Abstract
DNA barcodes developed for selected commercially important bamboo species can be utilized for the certification of planting stock in bamboo nurseries in absence of discriminatory features at the juvenile stage. Planting materials such as micropropagated plantlets, rhizome transplants and culm cuttings, generated at nursery level are directly procured for establishment of commercial plantations without any further verification. Very often misidentification and mixing up occur at nursery level and the error is not discovered until several years have passed. The present study evaluated the potentiality of seven Consortium for Barcode of Life (CBOL) recommended standard DNA barcode regions in commercially important bamboo species of India. Among the analyzed barcode regions, multiple sequence alignment (MSA) of psbA-trnH barcode region showed species-specific nucleotide differences in the studied bamboo taxa. The major nucleotide changes observed were transitions/transversions as well as insertions/deletions of nucleotides. Even though species-specific mononucleotide differences could be identified for most of the studied bamboo taxa, a small amount of sequence similarities were found in some of the Dendrocalamus and Bambusa species, which were grouped together in tree-based analysis. In subtribe Melocanninae, Ochlandra travancorica, Melocanna baccifera and M. clarkei showed unique species-specific psbA-trnH barcodes. Similarly, in the genus Oxytenanthera, unique species-specific psbA-trnH barcodes were obtained for O. monadelpha and O. parvifolia. Thus psbA-trnH barcode region generated distinct species-specific barcodes for commercial bamboo species in genera Bambusa, Dendrocalamus, Melocanna, Oxytenanthera as well as Ochlandra. Any national certification agency set up for the purpose can utilize psbA-trnH DNA barcode region to tag species identity and to establish the authenticity of multiplied planting materials in bamboos.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-2018-8) contains supplementary material, which is available to authorized users.
Keywords: Commercial bamboos species, Planting stock, Species certification, DNA barcoding
Introduction
Bamboos, woody perennials of the grass family Poaceae, provide livelihood for millions of people around the globe. India is well endowed with 136 species of bamboo resources in 23 genera, extending over 13.96 million ha and is the second largest bamboo reserve in the world (FSI 2011). Bamboo is highly adaptable to a wide range of climatic and rainfall conditions and is one of the most suitable species for commercial forestry. Along with traditional uses of bamboo poles for rural building construction, fencing, agricultural purposes and household articles, in recent times, the ‘green gold’ has got immense demand as an industrial raw material (Khan and Hazra 2007). The multiple harvests possible around the year along with tremendous growth rate contribute towards wide-scale acceptability and commercial potential of bamboo species among the farming community. In India, the last few decades have witnessed a considerable interest in bamboo cultivation owing to the huge demand of source material from bamboo-based industries.
Even though India possesses 45% of global bamboo growth, the country contributes only 4% towards the global trade with an annual productivity of only 1 MT/ha (Tripathi et al. 2006). Overexploitation of available resources, poor performance of unsuitable species, forest fires, grazing, gregarious flowering, among others contributed to productivity decline albeit the existence of significant species diversity/growing habitats of bamboos in India (NBM 2013). National Bamboo Mission (NBM), Government of India, is trying to achieve a global share of 27% through improved productivity of bamboo plantations (Kumar et al. 2005). In this regard, NBM has recommended priority species based on their annual productivity and suitability in different agro-climatic zones throughout the country (https://www.nbm.nic.in). Guidelines issued by NBM aim at ensuring the use of superior planting stock for raising bamboo plantations through a network of certified high-tech bamboo nurseries in India. There are more than thousand nurseries in the country which provide planting stock to farmers for establishment of plantations. The crucial step in any productivity improvement program is proper identification, multiplication and supply of high-quality planting materials of suitable species with guaranteed productivity. It is a common observation, however, that plants being propagated in these nurseries are misidentified or that mixing of species occurs inadvertently. It is imperative to ensure correct species identity along with quality planting stock so as to make certain that it is suitable for a given site or purpose.
Unlike other plants, most bamboo species are semelparous, resulting in unpredictable flowering at long cycles after which all plants in the population die. Field identification of bamboo species thus traditionally rely on morphological characteristics of vegetative parts, mainly culm/culm sheath characteristics (Ohrnberger and Georrings 1986; Clark et al. 2007). Generally, in bamboo species, these characteristics manifest only when new culms emerge and juvenile plants do not possess these characteristics until after the 1st year or even later. Thus, identification of species is rendered difficult even for the best of nursery men. Further compounding the problem is the presence of variation between accessions within same species. Planting materials of bamboos handled in a typical bamboo nursery consist of plants in various age classes derived from seeds, macroproliferated seedlings (Kumar 1991), rooted culm or branch cuttings, rhizome transplants and micropropagated plantlets. Due to the absence of any consistent vegetative characteristics, unintentional mixing of species/clones is quite common at the nursery level. Therefore, to ensure that material used for planting conforms to the intended quality, NBM envisages a certification framework under which planting materials are characterized using various state-of-the-art tools (BTSG 2014). Methods to identify plant material at clonal level as well as at species level are equally important. Ideally these methods should be of the kind that is applicable to planting material at all stages of propagation, plantation and the harvested products. Morphology-based identification keys are very useful for quick identification at field, but it is well known that environmental plasticity in these characters is a serious limiting factor that prevents precise identification. DNA-based molecular tools are devoid of such environmental or developmental influences and can bring in more precision to protect the genuine interests of bamboo growers and industries.
The tremendous advancement of molecular marker technologies holds promise to address this issue and yet only limited progress has been achieved with regard to traceability systems in forest reproductive material employing molecular markers (Botta et al. 2001, 2004; Konnert and Behm 2006; Degen et al. 2010). The last decade had witnessed unraveling of potential of molecular techniques as a tool to supplement species identification methods in plants as well as in animals. DNA barcoding, process of species identification based on short standard conserved region of the genome (Hebert et al. 2004; CBOL 2009) is foremost when it comes to identification at species level and to resolve taxonomic problems. Plastid gene sequences such as rbcL, matK, rpS4, rpL16, among others have contributed immensely to the current understanding of bamboo systematics and phylogeny (Kelchner and Clark 1997). However, the feasibility of recommended conserved plastid barcode regions for species discrimination in bamboos has been reported only in a few instances. In temperate woody bamboos, four barcoding loci, namely matK, rbcL, psbA-trnH and ITS2 were analyzed and the combination of rbcL + ITS2 suggested as a potential barcode region for species discrimination (Cai et al. 2012). Failure of matK to discriminate Bambusa species due to interspecific hybridization and polyploidy was recently reported (Das et al. 2013). Low discriminatory power of core barcode region (rbcL + matK) as well as greater discriminatory power of trnG-trnT spacer in bamboos was also suggested (Zhang et al. 2013). Sosa et al. (2013) recommended matK + psbI-psbK as discriminant barcode loci in some temperate bamboos.
