Abstract
Background and study aims Electrochemotherapy is an anticancer treatment that uses electric pulses to facilitate uptake of chemotherapeutic drugs in tumor cells and has proven to have a high local cytotoxic effect with minimal adverse events. Electrochemotherapy has mostly been used in treatment of cutaneous metastases but development of a new endoscopic electrode device has made treatment of colorectal tumors possible. This first-in-man multicenter phase I study investigated safety and efficacy of electrochemotherapy using endoscopic electroporation in patients with colorectal tumors.
Patients and methods Seven patients with colorectal tumors who were deemed ineligible for or had declined standard treatment were included. They were treated with bleomycin either intratumorally or intravenously and the electric pulses were delivered through the endoscopic electrode device. Safety and efficacy were assessed clinically and by scans immediately after treatment and adverse events were reported. Response was evaluated up to 6 months after treatment by scans (magnetic resonance imaging or computed tomography) and endoscopic examinations.
Results Seven patients aged 62 to 88 years with multiple comorbidities were included and had one or two treatments each. Post-treatment scans showed tumor responses in the treated areas and no damage to surrounding tissues. Only a few grade one adverse events were reported. Three patients had preoperative rectal bleeding, of which two reported cessation of bleeding and one reported decreased bleeding.
Conclusion This first-in-man study shows that electrochemotherapy for colorectal tumors using the endoscopic electrode device can induce local tumor response and is safe also for fragile elderly patients with comorbidities.
Introduction
Colorectal cancer is the third most commonly diagnosed cancer in both men and women worldwide with approximately 1.4 million new cases every year 1 . Standards of treatment include surgery, radiotherapy, and oncological treatment (chemotherapy and antineoplastic antibodies). Most new cases of colorectal cancers are in older patients. In 2014, 64 % to 68 % of new colorectal cases in the United States were men and women aged 65 years and older 2 .
Electrochemotherapy is an emerging and effective treatment that utilizes the effect of electric pulses to increase uptake of chemotherapy in cancer cells. Short high-voltage pulses are delivered locally to the tumor, which transiently permeabilizes cell membranes, enabling otherwise non-permeant chemotherapeutic drugs to enter the cancer cells 3 4 5 . The most widely used chemotherapeutic drug for electrochemotherapy is bleomycin. Bleomycin is a large, hydrophilic and charged molecule without a specific cellular uptake mechanism, but intracellularly, it is highly cytotoxic because of its ability to create several single- and double-strand DNA breaks 6 . When electric pulses facilitate the uptake of bleomycin, the cytotoxic effect is enhanced by more than 300-fold 7 8 9 .
Electrochemotherapy has mostly been used in treatment of cutaneous and subcutaneous tumors as these are easily accessible for electrodes. This has shown an objective response in approximately 85 % for tumors 3 cm or less after only one treatment and regardless of histology 10 11 12 13 14 15 16 17 18 19 . Electrochemotherapy has also been shown to be an easily tolerated treatment, especially for elderly patients 20 21 .
Development of new electrodes has now made it possible to start investigations of electrochemotherapy to internal tumors as well 22 23 24 25 . A new endoscopic vacuum electrode device has been developed to treat gastrointestinal tumors with electric pulses 26 . This device has been tested in preclinical studies in porcine and canine models and has demonstrated that it is safe and resulted in complete regression of colorectal tumors in two out of two treated dogs with no relapse after 2 years 26 .
This was a first-in-man clinical study using an endoscopic electrode device for electrochemotherapy on inoperable colorectal tumors. This article describes the experiences and results from the first seven treated patients.
Patients methods
Setting
This was an exploratory phase 1 multicenter study examining electrochemotherapy in seven patients with inoperable colorectal cancer using an endoscopic electrode device in combination with bleomycin. Endpoints were to investigate tumor regression and assess the safety of the treatment. Patients were recruited at Mercy University Hospital, Cork, Ireland, St Vincent’s Hospital, Dublin and Copenhagen University Hospital Herlev and Gentofte, Denmark. The protocol was approved by the Irish Medicines Board and the Danish Medicines Agency, the Regional Ethics Committees and the Danish Data Protection Agency. Clinicaltrials.gov identifier: NCT01172860.
Endpoints
Endpoints were to investigate tumor regression via magnetic resonance imaging (MRI) (If MR not possible then computed tomography [CT] or endoscopic images) at 3 and 6 months post the initial procedure and assess the safety of the treatment by reviewing adverse events (AEs) as they arose. Safety evaluation included report of tissue perforation, inflammatory response, electrode device issues and any AEs.
