensional laparoscopic system.
Evaluation for the presence of metastatic disease in all SLNs was performed with ultra‐staging. The SLNs were sliced at 3‐mm intervals and embedded in paraffin blocks. Six paraffin‐embedded slides were created from each section, 40 μm apart. The first two slides were hematoxylin and eosin (H&E) stained. The third and fourth slides were stained for pan‐cytokeratin AE1 and AE3 (DAKO; Agilent, Santa Clara, CA). The remaining two slides were reserved for further investigation. Metastatic disease was categorized and reported according to American Joint Committee on Cancer definitions [16], with macrometastases defined as foci of metastasis measuring more than 2 mm, micrometastases as disease volume of 0.2–2 mm, and isolated tumor cells (ITCs) as foci of disease measuring less than 0.2 mm in the greatest dimension or as individual pathological cells positive for pan‐cytokeratin AE1 or AE3 staining. Non‐SLNs were classified as positive or negative for metastases based on the results of routine sectioning and examination of a single H&E‐stained slide according to standard protocol.
Statistical Analysis
This study aimed to estimate the NPV of pelvic SLNs in endometrial cancer. Sensitivity was defined as the proportion of patients with node‐positive disease for which SLN mapping (either unilateral or bilateral) was successful and who had metastatic disease correctly identified in the SLNs. The negative predictive value was defined as the proportion of negative SLN specimens associated with negative non‐SLN specimens. False negative was defined as bilateral SLN biopsies that were negative and a non‐SLN that was positive. False‐positive results were not possible by definition. The detection rate was defined as the product of the number of patients with one or more detected pelvic SLN divided by the total number of patients who underwent labeling and SLN mapping. The 95% confidence intervals (CIs) of the proportions were calculated, and subgroup analysis was performed with log‐rank or Fisher's exact tests (α = 0.05). Data were managed in a Microsoft Excel database and analyzed using SPSS Version 13.1 (SPSS, Inc., Chicago, IL).
Results
Clinical and Pathological Data
A total of 131 patients underwent surgical management with SLN mapping during the study period. Figure 1 shows the interventions for all patients. The median patient age was 55 (range 35–76) years, and the median body mass index (BMI) was 24.8 (range 18.5–37.8) kg/m2. The clinical‐pathological features for the 131 patients who received interventions are shown in Table 1. Twenty‐five (19.1%) patients had high‐grade endometrial histologies. Most patients (114/131, 87%) were diagnosed as stage IA or IB as the final pathology.
Figure 1.
Trial profile.
Abbreviations: NPV, negative predictive value; SLN, sentinel lymph node.
Table 1. Clinical‐pathological features.

Abbreviations: BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics.
Surgical Staging Outcomes and SLN Mapping Results
The SLN mapping results are shown in Table 2. After mapping, pelvic lymphadenectomy was performed in all patients. Para‐aortic lymphadenectomy and omentectomy were performed in all 25 patients with high‐risk histologies. For four patients with stage II endometrial cancer, two of four received radical hysterectomy, bilateral salpingo‐oophorectomy, and pelvic lymphadenectomy. The other two patients with high‐risk histology endometrial cancer received additional para‐aortic lymphadenectomy. None of the patients had serious adverse events.
Table 2. Surgical results and SLN mapping results.

Data are n (%) or median (range).
Abbreviation: SLN, sentinel lymph node.
Mapping identified at least one SLN in 122 (93.1%) of 131 patients (Table 2). The bilateral and unilateral detection rates were 61.8% (81/131) and 31.3% (41/131), respectively. Details of patients with no SLN mapping are shown in supplemental online Table 1. The average visualization time was 17.8 minutes (range 9–30). A total of 290 SLNs were identified in 122 patients (median: 2, range 1–7). Sentinel nodes were mapped in the following locations by frequency: external iliac (155, 53.3%), obturator (76, 26.1%), internal iliac (47, 16.2%), common iliac (8, 2.8%), and para‐aortic (4, 1.3%).