The present study therefore envisages developing DNA barcodes for commercially important priority species of bamboos recommended by NBM, Government of India for large-scale multiplication in accredited bamboo nurseries.
Materials and methods
Plant sampling
Leaf samples were collected from mature clumps of bamboo species from reserve forests and protected areas throughout the distribution zones in India (Table 1). For the preparation of voucher specimens, twigs with a few leaves and culm sheaths were collected. Thirteen commercially important bamboo species, viz., Bambusa balcooa Roxb., B. bambos Voss, B. nutans Wall ex Munro, B. pallida Munro, B. tulda Roxb, B. vulgaris var. vulgaris Schrad ex Wendle, Dendrocalamus asper Baker ex Heyne, D. giganteus Munro, D. hamiltonii Nees and Arn ex Munro, D. strictus Nees, Melocanna baccifera Kurz., Ochlandra travancorica Benth and Oxytenanthera parvifolia Brandis ex Gamble were selected for the development of DNA barcodes. Leaf samples from multiple accessions were collected and dried in silica gel. In addition to NBM listed priority species, several species distributed in Northeastern India such as those of genera Bambusa (B. jaintiana, B. mohanramii, B. teres, B. multiplex), Dendrocalamus (D. brandisii, D. hookeri, D. longispathus), Oxytenanthera (O. monadelpha) as well as Melocanna clarkei, which are generally in cultivation were also considered for the development of DNA barcodes. Samples were authenticated at Kerala Forest Research Institute (KFRI) and voucher specimens were deposited at KFRI herbarium.
Table 1.
Details on bamboo species and their distribution included in the present study
| Name of species | No. of samples/species | Distribution | Propagules used |
|---|---|---|---|
| Bambusa balcooa Roxb.a | 7 | Northeastern India, Tripura, Nagaland, Meghalaya, Assam, West Bengal, Uttar Pradesh |
Branch/culm cuttings Tissue culture plantlets |
| B. bambos Vossa | 10 | Wide distribution in India | Seedlings |
| B. nutans Wall ex Munroa | 5 | Himachal and Northeastern states, West Bengal, Orissa, Sikkim, UP | Culm/branch cuttings, Offset plantings |
| B. pallida Munroa | 6 | Northeast India, Bhutan, Myanmar |
Seedlings Culm cuttings, rhizome/ Offset plantings |
| B. tulda Roxba | 10 | Assam, Bihar, Meghalaya, Meghalaya, Mizoram, Tripura |
Seedlings Culm/ branch/ rhizome cuttings Tissue culture plantlets |
| B. vulgaris var. vulgaris Schrad ex Wendlea | 7 | Northeast and central India | Culm/branch cuttings |
| B. mohanramii P. Kumari and P. Singh | 5 | Meghalaya | Culm/branch cuttings |
| B. jaintiana R.B. Majumdar | 5 | Assam, Meghalaya, Bangladesh, Myanmar, Bhutan | Culm/branch cuttings |
| B. multiplex (Lour.) Raeusch. ex Schult | 5 | Meghalaya, Assam | Culm/branch cuttings/Offset plantings |
| B. teres Munro | 5 | Meghalaya, Arunachal Pradesh, Assam, Manipur, West Bengal | Culm/branch/rhizome cuttings |
| Dendrocalamus asper Baker ex Heynea | 6 | Exotic and cultivated in Northeast India |
Culm/branch cuttings Tissue culture plantlets |
| D. giganteus Munroa | 7 | Exotic and cultivated in Northeastern India and West Bengal |
Seedlings Culm/branch cuttings, rhizome/offset plantings |
| D. hamiltonii Ness and Arn ex Munroa | 8 | Central, Northeast India, Sikkim, West Bengal, Assam |
Seedlings Culm/branch cuttings, rhizome/offset plantings |
| D. strictus Nessa | 10 | Throughout India |
Seedlings Culm/branch cuttings, rhizome cuttings |
| D. stocksii (Munro) M. Kumar, Remesh and Unnikrishnan | 6 | Maharashtra, Karnataka, Kerala, Goa | Culm cuttings |
| D. longispathus (Kurz) Kurz | 5 | Meghalaya, Assam, Manipur, Mizoram, Tripura, West Bengal | Culm cuttings |
| D. brandisii (Munro) Kurz | 7 | Manipur, Burma | Culm/branch cuttings, rhizome/offset plantings |
| D. hookeri (Munro) | 7 | Meghalaya, Arunachal Pradesh, Assam, Manipur, Nagaland | |
| Melocanna baccifera Kurza | 8 | Northeast India |
Seedlings Rhizome cuttings |
| M. clarkei (Gamble ex Brandis) P. Kumari and P. Singh | 6 | Meghalaya, Assam, Manipur, Nagaland | Rhizome cuttings |
| Ochlandra travancorica Bentha | Kerala, Tamil Nadu |
Seedlings Rhizome cuttings |
|
| Oxytenanthera parviflora Brandis ex Gamblea | 5 | Assam, Mizoram | Rhizome cuttings |
| O. monadelpha (Thwaites) Alston | 4 | Maharashtra, Karnataka, Kerala, Tamil Nadu | Culm cuttings |
| O. stocksii (Munro) | 5 | Kerala, Karnataka, Maharashtra | Culm cuttings |
aNational Bamboo Mission (NBM) recommended priority bamboo species
DNA extraction, PCR amplification and sequencing
Total genomic DNA was extracted from either fresh or silica dried leaves using modified cetyltrimethylammonium bromide (CTAB) method (Doyle and Doyle 1990) as well as using DNeasy Plant Mini Kit (Qiagen, Germany) according to the manufacturer’s protocol. Seven candidate barcode loci of the plastid genome (four coding regions such as matK, rbcL, rpoB, rpoC1 and three intergenic spacers namely psbA-trnH, psbK-psbI and atpF-atpH were evaluated to identify discriminant DNA barcodes for bamboo species. Primer details and reaction conditions standardized for DNA amplification through polymerase chain reaction (PCR) are listed in Table S1.
Amplification of genomic DNA was performed in a PTC-100 thermocycler (BIO-RAD, India) in a final volume of 20 μL reaction mixture containing 50–100 ng DNA, 10X Taq buffer with 1.5 mM MgCl2, 200 μM dNTPs, 10 pm of each primer, and 2U Taq DNA polymerase (Invitrogen, Bangalore). The amplified products were resolved in 2 % agarose gel and documented using a gel documentation system (Syngene, UK). PCR products were further purified using a Nucleospin Gel and PCR Clean-up kit (Macherey Nagel, USA) and quantified using Nanodrop (Thermo Scientific, USA). Sequencing was performed using Sanger dideoxy chemistry in both forward and reverse directions (Chromous, Bangalore).
Sequence analysis
Chromatograms were edited and trimmed using BioEdit software (Hall 1999). Edited sequences were aligned using ClustalX of Clustal W packages (Thompson et al. 1994) and submitted to GenBank (https://www.ncbi.nlm.nih.gov/genbank/) as well as BOLD https://www.barcodinglife.org.