Patients
Eligible patients had a histologically verified colorectal tumor and were reviewed by a multidisciplinary team who found no further standard treatments available and considered enrolment in this study to be the most appropriate option. Patients could also be included if they refused to undergo offered standard treatment options. Other inclusion criteria were: age ≥ 18 years, WHO performance status ≤ 2 27 or Karnofsky > 60 % 28 , treatment-free interval of at least 2 weeks (4 weeks if last treatment included bevacizumab), patients deemed capable of understanding the given information and written informed consent. Ineligiblity criteria included non-correctable coagulation disorder, s-creatinine > 150 µmol/L and with chrome-EDTA-clearance < 40 mL/min, severe lung disease, highly inflamed colon tissue with ulceration and bleeding, implanted stent in colon, previously allergic reaction to bleomycin, previous treatment with bleomycin with a cumulative dose of 200,000 units/m 2 , participation in another clinical trial, any other clinical condition or previous treatment that, in the investigators opinion, made the patient ineligible, and pregnancy or lactation in women (s-HCG mandatory). Women of childbearing potential and sexually active men had to use adequate contraception during and up to 6 months after last treatment.
Screening
When informed consent was signed, patients were screened with blood samples, chrome-EDTA clearance (only if s-creatinine was > 150 µmol/L), and endoscopic examination was performed. If screening met the inclusion criteria and an anesthetist assessed the patients eligible for anesthesia, the patients were included.
Bowel preparation
Bowel preparation was performed with either enema or Citrafleet and Toilax.
Anesthesia
Patients were anesthetized to alleviate discomfort from manipulation and pain from muscle contractions created by the electric pulses. They were sedated with propofol and fentanyl if not recommended otherwise by the anesthetist. To prevent lung toxicity from bleomycin, the fraction of inspired oxygen was limited to 30 % during anesthesia as standard operating procedure 29 .
Injection of chemotherapy
Bleomycin was administered intravenously (IV) or intratumorally (IT) depending on what was considered most suitable for the patient by the treating doctors. IV bleomycin was administered in fast drip; 15,000 IU/m 2 body surface (Denmark: bleomycin Baxter; Ireland: MaynePharma) diluted in 250 mL isotonic sodium chloride. Pulses were delivered, starting from 8 minutes after injection, according to ESOPE (European Standard Operating Procedure on Electrochemotherapy) 29 30 . IT bleomycin was administered intratumorally; 1,000 IU/mL with dose of 250 IU/cm 3 for tumors larger than 1 cm 3 , 500 IU for tumors smaller than 1 cm 3 but larger than 0.5 cm 3 , and 1000 IU/cm 3 for tumors smaller than 0.5 cm 3 , to compensate for loss to the circulation relative to injected volume. This was in accordance to ESOPE 29 30 .
Endoscopic electrode device (EndoVe), Cliniporator and pulses
The EndoVe device (700209, Mirai Medical, Galway, Ireland) is a single-use device developed to attach to a standard endoscope. The treatment head is a chamber (approximately 2.5 cm 3 ) containing two plate electrodes (gold plated, 25-micron thick, on flexible polyimide L 20 mm W 8 mm) and connected to a vacuum system to draw the tumor tissue into the chamber allowing electric field application ( Fig. 1 ).
Fig. 1 a.
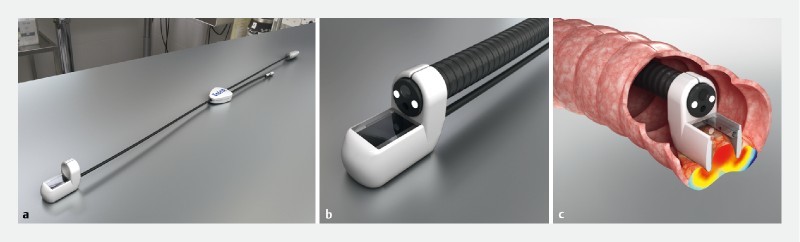
Illustration of the endoscopic vacuum electrode (EndoVe), an electrode developed to attach to a standard endoscope and vacuum system. b Illustration of the treatment head; a chamber approximately 2.5 cm 3 containing two plate electrodes 34 . c Illustration of the EndoVe device in colon. The treatment head is placed on tumor tissue which is drawn by negative pressure into the electrode chamber allowing application of the electric field. Source: Cork Cancer Research Centre
The device was connected to a square wave pulse generator (In Denmark: Cliniporator, type ref EPS01, IGEA, Carpi, Italy; In Ireland: Cliniporator, IGEA Carpi, Italy & ePORE, Mirai medical, Galway Ireland) which delivered eight pulses of 0.1 ms duration with a frequency of 5 kHz and an amplitude of 1 kV/cm (voltage to electrode distance ratio). A negative pressure between –100 to –760 mmHg was used to draw tissue into the chamber delivered by a surgical suction pump (HCPCS E0600, Atmos Medical, Germany).
Endoscopic procedure and administration of electric pulses
Before treatment, the tumor was inspected endoscopically and biopsies were collected from the intended treatment area. After injection of bleomycin, the electrode device was attached to the tip of the endoscope and placed on the tumor. Vacuum was applied to draw tumor tissue into the electrode chamber while electric pulses were delivered. Tumor area was treated by repeating positioning, vacuum, and pulsing until the treatment volume was covered. The treatment was performed by a specialist surgical endoscopist, and clinical experience was used to define treatment volume, assisted by preoperative scans and endoscopy.