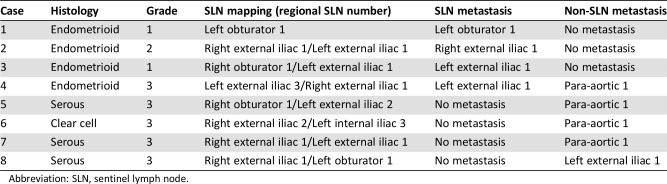
Of the 131 patients, 8 (6%) had positive lymph nodes, all of which occurred in the 122 patients with successful mapping of at least one SLN. Altogether, four positive sentinel nodes (one macrometastases, two micrometastases, one ITCs) were identified in four patients. In three (75%) of the four, the SLN was the only positive node, whereas para‐aortic lymph node metastasis was found in the systematic lymphadenectomy specimen of the fourth patient. In the other four patients, lymph node metastases were found only in systematic lymphadenectomy samples and not in SLNs. The details are shown in Table 3.
Table 3. The details of lymph node metastasis in all eight patients.
Abbreviation: SLN, sentinel lymph node.
Diagnostic Performance of SLN Mapping in High‐Risk and Low‐Risk Endometrial Cancer
The patients were stratified into two groups according to histology, resulting in 106 patients with low‐risk (endometrioid type, grade [G] 1–2) and 25 with high‐risk (endometrioid G3, serous, clear‐cell, and carcinosarcoma) histologies. Of the 106 patients with low‐risk disease, 3 (2.8%) had metastases in the pelvic SLNs, and all non‐SLNs were negative in the final histology. Among the 25 high‐risk endometrial patients, 1 (4%) had a metastatic pelvic SLN, with 1 metastatic non‐SLN in the para‐aortic area at the final histology. Another four (16%) patients had metastatic disease without positive SLNs (supplemental online Table 2); three had metastatic nodes in the para‐aortic area and one in the left external iliac area (Table 3).
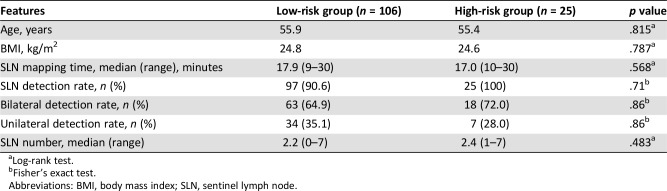
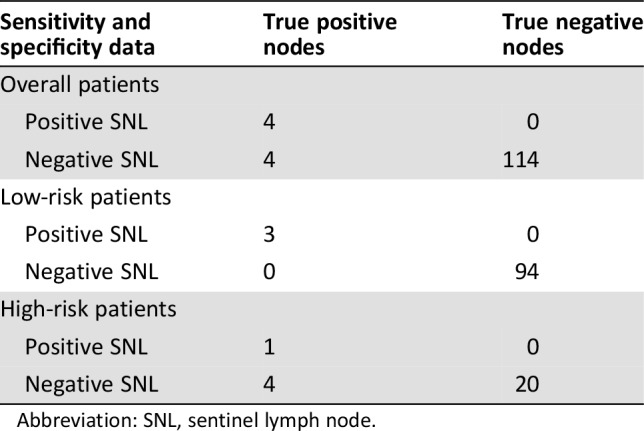
There were no significant differences in age, BMI, SLN detection rate, SLN visualization time, or SLN numbers between the two groups (Table 4). The lymph node metastasis rate was 2.8% (3/106) in the low‐risk group, significantly lower than that in the high‐risk group (20%, 5/25). The sensitivity and specificity data of 122 patients with successful mapping of at least one SLN are shown in Table 5. The overall sensitivity of the SLN technique to identify nodal metastatic disease was 50% (95% CI 17.4–82.5), the NPV was 96.6% (95% CI 91.0–98.9), and the FN rate was 50%. The values in the low‐risk group were 100% (95% CI 31.0–100), 100% (95% CI 95.1–100), and 0%; and 20% (95% CI 1.0–70.1), 83.3% (95% CI 61.8–94.5), and 80%, respectively, in the high‐risk group.