For pairwise genetic distance (PWG) method, interspecific as well as intraspecific genetic distances were determined by MEGA v.6.0 using Kimura two-parameter distance model (K2P) adopting complete deletion option (Tamura et al. 2013). The interspecific divergence between species was calculated using three parameters: (1) average interspecific distance, (2) average theta prime (θ') and (3) minimum interspecific distances. Intraspecific parameters such as (4) average intraspecific distance, (5) theta (θ) and (6) coalescent depth were also calculated to characterize intraspecific divergences (Meyer and Paulay 2005). Barcoding gap was calculated by plotting intraspecific distances against interspecific divergences for each species (Meier et al. 2006). A blind sampling test was performed with twelve samples of unknown identity to check the efficiency of the selected barcode region in discriminating bamboo species.
In the tree-based analysis, neighbor-joining (NJ) trees were constructed for the studied bamboo taxa using most discriminant psbA-trnH spacer barcode region, adopting K2P parameter in MEGA v.6.0. Bootstrap support was estimated with 1000 heuristic replicates (100 random addition cycles per replicate, with tree bisection reconnection and branch swapping) to test the reliability of inferred phylograms. All positions containing gaps and missing data were eliminated from dataset (complete deletion option).
Results
DNA barcode amplification, sequencing and alignment
An ideal DNA barcode is expected to have adequate conserved regions, high PCR amplification efficiency, and enough variability for species identification (CBOL Plant Working Group 2009). All the evaluated DNA barcode regions (matK, rbcL, rpoC1, rpoB, psbK-psbI, atpF-atpH and psbA-trnH) were successfully amplified with 100 % PCR efficiency using CBOL (2009) recommended primers (Fig. S1). The edited sequences after homology searches were deposited in the GenBank and accession numbers were provided (Table 2).
Table 2.
GenBank accession numbers and barcode regions of Dendrocalamus, Bambusa, Melocanna, Oxytenanthera genera
| Sl. no. | Barcode regions | Accession number | |||
|---|---|---|---|---|---|
| Genus Dendrocalamus | Genus Bambusa | Genus Melocanna | Genus Oxytenanthera | ||
| 1 | RbcL | MH1855639–MH185696 | MH170547–MH170611 | MH185450–MH185463 | MH256570–MH256587 |
| 2 | matK | MH185581–MH185638 | MH170482–MH170546 | MH238490–MH238503 | MH249834–MH249851 |
| 3 | rpoB | MH185523–MH185580 | MH185697–MH185761 | MH189392–MH189405 | MH249852–MH249869 |
| 4 | rpoC1 | MH185464–MH185522 | MH304513–MH304578 | MH241037–MH241050 | MH256588–MH256605 |
| 5 | atpF-atpH | MH185392–MH185449 | MH185269–MH185333 | MH240992–MH241005 | MH256606–MH256623 |
| 6 | psbK-psbI | MH185334–MH185391 | MH185204–MH185268 | MH240978–MH241005 | MH256624–MH256641 |
| 7 | psbA-trnH | MH230004–MH230061 | MH240913–MH240977 | MH234677–MH234690 | MH249816–MH249833 |
Six of the DNA barcode regions, viz., rbcL, matK, rpoB, rpoC1, psbK-psbI and atpF-atpH displayed exactly identical sequences in all analyzed species of Bambusa, Dendrocalamus, Oxytenanthera as well as Melocanna and Ochlandra. Hence, these DNA barcode regions cannot be useful for species certification of planting materials in bamboos. Multiple sequence alignment (MSA) of psbA-trnH intergenic spacer barcode region showed species-specific nucleotide differences in most studied bamboo taxa, viz., Bambusa, Dendrocalamus, Melocanna and Ochlandra. The blind sampling test carried out proved the effectiveness of this barcode region. Two specimens from each of the six bamboo species (Bambusa tulda, B. vulgaris, B. bambos, Dendrocalamus strictus, D. stocksii, Melocanna baccifera) randomly collected by a third party from a bamboo nursery maintained at KFRI, Peechi Campus were correctly identified to the species level. Additionally, the intergenic spacer psbK-psbI also showed species discrimination in the genus Melocanna.
Sequence length and basic sequence statistics like conserved sites, variable sites, singletons and informative sites of psbA-trnH spacer region based on CLUSTALX alignment as well as with alignment explorer in MEGA v.6.0 are provided in Table S2. The maximum number of variable sites among species was in genus Bambusa (23) followed by Dendrocalamus (12) and the least was in genus Melocanna (8).
Sequence analysis of Bambusa
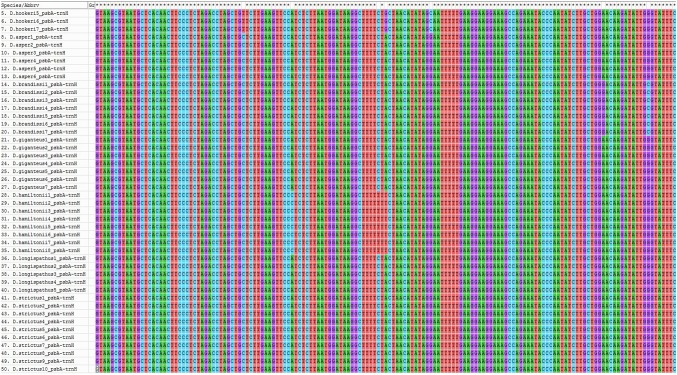
Multiple sequence alignment (MSA) of psbA-trnH barcode showed nucleotide differences unique to species in most cases (Fig. 1). The major nucleotide changes were transitions/transversions as well as insertions/deletions of nucleotides in the analyzed barcode region. In most species, deletions of mononucleotide thymine repeats in various numbers were obvious. Among ten species of the genus Bambusa, B. multiplex had maximum level of unique nucleotide changes. B. balcooa/B. vulgaris/B. pallida as well as B. nutans/B. teres shared the type of deletion of thymine mononucleotides along with other specific nucleotide changes. B. balcooa, B. tulda and B. vulgaris had a similar transition event (G>A). Similarly, B. nutans, B. tulda, B. vulgaris, B. multiplex and B. jaintiana had a similar transition event (C>T) (Table 3).
Fig. 1.
Multiple sequence alignment of psbA-trnH barcode region in the genus Bambusa
Table 3.
