Abstract
Background and study aims Preoperative biliary drainage of hilar cholangiocarcinoma (HC) is controversial. The goal of this study was to compare the clinical outcome and associated complications for types II, III, and IV HC managed by percutaneous transhepatic biliary drainage (PTBD) and endoscopic retrograde cholangiopancreatography (ERCP).
Patients and methods Between January 2011 and June 2017, a total of 180 patients with II, III, and IV HC were enrolled in this retrospective cohort study. According to the drainage method, patients were divided into two groups: PTBD (n = 81) and ERCP (n = 99). This study was registered with ClinicalTrials.gov, NCT03104582, and was completed.
Results Compared with the PTBD group, the ERCP group had a higher incidence of post-procedural cholangitis (37 [37.37 %] vs. 18 [22.22 %], P = 0.028) and pancreatitis (17 [17.17 %] vs. 2 [2.47 %], P = 0.001); required more salvaged biliary drainage (18 [18.18 %] vs. 5 [6.17 %], P = 0.029), and incurred a higher cost ( P < 0.05). Patients with type III and IV HC in the ERCP group had more cholangitis than those in the PTBD group (26 [36.62 %] vs. 11 [18.03 %], P = 0.018). The rate of cholangitis in patients who received endoscopic bilateral biliary stents insertion was higher than patients with unilateral stenting (23 [50.00 %] vs. 9 [26.47 %], P = 0.034), and underwent PTBD internal-external drainage had a higher incidence of cholangitis than those with only external drainage (11 [34.36 %] vs. 7 [14.29 %], P = 0.034). No significant difference in the rate of cholangitis was observed between the endoscopic unilateral stenting group and the endoscopic nasobiliary drainage group (9 [26.47 %] vs. 5 [26.32 %], P = 0.990).
Conclusion Compared to ERCP, PTBD reduced the rate of cholangitis, pancreatitis, salvage biliary drainage, and decreased hospitalization costs in patients with types II, III, and IV HC. Risk of cholangitis for patients with types III and IV was significantly lower in the PTBD group.
Introduction
Hilar cholangiocarcinoma (HC, or Klatskin tumor) is considered highly malignant due to its aggressive nature with early invasion into the adjacent liver and thus a poor prognosis. It accounts for 40 % to 60 % of all extrahepatic bile duct carcinomas 1 . Currently, radical resection is still considered one of the most effective treatments, especially for Bismuth-Corlette type II, III, and IV lesions. Due to the slow-growing nature of the tumor, most lesions are found in an advanced stage when patients present with obstructive jaundice, involvement of the segmental bile ducts, and invasion into the liver, which makes complete resection difficult and, thus, radical resection rates are low 2 3 4 5 . Tumor involvement of the liver hilum leads to extensive intrahepatic bile duct obstruction, resulting in damage to the liver, nervous system, and cardiovascular and urinary systems. Tumor invasion could involve the cystic duct and extension to the intrahepatic bile ducts. Resection of the hilar tumor with extended hepatectomy offers the best chance for cure. Although HC can be diagnosed at an early stage, the current reported R0 resection rate is less than 30 %, and a reported 5-year postoperative survival rate ranges from 30 % to 40 % 6 7 .
Current consensus indicates that preoperative biliary drainage is beneficial to improve postoperative liver function 8 . Endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic biliary drainage (PTBD) are frequently used to provide biliary drainage because both methods are effective in relieving the obstructive jaundice. However, these invasive procedures are associated with an increase in postprocedural complications, including cholangitis, pancreatitis, and hemobilia. Recent publications have suggested that cholangitis could negatively affect clinical outcome and prognosis. So far it is still unclear whether ERCP or PTBD is the preferred preoperative drainage method. Our study aimed to investigate the optimal preoperative biliary drainage methods to prevent post-procedural cholangitis in type II, III, and IV hilar cholangiocarcinoma.
Patients and methods
Study design and patients
Between January 2011 and June 2017, 339 patients with hilar bile duct stricture who required bile drainage were recruited into the study. Diagnosis of HC depends on results of its clinical manifestation, serum biomarkers and imaging examination. Patients with malnutrition, hypoalbuminemia, suspected cholangitis, long-term jaundice, planned preoperative anti-neoplastic therapy and future liver remnant (FLR) < 30 % were considered to need preoperative bile drainage. One hundred fifty-nine patients were excluded because of type I HC, hepatic duct stones with stenosis, gallbladder carcinoma, metastatic tumors of hilar, primary sclerosing cholangitis (PSC), IgG4-Related sclerosing cholangitis (IgG4-SC), hepatic echinococcosis, inability to undergo percutaneous biliary drainage, or failure of ERCP or placement of a percutaneous transhepatic cholangiography. Eventually, 180 eligible patients with type II, III, and IV hilar cholangiocarcinoma were included in this study ( Fig. 1 ). According to the drainage method, the patients were divided into two groups: ERCP group and PTBD group. The ERCP group included patients who underwent endoscopic biliary stenting (EBS) and endoscopic nasobiliary drainage (ENBD).