Table 4. Clinical features and SLN mapping outcome in high‐risk and low‐risk endometrial cancer.
Log‐rank test.
Fisher's exact test.
Abbreviations: BMI, body mass index; SLN, sentinel lymph node.
Table 5. Sensitivity and specificity data for overall patients, low‐risk patients, and high‐risk patients.
Abbreviation: SNL, sentinel lymph node.
Discussion
The present study investigated the feasibility of NIR‐ICG SLN mapping in endometrial cancer. Although our study revealed promising sensitivity, NPV, and FN rates in patients with low‐risk endometrial cancer, these results were astonishingly lower in high‐risk patients, at 20%, 83.3%, and 80%, respectively, results that are barely acceptable for practical clinical use.
SLN mapping with ultra‐staging may identify lymph node metastasis with high sensitivity and low FN rates [8], [9], [17], [18]. Therefore, SLN mapping has been gradually accepted as a trade‐off between none and complete pelvic lymphadenectomy. However, these studies included patients with endometrial cancer at low risk for lymph node metastasis, which may lead to an underestimation of the FN rate. Recently, the focus has shifted to high‐risk endometrial cancer cohorts with higher possibilities of lymph node metastasis.
There remains controversy concerning the efficacy of SLN mapping in patients with high‐risk endometrial cancer. As shown in the SENTI‐ENDO trial, all three FN cases occurred in high‐risk, type 2 patients [17]. A retrospective study by Naoure et al. reported an FN rate of 20% in patients with high‐risk endometrial cancer. In contrast, the FN rate in patients with low‐risk endometrial cancer in the same study was 6% [11]. These results, together with ours, indicate that SLN mapping should be undertaken cautiously in patients with high‐risk histologies.
Conversely, eight studies reported promising results [8], [14], [19], [20], [21], [22], [23], [24]. The most convincing results were demonstrated by the FIRES trial, with an overall sensitivity, FN rate, and NPV of 97.2%, 2.7%, and 99.6% respectively. Among the 340 total patients, 102 (30%) had either grade 3 endometrioid or type 2 carcinomas. Para‐aortic lymphadenectomy was performed in 75% of high‐risk patients, with a median of 19.8 pelvic and para‐aortic lymph nodes removed. Thus, even for the high‐risk subtype, SLN mapping may perform well. However, the feasibility of SLN mapping in high‐risk patients was not evaluated separately. The other seven studies included retrospective investigations or prospective validation trials with relatively small sample sizes. For example, in a cohort of 69 patients with endometrial cancer. Rajanbabu et al. reported that NIR‐IG SLN mapping without ultra‐staging in high‐risk patients (25/69, 36.2%) had a sensitivity of 57.1% and an FN rate of 42.9% [23]. However, the sensitivity increased to 100% when using an SLN algorithm such as that proposed by the Memorial Sloan Kettering Cancer Center (MSKCC) [25]. Soliman et al. reported a sensitivity of 95% and FN rate of 5% in a prospective validation trial of 101 patients with grade 3 and type 2 endometrial cancer undergoing full pelvic and para‐aortic lymphadenectomy after SLN mapping [14]. Based on these results, the suggestion in the NCCN guideline concerning this issue was changed from “be undertaken with particular caution” to “may perform well in high‐risk histologies.”
