Abstract
Few studies show the potential changing effect of fat-mass and obesity-associated ( FTO ) rs9939609 gene on cardiometabolic risk after a lifestyle intervention. This study aims to evaluate whether overweight and obese adolescents, carriers of the risk genotypes for obesity of the FTO rs9939609 gene polymorphism, have different anthropometric and biochemical responses to an interdisciplinary intervention program. The quasi-experimental study involved 34 adolescents aged 10 to 15 years. Schoolchildren with AA/AT genotype decreased glucose, total cholesterol, low-density lipoprotein cholesterol, and increased high-density lipoprotein cholesterol. However, there were no differences between the genotypes, suggesting that the “A” allele did not modify the subject's response to the intervention program.
Keywords: clinical trial, obesity, genetic polymorphism
Introduction
The prevalence of childhood overweight and obesity has increased in both developed and developing countries. 1 2 The etiology of accumulating fat mass is likely to be a result of imbalances between energy intake and energy expenditure. However, nutritional behavior and physical inactivity are not the only determinants of obesity. 3 4 The environmental and genetic aspects linked to obesity are being increasingly investigated. The fat-mass and obesity-associated ( FTO ) gene has been associated with body mass index (BMI) of children and adolescents. 5 6 For FTO rs9939609 specifically, one of the most studied polymorphisms in this gene, the A allele is associated with obesity. 7 8 This gene has an effect on the hypothalamus indicating a possible influence in energetic homeostasis control, possibly on regulation of body fat accumulation. 9 10
Children and adolescents carriers of the A allele of rs9939609 FTO polymorphism eat more frequently, ingest more food, and prefer foods with a higher fat content, 11 presenting a higher total energy intake. 12 Yang et al 8 also highlighted differences in food preferences in different FTO genotypes, such as the preference of AA genotype carriers for meat-based diets. In addition, physical inactivity accentuates the rs9939609 polymorphism effect on body fat accumulation, 13 suggesting that lifestyle may attenuate the impact of FTO genotype on obesity. However, a potential modifier effect of FTO gene on body weight changes and parameters related to obesity reached by lifestyle intervention is limited: current studies are heterogeneous and with imprecise results.
This study aims to investigate whether adolescents with overweight and obesity, carriers of the risk genotypes for obesity (AA and AT) of the FTO rs9939609 gene polymorphism, have different anthropometric and biochemical responses to an interdisciplinary intervention program when compared with TT genotype.
Materials and Methods
Subject Selection
Overweight and obese adolescents aged 10 to 15 years from the municipality of Santa Cruz do Sul, State of Rio Grande do Sul, Brazil, participated in this quasi-experimental study. Subjects were divided into a control group (CG) and experimental group (EG). Subjects classified as overweight or obese from our previous cross-sectional study were invited to participate in this study. The intervention program was highlighted on local radio and newspapers, social networks, and in schools.
Subjects in the EG participated in an interdisciplinary intervention program based on physical exercises as well as nutritional and psychological counseling. Subjects allocated to the CG did not receive any kind of intervention. EG and CG were matched by age, gender, anthropometric variables such as BMI, waist circumference (WC), and body fat percentage (BF%).
Inclusion criteria to participate in this study were age between 10 and 17 years; present the consent form signed by parents or guardians and the assent term signed by the schoolchildren; current BMI percentile ≥ 85 associated with a WC classified as elevated or to a high BF% (classified as moderately high, high, or very high). Exclusion criteria were carrier of any disease, abnormality or health problem that precludes the participation in the intervention program, and evaluations of pre- and postinterventions; a frequency of participation less than 70% in all intervention sessions; and a long-term advice against practicing any physical activity during the intervention.
In this study, adolescence was defined according to the World Health Organization, 14 beginning at 10 and finishing at completion of 19 years of age. Sample size calculation was performed using the software G*Power by de Faul et al, 15 based on estimates of Hulley et al. 16 For a test power of 0.8, an effect of 0.9 and an experimental significance level of 95% calculate the need of at least 17 subjects in each group (CG and EG) ( Fig. 1 ).
Fig. 1.
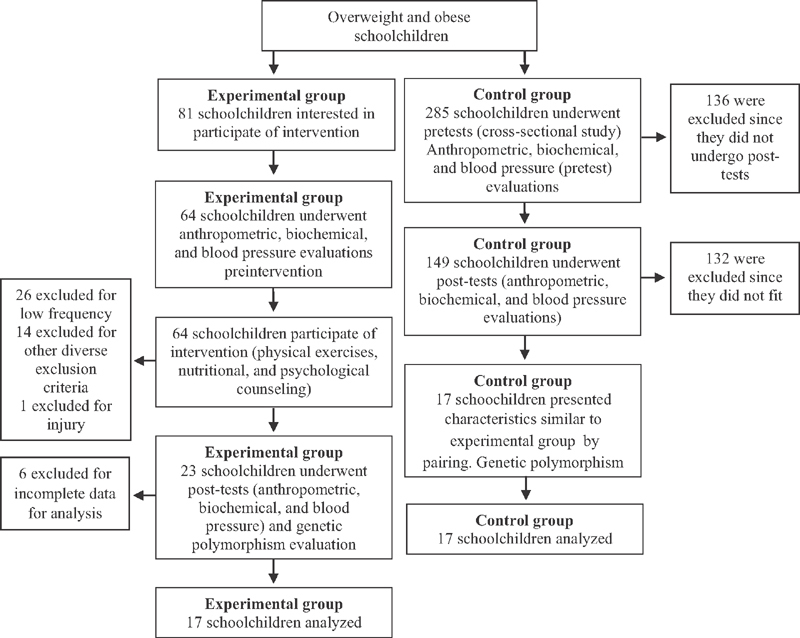
Distribution flowchart of subjects.