Follow-up
If the patients were able, they had a follow-up endoscopy 6, 12, 18 and 24 weeks after initial treatment to evaluate change in tumor size and shape and to collect biopsies from the treated area for histological analysis. The patients were offered retreatment in case of remaining tumor tissue and if they were deemed suitable by the investigator. A maximum of two repeat treatments were performed in a minimum interval of 6 weeks ( Fig. 2 ).
Fig. 2 .
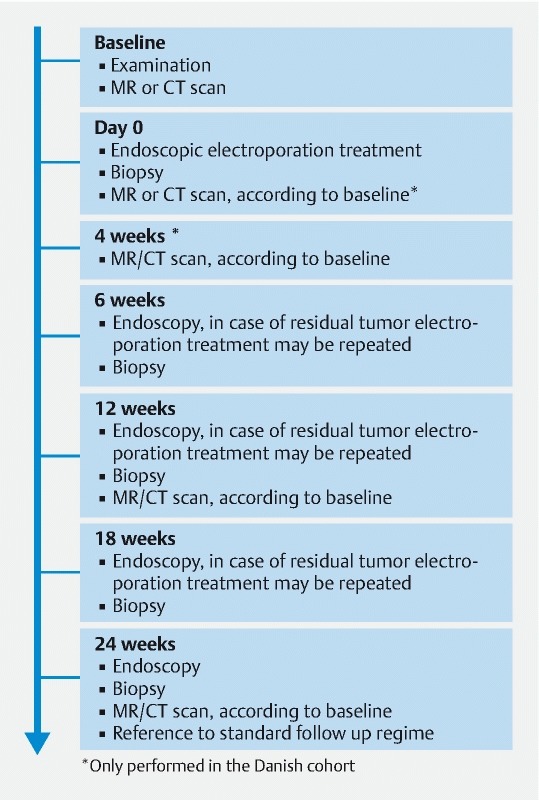
Timeline illustrating treatment plan and follow-up for patients treated at Mercy University Hospital, Cork Ireland and Herlev hospital, University of Copenhagen, Denmark. Additional scans were performed immediately after treatment and at 4-month follow-up at Herlev Hospital.
Assessment of safety
Safety was evaluated on reported AEs.
Assessment of response
Methods for monitoring treatment efficacy included CT or MRI for tumor volume assessment and endoscopy visual assessment.
Imaging was performed to evaluate response at baseline, 4 weeks (only in Denmark), 12 weeks and 24 weeks after first treatment ( Fig. 2 ). In Denmark: CT scans were performed with IV contrast Iomeron 350 mg Iodine/mL. Slice thickness 3 mm, KVP 100, mA 426 FOV 31.4. MRI was performed as anatomic standard using T2 and diffusion sequences. The MRI protocol comprised T2 scans in three planes, sagittal, para-coronal (parallel to the axis along the length a the affected tissue) and transversal (perpendicular to the aforementioned axis and hence the para-coronal images). All three scans had TR/TE = 1946 /120, flip angle (FA) = 90, an in-plane resolution of 0.5mm × 0.5 mm and a slice thickness of 3 mm. The DWI scans were recorded with spin-echo acquisition, DWI-SE, to avoid the rather large susceptibility artifacts usually seen with the more common DWI-EPI approach, although this necessitates further repeats of the sequence to gather sufficient signal. Parameters for the DWI-SE were TR/TE = 9289 /74, FA = 90, resolution of 1.8mm × 2.0 mm, thickness 3 mm, SPIR fat-sat and five b-values 0, 50, 150, 750 and 1000. All scans were performed on a 1.5 T Achieva clinical scanner (Philips Healthcare, Best, the Netherlands). To minimize intestinal peristalsis during MRI, patients had Buscopan 20 mg intramuscularly. All MRI and CT scans were evaluated by the same radiologist.
Statistics
This was an exploratory first-in-man trial and only descriptive statistics were used.
Results
The study was initiated at Cork Cancer Research Centre in Ireland but became affected by staff changes and for this reason, the protocol was extended to Copenhagen University Hospital Herlev and Gentofte, Denmark.
Patients
A total of seven patients were included and treated. Four patients were included at Mercy University Hospital, Cork, Ireland from March 2010 to December 2012 and three patients were included at Copenhagen University Hospital Herlev and Gentofte, Denmark from July 2015 to September 2015. Three men and four women aged 62 to 88 with WHO performance status from 1 to 2 and with diverse comorbidity as early Alzheimer’s disease, diabetes, nephropathy, respiratory and cardiac disease (arrhythmia, implanted pacemaker, biological aortic valves, previous coronary artery bypass grafting and previous apoplexy) were included. Five patients had local disease (T1–4 N0 M0) and 2 patients had loco regional disease (T1–4 N1–2 M0). Five of seven patients had received treatment for their colorectal cancer before inclusion ( Table 1 ).