However, several weaknesses in these studies may limit the accuracy of their results. For instance, various SLN mapping techniques, such as blue dye and Tc‐99, ICG, or their combination, have been used in different trials or even in the same cohorts. Moreover, different surgical approaches, including laparoscopy, robotic surgery, or laparotomy, have been performed, with differing rates of para‐aortic lymphadenectomy. The extents of para‐aortic lymphadenectomy also vary greatly, from below the inferior mesenteric artery to up to the renal vein. Moreover, the learning curves of different surgeons in the same trial may also affect the results. The definition of high‐risk endometrial cancer varies between series. In all, scarce studies and a lack of standardization make it difficult to provide surgeons with data with a high level of evidence.
We adopted several measures to standardize our trial. All surgeries were performed by one highly experienced doctor, thus eliminating possible discrepancies between different doctors; NIR‐ICG SLN, which showed a distinct advantage over blue dye in identifying sentinel nodes [26], was used in every patient, and para‐aortic lymphadenectomy up to the inferior mesenteric artery was performed in every patient with high‐risk histology but not in low‐risk patients. These measures were intended to minimize intrinsic bias.
Our overall and bilateral detection rates were 93.1% and 61.8%, respectively, slightly higher rates than others reported for ICG SLN mapping [8], [9]. This relatively high detection rate may be due to the experience of the surgeon in SLN mapping. We also achieved excellent performance results for this technique in low‐risk endometrial cancers, with a sensitivity, NPV, and FN rate of 100%, 100%, and 0%, respectively. Moreover, a median of 28 (range 12–60) and 10 (range 2–18) pelvic and para‐aortic lymph nodes were removed. These results confirmed the high quality of our trial.
In our study, poor results were achieved in evaluating the feasibility of NIR‐ICG SLN mapping in high‐risk histologies. The sensitivity and NPV were extremely low and the FN rate was extremely high compared with those in other studies. One possible explanation for this discrepancy was the high rate of isolated para‐aortic lymph node metastasis in our high‐risk patients. The incidence of isolated para‐aortic lymph node metastasis in endometrial cancer is rare, which is around 1%–3% [27]. However, in our cohort, among 25 patients with high‐risk endometrial cancer, 3 (12%) had isolated metastasis para‐aortic non‐SLN (NSLN) with bilaterally negative pelvic SLNs. In addition, the difficulty of SLN mapping using cervical injection to identify para‐aortic SLNs may have contributed to our suboptimal results [28].
Uterine or fundus injections for SLN mapping may help to identify isolated para‐aortic metastases [29], [30], especially in high‐risk endometrial cancers [31]. However, contradictory results were reported in the FIRES trial [8] and other studies [32]. Furthermore, uterine or fundus injections are inconvenient compared with cervical injection. On the other hand, the MSKCC SLN mapping algorithm may greatly improve diagnostic performance [25]. If applied to our series, all four isolated para‐aortic metastasis cases could have been identified based on the algorithm requirement for “para‐aortic lymphadenectomy at attending discretion.” However, this improvement is only achieved by universal application of para‐aortic lymphadenectomy for all high‐risk patients, thus decreasing the significance of SLN mapping.
The limitations of our study include the single‐institution design, relatively small sample size, especially of high‐risk histologies, and small number of patients with metastasis. The strengths of the study include its prospective nature and rigorous methodologic approach intended to minimize intrinsic bias. The results showed that SLN mapping should be used with caution to avoid missed diagnoses of isolated para‐aortic lymph node metastasis in high‐risk endometrial cancer. Given the large proportion of patients with para‐aortic NSLN metastasis and negative pelvic SLN in our study, future trials should investigate whether para‐aortic lymphadenectomy should be performed in all high‐risk endometrial patients despite the results of the SLN mapping by cervical injection.
Conclusion
Cervical injection of ICG and SLN mapping yielded a low sensitivity and a high FN rate for the identification of node metastasis in endometrial cancer with high‐risk histologies. The SLN strategy in these patients may increase the risk of missed diagnosis of isolated para‐aortic node metastases and seems to be unacceptable in clinical practice. However, a large‐scale, multi‐institutional investigation including more patients with high‐risk endometrial cancer is needed to further clarify this issue.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81472427, 81672574), the Science and Technology Commission of Shanghai Municipality (17411951600), Shanghai ShenKang Hospital Development Center (SHDC12015110), and Shanghai Municipal Medical and Health Discipline Construction Projects (2017ZZ02015) to X.P.W.