Intervention Program
This intervention study is part of a wider research project entitled “Obesity in schoolchildren from basic education: an interdisciplinary intervention study” approved by the research ethics committee of the University of Santa Cruz do Sul under number 357.403. This study is also registered on ClinicalTrials.gov (54985316.0.0000.5343).
Interdisciplinary intervention program sessions took place three times per week (Mondays, Wednesdays, and Fridays) in campus and lasted for 2 hours, beginning on May and ending on November, totalizing 6 months of intervention. The intervention was based on exercise sessions. On Mondays, warm up, walking, stretching, and sports (soccer, basketball, and handball) were held. On Wednesdays, aquatic activities including water aerobics, recreational activities, and swimming were held. On Fridays, walking, resisted and aerobic exercises, circuit and respiratory exercises were basically held. Heart rate (HR) during exercises was monitored through HR monitors (Polar FT1), maintaining the frequency between 50 and 70% of maximum HR. The values were previously defined using the calculation of Karvonen (maximum HR = 220 − age).
Schoolchildren also received group nutritional and psychological counseling. Nutritional intervention sessions took place on Wednesday with 1-hour duration, focusing on food re-education through conversation circles, interactive games, movies, lectures, and workshops for making healthy recipes. Group psychological intervention was performed once a week during 1 hour (Monday), comprising cognitive orientation and training in groups, using directed techniques in the handling of thoughts related to obesity.
Anthropometric, Biochemical, Blood Pressure, and Genetic Polymorphism Evaluations
All these evaluations were performed on two separate occasions, pre- and postinterventions, except for genetic polymorphism, that was performed only preintervention. BMI was calculated by the formulae: BMI = weight/height 2 (kg/m 2 ) using calibrated anthropometric scale with coupled stadiometer and the results were classified according to the World Health Organization 17 percentile curves. Waist and hip circumference (HC) were performed using an inflexible tape measure. For groups pairing, WC was classified according to Fernández et al's 18 protocols and HC according to the established criteria of Picon et al. 19 Waist–hip ratio (WHR) was obtained through the equation: WHR = waist (cm)/hip (cm). BF% was determined by measuring triceps and subscapular skinfolds using a skinfold compass model Lange. For calculations, we use Slaughter et al's 20 equation and later, for pairing, the classification according to Lohman. 21
For biochemical tests, peripheral venous blood was collected from each subject following a 12-hour (overnight) fast. Total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides (TG), and glucose were determined using Miura 200 (I.S.E., Rome, Italy) automated system and commercial kits Kovalent/DiaSys (DiaSys Diagnostic Systems, Germany). Insulin was determined by chemiluminescence on ARCHITECT i 2000SR (Abbott Park, Illinois, United States). HOMA-IR (Homeostatic Model Assessment) was calculated through the formulae: fasting glucose (mmol/L) × insulin (µU/L)/22.5. 22
Genetic polymorphism determination of carriers of risk genotypes for obesity (AA or AT) and of TT genotype of FTO rs9939609 gene was performed through DNA extraction, quantification, and genotyping. DNA extraction was performed using whole blood samples through the salting out method described by Miller et al. 23 DNA quantification was performed using NanoDrop 2000c Spectrophotometer (Thermo Scientific, Wilmington, United States), and later the samples were diluted with ultrapure water to the concentration of 10 mg/dL. Genotyping was performed through allele discrimination assays using quantitative polymerase chain reaction with TaqMan probes (Applied Biosystems, Foster City, California, United States) on StepOnePlus (Applied Biosystems).
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were determined according to Brazilian guidelines (SBC, 2010). 24 Puberty was determined using the adapted staging method of Tanner, 25 which consists in a self-assessment test with pictures representing the stages of developing pubic hairiness. Socioeconomic status was evaluated according to the self-reported questionnaire of the Brazilian Market Research Association, which divides the socioeconomic classes in A–B (high), C (middle), and D–E (low). 26 Skin color was determined according to self-reported questionnaire, being posteriorly regrouped in white and nonwhite.
Data Analysis
Statistical analysis was performed using SPSS (IBM, Armonk, New York, United States) version 23.0. Normality of sample was tested using the Shapiro–Wilk's test. Normal distribution data were analyzed using t -test for independent samples and paired t -test. For data without normal distribution, we use Mann–Whitney and Wilcoxon's tests. The effect size calculation considering the Cohen's d test was performed according to Sullivan and Feinn. 27 Statistical significance was considered for p < 0.05. Hardy–Weinberg's equilibrium was tested for the rs9939609 polymorphism using the same statistical software ( p = 0.356). Moraes et al 28 described the genotype distribution and allele frequency of the rs9939609 polymorphism in EG and CG.