Nucleotide differences in psbA-trnH spacer region in the genus Bambusa
| Sl. no. | Species | Nucleotide changes in psbA-trnH sequence |
|---|---|---|
| 1 | B. nutans | Deletion of TTTTTT mononucleotide repeats |
| Transition—C > T | ||
| Transversion—T > G at 2 places | ||
| 2 | B. tulda | Deletion of TTTTT mononucleotide repeats |
| Transition—G > A at 2 places | ||
| Transversion—T > G at 2 places | ||
| 3 | B. balcooa | Deletion of TT mononucleotide repeats |
| Transition—G > A | ||
| 4 | B. vulgaris | Deletion of TT mononucleotide repeats |
| Transition—G > A | ||
| Transition—C > T | ||
| 5 | B. bambos | Deletion of TTT mononucleotide repeats |
| 6 | B. pallida | Deletion TT mononucleotide repeats |
| Insertion of mononucleotide T at 2 places | ||
| 7 | B. multiplex | Deletion of TTTGTTTGTTT sequences |
| Transition—C > T | ||
| Transversion—G > C | ||
| Transversion—C > A | ||
| 8 | B. jaintiana | Insertion of TTT in place of GTA |
| Transition C > T | ||
| Transversion G > C | ||
| 9 | B. teres | Deletion of TTTTTT mononucleotide repeats |
| 10 | B. mohanramii | Insertion of TT mononucleotide repeats |
| Transversion T > G at 2 places |
In distance-based analysis using psbA-trnH, basic statistical parameters (average interspecific distance, theta prime and minimum interspecific distance) were employed to characterize interspecific divergence. The intraspecific variations were calculated by employing average intraspecific distance, mean theta and coalescent depth (Table 4). Average interspecific distance was 0.0509 and DNA barcoding gap was 0.0485 for the genus Bambusa. Even though species-specific nucleotide differences could be identified for each Bambusa species, differences were located in the non-coding intergenic spacer regions or in mononucleotide repeats.
Table 4.
Genetic divergence parameters in the genus Bambusa, Dendrocalamus and Oxytenanthera using MEGA v 6.0
| Parameters | psbA-trnH | ||
|---|---|---|---|
| Bambusa | Dendrocalamus | Oxytenanthera | |
| Average intraspecific distance | 0.0024 ± 0.0010 | 0.0003 ± 0.0002 | 0.0000 ± 0.0000 |
| Average theta | 0.0008 ± 0.0003 | 0.0015 ± 0.0001 | 0.0000 ± 0.0000 |
| Average coalescent depth | 0.0004 ± 0.0001 | 0.0022 ± 0.0026 | 0.0000 ± 0.0000 |
| Average interspecific divergence | 0.0509 ± 0.0012 | 0.0267 ± 0.0006 | 0.0014 ± 0.0006 |
| Minimum interspecific distance | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 |
| Average theta prime | 0.0086 ± 0.0033 | 0.0043 ± 0.0021 | 0.0060 ± 0.0002 |
| Barcoding gap | 0.0485 ± 0.0002 | 0.0264 ± 0.0004 | 0.0014 ± 0.0006 |
Sequence analysis of Dendrocalamus
Out of the seven analyzed barcode regions, only psbA-trnH showed species-specific nucleotide differences in the genus Dendrocalamus (Fig. 2). Basic statistical parameters used to characterize interspecific and intraspecific distances are provided in Table 4. The average interspecific distance was 0.0267 and DNA barcoding gap was 0.0264 for the genus Dendrocalamus.
Fig. 2.
Multiple sequence alignment of psbA-trnH barcode region in the genus Dendrocalamus
Unlike in Bambusa, Dendrocalamus species had a lower number of nucleotide changes and also shared some of the nucleotide changes. Both transitional and transversional nucleotide changes were present only in D. hookeri in addition to two major deletions. Transversion of G > C was observed both in D. hookeri and D. brandisii. An inversion of GTA nucleotides was specifically observed in psbA-trnH sequence of D. stocksii. Both D. asper and D. longispathus showed only a deletion of T mononucleotide (Table 5).
Table 5.
Nucleotide differences in psbA-trnH spacer region in the genus Dendrocalamus using MEGA v 6.0
| Sl. no. | Species | Nucleotide differences |
|---|---|---|
| 1 | D. strictus | Deletion of G nucleotide |
| 2 | D. stocksii | Insertion of AA nucleotides |
| Inversion of GTA to ATG | ||
| 3 | D. hookeri | Deletion of GTATTTG nucleotides |
| Deletion of GTTTT nucleotides | ||
| Insertion of T nucleotide | ||
| Transition—A>G | ||
| Transversion—G> C | ||
| 4 | D. giganteus | Deletion of GTTTTT mononucleotide repeats |
| 5 | D. hamiltonii | Deletion of TTT mononucleotide repeats |
|
6 7 |
D. asper D. longispathus |
Deletion of T in mononucleotide repeats Deletion of T in mononucleotide repeats |
| 8 | D. brandisii | Deletion of GTTTTT mononucleotide repeats |
| Insertion of TG nucleotides | ||
| Transversion G > C |
Sequence analysis of Melocanna and Ochlandra
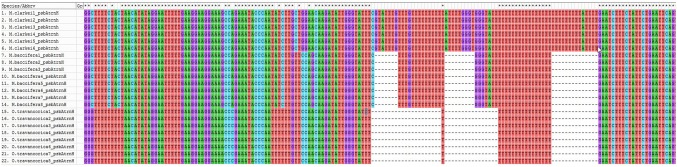
Among genus Melocanna and Ochlandra of subtribe Melocanninae, only psbA-trnH and atpF-atpH spacer regions showed nucleotide differences which were species specific out of the seven analyzed barcode regions. Both M. baccifera and M. clarkei differed in terms of C>T and T>C transitions and G>C transversion in their psbA-trnH sequences. Additionally, M. baccifera had two specific deletions of GTATTG and TTATTTT sequences (Fig. 3). psbA-trnH sequence of O. travancorica was more similar to M. clarkei than M. baccifera. Both O. travancorica and M. clarkei shared G nucleotide at two sites, whereas it had undergone a transversional change (G>C) in M. baccifera. Similarly, a cytosine and an adenine nucleotide present in O. travancorica and M. clarkei, respectively, had undergone transitional changes in M. baccifera (C>T and A>G). On the contrary, both O. travancorica and M. baccifera shared three major deletions such as GTATTTG, ATT and GTGGGTATTTTTTTTTT (Fig. 3). Even though, two genera shared many nucleotide changes among them, each of them had unique species-specific nucleotide changes as well. Statistical parameters were employed to characterize interspecific divergence and average intraspecific distances (Table 6).
Fig. 3.
Multiple sequence alignment of psbA-trnH barcode region in the genera Ochlandra and Melocanna
Table 6.