Contributed equally.
Contributor Information
Qin Yan, Email: yanqin76@126.com.
XiaoPing Wan, Email: wanxiaoping@tongji.edu.cn.
Author Contributions
Conception/design: Lei Ye, Wen Lu, YiRan Li, Qin Yan, XiaoPing Wan
Provision of study material or patients: Lei Ye, Wen Lu, QiZhi He, YiRan Li, BiLan Li, XiaoJun Wang, Qin Yan, XiaoPing Wan
Collection and/or assembly of data: Lei Ye, ShuangDi Li, Wen Lu, YiRan Li, Qin Yan
Data analysis and interpretation: ShuangDi Li, Qin Yan
Manuscript writing: ShuangDi Li, Qin Yan
Final approval of manuscript: Lei Ye, ShuangDi Li, Wen Lu, QiZhi He, YiRan Li, BiLan Li, XiaoJun Wang, Qin Yan, XiaoPing Wan
Disclosures
The authors indicated no financial relationships.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 3.Morice P, Leary A, Creutzberg C et al. Endometrial cancer. Lancet 2016;387:1094–1108. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti Panici P, Basile S et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early‐stage endometrial carcinoma: Randomized clinical trial. J Natl Cancer Inst 2008;100:1707–1716. [DOI] [PubMed] [Google Scholar]
- 5.ASTEC study group , Kitchener H, Swart AM et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009;373:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu‐Rustum NR, Alektiar K, Iasonos A et al. The incidence of symptomatic lower‐extremity lymphedema following treatment of uterine corpus malignancies: A 12‐year experience at Memorial Sloan‐Kettering Cancer Center. Gynecol Oncol 2006;103:714–718. [DOI] [PubMed] [Google Scholar]
- 7.Suh DH, Kim M, Lee KH et al. Major clinical research advances in gynecologic cancer in 2017. J Gynecol Oncol 2018;29:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi EC, Kowalski LD, Scalici J et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol 2017;18:384–392. [DOI] [PubMed] [Google Scholar]
- 9.Bodurtha Smith AJ, Fader AN, Tanner EJ. Sentinel lymph node assessment in endometrial cancer: A systematic review and meta‐analysis. Am J Obstet Gynecol 2017;216:459–476.e10. [DOI] [PubMed] [Google Scholar]
- 10.Holloway RW, Abu‐Rustum NR, Backes FJ et al. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol Oncol 2017;146:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naoura I, Canlorbe G, Bendifallah S et al. Relevance of sentinel lymph node procedure for patients with high‐risk endometrial cancer. Gynecol Oncol 2015;136:60–64. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network . Clinical Practice Guidelines in Oncology. Uterine Neoplasms, Version 3.2017. Available at http://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed July 18, 2017.