Results
General characteristics of subjects participating in this study regarding age, gender, BMI, pubertal stage, skin color, socioeconomic level, and rs9939609 FTO genotype are shown in Table 1 . No differences were found in the comparison of the odds ratio (OR) values of the genotypic frequencies for the experimental and control groups (OR: 2.06; confidence interval: 0.52–8.17; p = 0.303; data are not shown in the table).
Table 1. General characteristics of all subjects' participants of this study.
| Variables | Experimental group | Control group | Total |
|---|---|---|---|
| n | n | N | |
| Sex | |||
| Male | 4 | 5 | 9 |
| Female | 13 | 12 | 25 |
| Age (y) | |||
| 10–12 | 12 | 10 | 22 |
| 13–15 | 5 | 7 | 12 |
| BMI | |||
| Overweight | 1 | 1 | 2 |
| Obesity | 16 | 16 | 32 |
| Maturational stage | |||
| Prepubertal | 8 | 6 | 14 |
| Continuous maturation | 8 | 9 | 17 |
| Matured | 1 | 2 | 3 |
| Skin color | |||
| White | 11 | 10 | 21 |
| Nonwhite | 6 | 7 | 13 |
| Socioeconomic level | |||
| AB | 4 | 3 | 7 |
| C | 11 | 12 | 23 |
| DE | 2 | 2 | 4 |
| rs9939609 FTO genotype | |||
| AA/AT | 11 | 8 | 19 |
| TT | 6 | 9 | 15 |
Abbreviations: BMI, body mass index; FTO, fat-mass and obesity-associated.
Anthropometric, biochemical, and blood pressure variables pre- and postinterventions according to genotype AA/AT and TT are shown in Table 2 . The levels of HDL cholesterol and glucose were significantly different between the CG and EG after the intervention in the TT and AA/AT genotypes, respectively. Insulin and HOMA-IR levels, which were statistically different between the CG and EG groups before intervention in the AA/AT genotype carriers, did not differ after the intervention.
Table 2. Biochemical, anthropometric, and blood pressure variables pre- and postinterventions with subjects divided according to genotype AA/AT and TT.
| rs9939609 FTO genotype | Preintervention | Postintervention | ||||
|---|---|---|---|---|---|---|
| Control | Experimental | p -Value | Control | Experimental | p -Value | |
| TT | n = 9 | n = 6 | n = 9 | n = 6 | ||
| BMI (kg/m 2 ) | 30.21 (4.51) | 29.81 (4.76) | 0.556 | 29.91 (3.87) | 29.20 (5.03) | 0.637 |
| WC (cm) | 86.67 (7.60) | 84.33 (5.91) | 0.409 | 86.90 (7.44) | 83.68 (5.94) | 0.346 |
| Waist–hip ratio | 0.83 (0.05) | 0.80 (0.03) | 0.259 | 0.84 (0.04) | 0.79 (0.04) | 0.124 |
| Fat percentage (%) | 41.83 (6.14) | 41.04 (1.68) | 0.814 | 34.60 (7.96) | 42.10 (6.83) | 0.077 |
| Glucose (mg/dL) | 92.44 (5.87) | 94.83 (7.75) | 0.636 | 86.22 (8.10) | 93.00 (8.07) | 0.098 |
| Insulin (ng/mL) | 15.02 (8.64) | 16.45 (8.29) | 0.637 | 13.28 (8.08) | 15.68 (6.33) | 0.289 |
| HOMA-IR | 3.45 (2.06) | 3.88 (2.07) | 0.637 | 2.88 (1.94) | 3.60 (1.47) | 0.289 |
| Total cholesterol (mg/dL) | 161.00 (37.09) | 170.16 (20.96) | 0.345 | 149.55 (50.08) | 162.16 (19.03) | 0.126 |
| HDL cholesterol (mg/dL) | 45.98 (7.00) | 51.19 (9.31) | 0.239 | 46.65 (9.85) | 58.78 (8.33) | 0.045 |
| LDL cholesterol (mg/dL) | 97.57 (33.01) | 100.81 (22.86) | 0.556 | 74.83 (38.97) | 86.22 (22.36) | 0.239 |
| Triglycerides (mg/dL) | 90.88 (33.26) | 90.03 (33.11) | 0.906 | 82.57 (35.57) | 85.81 (31.44) | 0.556 |
| SBP (mm Hg) | 119.11 (16.52) | 110.33 (11.34) | 0.203 | 122.44 (13.37) | 113.67 (11.69) | 0.246 |
| DBP (mm Hg) | 80.67 (14.45) | 71.17 (10.59) | 0.227 | 81.11 (11.66) | 71.67 (11.69) | 0.145 |
| AT/AA | n = 8 | n = 11 | n = 8 | n = 11 | ||
| BMI (kg/m 2 ) | 28.08 (6.23) | 30.32 (5.87) | 0.283 | 27.23 (6.73) | 29.69 (6.48) | 0.364 |
| WC (cm) | 82.62 (10.23) | 89.07 (11.97) | 0.186 | 79.96 (12.67) | 87.48 (13.24) | 0.126 |
| Waist–hip ratio | 0.84 (0.04) | 0.86 (0.07) | 0.707 | 0.82 (0.03) | 0.84 (0.07) | 0.901 |
| Fat percentage (%) | 40.43 (6.92) | 38.30 (5.76) | 0.649 | 38.88 (11.30) | 38.36 (9.75) | 0.804 |
| Glucose (mg/dL) | 90.62 (6.02) | 97.72 (7.12) | 0.035 | 86.37 (7.68) | 93.18 (5.13) | 0.038 |
| Insulin (ng/mL) | 11.26 (5.31) | 19.69 (6.13) | 0.009 | 10.95 (5.69) | 17.44 (8.99) | 0.099 |
| HOMA-IR | 2.51 (1.20) | 4.72 (1.38) | 0.004 | 2.38 (1.36) | 3.99 (2.03) | 0.069 |
| Total cholesterol (mg/dL) | 143.25 (26.59) | 161.72 (38.62) | 0.076 | 143.37 (38.16) | 145.27 (28.38) | 0.967 |