Table 1. Demographics.
| Patient | Country | Sex | Age | Performance status (WHO) | Tumor stage | Comorbidity | Previous treatment |
| 1 | Ireland | M | 78 | 2 | T3 N2 M0 | None reported | None |
| 2 | Ireland | M | 77 | 2 | T4 N2 M0 | Respiratory compromise | Chemotherapy FOLFOX × 4 cycles |
| 3 | Ireland | F | 74 | 1 | T3 N0 M0 | Early Alzheimers | None |
| 4 | Ireland | F | 62 | 1 | T4 N0 M0 | None reported | Insulin potentiation therapy |
| 5 | Denmark | F | 85 | 2 | T2 N0 M0 | Cardiovascular disease (biological aortic valve, pacemaker, arrhythmia, previous apoplexy) | Radiotherapy 5 Gy × 3 |
| 6 | Denmark | F | 88 | 2 | T2 N0 M0 | Arrhythmia | Argon beam |
| 7 | Denmark | M | 71 | 1 | T1 N0 M0 | Diabetes, chronic nephropathy Cardiovascular disease (biological aortic valve, pacemaker) | Radiotherapy 1.8 Gy × 25 |
FOLFOX, fluorouracil, leucovorin and oxaliplatin; Gy,Gray.
Treatment procedure
All patients were treated with IV bleomycin, and one patient in Ireland was treated with both IV and IT bleomycin infusion in same treatment due to exophytic component of the tumor. A median of 16 pulses (range 7–34 pulses) was applied in each treatment. Immediately as the pulses were applied the treated tumor tissue appeared ischemic. The cliniporator was set to deliver 1000 V, but in two cases the voltage was decreased, one to 900 V due to generator limitations (patient 6) and one to 650 due to discomfort from the electrical pulses (patient 3). Median time from start of bleomycin infusion to last pulse was 48 minutes (range 18–80 min).
Four patients had retreatment and mean time between treatments was 51 days (range 43–57 days)( Table 2 ).
Table 2. Treatments.
| Patient | Country | Treatment number | Bleomycin (IE) | Pulses (n) | Highest current (A) | Treated tumor surface | Treatment duration (min) | Comment to treatment | Treatment outcome | |
| 1 | Ireland | 1 | 31,500 | IV | 9 | 7 | > 50 % | 80 | Partial response | |
| 2 | 31,500 | IV | 12 | 11 | ~ 100 % | 18 | Complete response | |||
| 2 | Ireland | 1 | 20,000 | IV | 17 | 16 | > 25 % | 25 | Partial response | |
| 2 | 20,000 | IV | 31 | ~ 100 % | 39 | Complete response | ||||
| 3 | Ireland | 1 | 15,000 | IV | 8 | 4 | > 75 % | 22 | Voltage decreased to 650 V due to discomfort from muscular contractions. | Partial response |
| 4 | Ireland | 1 | 25,000 5,000 | IV IT | 11 | 16 | > 25 % | 32 | Partial response | |
| 5 | Denmark | 1 | 27,450 | IV | 34 | 13 | > 25 % | 62 | Partial response | |
| 6 | Denmark | 1 | 27,000 | IV | 7 | 21 | > 50 % | 48 | Voltage decreased to 900 V due to lack of capacity of the cliniporator. | Partial response |
| 2 | 27,000 | IV | 18 | 21 | > 75 % | 24 | Voltage decreased to 900 V due to lack of capacity of the cliniporator. | Partial response | ||
| 7 | Denmark | 1 | 30,000 | IV | 16 | 21 | ~ 100 % | 31 | Complete response | |
| 2 | 30,000 | IV | 15 | 21 | > 75 % | 49 | ||||
IV, intravenous; IT, intratumoral
Response evaluation
Response evaluation was not uniform due to different imaging and different follow-up and several patients had treatment in only part of the tumor, thus response and follow-up is reported separately for each patient.
Patient 1 (Ireland)
A 78-year-old man, diagnosed with bleeding rectal tumor. Baseline MRI scan identified a T3N0 rectal tumor estimated to 3.0 × 2.5 cm. He refused perineal resection, radiotherapy and chemotherapy. He underwent two treatments: At the initial treatment, difficulties were encountered with placement of the electrode device due to tumor location and approximately 50 % of tumor surface area was treated. At second treatment, the remaining portion of the tumor was successfully treated with a reported cessation of rectal bleeding and the follow-up endoscopy revealed a complete endoluminal response. After 6 months the MRI scan indicated potential for residual disease and a subsequent rectal bleed resulted in the patient agreeing to an abdominal perineal resection.
Patient 2 (Ireland)
A 77-year-old man diagnosed with a T4N2 colorectal tumor was deemed unfit for operation and general anesthesia due to respiratory compromise and was previously treated with chemotherapy. He underwent two treatments. The initial treatment was only partially possible due to an obstructive component of the tumor, however, an estimated 90 % reduction endoluminally was observed endoscopically and allowed for complete treatment at the second electrochemotherapy session. Response was only evaluated endoscopically. Post-treatment the patient reported excellent quality-of-life improvements with cessation of rectal bleeding and elimination of pressure and pain in the bladder. He succumbed to an unrelated disease 5 months after the initial procedure.
Patient 3 (Ireland)
A 77-year-old woman with early Alzheimer’s disease (consent made by legal guardian), diagnosed with a T3N0 rectal tumor was deemed unsuitable for the standard of care (operation, chemotherapy and radiotherapy). She underwent one treatment that was performed under light sedation and was found to be distressing for the patient. Consequently, the applied voltage was reduced to 650 V to minimize discomfort from the muscular contractions associated with the procedure. Response was only evaluated endoscopically and partial response was observed at 12 weeks follow-up. She was deemed unsuitable for a second treatment.