Fig. 1.
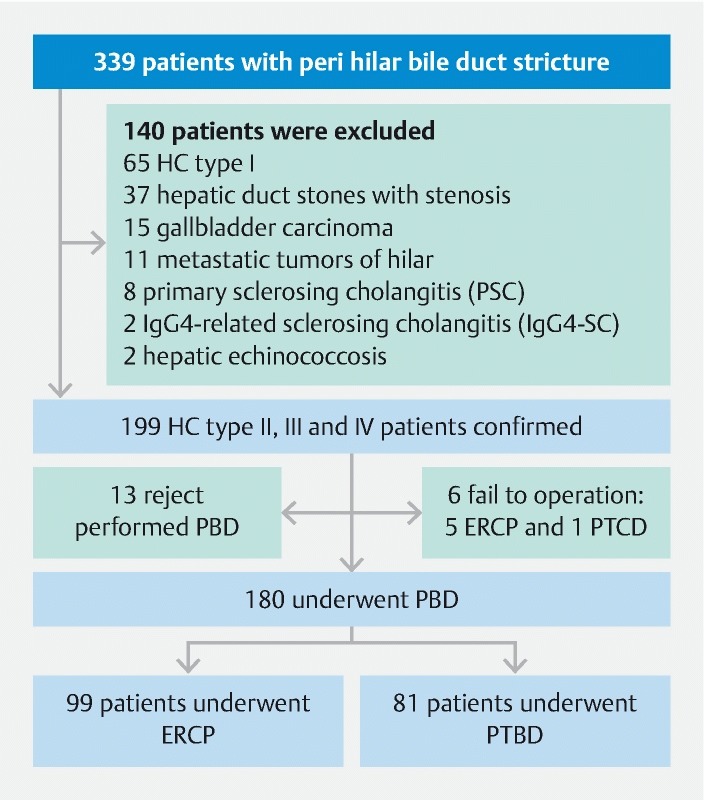
Flow diagram of the study.
Drainage method
ERCP
When performing ERCP, bile duct cannulation and cholangiogram procedures were completed by using a duodenoscope (TJF-260V, Olympus, Japan). In a general way, the guide wire was inserted and crossed over the malignant obstruction site to the proximal end of the hepatic bile duct. Considering that patients may undergo radical cholangiocarcinoma resection when jaundice reduction is satisfactory, one or two plastic biliary stents or endoscopic nasobiliary drains were placed (8.5 Fr; Boston Scientific, United States or Olympus, Japan). If the guide wire first entered the bile duct on the side of the future remnant lobe, a unilateral plastic stent or ENBD was usually placed; if the guide wire entered the bile duct of the non-future remnant lobe first and then the bile duct of the future remnant lobe was selected, bilateral biliary stents were placed side by side ( Fig. 2a , Fig. 2b ). Generally speaking, ENBD was performed when patients had difficulty during contrast agent excretion, or when bile was thick or full of dregs, which is easy to clog drainage tubes. The upper edge of plastic stents and the tip of the nasobiliary catheter (8 Fr/10.2 Fr; Cook Medical, Winston-Salem, North Carolina, United States) should cross over the upper margin of the malignant obstruction site by 2 cm.
Fig. 2.