| HDL cholesterol (mg/dL) | 46.24 (8.49) | 45.47 (7.34) | 0.620 | 48.33 (12.68) | 50.38 (10.24) | 0.364 |
| LDL cholesterol (mg/dL) | 82.36 (23.12) | 95.18 (30.92) | 0.322 | 81.24 (27.69) | 77.46 (23.55) | 0.804 |
| Triglycerides (mg/dL) | 73.22 (25.01) | 99.64 (41.85) | 0.186 | 68.97 (26.11) | 86.98 (27.46) | 0.099 |
| SBP (mm Hg) | 111.25 (15.81) | 110.45 (12.93) | 0.934 | 106.25 (13.91) | 111.55 (16.56) | 0.530 |
| DBP (mm Hg) | 69.38 (14.25) | 74.18 (11.24) | 0.357 | 67.50 (24.23) | 70.18 (17.33) | 0.895 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FTO, fat-mass and obesity-associated; HDL, high-density lipoprotein; HOMA-IR, Homeostatic Model Assessment; LDL, low-density lipoprotein; SBP, systolic blood pressure; WC, waist circumference.
Note: Statistic, data are mean (standard deviation). t -test for independent samples or Mann–Whitney's test.
Table 3 shows the mean difference of anthropometric, biochemical, and blood pressure variables pre- and post-interventions for EG and CG according to rs9939609 FTO genotype. Schoolchildren from EG with the risk allele A had a significant decrease in glucose ( p = 0.014), TC ( p = 0.004), LDL cholesterol ( p = 0.003), and an increase of HDL cholesterol ( p = 0.033). Among schoolchildren of EG (genotype TT) there was significant increase for HDL cholesterol ( p = 0.028) only. However, the differences between AA/AT and TT genotypes were not significant.
Table 3. Mean difference of pre- and postintervention variables.
| Experimental group | TT | AT/AA | p b | ||||
| Δ (SD) | Δ (%) | p a | Δ (SD) | Δ (%) | p a | ||
| BMI (kg/m 2 ) | −0.61 (0.93) | −2.04 | 0.249 | −0.62 (2.82) | −2.07 | 0.477 | 0.763 |
| WC (cm) | −0.65 (2.89) | −0.77 | 0.500 | −1.59 (4.74) | −1.78 | 0.213 | 0.651 |
| Waist–hip ratio | −0.008 (0.03) | −1.25 | 0.588 | −0.02 (0.03) | −2.32 | 0.087 | 0.448 |
| Fat percentage (%) | 1.05 (6.97) | 2.58 | 0.600 | 0.06 (11.95) | 0.15 | 0.722 | 1.000 |
| Glucose (mg/dL) | −1.83 (7.08) | −1.92 | 0.500 | −4.54 (4.92) | −4.64 | 0.014 | 0.267 |
| Insulin (ng/mL) | −0.76 (10.64) | −4.68 | 0.917 | −2.24 (6.19) | −11.42 | 0.197 | 0.840 |
| HOMA-IR | −0.28 (2.62) | −7.21 | 0.753 | −0.72 (1.53) | −15.46 | 0.131 | 0.841 |
| Total cholesterol (mg/dL) | −8.00 (22.56) | −4.70 | 0.463 | −16.45 (14.19) | −10.17 | 0.004 | 0.614 |
| HDL cholesterol (mg/dL) | 7.58 (4.93) | 14.82 | 0.028 | 4.91 (5.83) | 10.79 | 0.033 | 0.482 |
| LDL cholesterol (mg/dL) | −14.59 (16.73) | −14.47 | 0.116 | −17.71 (10.12) | −18.61 | 0.003 | 0.763 |
| Triglycerides (mg/dL) | −4.21 (29.80) | −4.68 | 0.600 | −12.66 (36.85) | −12.70 | 0.424 | 0.841 |
| SBP (mm Hg) | 3.33 (13.42) | 3.02 | 0.416 | 1.09 (10.63) | 0.99 | 0.591 | 0.649 |
| Control group | TT | AT/AA | pb | ||||
| Δ (SD) | Δ (%) | pa | Δ (SD) | Δ (%) | pa | ||
| BMI (kg/m 2 ) | −0.29 (1.26) | −0.99 | 0.953 | −0.84 (0.83) | −3.02 | 0.012 | 0.068 |
| WC (cm) | 0.22 (0.78) | 0.26 | 0.482 | −2.66 (3.38) | −3.21 | 0.063 | 0.060 |
| Waist–hip ratio | 0.003 (0.02) | 1.20 | 0.394 | −0.02 (0.04) | −2.38 | 0.175 | 0.133 |
| Fat percentage (%) | −7.23 (10.67) | −17.28 | 0.066 | −1.55 (7.19) | −3.83 | 0.674 | 0.178 |
| Glucose (mg/dL) | −6.22 (5.06) | −6.72 | 0.011 | −4.25 (8.56) | −4.68 | 0.340 | 0.122 |
| Insulin (ng/mL) | −1.73 (3.31) | −11.58 | 0.173 | −0.31 (4.09) | −2.75 | 1.000 | 0.630 |
| HOMA-IR | −0.56 (0.74) | −16.52 | 0.086 | −0.12 (0.94) | −5.17 | 0.779 | 0.336 |
| Total cholesterol (mg/dL) | −11.44 (17.43) | −7.11 | 0.086 | 0.12 (18.82) | 0.08 | 0.441 | 0.123 |
| HDL cholesterol (mg/dL) | 0.67 (9.35) | 1.45 | 0.441 | 2.09 (12.12) | 4.51 | 1.000 | 0.773 |
| LDL cholesterol (mg/dL) | −22.73 (38.41) | −23.30 | 0.021 | −1.12 (8.85) | −1.38 | 0.674 | 0.773 |
| Triglycerides (mg/dL) | −8.31 (35.77) | −9.14 | 0.441 | −4.25 (34.19) | −5.80 | 0.889 | 0.630 |
| SBP (mm Hg) | 3.33 (13.85) | 2.79 | 0.438 | −5.00 (13.00) | −4.49 | 0.223 | 0.207 |
| DBP (mm Hg) | 0.44 (10.08) | 0.54 | 1.000 | −1.87 (6.51) | −2.70 | 0.450 | 0.617 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HDL, high-density lipoprotein; HOMA-IR, Homeostatic Model Assessment; LDL, low-density lipoprotein; SBP, systolic blood pressure; SD, standard deviation; WC, waist circumference.