Patient 4 (Ireland)
A 58-year-old woman diagnosed with a T4 anorectal tumor. At baseline MRI, tumor was estimated to 5.4 × 4.7 × 7.9 cm and with a large exophytic component. She underwent one treatment. The exophytic component was successfully treated along with a portion of the anorectal component. Unfortunately, endoscopic follow-up and treatment was not feasible due to the hardened and compact nature of the rectal component of the tumor. The patient underwent CyberKnife radiotherapy on the untreated rectal component 3 months after electrochemotherapy. Follow-up scan 3 years post-treatment indicated complete response and the patient has regained her original weight and reports excellent quality of life.
Patient 5 (Denmark)
An 85-year-old woman was diagnosed with a bleeding rectal tumor causing need of several blood transfusions. She had severe cardiovascular disease and was ineligible for surgery or chemotherapy. She underwent palliative irradiation (5 Gy × 3) but bleeding relapsed. At baseline CT scan, the tumor was estimated to be 3.6 × 5.6 × 5.3 cm (MRI deemed ineligible due to pacemaker). She underwent one treatment. Due to the large tumor only 25 % to 50 % of tumor surface area was treated. At 1-month follow-up the CT scan showed regression in the treated area with estimated tumor size of 1.5 × 1.2 × 2 cm ( Fig. 3 ) and bleeding had decreased markedly. Retreatment was planned but cancelled twice due to other medical diseases. Five months after treatment, tumor progressed and a rectal stent was implanted.
Fig. 3.
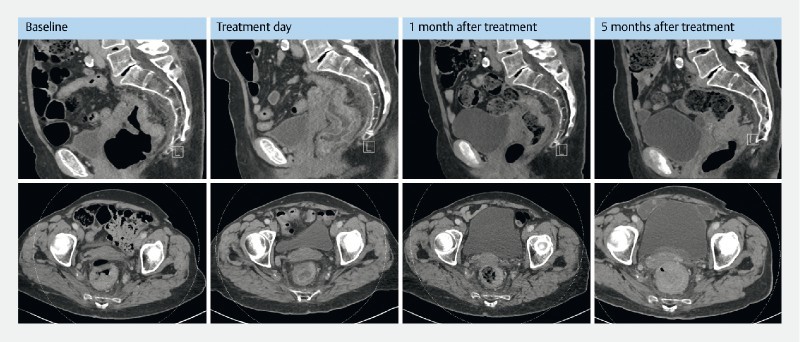
Pre and post treatment CT scans from patient no. 5 . CT evaluation from patient no. 5 treated at Herlev hospital, University of Copenhagen, Denmark. The patient only had one treatment. Baseline: Shows a rectal tumor, mainly on the posterior wall with maximum measurements of 3.6 × 5.9 cm and a craniocaudal extension of 5.3 cm. Treatment day: Performed a few hours after the procedure and shows no change in tumor volume but massive edema of the treated area. There is no free fluid, no sign of perforation and surrounding intestinal loops appear unaffected. One-month follow- up: Shows decreasing tumor 1.5 × 3.2 cm and craniocaudal extension 4.3 cm. There is still edema of the mucosa. Surrounding intestinal loops still unaffected. Five-month follow-up: Shows marked increase of tumor volume with mechanical obstruction of colon.
Patient 6 (Denmark)
An 87-year-old woman was diagnosed with a T2N0 rectal tumor. She was assessed ineligible for surgery and chemotherapy due to poor performance status and refused radiotherapy. She was treated with repetitive argon beaming but tumor progressed. Baseline MRI scan showed a circumferential tumor estimated to be 1.5 cm thick. She underwent two treatments: At the initial treatment, the patient appeared with arrhythmia which resulted in non-sufficient anesthesia/sedation and treatment was discontinued after treatment of 50 % to 75 % of tumor surface area. In addition, voltage was decreased to 900 V due to generator limit. At 1-month follow-up, MRI showed disappearance of tumor tissue equivalent to treatment area ( Fig. 4 ). A second treatment was performed, again with successful regression of treatment area, but tumor progressed profound the treatment area. Five months after the first treatment, she was referred for radiation therapy.
Fig. 4.
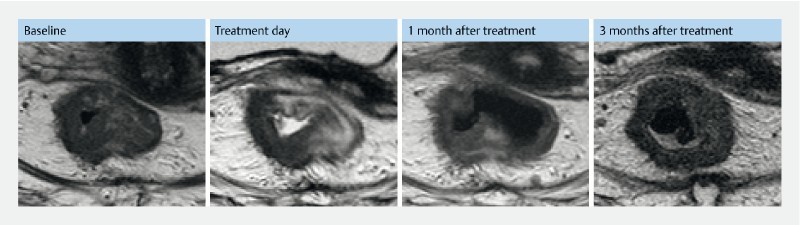
Pre- and post-MRI from patient no. 6. MR evaluation from patient no. 6 treated at Herlev hospital, Denmark. The patient had two treatments. Baseline: MRI shows a circumscript rectum tumor, thickest part 1.5 cm. Treatment day: MRI a few hours after first treatment. Only the right lateral wall was treated. The MRI shows edema in treated area. 1 month after treatment: One-month follow-up after initial treatment MRI shows decrease of tumor in the treated area. Three months after treatment: Two months after initial treatment, the patient was retreated to cover tumor in the left lateral wall. The MRI is 1 month after the second treatment and 3 months after the first treatment. The scan shows a decrease in the newly treated area but a progression in the right lateral wall.