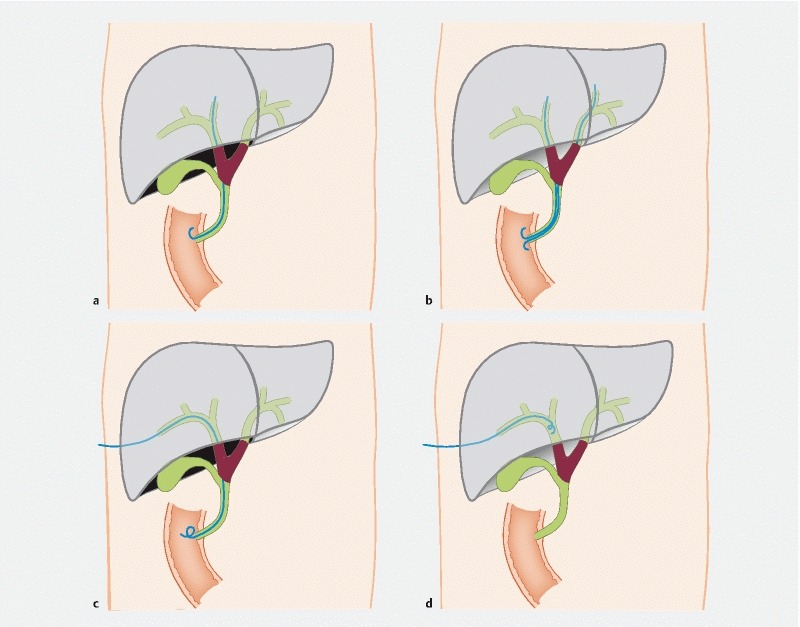
Various biliary drainage methods. a Unilateral EBS. b Bilateral EBS. c Internal-external PTBD. d External PTBD.
PTBD
The dilated intrahepatic bile duct of the expected retention liver was selected based on imaging findings. Ultrasound or x-ray-guided percutaneous transhepatic cholangiography was achieved by using a trocar to penetrate the target intrahepatic bile duct. The guidewire was then advanced through the trocar to lead the tube insertion. After the guidewire passed over the malignant stenosis site and the duodenal papilla, a catheter (7Fr; Olympus, Japan or Cook Medical, Winston-Salem, North Carolina, United States) was placed with its distal end along the guidewire in the duodenum for internal-external drainage. PTBD external drainage was performed if the guidewire failed to pass the malignant stenosis site of the intrahepatic bile duct ( Fig. 2c , Fig. 2 d ).
Data collection
Patient demographics, including age and sex, and biochemistry, including tumor marker CA19 – 9, white blood cell (WBC) count, serum amylase level, and electrolytes were collected before the drainage procedure. Total bilirubin (TBIL) level, WBC, serum amylase, and electrolyte levels were checked again at 24 and 48 hours and 1 and 2 weeks after biliary drainage. Data on body temperature, color of bile, and abdominal pain score (evaluated by numerical rating scale [NRS]) were also recorded. Complications, including acute cholangitis, pancreatitis, and hemobilia, were recorded 9 . Diagnostic criteria for acute cholangitis were based on the TG13 Tokyo Guidelines and included: (1) upper abdominal pain (NRS ≥ 4); (2) fever (> 38.0℃) and/or chills; (3) serum total bilirubin (TBIL) level > 34.2 μmol/L; and (4) inflammatory response (WBC > 10,000/μL, CRP > 1 mg/dL) 10 . The revised Atlanta classification of acute pancreatitis was used to assess: (1) abdominal pain (NRS > 4) > 24 hours after drainage; (2) amylase level more than three times normal level; and (3) gastrointestinal perforation, acute cholecystitis or acute peritonitis 11 . Clinical diagnosis of hemobilia consisted of: (1) blood mixed with bile after biliary drainage; (2) slight bleeding indicated by a small amount of black stool or positive occult blood test; and (3) moderate to severe bleeding, indicated by a drop in hemoglobin of 3 g/L, requiring blood transfusion, angiographic intervention, or surgery 12 13 . Successful drainage was defined as a reduction in serum bilirubin level to < 50 % of the pre-drainage value within 2 weeks or normalization after the procedure without any complications during the follow-up period 14 .
Statistical method
All statistical analyses were performed with Stata (version 12.0) statistical software. Continuous variable data were reported as mean ± SD for parametric data and median with interquartile ranges (IQR) for nonparametric data or as counts and percentages for categorical variables. Continuous variables were also expressed as ranges. We also compared baseline characteristics and post-drainage complications. Data were analyzed by t-test, and the χ2 test was used to investigate qualitative variables. Results were presented as relative risk (RR) with 95 % confidence intervals (CIs), and P < 0.05 was considered statistically significant.
Reported incidence of cholangitis after biliary drainage varied from 22 % to 46 % 12 . Our prior experience showed a cholangitis rate of 40 %. To detect a difference with a significance level (α) of 0.05 and a power of 80 % with a two-tailed test, and to account for a 10 % loss to follow-up, it was estimated that 150 patients were needed for the study. Therefore, we enrolled a total of 180 patients.