Note: Statistic, data are mean (standard deviation).
Paired t -test or Wilcoxon.
Paired t -test for independent samples or Mann–Whitney.
Discussion and Conclusion
This study verified that after the intervention, schoolchildren from EG and carriers of A allele had significant decrease in glucose, TC and LDL cholesterol, and increase in HDL cholesterol. Among schoolchildren from EG with TT genotype, there was a significant increase of HDL cholesterol, showing the effectiveness of an intervention program on these biochemical parameters in overweight and obese adolescents. However, when comparing the AA/AT with the TT genotypes after the intervention, there were no significant differences, showing that both groups had a similar response to the intervention program.
A 4-month intervention study from southern Brazil with 36 schoolchildren aged 8 to 16 years observed that after the intervention with physical exercises and nutritional and oral health counseling, there was an absolute improvement of WC, HC, and C-reactive protein in subjects with the presence of A allele and of HC and uric acid for the TT genotype. However, there was no difference in other biochemical parameters (glucose, insulin, and lipid profile) and blood pressure (SBP and DBP), disagreeing of our study, which presented improvement in biochemical profile, but not in anthropometric parameters. Similar to our study, they also did not find statistical significance when comparing the AA/AT and TT genotypes after the intervention. 28
A study with 136 overweight/obese children and adolescents and 172 normal weight subjects observed that after a 12-week program of physical exercises (aerobic, high-intensity interval training, combined training, and walking in water), FTO rs9939609 gene alleles did not show interaction with changes in anthropometric parameters. 29
As well as in our study, a study with a subset of 207 overweight and obese individuals (94 males, mean age 10.79 ± 2.52 years) performed in Germany reported that after an intervention program based on physical exercises and nutritional education and behavioral therapy (individual psychological care of the child/adolescent and his or her family), there was no association of the FTO rs9939609 gene alleles with weight loss, fasting glucose, TG, and LDL and HDL cholesterol. 30 Likewise, Lappalainen et al, 31 in a study with 502 adult subjects with overweight and impaired glucose tolerance from Finland, performed an intervention of lifestyle that recommends and follows the increase in physical activities practice and proposed a personalized diet, and did not observe differences between the FTO rs9939609 markers regarding the weight reduction after the intervention. Both studies suggest that subjects with genetic predisposition to obesity may also benefit from lifestyle changes.
The same conclusion of the above paragraph was reported in a systematic review that evaluated interventions aiming weight loss on overweight adults; differences between FTO genotypes regarding BMI, body weight, and WC after intervention with dietary, physical activity, or drug-based weight loss were not observed. 32
However, a study on 138 Chinese adolescents aged 10 to 18 years from Shanghai and submitted to a dietary (individual and supplied according to the basal metabolic rate) and physical exercises intervention (such as ball games, swimming, and aerobics) for 4 weeks concluded that the subjects with genotype AA or AT decreased significantly the levels of total and LDL cholesterol when compared with the TT genotype ( p < 0.05). 33
In this sense, there is contradictory evidence about the association of the genetic variability of FTO gene with weight loss and cardiometabolic improvements following increased physical activity. Future research is required to understand the role of FITT principles (frequency, intensity, time, and type) in obese children with different genetic profiles and susceptibility to premature disease.