Patient 7 (Denmark)
A 71-year-old man with diabetes, chronic nephropathy, and severe cardiovascular disease had relapse of rectal cancer after radiotherapy (1.8 Gy × 27). Due to comorbidity he was deemed ineligible for chemotherapy and he refused surgery. Baseline CT scan showed a small tumor on the posterior wall of the sigmoid colon measuring 2.0 × 1.0 × 1.2 cm (MRI deemed not possible due to pacemaker). He underwent two treatments. At the initial treatment ~100 % of tumor surface area was treated ( Fig. 5 ). At 1-month follow-up, CT scan showed disappearance of the tumor. At 7 weeks follow-up, endoscopy showed tumor tissue at the lateral margins and he was retreated. The tumor relapsed after 5 months and the patient then agreed to undergo surgery.
Fig. 5 .
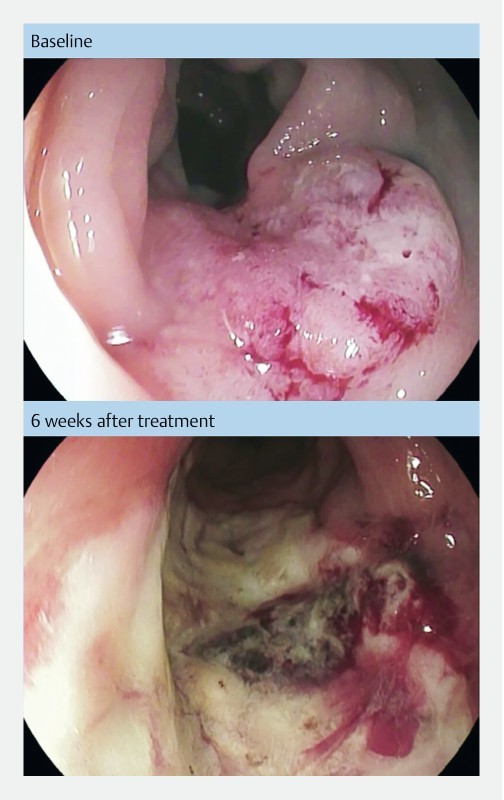
Pictures from endoscopic examination before and after treatment. Endoscopic pictures from patient no. 7 treated at Herlev hospital, Denmark. Baseline picture shows a rectal tumor estimated 3 × 2 cm. Six weeks after treatment there is a necrotic tumor decreased in size and surrounded by fibrotic tissue.
Safety
Overall, no severe AEs were reported. In the Irish cohort, two patients had temperature rise 24 hours post-treatment and one had rectal bleeding 4 weeks post-treatment. In the Danish cohort, none of the patients reported post-treatment pain 6, 12 and 24 hours after treatment. Three patients had altered bowel movements up to 2 weeks after treatment and minor problems with both loose and hard stool were reported. One patient reported mild nausea lasting 2 days after treatment and one patient reported headaches and pain in joints the day after treatment.
No device issues were reported regarding concerns about safety. In the three Danish patients who had MRI or CT scans 1 to 2 hours after treatment, the scans showed edema in the treated area, but no AEs, bleeding or perforation. Three patients had preoperative rectal bleeding, whereas two reported cessation of bleeding (2 Irish patients) and one markedly decrease of bleeding post treatment (Danish patient).
Discussion
In this first human study, seven patients with colorectal cancer were successfully treated with electrochemotherapy delivered by an endoscopic electrode device, resulting in local tumor responses and only limited AEs.
The patients seen in this study were elderly fragile patients with multiple comorbidities and were deemed ineligible for surgery or other standard treatments. Despite this, tumor response was seen after only one treatment in all patients and the procedure was well tolerated. The procedure was performed as an outpatient procedure, so the patients went home a few hours after the treatment and could maintain everyday life.
Electrochemotherapy is widely used for cutaneous metastases 10 11 12 13 14 15 16 17 18 and high response rates have consistently been reported across tumor histologies. This finding may be explained by the local enhanced effect of bleomycin of over 300-fold 7 8 9 making it possible to achieve tumor response even after a single treatment. In this study, the effect of treatment was seen during the procedure as tumor vessels in the treated area stopped bleeding and the tumor tissue attained a pale or cyanotic color.