Results
Patient characteristics
A total of 180 patients were selected for the study, 99 patients received ERCP, and 81 patients underwent PTBD. There were no significant differences in patient demographics, including age, sex, and Bismuth classification distribution. No significant differences were noted in WBC count, TBIL, or tumor marker CA19 – 9 between the two groups ( P > 0.05) ( Table 1 ).
Table 1. Baseline characteristics.
| ERCP group (n = 99) | PTBD group ( n = 81) | P | |
| Age (y) | |||
| < 65 | 26 | 32 | 0.13 |
| 65 – 75 | 48 | 29 | |
| 75 > | 25 | 20 | |
| Sex | |||
| Male | 55 | 43 | 0.74 |
| Female | 44 | 38 | |
| Types | |||
| II | 28 | 20 | 0.75 |
| III | 27 | 26 | |
| IV | 44 | 35 | |
| WBC (× 10 2 /L)(mean ± SD) | 7.33 ± 2.81 | 7.91 ± 3.60 | 0.343 |
| TBIL (umol/L)(mean ± SD) | 303.17 ± 182.72 | 332.14 ± 186.85 | 0.242 |
| CA19 – 9 (kU/L)(mean ± SD) | 700.52 ± 457.87 | 681.34 ± 405.27 | 0.769 |
| Hospitalization time (days)(mean ± SD) | 14.32 ± 7.84 | 14.86 ± 9.19 | 0.671 |
| Hospitalization costs (RMB)(mean ± SD) | 44406.67 ± 12407.3 | 22300.5 ± 8567.9 | 0.005 |
ERCP, endoscopic retrograde cholangiopancreatography; PTBD, percutaneous transhepatic biliary drainage; WBC, white blood cell; TBIL, total bilirubin; SD, standard deviation; RMB, renminbi
Impact of ERCP and PTBD drainage on serum total bilirubin
Of the 99 patients in the ERCP group, 34 underwent endoscopic unilateral drainage with a single biliary stent, 46 patients had bilateral biliary stent placement, and another 19 patients received nasobiliary drainage. Forty-nine of the 81 patients in the PTBD group had external drainage with the catheter tip left above the obstruction and 32 had internal-external drainage with the tip of the catheter in the duodenum. Compared with pre-drainage, total bilirubin decreased significantly both in the ERCP group (303.17 ± 182.72 vs. 126.87 ± 75.96, P = 0.001) and the PTBD group (332.14 ± 186.85 vs. 161.42 ± 93.11, P = 0.001) after 2 weeks of drainage. But there was no statistical difference in decrease in total bilirubin between the two groups (176.30 ± 106.76 in ERCP group vs. 170.72 ± 93.74 in PTBD group, P = 0.81) ( Table 2 ).
Table 2. Effects of different drainage methods on serum total bilirubin.
| TBIL (umol/L) | P | |
| ERCP group (n = 99) | ||
|
303.17 ± 182.72 | 0.001 |
|
126.87 ± 75.96 | |
| PTBD group (n = 81) | ||
|
332.14 ± 186.85 | 0.001 |
|
161.42 ± 93.11 | |
| ERCP group for decreasing of TBIL (mean±SD) | 176.30 ± 106.76 | 0.810 |
| PTBD group of decreasing of TBIL (mean±SD) | 170.72 ± 93.74 | |
TBIL, total bilirubin; ERCP, endoscopic retrograde cholangiopancreatography; PTBD, percutaneous transhepatic biliary drainage; SD, standard deviation
Complications
Fifty-five of 180 patients (30.56 %) developed post-drainage cholangitis: 37 in the ERCP group and 18 in the PTBD group. Among them, 46 cases (83.64 %) of post-procedure acute cholangitis were considered as bacterial contamination originating from the duodenum, eight cases (14.55 %) with drainage tube migration and 1 case (1.81 %) with blockage of drainage tube caused by hemobilia. Most fevers occurred within 24 hours after intervention.
Incidence of cholangitis was significantly higher in the ERCP group than in the PTBD group (37.37 % vs. 22.22 %, P = 0.028). Subgroup analysis showed similar results between the two groups when comparing patients with types III and IV hilar obstruction (36.62 % in ERCP group vs. 18.03 % in PTBD group, P = 0.018). There was no significant difference in patients with type II obstruction between the two groups (39.29 % in ERCP group vs. 35.00 % in PTBD group, P = 0.762). Four of 180 patients (2.22 %) had hemobilia, and there was no statistical difference between the two groups (2.02 % in ERCP group vs. 2.47 % in PTBD group, P = 0.76). Nineteen of 180 patients (10.56 %) developed post-procedure pancreatitis, with significantly more patients in the ERCP group than in the PTBD group (17 [17.17 %] vs . 2 [2.47 %], P = 0.001). Twenty-three of 180 (12.78 %) patients required salvage biliary drainage, again with significantly more patients in the ERCP group than in the PTBD (18 [18.18 %] vs. 5 [6.17 %], P = 0.029) ( Fig. 3 ). The type II HC patients who received unilateral stent placement had the lowest rate of post-drainage cholangitis and types III or IV HC patients with PTBD external drainage had the lowest incidence of cholangitis ( Fig. 4 ).