In 2009, Rendo et al 34 reported that few studies investigated the influence of FTO gene variables through intervention studies with physical exercise and that it was not elucidated if the increment of the physical exercise modifies the interaction of the risk allele A in the excess fat of the body. Recently, Leońska-Duniec et al 35 reinforced that this fact is not solved yet and there is few knowledge on the genetic variability and its interaction with physical activity.
Analysis of the studies so far shows that they are heterogeneous regarding the evaluated populations, intervention period, intervention methods, and sample size. Few studies indicate the type, intensity, and frequency of proposed exercises. This is also observed in the systematic review of Xiang et al 36 that evaluated the relationship between FTO genotypes and the response to the obesity treatment; the studies included had differences regarding intervention type, study period (3 months–4 years), sample size, and breed/ethnicity, indicating the need of other studies that consider these characteristics. Livingstone et al 32 showed that the relationship was influenced by the kind of study (diet, physical exercise, or drugs), study period (8 weeks–3 years), breed/ethnicity, gender, or BMI; however, the study did not evaluate the kind of diet or physical exercises proposed and these can be a possible contributing factor for answering in the intervention programs.
Since there are few intervention studies verifying the FTO gene variability and its response to physical exercise, we look other nonexperimental studies and notice that the presence of physical activity can attenuate the effects of FTO gene. Celis-Morales et al, 37 in a cross-sectional study with 1,280 European adults, reported that the effect size of the FTO associations on BMI and WC for active subjects were lower than for inactive subjects, indicating that the genetic susceptibility to overweight can be diminished with the implementation of physical exercises. By the same way, in the Lithuanian adult population, Petkeviciene et al 38 observed that the physical activity could weaken the effect of FTO polymorphism on the body weight and metabolic syndrome. The same was found in Nigeria, where a case-control pilot study conducted in young adults with a mean age of 22.6 years (103 subjects with obesity and 98 controls), verified that environmental factors such as physical inactivity mediated the relationship between polymorphism and obesity. 39
The earlier association is also observed in the adult–juvenile population. In a cross-sectional study with 752 European adolescents, Ruiz et al 40 reported that the effect of FTO gene polymorphism on body fat parameters (BMI, WC, and BF%) is attenuated when the daily recommendations of physical activity are fulfilled (>60 minutes of moderate to vigorous physical activity). Xi et al, 41 in a population study with 3,503 children and adolescents aged 6 to 18 years from Pequin (China), reported interaction between the rs9939609 FTO genotype and physical activity, in which the effect of the A allele on BMI was reduced as increased physical activity intensity (low, moderate, and severe). However, a meta-analysis with 218,166 adults and 19,268 children and adolescents verified that, in adults, the physical activity attenuated the risk effect of the A allele for obesity; however, this was not found in children and adolescents. 42
Thus, although association studies shows that physical exercise practice can attenuate the effect of FTO gene on obesity, experimental studies are yet few and contradictory. Knowing the relationship of FTO rs9939609 gene polymorphism with obesity, we need more studies like this, which aims to identify whether subjects with genetic predisposition to obesity also can benefit of intervention program focused on physical exercise practice, improving your overall health. In this way, future intervention programs can be designed according to the subject's genetic variability, offering more effective personalized intervention programs.
The strengths of this study are as follows: first, including genetic analysis in conducting a clinical trial and supplying the lack of intervention studies which evaluate genetic interference in weight loss programs focused on physical exercises; second, group pairing, in which the study subjects were paired by gender, age, and anthropometric variables (BMI, WC, and BF%). To decrease the natural differences among subjects, we organized pairs with features as similar as possible.
Among the limitations of this study are the analyses of only the rs9939609 genetic variant, since the subjects of this study can carry other different genetic alterations that predispose the body fat accumulation. However, we chose the FTO rs9939609 gene polymorphism because it was strongly related with the overweight presence in schoolchildren from the same city in a previous cross-sectional study. 43 The study also has few subjects. However, a sample size calculation was performed to make the inferences with a stipulated test power. The loss of subjects in the intervention program was a challenge for our study. However, it is known the difficulty of permanence of the subjects in intervention programs 44 as well as that biological and psychosocial factors might be associated with desistance of obese adolescents from intervention programs. 45
In conclusion, we demonstrate that the interdisciplinary intervention program with physical exercises and nutritional and psychological counseling was effective for decreasing glucose, TC, and LDL cholesterol levels and increasing HDL cholesterol in overweight/obese adolescents with the A allele of FTO rs9939609 gene polymorphism, as well as the increasing of HDL cholesterol in TT genotype adolescents. However, adolescents with AA and AT genotype have the same answer to intervention when comparing with TT genotype adolescents, suggesting that the presence of the risk allele for obesity did not modify the subject's answer to anthropometric, biochemical, and blood pressure variables after an interdisciplinary intervention program. This indicates that subjects who had genetic predisposition to overweight also can be beneficiated for changes in lifestyle.
Footnotes
Conflict of Interest None declared.