Genetic divergence parameters in the genus Melocanna using MEGA v 6.0
| Parameters | psbA-trnH | atpF-atpH |
|---|---|---|
| Average intraspecific distance | 0.0062 ± 0.0060 | 0.0012 ± 0.0009 |
| Average theta | 0.0015 ± 0.0015 | 0.0013 ± 0.0011 |
| Average coalescent depth | 0.0034 ± 0.0023 | 0.0016 ± 0.0007 |
| Average interspecific divergence | 0.0125 ± 0.0045 | 0.0022 ± 0.0015 |
| Minimum interspecific distance | 0.0000 ± 0.0000 | 0.0000 ± 0.0000 |
| Average theta prime | 0.0073 ± 0.0031 | 0.0020 ± 0.0016 |
| Barcoding gap | 0.0063 ± 0.0045 | 0.0010 ± 0.0006 |
Sequence analysis of Oxytenanthera
Among seven analyzed barcode regions, only psbA-trnH barcode showed species-specific nucleotide differences in the genus Oxytenanthera. Species-specific differences were mostly in mononucleotide repeats of psbA-trnH, which consist of three base pair and single base pair deletions, respectively, in O. monadelpha and O. parvifolia (Fig. 4). Statistical parameters to characterize interspecific/intraspecific distances were employed (Table 4).
Fig. 4.
Multiple sequence alignment of psbA-trnH barcode region in the genus Oxytenanthera
Tree-based analysis
In the tree-based analysis, ten species of the genus Bambusa showed species-specific clustering with two major groups except for B. balcooa and B. teres (Fig. 5). Similarly, out of the seven species in genus Dendrocalamus, three species such as D. asper, D. giganteus and D. longispathus were clustered together as a complex, while the remaining species formed monophyletic clusters with greater than 60 % bootstrap support (Fig. S2). Three taxa of Melocanninae subtribe such as M. clarkei, M. baccifera and O. travancorica formed monophyletic clusters with 64 %, 96 % and 99 % bootstrap support values, respectively, thus psbA-trnH spacer region can be effectively utilized as a discriminant barcode for these species (Fig. S3). Similarly, O. parviflora and O. monadelpha grouped into two separate monophyletic clusters with 100 % bootstrap support (Fig. S4).
Fig. 5.
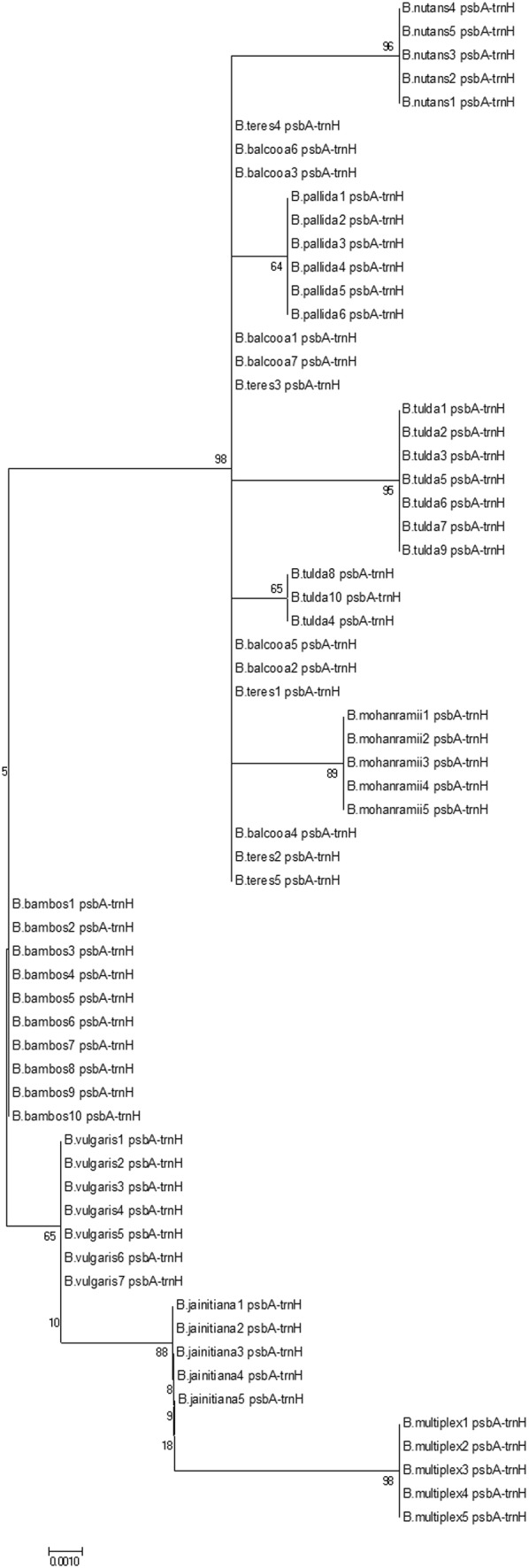
Neighbor-joining tree of selected Bambusa species
Discussion
Bamboo is very fast growing with 20 times more yield than any other timber tree species and is the most preferred industrial raw material (https://life.gaiam.com/article/how-eco-friendly-bamboo). Over the last few decades, commercial bamboo plantations in India have significantly increased and the planters are really concerned about reliability and identity of species/clonal material multiplied in nurseries all over India. Among the hurdles that nursery managers face in producing quality planting material, the difficulty in precise identification of species is particularly vexing. A further confounding problem is the relative lack of discriminatory morphological features in juvenile plants maintained in nurseries. The misidentification of species suitable for different agro-climatic zones can lead to a significant decline in the productivity (Sharma 2008). It can also lead to significant loss to farmers if wrong species identity is discovered much later especially when the species is unsuitable for a specific end use for which it was grown. This has necessitated the need for a certification agency at national level and with Bamboo Technical Support Group (BTSG), Kerala Forest Research Institute (KFRI) proposed to National Bamboo Mission (NBM), Government of India, a certification framework and guidelines and recommends an integrated approach which includes DNA barcoding for precise species identification (BTSG 2014). A species-specific DNA barcode thus can serve as a valid certification tool to ensure species identity and productivity in the commercial bamboo plantations.
Out of the seven recommended DNA barcode regions evaluated, six DNA barcodes were substantially the same across all species of genera Bambusa, Dendrocalamus, Ochlandra and Melocanna. Because of low ability for species discrimination, most working groups had suggested the use of rbcL in conjunction with other gene regions (Chase et al. 2007; Hollingsworth et al. 2009). Similarly, matK had proved its utility as a potential barcode in closely related groups, such as Compsoneura (Newmaster et al. 2007), orchids (Lahaye et al. 2008), sedges (Starr et al. 2009) and Acacia (Newmaster and Ragupathy 2009), but universality of this barcode region remains uncertain in various taxa. In this study, rbcL and matK could not differentiate bamboo species. Even though rpoB and rpoC1 has been recommended as suitable barcodes, due to low interspecific divergence, these barcode regions were reported as inappropriate supplementary barcode loci (Lahaye et al. 2008). The present barcode analysis also revealed low discriminatory power of rpoB and rpoC1. In addition to the candidate barcode regions described above, other plastid barcoding regions such as atpF-atpH, psbK-psbI and trnT-trnL were also recommended for species identification (Taberlet et al. 2007). psbA-trnH has the potentiality as a suitable marker for species discrimination between closely related taxa due to high rate of sequence variation present generally in this intergenic spacer region (Kress and Erickson 2007; Newmaster et al. 2007). It has also been recommended as one of the best performing loci for various taxa in terms of PCR amplification success, sequencing and species resolution (Lahaye et al. 2008). In the present analysis, species-specific nucleotide differences were observed in psbA-trnH barcode region of genera, Bambusa and Dendrocalamus, Ochlandra and Melocanna. Thus, psbA-trnH can serve as a DNA barcode region for species identification of various bamboo taxa taken up for this study.