Electrochemotherapy is known to have an antivascular effect with reflexive vascular constriction followed by destruction of tumor vessels and has also shown immediately and long-term cessation of bleeding in treatment of cutaneous metastases 31 32 33 . This is in line with the finding from this study that three patients with preoperative rectal bleeding reported marked reduction or complete cessation of bleeding. Bleeding persisted, although at a markedly reduced level, in one patient, and she had treatment of only 25 % to 50 % of the tumor surface area. This finding highlights the potential use of electrochemotherapy as a palliative treatment for colorectal cancer, as rectal bleeding is a frequent symptom in this patient group.
Tumor reduction was only seen in the tumor tissue covered by the electric field. In several patients it was not possible to reach the tumor margins either because of limited visibility due to the electrode device or because of tumor thickness. One patient had relapse in the treated tissue and three patients had relapse or progression in the untreated tumor tissue but none had visual progression in the treated tissue (CT, MR or endoscopic).
No safety issues were reported with the electrode device, although the voltage was decreased in two patients. One patient was only lightly sedated and experienced discomfort from the muscular contractions associated with the procedure; fully sedated patients did not report any discomfort. In the second case, voltage was reduced due to generator limitation. In this study we used a generator developed for treatment of cutaneous electrodes, the first version of the cliniporator (cliniporator EPS01), and this may explain why the generator was working close to its limit.
This was the first phase 1 clinical study to investigate the feasibility and safety of endoscopically delivered electrochemotherapy. The effect was observed endoscopically after a very short interval and had a palliative effect with immediate cessation of bleeding and reduction of intraluminal tumor masses as verified by respectively MR and CT scans.
A similar system with an endoscopic electrode device has been used in a phase 1 study to treating patients with esophageal cancer successfully with electrochemotherapy 34 . Also here the treatment was well tolerated and tumor response was visualized in five of six patients.
Standard therapeutic options for unresectable colorectal cancer include chemotherapy, radiotherapy, laser ablation or stenting. Electrochemotherapy adds an additional treatment option that is performed once or only a few times and can be used in refractory cases or where symptom palliation is needed, e. g. during other types of systemic chemotherapy regimens.
Interestingly, novel preclinical data indicate an immunostimulatory effect of electrochemotherapy in a murine colorectal cancer model 35 . Further studies may clarify if electrochemotherapy can be used as part of an immunostimulatory procedure giving rise to local tumor cell kill as well as release of tumor antigens.
Conclusion
Electrochemotherapy is a simple and safe treatment and has the potential to be a palliative option for patients with colorectal tumors deemed ineligible for standard treatments. It could also be advantageous in combination with standard treatments to perform tumor debulking or to stop symptomatic rectal bleeding.
Footnotes
Competing interests Dr. Soden reports a conflict of interest regarding a patent on the EndoVe electrode as well as owner interests in Mirai Medical.
References
- 1.Torre L A, Bray F, Siegel R L et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 3.Mir L M, Orlowski S. Mechanisms of electrochemotherapy. Adv Drug Deliv Rev. 1999;35:107–118. doi: 10.1016/s0169-409x(98)00066-0. [DOI] [PubMed] [Google Scholar]
- 4.Mir L M. Bases and rationale of the electrochemotherapy. EJC Suppl. 2006;4:38–44. [Google Scholar]
- 5.Yarmush M L, Golberg A, Sersa G et al. Electroporation-based technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng. 2014;16:295–320. doi: 10.1146/annurev-bioeng-071813-104622. [DOI] [PubMed] [Google Scholar]
- 6.Gothelf A, Mir L M, Gehl J. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev. 2003;29:371–387. doi: 10.1016/s0305-7372(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 7.Orlowski S, Belehradek J, Jr., Paoletti C et al. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem Pharmacol. 1988;37:4727–4733. doi: 10.1016/0006-2952(88)90344-9. [DOI] [PubMed] [Google Scholar]
- 8.Gehl J, Skovsgaard T, Mir L M. Enhancement of cytotoxicity by electropermeabilization: an improved method for screening drugs. Anticancer Drugs. 1998;9:319–325. doi: 10.1097/00001813-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Jaroszeski M J, Dang V, Pottinger C et al. Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer Drugs. 2000;11:201–208. doi: 10.1097/00001813-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Marty M, Sersa G, Garbay J R et al. Electrochemotherapy - An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. EJC Suppl. 2006;4:3–13. [Google Scholar]
- 11.Matthiessen L W, Chalmers R L, Sainsbury D C et al. Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 2011;50:621–629. doi: 10.3109/0284186X.2011.573626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthiessen L W, Johannesen H H, Hendel H W et al. Electrochemotherapy for large cutaneous recurrence of breast cancer: A phase II clinical trial. Acta Oncol. 2012;51:713–721. doi: 10.3109/0284186X.2012.685524. [DOI] [PubMed] [Google Scholar]