Fig. 3.
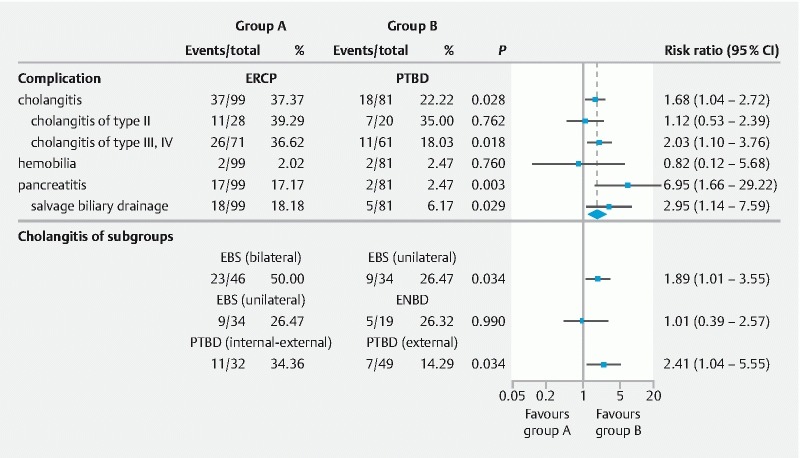
Incidence of complications with various types of drainage.
Fig. 4.
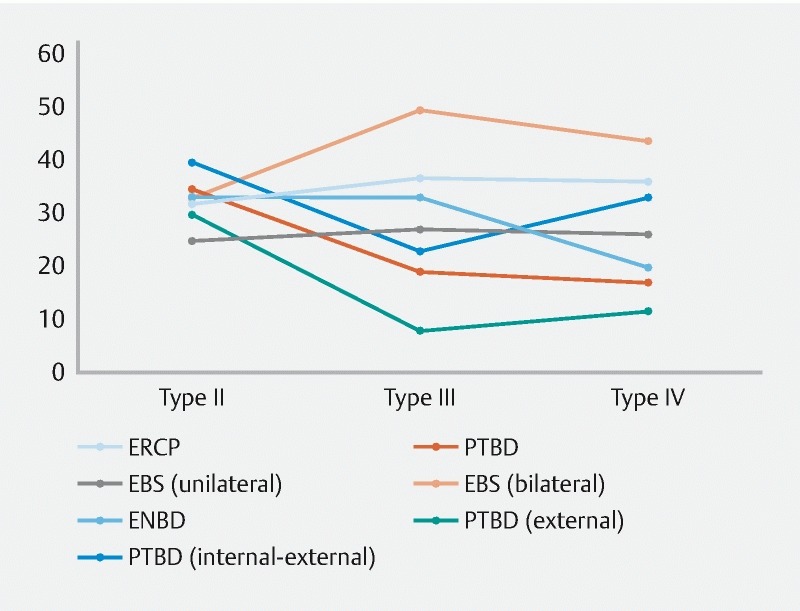
The tendency of post-drainage cholangitis after ERCP/PTBD in types II, III and IV HC.
Incidence of cholangitis in various subgroups
In the ERCP group, patients with endoscopic bilateral biliary stent placement had a higher rate of post-drainage cholangitis than those who received unilateral biliary stent placement (50.00 % vs. 26.47 %, P = 0.034). There was no significant difference between those who had endoscopic unilateral stenting and those who had endoscopic nasobiliary drainage (ENBD) (26.47 % vs. 26.32 %, P = 0.99). For the PTBD group, patients who underwent PTBD internal-external drainage had a higher rate of cholangitis than those with only external drainage (34.36 % vs. 14.29 %, P = 0.034) ( Fig. 3 ).