References
- 1.Ogden C L, Carroll M D, Flegal K M. High body mass index for age among US children and adolescents, 2003-2006. JAMA. 2008;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1(01):11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 3.Cárdenas Fuentes G, Bawaked R A, Martínez González MÁ et al. Association of physical activity with body mass index, waist circumference and incidence of obesity in older adults. Eur J Public Health. 2018;28(05):944–950. doi: 10.1093/eurpub/cky030. [DOI] [PubMed] [Google Scholar]
- 4.Schröder H, Mendez M A, Ribas L et al. Caloric beverage drinking patterns are differentially associated with diet quality and adiposity among Spanish girls and boys. Eur J Pediatr. 2014;173(09):1169–1177. doi: 10.1007/s00431-014-2302-x. [DOI] [PubMed] [Google Scholar]
- 5.Luczyński W, Fendler W, Ramatowska A et al. Polymorphism of the FTO gene influences body weight in children with type 1 diabetes without severe obesity . Int J Endocrinol. 2014;2014:630712. doi: 10.1155/2014/630712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M, Zhao X, Xi B et al. Impact of obesity-related gene polymorphism on risk of obesity and metabolic disorder in childhood [in Chinese] Zhonghua Yu Fang Yi Xue Za Zhi. 2014;48(09):776–783. [PubMed] [Google Scholar]
- 7.Quan L L, Wang H, Tian Y, Mu X, Zhang Y, Tao K. Association of fat-mass and obesity-associated gene FTO rs9939609 polymorphism with the risk of obesity among children and adolescents: a meta-analysis . Eur Rev Med Pharmacol Sci. 2015;19(04):614–623. [PubMed] [Google Scholar]
- 8.Yang M, Xu Y, Liang L et al. The effects of genetic variation in FTO rs9939609 on obesity and dietary preferences in Chinese Han children and adolescents. PLoS One. 2014;9(08):e104574. doi: 10.1371/journal.pone.0104574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredriksson R, Hägglund M, Olszewski P K et al. The obesity gene, FTO , is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain . Endocrinology. 2008;149(05):2062–2071. doi: 10.1210/en.2007-1457. [DOI] [PubMed] [Google Scholar]
- 10.Gerken T, Girard C A, Tung Y Cet al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase Science 2007318(5855):1469–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanofsky-Kraff M, Han J C, Anandalingam K et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating . Am J Clin Nutr. 2009;90(06):1483–1488. doi: 10.3945/ajcn.2009.28439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi Q, Downer M K, Kilpeläinen T O et al. Dietary intake, FTO genetic variants, and adiposity: a combined analysis of over 16,000 children and adolescents. Diabetes. 2015;64(07):2467–2476. doi: 10.2337/db14-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreasen C H, Stender-Petersen K L, Mogensen M S et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57(01):95–101. doi: 10.2337/db07-0910. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization.Young People's Health – a Challenge for Society. Report of a WHO Study Group on Young People and Health for All. Technical Report Series 731 Geneva: WHO; 1986 [PubMed] [Google Scholar]
- 15.Faul F, Erdfelder E, Lang A G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(02):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 16.Hulley S B, Cummings S R, Browner W S . Philadelphia, PA: Lippincott Williams & Wilkins; 2013. Designing Clinical Research: An Epidemiologic Approach, 4th ed. [Google Scholar]
- 17.World Health Organization.Growth reference 5–19 years 2007. Available at:http://www.who.int/growthref/who2007_bmi_for_age/en/. Accessed December 10, 2018
- 18.Fernández J R, Redden D T, Pietrobelli A, Allison D B. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145(04):439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 19.Picon P X, Leitao C B, Gerchman F et al. Waist measure and waist-to-hip ratio and identification of clinical conditions of cardiovascular risk: multicentric study in type 2 diabetes mellitus patients. Arq Bras Endocrinol Metab. 2007;51(03):443–449. doi: 10.1590/s0004-27302007000300013. [DOI] [PubMed] [Google Scholar]
- 20.Slaughter M H, Lohman T G, Boileau R A et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60(05):709–723. [PubMed] [Google Scholar]
- 21.Lohman T G. The use of skinfold to estimate body fatness on children and youth. JOPERD. 1987;58(09):98–102. [Google Scholar]
- 22.Matthews D R, Hosker J P, Rudenski A S, Naylor B A, Treacher D F, Turner R C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(07):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Miller S A, Dykes D D, Polesky H F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(03):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sociedade Brasileira de Cardiologia; Sociedade Brasileira de Nefrologia; Sociedade Brasileira de Hipertensão.VI Brazilian Guidelines on Hypertension Arq Bras Cardiol 20109501011–51. [PubMed] [Google Scholar]
- 25.Tanner J M. Oxford: Blackwell Scientific; 1962. Growth at Adolescence, 2nd.ed. [Google Scholar]
- 26.ABEP.Brazilian Market Research Association. Brazilian Criteria 2016Available at:http://www.abep.org/criterio-brasil. Accessed December 10, 2018