- 13.Belehradek M, Domenge C, Luboinski B et al. Electrochemotherapy, a new antitumor treatment. First clinical phase I-II trial. Cancer. 1993;72:3694–3700. doi: 10.1002/1097-0142(19931215)72:12<3694::aid-cncr2820721222>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Campana L G, Mocellin S, Basso M et al. Bleomycin-based electrochemotherapy: clinical outcome from a single institution's experience with 52 patients. Ann Surg Oncol. 2009;16:191–199. doi: 10.1245/s10434-008-0204-8. [DOI] [PubMed] [Google Scholar]
- 15.Curatolo P, Quaglino P, Marenco F et al. Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: a two-center prospective phase II trial. Ann Surg Oncol. 2012;19:192–198. doi: 10.1245/s10434-011-1860-7. [DOI] [PubMed] [Google Scholar]
- 16.Heller R, Jaroszeski M J, Reintgen D S et al. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer. 1998;83:148–157. doi: 10.1002/(sici)1097-0142(19980701)83:1<148::aid-cncr20>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 17.Kis E, Olah J, Ocsai H et al. Electrochemotherapy of cutaneous metastases of melanoma--a case series study and systematic review of the evidence. Dermatol Surg. 2011;37:816–824. doi: 10.1111/j.1524-4725.2011.01951..x. [DOI] [PubMed] [Google Scholar]
- 18.Quaglino P, Mortera C, Osella-Abate S et al. Electrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastases. Ann Surg Oncol. 2008;15:2215–2222. doi: 10.1245/s10434-008-9976-0. [DOI] [PubMed] [Google Scholar]
- 19.Clover A JP, Bertino G, Curatolo P Budapest: ESSO38 (European Society of Surgical Oncology, 38th Congress); 2018. Electrochemotherapy in the treatment of cutaneous malignancy; outcomes and subgroup analysis from the cumulative results from the pan- European InspECT Database for 1478 lesions in 691 patients (2008-2018)
- 20.Matthiessen L W, Chalmers R L, Sainsbury D C et al. Management of cutaneous metastases using electrochemotherapy. Acta Oncol. 2011;50:621–629. doi: 10.3109/0284186X.2011.573626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benevento R, Santoriello A, Perna G et al. Electrochemotherapy of cutaneous metastastes from breast cancer in elderly patients: a preliminary report. BMC Surg. 2012;12 01:S6. doi: 10.1186/1471-2482-12-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linnert M, Iversen H K, Gehl J. Multiple brain metastases – current management and perspectives for treatment with electrochemotherapy. Radiol Oncol. 2012;46:271–278. doi: 10.2478/v10019-012-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edhemovic I, Gadzijev E M, Brecelj E et al. Electrochemotherapy: a new technological approach in treatment of metastases in the liver. Technol Cancer Res Treat. 2011;10:475–485. doi: 10.7785/tcrt.2012.500224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campana L G, Edhemovic I, Soden D et al. Electrochemotherapy - Emerging applications technical advances, new indications, combined approaches, and multi-institutional collaboration. Eur J Surg Oncol. 2019;45:92–102. doi: 10.1016/j.ejso.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Miklavcic D, Sersa G, Brecelj E et al. Electrochemotherapy: technological advancements for efficient electroporation-based treatment of internal tumors. Med Biol Eng Comput. 2012;50:1213–1225. doi: 10.1007/s11517-012-0991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forde P F, Sadadcharam M, Bourke M G et al. Preclinical evaluation of an endoscopic electroporation system. Endoscopy. 2016;48:477–483. doi: 10.1055/s-0042-101343. [DOI] [PubMed] [Google Scholar]
- 27.Oken M M, Creech R H, Tormey D C et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 28.Karnofsky D A, Abelmann W H, Craver L F et al. The use of the nitrogen mustards in the palliative treatment of carcinoma – with particular reference to bronchogenic carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 29.Gehl J, Sersa G, Matthiessen L W et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018;57:874–882. doi: 10.1080/0284186X.2018.1454602. [DOI] [PubMed] [Google Scholar]
- 30.Mir L M, Gehl J, Sersa G et al. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. EJC Suppl. 2006;4:14–25. [Google Scholar]
- 31.Markelc B, Sersa G, Cemazar M. Differential mechanisms associated with vascular disrupting action of electrochemotherapy: intravital microscopy on the level of single normal and tumor blood vessels. PLoS One. 2013;8:e59557. doi: 10.1371/journal.pone.0059557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sersa G, Cemazar M, Miklavcic D et al. Tumor blood flow modifying effect of electrochemotherapy with bleomycin. Anticancer Res. 1999;19:4017–4022. [PubMed] [Google Scholar]
- 33.Jarm T, Cemazar M, Miklavcic D et al. Antivascular effects of electrochemotherapy: implications in treatment of bleeding metastases. Expert Rev Anticancer Ther. 2010;10:729–746. doi: 10.1586/era.10.43. [DOI] [PubMed] [Google Scholar]
- 34.Egeland C, Baeksgaard L, Johannesen H H et al. Endoscopic electrochemotherapy for esophageal cancer - a phase I clinical study. Endosc Int Open. 2018;6:E727–E734. doi: 10.1055/a-0590-4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falk H, Forde P F, Bay M L et al. Calcium electroporation induces tumor eradication, long-lasting immunity and cytokine responses in the CT26 colon cancer mouse model. Oncoimmunology. 2017;6:e1301332. doi: 10.1080/2162402X.2017.1301332. [DOI] [PMC free article] [PubMed] [Google Scholar]