Discussion
Preoperative biliary drainage plays a positive role in enhancing surgical safety for patients with types II, III, and IV potentially resectable hilar cholangiocarcinoma with jaundice. This method not only improves retention of liver function after radical surgery, but also speeds regeneration of the remnant liver 15 . It also should be noted that not all patients can benefit from preoperative biliary drainage, for instance, those with drainage-associated cholangitis, a critical predictor that can result in liver insufficiency and liver failure and could even increase the chance of mortality after radical surgery 16 17 18 . Currently, there is no consensus about how preoperative biliary drainage should be performed worldwide. Kloek JJ et al 19 found that preoperative PTBD could outperform EBS in patients with resectable HC, because PTBD was associated with fewer infectious complications and involved fewer procedures. A Japanese clinical practical guideline 20 , however, recommended ERCP as the first choice for preoperative drainage of biliary tract cancers. It is important to note that the guideline was aimed at all biliary tract cancers rather than HC. How to define the optimal method is still debated.
The basis of an ideal drainage plan is maximization of drainage volume of the expected retention liver and minimization of drainage-associated cholangitis at the same time 21 . To drain bile from the future remnant lobe adequately, bilateral plastic stents (t2 stents) were inserted in the bile duct of the retained liver during our ERCP operations. Vienne et al reported that they needed double-sided stent insertion if more than 50 % of the liver drainage volume was expected, but our results showed that single-sided stent insertion could also produce a good jaundice reduction effect 22 . Both the double-sided stent insertion group and the single-sided stent insertion group with types III and IV potentially resectable hilar cholangiocarcinoma exhibited obvious bilirubin number reduction and similar levels of bilirubin drop ( P = 0.01). Besides this, jaundice reduction effects were also similar in the ERCP and PTBD groups and in their corresponding subgroups ( P > 0.05).
The US consensus statement on hilar cholangiocarcinoma published in 2013 suggested that PTBD is superior in reducing risks of complications 23 . We recently published results of a meta-analysis that testify to PTBD as the preferred initial drainage method for patients with types II, III, and IV tumor obstruction 24 . Many centers also reported a higher incidence of acute cholangitis after ERCP. Min et al. reported 106 cases of hilar cholangiocarcinoma, with 44 undergoing ERCP versus 62 with PTBD. They found higher rates of cholangitis in the ERCP group than in the PTBD group (54.5 % vs . 22.6 %, P < 0.05) 25 . In our research, we found that incidence of post-drainage cholangitis, pancreatitis, and secondary remedial biliary drainage were greater in the ERCP group than in the PTBD group ( P < 0.05). We analyzed the relationship between post-drainage cholangitis and Bismuth-Corlette classification and found that type II HC patients showed no difference in incidence of post-drainage cholangitis (39.29 % vs. 35.00 %, P = 0.762), only with types III and IV HC was incidence of post-drainage cholangitis higher in the ERCP group than in the PTBD group (36.62 % vs. 18.03 %, P = 0.018).
Choice of endoscopic unilateral stenting versus bilateral stenting remains controversial. De Palma found that rates of cholangitis were higher with bilateral stenting than with unilateral stenting (16.6 % vs. 8.8 %, P < 0.05) 26 . Iwano reported that liver abscesses occurred more frequently in the bilateral stenting group than in the unilateral stenting group (17.6 % vs. 1.5 %, P < 0.05) 27 . In our study, incidence of cholangitis was significantly higher among patients with endoscopic bilateral drainage than in those with endoscopic unilateral drainage (50.00 % vs. 26.47 %, P = 0.034). Considering patients with types II, III, and IV potentially resectable HC whose operative treatment should be performed soon after the diagnosis is ascertained, endoscopic unilateral stenting and endoscopic bilateral biliary stents were no different in drainage effect. Besides, incidence of post-drainage cholangitis from endoscopic bilateral biliary stent insertion was higher, so there were more advantages to unilateral endoscopic biliary stenting for radical resection of hilar cholangiocarcinoma, particularly in type II or III cases.
Some endoscopists have advocated use of ENBD for preoperative biliary drainage for hilar cholangiocarcinoma. Kawakami reported that cholangitis secondary to stent occlusion was significantly more common in the stenting group than in the ENBD group (60.0 % vs. 10.0 %, P < 0.05). The nasobiliary catheter may have negative pressure, making the bile flow more easily 28 . However, we observed that the cholangitis rate was comparable between the group with endoscopic unilateral stenting insertion and the ENBD group (26.47 % vs. 26.32 %, P = 0.99). ENBD has the disadvantage of causing nose discomfort and is at risk of dislocation. Because external bile loss leads to water-electrolyte imbalance, radical resection of hilar tumor is recommended within 4 weeks of ENBD placement. An alternative option to provide internal drainage is cutting the ENBD tube, using a pair of endoscopic scissors, and leaving the tip of the drain in the stomach to minimize risk of external bile loss.