- 13.Schiavone MB, Zivanovic O, Zhou Q et al. Survival of patients with uterine carcinosarcoma undergoing sentinel lymph node mapping. Ann Surg Oncol 2016;23:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soliman PT, Westin SN, Dioun S et al. A prospective validation study of sentinel lymph node mapping for high‐risk endometrial cancer. Gynecol Oncol 2017;146:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network . (NCCN) Clinical Practice Guidelines in Oncology. Uterine Neoplasms, Version 1.2019. Available at http://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf. Accessed October 17, 2018. [DOI] [PubMed]
- 16.AJCC . AJCC Cancer Staging Manual. 8th ed New York: Springer, 2017. [Google Scholar]
- 17.Ballester M, Dubernard G, Lecuru F et al. Detection rate and diagnostic accuracy of sentinel‐node biopsy in early stage endometrial cancer: A prospective multicentre study (SENTI‐ENDO). Lancet Oncol 2011;12:469–476. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Liu F. Meta‐analysis of laparoscopy sentinel lymph node mapping in endometrial cancer. Arch Gynecol Obstet 2018;298:505–510. [DOI] [PubMed] [Google Scholar]
- 19.Papadia A, Gasparri ML, Radan AP et al. Retrospective validation of the laparoscopic ICG SLN mapping in patients with grade 3 endometrial cancer. J Cancer Res Clin Oncol 2018;144:1385–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Touhami O, Gregoire J, Renaud MC et al. Performance of sentinel lymph node (SLN) mapping in high‐risk endometrial cancer. Gynecol Oncol 2017;147:549–553. [DOI] [PubMed] [Google Scholar]
- 21.Ehrisman J, Secord AA, Berchuck A et al. Performance of sentinel lymph node biopsy in high‐risk endometrial cancer. Gynecol Oncol Rep 2016;17:69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holloway RW, Ahmad S, Kendrick JE et al. A prospective cohort study comparing colorimetric and fluorescent imaging for sentinel lymph node mapping in endometrial cancer. Ann Surg Oncol 2017;24:1972–1979. [DOI] [PubMed] [Google Scholar]
- 23.Rajanbabu A, Agarwal R. A prospective evaluation of the sentinel node mapping algorithm in endometrial cancer and correlation of its performance against endometrial cancer risk subtypes. Eur J Obstet Gynecol Reprod Biol 2018;224:77–80. [DOI] [PubMed] [Google Scholar]
- 24.Buda A, Gasparri ML, Puppo A et al. Lymph node evaluation in high‐risk early stage endometrial cancer: A multi‐institutional retrospective analysis comparing the sentinel lymph node (SLN) algorithm and SLN with selective lymphadenectomy. Gynecol Oncol 2018;150:261–266. [DOI] [PubMed] [Google Scholar]
- 25.Barlin JN, Khoury‐Collado F, Kim CH et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: Beyond removal of blue nodes. Gynecol Oncol 2012;125:531–535. [DOI] [PubMed] [Google Scholar]
- 26.Frumovitz M, Plante M, Lee PS et al. Near‐infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): A randomised, phase 3, multicentre, non‐inferiority trial. Lancet Oncol 2018;19:1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baiocchi G, Faloppa CC, Mantoan H et al. Para‐aortic lymphadenectomy can be omitted in most endometrial cancer patients at risk of lymph node metastasis. J Surg Oncol 2017;116:220–226. [DOI] [PubMed] [Google Scholar]
- 28.Khoury‐Collado F, Murray MP et al. Sentinel lymph node mapping for endometrial cancer improves the detection of metastatic disease to regional lymph nodes. Gynecol Oncol 2011;122:251–254. [DOI] [PubMed] [Google Scholar]
- 29.Martinelli F, Ditto A, Signorelli M et al. Sentinel node mapping in endometrial cancer following Hysteroscopic injection of tracers: A single center evaluation over 200 cases. Gynecol Oncol 2017;146:525–530. [DOI] [PubMed] [Google Scholar]
- 30.Kimmig R, Aktas B, Buderath P et al. Intraoperative navigation in robotically assisted compartmental surgery of uterine cancer by visualisation of embryologically derived lymphatic networks with indocyanine‐green (ICG). J Surg Oncol 2016;113:554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cormier B, Rozenholc AT, Gotlieb W et al. Sentinel lymph node procedure in endometrial cancer: A systematic review and proposal for standardization of future research. Gynecol Oncol 2015;138:478–485. [DOI] [PubMed] [Google Scholar]
- 32.Frumovitz M, Bodurka DC, Broaddus RR et al. Lymphatic mapping and sentinel node biopsy in women with high‐risk endometrial cancer. Gynecol Oncol 2007;104:100–103. [DOI] [PubMed] [Google Scholar]