Bambusa balcooa and B. vulgaris shared the same type of deletion in thymine mononucleotides. These species are widely cultivated and morphological features are greatly influenced by selection process. Both the species are different in the absence of transverse veinlets in lemma with ovate oblong lodicules in the former and presence of transverse veinlets in lemma with narrowly oblong lodicules in the latter. B. vulgaris formed a very distinct cluster in tree-based analysis. Likewise, B. balcooa and B. tulda had a similar transversion event (G > A). In B. balcooa and B. tulda, inflorescence is clustered at nodes and glumes persistent and shorter than spikelet. In NJ tree, B. balcooa, B. tulda and B. teres grouped together into a complex. Both B. teres and B. balcooa are arborescent densely tufted clump-forming species with glabrous culm sheaths. Even though B. teres is distinct in having glabrous culm sheath proper, similar auricles erect at top of sheath proper, long ciliate ligule having white hair underneath blade, and dense hair at incurved leaf apex, Majumdar (1989) treated B. teres under synonym of B. tulda. B. tulda is a widely distributed species and morphologically highly variable in vegetative and reproductive characters.
Morphologically distinct species like B. multiplex, B. mohanramii, B. jaintiana as well as B. pallida displayed unique barcodes and also showed distinct species-specific clusters in the derived NJ tree. In mature state, B. pallida is a quite distinct species with triangular culm sheath with sheath proper truncately cut at top, long imperfect blade as broad as top of sheath proper and lanceolate spikelets with 3–8 fertile florets. B. jaintiana is a shrubby erect bamboo found in loose clumps. Culms are green becoming orange with age and while young ones are white powdery. B. mohanramii differs remarkably in other vegetative and floral aspects like culm sheath short than internodes, auricles with short rounded shape with somewhat matching culm sheath to B. balcooa (Kumari and Singh 2009). Bambusa multiplex is a morphologically variable widely cultivated perennial species with slender and erect woody culms, nodal roots, bractiferous inflorescence and caryopsis fruit. Among ten species of the genus, B. multiplex had maximum level of unique nucleotide changes.
Dendrocalamus brandisii and D. giganteus had same type of GTTTTT nucleotide deletions and both of which have morphologically prominent auricles in culm sheath and its sheath is glabrous. Some unique nucleotide changes present in D. stocksii such as inversion of GTA to ATG and insertion of AA nucleotides are absent in other Dendrocalamus species. D. stocksii was initially known as Oxytenanthera stocksii which was shifted later on to genus Dendrocalamus based on similar morphological features such as basal nodes bearing aerial roots, erect culms and short internodes, large panicle of spicate heads, keeled palea, among others (Kumar et al. 2004). D. brandisii with its unique nucleotide changes is a morphologically distinct species with its mature culm smooth ashy-gray to greenish-gray colored, loosely spaced and thornless which formed a well-defined clade in the tree-based analysis. D. asper, D. giganteus and D. longispathus have morphologically distinct characteristics but they grouped together in the phylogenetic tree generated.
So far, only universal molecular markers were used for species/cultivar certification of tree species. For example, RAPD markers were employed for certification of Populus species (Sanchez et al. 1998), ISSR markers for Picea species (Nkongolo et al. 2005), Eucalyptus species (Balasaravanan et al. 2006), certification of lupine cultivars (Nam et al. 2014) as well as for genetic fidelity testing in Saccharum officinarum (Thorat et al. 2018). A reliable and affordable certification tool based on SSRs was reported for the certification of chestnut varieties to prevent its commercial misuse (Botta et al. 2001), commercial cultivars of Populus (de-Lucas et al. 2007), characterization of olive cultivars (Muzzalupo et al. 2009), registration and certification of planting materials in Eucalyptus (Torres-Dini et al. 2011), to differentiate Chilean and foreign commercial rice varieties (Becerra et al. 2015), discrimination of Panax species/cultivars (Jo et al. 2016) and for the certification of Albania olive (Muzzalupo et al. 2018).
Forest certification schemes, state agencies such as customs offices, forest enterprises producing timber have relied on molecular methods to improve the traceability of timber and offering opportunities to identify false declarations of timber origin (Finkeldey et al. 2010). Even though, the necessity of a viable molecular method for the certification of planting material through vegetative propagation has been discussed and suggested by various research groups (Alvarez et al. 2001; Rajora and Rahman 2003; Fossati et al. 2005), no reports are available on actual use and implementation of DNA barcode tool for certification of vegetative propagation material.
Conclusion
A molecular tool that is not influenced by age, phenological and physiological status, is useful for species certification of planting stock produced in bamboo nurseries. This study could demonstrate the efficiency of DNA barcoding as a reliable supplementary tool in an integrated approach for the proposed certification system in bamboos. psbA-trnH DNA barcode region can thus be utilized to authenticate species identity against a database of barcodes of bamboo species generated for this purpose. The tool can be integrated into the framework to comply with requirement for confirming species identity under the certification program and give farmers an assurance of the quality intended in label issued by accredited nurseries. To achieve assured productivity in bamboo species, any national certification agency set up for this purpose can utilize psbA-trnH DNA barcode region to tag species identity and to prove the authenticity of multiplied planting materials in all NBM recommended priority bamboo species.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1 PCR amplification of psbA-trnH [Lane: 1—Bambusa, 2—Dendrocalamus, 3—Oxytenanthera, 4—Melocanna, 5—Ochlandra, 6-100 bp ladder (M)] (TIF 3525 kb)
Figure S2 Neighbor-joining tree of selected Dendrocalamus species (TIF 25821 kb)
Figure S3 Neighbor-joining tree of selected Ochlandra and Melocanna taxa (TIF 11168 kb)
Figure S4 Neighbor-joining tree of selected Oxytenanthera species (TIF 10374 kb)
Acknowledgements
We are grateful to the Forest Department (KFD), Govt. of Kerala and Karnataka as well as Forest Departments of the States of Arunachal Pradesh, Meghalaya, Mizoram for their permission to collect leaf samples. The financial support received from National Bamboo Mission (NBM), Govt. of India is gratefully acknowledged.
Author contributions
SAD: developing the concept, getting financial support, designing the wet lab experiments and writing paper. SK: conducting wet lab experiments and writing paper. PPS: conducting wet lab experiments. SVB: involved in sample collection and writing paper. EMM: involved in sample collection and writing paper.
Funding
National Bamboo Mission (NBM), Government of India Grant (F.NO. 44-73/2018-NBM/KFRI 688.12/2014).