In patients with PTBD, internal drainage is achieved by leaving the tip of the drain catheter in the duodenum. Internal drainage minimizes external bile loss and maintains water-electrolyte balance, which also improves nutritional status 29 . In our study, incidence of cholangitis in those with external/internal drainage was higher than in those with external drainage alone (34.36 % vs. 14.29 %, P = 0.034). We hypothesized that the difference in incidence of cholangitis was secondary to reflux of duodenal content into the biliary system through the catheter when the tip is placed in the duodenum. Comparing patients with endoscopic single stent placement, ENBD, and those with external drainage alone in the PTBD group, incidence of cholangitis was 26.47 %, 26.32 %, and 14.29 %, respectively. Although endoscopic unilateral stenting insertion and ENBD can reduce incidence of cholangitis, PTBD with external drainage resulted in the lowest incidence of cholangitis. PTBD with external drainage alone may be the preferred drainage method, especially for salvage biliary drainage when endoscopic stenting fails.
Many aspects of preoperative biliary drainage need to be considered for patients with types II, III, and IV HC. Our results appeared to show that PTBD external drainage for types III and IV HC has more advantages. Individualized experience with drainage operation is also a necessity, and the competency of the hospital’s biliary intervention or endoscopic techniques should be considered comprehensively. The study also has some limitations. It was single-center and retrospective with a relatively small number of patients. Data on long-term clinical outcomes, including stent patency and patient surgical mortality during follow-up are limited. A prospective, randomized controlled trial with a larger number of patients is needed.
Conclusion
In conclusion, percutaneous transhepatic biliary external drainage may be the preferred preoperative drainage method for hilar cholangiocarcinoma because of its low incidence of cholangitis and pancreatitis. If a patient is not suitable for PTBD, unilateral EBS or ENBD can be used as a secondary option.
Acknowledgements
This work was supported by the Free Exploration Project of the Central Universities, Lanzhou University (No. 18LZUJBWZY054) and Teaching Research Project of Lanzhou University (No. 2017218). Clinical trial registration: NCT03104582
Competing interests None
Yongjiang Ba and Ping Yue make the same contribution to this work.
References
- 1.Polistina F A, Guglielmi R, Baiocchi C et al. Chemoradiation treatment with gemcitabine plus stereotactic body radiotherapy for unresectable, non-metastatic, locally advanced hilar cholangiocarcinoma. Results of a five year experience. Radiother Oncol. 2011;99:120–123. doi: 10.1016/j.radonc.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Paik W H, Park Y S, Hwang J H et al. Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc. 2009;69:55–62. doi: 10.1016/j.gie.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Byrnes V, Afdhal N. Cholangiocarcinoma of the hepatic hilum (Klatskin tumor) Curr Treat Options Gastroenterol. 2002;5:87–94. doi: 10.1007/s11938-002-0055-5. [DOI] [PubMed] [Google Scholar]
- 4.Castellano-Megías V M, Andrés I D, Colina-Ruizdelgado F. Pathological aspects of so called “hilar cholangiocarcinoma”. World J Gastrointest Oncol. 2013;5:159–170. doi: 10.4251/wjgo.v5.i7.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramos E. Principles of surgical resection in hilar cholangiocarcinoma. World J Gastrointest Oncol. 2013;5:139–146. doi: 10.4251/wjgo.v5.i7.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Govil S, Reddy M S, Rela M. Surgical resection techniques for locally advanced hilar cholangiocarcinoma. Langenbeck Arch Surg. 2014;399:707–716. doi: 10.1007/s00423-014-1216-4. [DOI] [PubMed] [Google Scholar]
- 7.Rd V V, Cosgrove D, Herman J M et al. Management of perihilar cholangiocarcinoma in the era of multimodal therapy. Expert Rev Gastroent. 2012;6:481–495. doi: 10.1586/egh.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soares K C, Kamel I, Cosgrove D P et al. Hilar cholangiocarcinoma: diagnosis, treatment options, and management. Hepatobil Surg Nutr. 2014;3:18–34. doi: 10.3978/j.issn.2304-3881.2014.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel B, Bolus R, Harris L A et al. Measuring irritable bowel syndrome patient-reported outcomes with an abdominal pain numeric rating scale. Aliment pharm therap. 2009;30:1159–1170. doi: 10.1111/j.1365-2036.2009.04144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiriyama S, Takada T, Strasberg S M et al. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos) J Hepatobiliary Pancreat Sci. 2013;20:24–34. doi: 10.1007/s00534-012-0561-3. [DOI] [PubMed] [Google Scholar]