- 27.Sullivan G M, Feinn R. Using effect size-or why the P value is not enough. J Grad Med Educ. 2012;4(03):279–282. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moraes G, Reuter C P, Renner J DP et al. Genotypic carriers of the obesity-associated FTO polymorphism exhibit different cardiometabolic profiles after an intervention. An Acad Bras Ciênc. 2016;88(04):2331–2339. doi: 10.1590/0001-3765201620160114. [DOI] [PubMed] [Google Scholar]
- 29.do Nascimento G A, Teixeira M D, Furtado-Allee L et al. FTO rs9939609 A allele influences anthropometric outcome in response to dietary intervention, but not in response to physical exercise program. Eur J Nutr. 2019;58(01):325–334. doi: 10.1007/s00394-017-1596-7. [DOI] [PubMed] [Google Scholar]
- 30.Müller T D, Hinney A, Scherag A et al. ‘Fat mass and obesity associated’ gene ( FTO ): no significant association of variant rs9939609 with weight loss in a lifestyle intervention and lipid metabolism markers in German obese children and adolescents . BMC Med Genet. 2008;9:85. doi: 10.1186/1471-2350-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lappalainen T J, Tolppanen A M, Kolehmainen M et al. The common variant in the FTO gene did not modify the effect of lifestyle changes on body weight: the Finnish Diabetes Prevention Study . Obesity (Silver Spring) 2009;17(04):832–836. doi: 10.1038/oby.2008.618. [DOI] [PubMed] [Google Scholar]
- 32.Livingstone K M, Celis-Morales C, Papandonatos G D et al. FTO genotype and weight loss: systematic review and meta-analysis of 9563 individual participant data from eight randomised controlled trials. BMJ. 2016;354:i4707. doi: 10.1136/bmj.i4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou Z C, Shi Y Y, Chen J H, Wang L S, Cai W. Effect of exercise combined with dietary intervention on obese children and adolescents associated with the FTO rs9939609 polymorphism. Eur Rev Med Pharmacol Sci. 2015;19(23):4569–4575. [PubMed] [Google Scholar]
- 34.Rendo T, Moleres A, Marti Del Moral A. Effects of the FTO gene on lifestyle intervention studies in children . Obes Facts. 2009;2(06):393–399. doi: 10.1159/000262296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leońska-Duniec A, Ahmetov I I, Zmijewski P. Genetic variants influencing effectiveness of exercise training programmes in obesity - an overview of human studies. Biol Sport. 2016;33(03):207–214. doi: 10.5604/20831862.1201052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiang L, Wu H, Pan Aet al. FTO genotype and weight loss in diet and lifestyle interventions: a systematic review and meta-analysisAm J Clin Nutr [DOI] [PMC free article] [PubMed]
- 37.Celis-Morales C, Marsaux C F, Livingstone K M et al. Physical activity attenuates the effect of the FTO genotype on obesity traits in European adults: the Food4Me study. Obesity (Silver Spring) 2016;24(04):962–969. doi: 10.1002/oby.21422. [DOI] [PubMed] [Google Scholar]
- 38.Petkeviciene J, Smalinskiene A, Klumbiene J, Petkevicius V, Kriaucioniene V, Lesauskaite V. Physical activity, but not dietary intake, attenuates the effect of the FTO rs9939609 polymorphism on obesity and metabolic syndrome in Lithuanian adult population. Public Health. 2016;135:23–29. doi: 10.1016/j.puhe.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Oyeyemi B F, Ologunde C A, Olaoye A B, Alamukii N A. FTO gene associates and interacts with obesity risk, physical activity, energy intake, and time spent sitting: pilot study in a Nigerian population . J Obes. 2017;2017:3.24527E6. doi: 10.1155/2017/3245270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz J R, Labayen I, Ortega F B et al. Attenuation of the effect of the FTO rs9939609 polymorphism on total and central body fat by physical activity in adolescents: the HELENA study. Arch Pediatr Adolesc Med. 2010;164(04):328–333. doi: 10.1001/archpediatrics.2010.29. [DOI] [PubMed] [Google Scholar]
- 41.Xi B, Shen Y, Zhang M et al. The common rs9939609 variant of the fat mass and obesity-associated gene is associated with obesity risk in children and adolescents of Beijing, China. BMC Med Genet. 2010;11:107. doi: 10.1186/1471-2350-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kilpeläinen T O, Qi L, Brage S et al. Physical activity attenuates the influence of FTO variants on obesity risk: a meta-analysis of 218,166 adults and 19,268 children. PLoS Med. 2011;8(11):e1001116. doi: 10.1371/journal.pmed.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reuter C P, Rosane De Moura Valim A, Gaya A R et al. FTO polymorphism, cardiorespiratory fitness, and obesity in Brazilian youth. Am J Hum Biol. 2016;28(03):381–386. doi: 10.1002/ajhb.22798. [DOI] [PubMed] [Google Scholar]
- 44.Burgess E, Hassmén P, Pumpa K L. Determinants of adherence to lifestyle intervention in adults with obesity: a systematic review. Clin Obes. 2017;7(03):123–135. doi: 10.1111/cob.12183. [DOI] [PubMed] [Google Scholar]
- 45.Fidelix Y L, Farias Júnior J C, Lofrano-Prado M C, Guerra R L, Cardel M, Prado W L. Multidisciplinary intervention in obese adolescents: predictors of dropout. Einstein (Sao Paulo) 2015;13(03):388–394. doi: 10.1590/S1679-45082015AO3339. [DOI] [PMC free article] [PubMed] [Google Scholar]