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
Data archiving statements
All the generated barcode gene sequences are submitted to GenBank and are available in the following (https://www.ncbi.nlm.nih.gov/genbank/). GenBank accession numbers are provided in Table 2.
References
- Alvarez A, Cervera MT, Agundez D, Alba N, Antonanzas GR, Zapater JM, Grau JM. Aplicacion de la tecnica AFLPs para la identificacion de clones del Género Populus. Zamora: Simposio del Chopo; 2001. pp. 381–389. [Google Scholar]
- Balasaravanan T, Chezhian P, Kamalakannan R, Yasodha R, Varghese M, Gurumurthi K, Ghosh M. Identification of species-diagnostic ISSR markers for six Eucalyptus species. Silvae Genetica. 2006;55:1–6. doi: 10.1515/sg-2006-0017. [DOI] [Google Scholar]
- Becerra V, Paredes M, Gutiérrez E, Rojo C. Genetic diversity, identification, and certification of Chilean rice varieties using molecular markers. Chilean J Agri Res. 2015 doi: 10.4067/S0718-58392015000400001. [DOI] [Google Scholar]
- Botta R, Marinoni D, Beccaro G, Akkak A, Bounous G. Development of a DNA typing technique for the genetic certification of the chestnut cultivars. For Snow Landsc Res. 2001;76:425–428. [Google Scholar]
- Botta R, Marinoni DT, Bounous G (2004) Molecular Markers and Certification, Proceedings from the Workshop Biotecnolegia Forestal, Global Biotechnology Forum, Chile, 63–72.
- BTSG (2014) Certification of Bamboo Planting Material for Area Expansion Programme under National Bamboo Mission, Bamboo Technical Support Group- KFRI, Kerala Forest Research Institute, Peechi. https://nbm.nic.in/PDF/CertificationNBM15.05.2014.pdf.
- Cai ZM, Zhang YX, Zhang LN, Gao LM, Li DH. Testing four candidate barcoding markers in temperate woody bamboos (Poaceae: Bambusoideae) J Syst Evol. 2012;50(6):527–539. doi: 10.1111/j.1759-6831.2012.00216.x. [DOI] [Google Scholar]
- Cbol PWG. A DNA barcode for land plants. Proc Natl Acad Sci USA. 2009;106(31):12794–12797. doi: 10.1073/pnas.0905845106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase Mark W., Cowan Robyn S., Hollingsworth Peter M., van den Berg Cassio, Madriñán Santiago, Petersen Gitte, Seberg Ole, Jørgsensen Tina, Cameron Kenneth M., Carine Mark, Pedersen Niklas, Hedderson Terry A.J., Conrad Ferozah, Salazar Gerardo A., Richardson James E., Hollingsworth Michelle L., Barraclough Timothy G., Kelly Laura, Wilkinson Mike. A proposal for a standardised protocol to barcode all land plants. TAXON. 2007;56(2):295–299. doi: 10.1002/tax.562004. [DOI] [Google Scholar]
- Clark LG, Dransfield S, Triplett JK, Sanchez-Ken JG. Phylogenetic relationships among the one-flowered, determinate genera of Bambuseae (Poaceae: Bambusoideae) Aliso. 2007;23(1):315–332. doi: 10.5642/aliso.20072301.26. [DOI] [Google Scholar]
- Das MM, Mahadani P, Singh R, Karmakar K, Ghosh SK. MatK sequence based plant DNA Barcoding failed to identify Bambusa (Family: Poaceae) species from Northeast India. J Env Sociobiol. 2013;10(1):49–54. [Google Scholar]
- Degen B, Holtken A, Rogge M. Use of DNA-fingerprints to control the origin of forest reproductive material. Silvae Genetica. 2010;59:268–273. doi: 10.1515/sg-2010-0038. [DOI] [Google Scholar]
- de-Lucas AI, Santana JC, Recio P, Hidalgo E. SSR-based tool for identification and certification of commercial Populus clones in Spain. Ann For Sci. 2007;65(1):107. doi: 10.1051/forest:2007079. [DOI] [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Finkeldey R, Leinemann L, Gailing O. Molecular genetic tools to infer the origin of forest plants and wood. Appl Microbiol Biotechnol. 2010;85:1251–1258. doi: 10.1007/s00253-009-2328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FSI . State of forest report. Dehradun: Forest Survey of India (Ministry of Environment & Forests); 2011. [Google Scholar]
- Fossati T, Zapelli I, Bisoffi S, Micheletti A, Vietto L, Sala F, Castiglione S. Genetic relationships and clonal identity in a collection of commercially relevant poplar cultivars assessed by AFLP and SSR. Tree Genet Genom. 2005;1:11–19. doi: 10.1007/s11295-004-0002-9. [DOI] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis: Department of Microbiology. Raleigh: North Carolina State University; 1999. [Google Scholar]
- Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci USA. 2004;101:14812–14817. doi: 10.1073/pnas.0406166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth PM, Clark A, Forrest LL, Richardson J, Pennington RT. Selecting barcoding loci for plants: evaluation of seven candidate loci with species-level sampling in three divergent groups of land plants. Mol Ecol Resour. 2009;9:439–457. doi: 10.1111/j.1755-0998.2008.02439.x. [DOI] [PubMed] [Google Scholar]
- Jo IH, Kim YC, Kim DH, Kim KH, Hyun TK, Ryu H, Bang KH. Applications of molecular markers in the discrimination of Panax species and Korean ginseng cultivars (Panax ginseng) J Ginseng Res. 2016;41(4):444–449. doi: 10.1016/j.jgr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelchner SA, Clark LG. Molecular evolution and phylogenetic utility of the rpl16 intron in Chusquea and the Bambusoideae (Poaceae) Mol Phylogenet Evol. 1997;8(3):385–397. doi: 10.1006/mpev.1997.0432. [DOI] [PubMed] [Google Scholar]
- Khan AU, Hazra A. Industrialisation of the bamboo sector: Challenges and Opportunities. India Development Foundation, Publication 15. New Delhi: Confederation of Indian Industry (CII); 2007. [Google Scholar]
- Konnert M, Behm A. Proof of identity reproductive material based on reference samples. Mitteilungen der Bundesforschungsanstalt für Forst- und Holzwirtschaft Hamburg. 2006;221:61–71. [Google Scholar]
- Kress WJ, Erickson DL. A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS ONE. 2007;2(1):e508. doi: 10.1371/journal.pone.0000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M. Mass production of field planting stock of Dendrocalamus strictus through macroproliferation—a technology. Ind For. 1991;117(12):146–152. [Google Scholar]