- 11.Banks P A, Bollen T L, Dervenis C et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 12.Cotton P B, Garrow D A, Gallagher J et al. Risk factors for complications after ERCP: a multivariate analysis of 11,497 procedures over 12 years. Gastrointest Endosc. 2009;70:80–88. doi: 10.1016/j.gie.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Parikh K, Ali M A, Wong R C. Unusual causes of upper gastrointestinal bleeding. Gastrointest Endosc Clin N Am. 2015;25:583–605. doi: 10.1016/j.giec.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Aljiffry M, Abdulelah A, Walsh M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. 2009;208:134–147. doi: 10.1016/j.jamcollsurg.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Farges O, Regimbeau J M, Fuks D et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surgery. 2013;100:274–283. doi: 10.1002/bjs.8950. [DOI] [PubMed] [Google Scholar]
- 16.Lafemina J, Jarnagin W R. Surgical management of proximal bile duct cancers. Langenbeck Arch Surg. 2012;397:869–879. doi: 10.1007/s00423-012-0928-6. [DOI] [PubMed] [Google Scholar]
- 17.Sakata J, Shirai Y, Tsuchiya Y et al. Preoperative cholangitis independently increases in-hospital mortality after combined major hepatic and bile duct resection for hilar cholangiocarcinoma. Langenbeck Arch Surg. 2009;394:1065–1072. doi: 10.1007/s00423-009-0464-1. [DOI] [PubMed] [Google Scholar]
- 18.Ribero D, Zimmitti G, Aloia T A et al. Preoperative cholangitis and future liver remnant volume determine the risk of liver failure in patients undergoing resection for hilar cholangiocarcinoma. J Am Coll Surg. 2016;223:87–97. doi: 10.1016/j.jamcollsurg.2016.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloek J J, Na V DG, Aziz Y et al. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg. 2010;14:119–125. doi: 10.1007/s11605-009-1009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazaki M, Yoshitomi H, Miyakawa S et al. Clinical practice guidelines for the management of biliary tract cancers 2015: the 2nd English edition. J Hepatobiliary Pancreat Sci. 2015;22:249–273. doi: 10.1002/jhbp.233. [DOI] [PubMed] [Google Scholar]
- 21.Rerknimitr R, Angsuwatcharakon P, Ratanachu-Ek T et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol. 2013;28:593–607. doi: 10.1111/jgh.12128. [DOI] [PubMed] [Google Scholar]
- 22.Vienne A, Hobeika E, Gouya H et al. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc. 2010;72:728–735. doi: 10.1016/j.gie.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 23.Yasuda I, Mukai T, Moriwaki H. Unilateral versus bilateral endoscopic biliary stenting for malignant hilar biliary strictures. Digest Endosc. 2013;25:81–85. doi: 10.1111/den.12060. [DOI] [PubMed] [Google Scholar]
- 24.Tang Z, Yang Y, Meng W et al. Best option for preoperative biliary drainage in Klatskin tumor: A systematic review and meta-analysis. Medicine. 2017;96:e8372. doi: 10.1097/MD.0000000000008372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min K K, Won P J, Kyun L J et al. A comparison of preoperative biliary drainage methods for perihilar cholangiocarcinoma: endoscopic versus percutaneous transhepatic biliary drainage. Gut Liver. 2015;9:791–799. doi: 10.5009/gnl14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Palma G D, Galloro G, Siciliano S et al. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53:547–553. doi: 10.1067/mge.2001.113381. [DOI] [PubMed] [Google Scholar]
- 27.Iwano H, Ryozawa S, Ishigaki N et al. Unilateral versus bilateral drainage using self-expandable metallic stent for unresectable hilar biliary obstruction. Digest Endosc. 2011;23:43–48. doi: 10.1111/j.1443-1661.2010.01036.x. [DOI] [PubMed] [Google Scholar]
- 28.Kawakami H, Kuwatani M, Onodera M et al. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J Gastroenterol. 2011;46:242–248. doi: 10.1007/s00535-010-0298-1. [DOI] [PubMed] [Google Scholar]
- 29.Xu C, Huang X E, Wang S X et al. Comparison of infection between internal-external and external percutaneous transhepatic biliary drainage in treating patients with malignant obstructive jaundice. Asian Pac J Cancer Prev. 2015;16:2543–2546. doi: 10.7314/apjcp.2015.16.6.2543. [DOI] [PubMed] [Google Scholar]