- Kumar M, Rajesh G, Sudheesh KG. Above ground biomass production and nutrient uptake of thorny bamboo [Bambusa bambos (L.) Voss] in the home gardens of Thrissur, Kerala. J Tropical Agri. 2005;43:51–56. [Google Scholar]
- Kumar M, Remesh N, Unnikrishnan N. A new combination in Dendrocalamus (Poaceae: Bambusoideae) SIDA Contrib Bot. 2004;21:93–96. [Google Scholar]
- Kumari P, Singh P. Two new species of Bambusa (Poaceae) from India. Kew Bull. 2009;64:565–569. doi: 10.1007/s12225-009-9140-4. [DOI] [Google Scholar]
- Lahaye R, van der Bank M, Bogarin D, Warner J, Pupulin F. DNA barcoding the floras of biodiversity hotspots. Proc Natl Acad Sci USA. 2008;105(8):2923–2928. doi: 10.1073/pnas.0709936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar RB. Bambusoideae. In: Karthikeyan S, Jain SK, Nayar MP, Sanjappa M, editors. Florae Indicae Enumeratio: Monocotyledoneae. Calcutta: Botanical Survey of India; 1989. pp. 254–274. [Google Scholar]
- Meier R, Shiyang K, Vaidya G, Ng PK. DNA barcoding and taxonomy in Diptera: a tale of high intraspecific variability and low identification success. Syst Biol. 2006;55(1):715–728. doi: 10.1080/10635150600969864. [DOI] [PubMed] [Google Scholar]
- Meyer CP, Paulay G. DNA barcoding: error rates based on comprehensive sampling. PLoS Biol. 2005;3(12):2229–2238. doi: 10.1371/journal.pbio.0030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzalupo I, Muto A, Badolati G, Veizi A, Chiappetta A. Genotyping of Albania olive (Olea europaea) germplasm by SSR molecular marker. Emir J Food Agric. 2018;30(7):573–580. doi: 10.9755/ejfa.2018.v30.i7.1740. [DOI] [Google Scholar]
- Muzzalupo I, Stefanizzi F, Perri E. Evaluation of olives cultivated in southern Italy by Simple Sequence Repeat Markers. Hort Science. 2009;44:582–588. doi: 10.21273/HORTSCI.44.3.582. [DOI] [Google Scholar]
- Nam IY, Zayakin VV, Artjuchova AV, Lukashevich MI, Kupzov NS. Identification and certification of lupine cultivars using molecular markers. World Appl Sci J. 2014;30:796–801. doi: 10.5829/idosi.wasj.2014.30.07.14076. [DOI] [Google Scholar]
- NBM (National Bamboo Mission) (2013) This information is retrieved on 17 December 2013 from https://www.nbm.nic.in
- Newmaster SG, Fazekas AJ, Steeves RAD, Janovec J. Testing candidate plant barcode regions in the Myristicaceae. Mol Ecol Notes. 2007;8(3):480–490. doi: 10.1111/j.1471-8286.2007.02002.x. [DOI] [PubMed] [Google Scholar]
- Newmaster SG, Ragupathy S. Testing plant barcoding in a sister species complex of pantropical Acacia (Mimosoideae, Fabaceae) Mol Ecol Res. 2009;9:172–180. doi: 10.1111/j.1755-0998.2009.02642.x. [DOI] [PubMed] [Google Scholar]
- Nkongolo KK, Michael P, Demers T. Application of ISSR, RAPD, and cytological markers to the certification of Picea mariana, P. glauca, and P. engelmannii trees, and their putative hybrids. Genome. 2005;48(2):302–311. doi: 10.1139/g04-118. [DOI] [PubMed] [Google Scholar]
- Ohrnberger D, Georrings J. The bamboos of the World. Dehra Dun: International Book Publishers; 1986. [Google Scholar]
- Rajora OP, Rahman MH. Microsatellite DNA and RAPD fingerprinting, identification and genetic relationships of hybrid poplar (Populus × canadensis) cultivars. Theoretical Appl Genet. 2003;106:470–477. doi: 10.1007/s00122-002-1082-2. [DOI] [PubMed] [Google Scholar]
- Sanchez N, Grau JM, Bueno MA. RAPD markers for the identification of Populous species. Silvae Genet. 1998;47:67–71. [Google Scholar]
- Sharma A (2008) Bamboo Industry Eyes Slice of $7.5 Bn World Market. The Financial Express, p,20. https://www.financialexpress.com/news/bamboo-industry-eyes-slice-of-7.5-bn-worldmkt/299457/
- Sosa V, Mejia-Saules T, Cullar MA. DNA barcoding in endangered Mesoamerican groups of plants. Bot Rev. 2013;79:469. doi: 10.1007/s12229-013-9129-4. [DOI] [Google Scholar]
- Starr JR, Naczi RFC. Plant DNA barcodes and species resolution in sedges (Carex, Cyperaceae) Mol Ecol Res. 2009 doi: 10.1111/j.1755-0998.2009.02640.x. [DOI] [PubMed] [Google Scholar]
- Taberlet P, Coissac E, Pompanon F, Gielly L, Miquel C. Power and limitations of the chloroplast trnL (UAA) intron for plant DNA barcoding. Nuc Acids Res. 2007;35(3):e14. doi: 10.1093/nar/gkl938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA 6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Dini D, Bennadji Z, Cabrera M, Centurion C, Resquin F, Balmelli G. Use of SSR-tools for clone certification in Uruguayan Eucalyptus grandis and Eucalyptus dunnii breeding programs. BMC Proc. 2011;5(Suppl 7):P58. doi: 10.1186/1753-6561-5-S7-P58. [DOI] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nuc Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorat AS, Sonone NA, Choudhari VV, Devarumath RM, Babu KH. Plant regeneration from direct and indirect organogenesis and assessment of genetic fidelity in Saccharum officinarum using DNA based markers. Biosci Biotech Res Commun. 2018 doi: 10.21786/bbrc/11.1/9. [DOI] [Google Scholar]
- Tripathi SK, Sumida A, Shibata H, Ono K, Uemura S, Kodama Y, Hara T. Leaf litter fall and decomposition of different above and below ground parts of birch (Betula ermanii) trees and dwarf bamboo (Sasa kurilensis) shrubs in a young secondary forest in Northern Japan. Biol Fertili Soils. 2006;43(2):237–436. doi: 10.1007/s00374-006-0100-y. [DOI] [Google Scholar]
- Zhang YX, Xu YX, Ma PF, Zhang LN, Li DZ. Selection of potential plastid DNA barcodes for Bambusoideae (Poaceae) Plant Divers Resour. 2013;35(6):743–750. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 PCR amplification of psbA-trnH [Lane: 1—Bambusa, 2—Dendrocalamus, 3—Oxytenanthera, 4—Melocanna, 5—Ochlandra, 6-100 bp ladder (M)] (TIF 3525 kb)
Figure S2 Neighbor-joining tree of selected Dendrocalamus species (TIF 25821 kb)
Figure S3 Neighbor-joining tree of selected Ochlandra and Melocanna taxa (TIF 11168 kb)
Figure S4 Neighbor-joining tree of selected Oxytenanthera species (TIF 10374 kb)