Abstract
Background
The clinical utility of patient and environmental surveillance screening for vancomycin-resistant enterococci (VRE) in the postacute care setting has not been definitively clarified. We assessed the longitudinal relationship between patient colonization and room contamination, and we established their association with unfavorable health outcomes.
Methods
Four hundred sixty-three postacute care patients were followed longitudinally from enrollment to discharge for up to 6 months. Multiple body and environmental sites were sampled at regular intervals to establish correlation between environmental contamination and patient colonization and with longer than expected stay, unplanned hospitalization, and infections adjusting for sex, age, race, Charlson’s comorbidity index, and physical self-maintenance score.
Results
New VRE acquisition was more likely in patients residing in contaminated rooms (multivariable odds ratio [OR] = 3.75; 95% confidence interval [CI], 1.98–7.11) and vice versa (OR = 3.99; 95% CI, 2.16–7.51). New acquisition and new contamination were associated with increased length of stay (OR = 4.36, 95% CI = 1.86–10.2 and OR = 4.61, 95% CI = 1.92–11.0, respectively) and hospitalization (OR = 2.42, 95% CI = 1.39–4.22 and OR = 2.80, 95% CI = 1.52–5.12). New-onset infections were more common with higher VRE burdens (15% in the absence of VRE, 20% when after VRE isolation only on the patient or only in the room, and 29% after VRE isolation in both the patient and the room).
Conclusions
Room contamination with VRE is a risk factor for patient colonization, and both are associated with future adverse health outcomes in our postacute care patients. Further research is warranted to establish whether VRE screening may contribute to better understanding of risk assessment and adverse outcome prevention in postacute care.
Keywords: contamination, Enterococcus, health outcome, nursing home, postacute
Postacute care facilities (PACs) host patients who are in need of Specialist care and/or rehabilitation procedures before they are ready to be discharged to their permanent residence, whereas a small percentage will transition to long-term care. The majority of such patients are admitted directly from hospitals. Although PACs are often burdened by a higher prevalence of multidrug-resistant organisms (MDROs) than acute care facilities [1, 2], knowledge of their epidemiology is lacking in this specific setting. Vancomycin-resistant enterococci (VRE) in particular are on the rise in PACs, and long-term care facilities and have been found to colonize up to one third of the patients, depending on geographic location and sampling protocols [2–5]. Colonization surveillance programs are often advocated but seldom adopted [6].
Environmental contamination has been implicated as a contributor to acquisition of MDROs by the patient [7–10]. We recently demonstrated a close relationship between patient colonization and concurrent contamination of their room environment with MDROs including VRE [11]. However, the time relationship between patient and environmental VRE contamination, and their influence on patient health outcomes, is largely unknown in this particular setting.
We investigated the existence and extent of a longitudinal cause-effect relationship between patient colonization and environmental contamination with VRE and vice versa in PAC patients. In addition, we were interested in how such events may reflect on adverse health outcomes, including prolonged stay at the facility, need of rehospitalization, and infections.
METHODS
Study Design
We analyzed multisite VRE patient colonization and environmental contamination data obtained during an observational study in 651 patients residing in 6 postacute care nursing facilities in southeast Michigan [2]. To assess the relationship and impact of baseline and new VRE acquisition, we focused on a subset of participants for which patient colonization and environmental contamination were assessed at baseline and at least 1 follow-up visit. Nares, roof of the mouth, groin, perianal, and dominant hand were sampled with patient consent, along with an average of 10 high-touch surfaces in the patient’s room. Sampling was performed upon admission and repeated after 14 days and every month for up to 6 months or discharge. Every newly admitted patient who consented to be in the study was included, regardless of comorbidities or other patient characteristics, unless they were receiving end-of-life care.
Data Collection
Demographics including age, race, sex, expected length of stay, were recorded for each participant at study enrollment. In addition, information on new infections, hospitalizations for any reason within the last 30 days, antibiotic use, Charlson’s comorbidity index [12], and physical self-maintenance score (PSMS) [13] were collected at each visit.
Specimen Collection and Laboratory Methods
Bacti-swabs (Remel, Lenexa, KS) were used by trained research personnel to obtain patient and environmental samples. Patients’ nares, oral cavity, groin, and perianal site swabs were cultured on bile-esculin plates with 6 mg/L vancomycin (BEV6) on the same day. Hands and environmental swabs (bed controls, side table top and bottom, nurse call button, curtain, toilet seat, door knob, TV remote control, wheelchair, and other equipment when available) were enriched overnight in Brain Heart Infusion broth at 36ºC before culturing on BEV6 plates. Growth suggestive of VRE was confirmed by pyrrolidonyl arylamidase testing (DrySlide; BD, Franklin Lakes, NJ).
Pulsed-field gel electrophoresis was performed to determine the relatedness of VRE isolates. Genomic deoxyribonucleic acid was prepared and digested with SmaI (New England BioLabs, Beverly, MA) using a previously described method [14]. SmaI fragments were separated using a CHEF DR III apparatus (Bio-Rad, Hercules, CA) and compared using BioNumerics software (Applied Maths, Kortrijk, Belgium). Similarity of isolates was calculated using Dice coefficient (BioNumerics software). Isolates were placed in the same pulsotype if their restriction patterns were ≥80% similar [14].
Health Outcomes
We longitudinally assessed patient colonization, environmental contamination, length of stay at the facility, requiring hospitalization, and new-onset of infection as described below.
Patient colonization at a specific visit was defined as VRE positivity in any 1 or more samples collected from that patient at that specific visit. Environmental contamination was defined as VRE positivity in any 1 or more samples collected from surfaces in that patient’s room at that specific visit. We also identified patient and environmental visits showing multiple site colonization (2 or more body sites and 2 or more room sites, respectively) in order to perform a sensitivity analysis of odds ratios for our target outcomes.
Upon patient admission, we collected information on the expected length of stay at the facility (less than 90 days or more than 90 days). This threshold was chosen because the majority of patients in our population are expected to stay for less than 2 months (average of less than 30 days), whereas some patients are expected to stay for more than 6 months (average length of stay was between 33 and 60 days in the 6 facilities). Patients that were expected, at admission, to stay in the facility for more than 90 days were excluded from analysis of this outcome. For each patient, we also noted the occurrence of requiring an unplanned transfer to a hospital. Patients who, upon admission, were expected to be transferred to acute care or to another facility for any reason (eg, planned procedures, family arrangements, etc) were excluded from analysis of this outcome. Infections were defined by the presence of the following: (1) a clinical note in the participant’s medical record documenting any type of infection as well as (2) prescription of a systemic antibiotic for at least 3 days to treat that infection. In addition, failure to be discharged home within 90 days was reported as an outcome if either a length of stay more than 90 days or an unplanned hospitalization had been verified for that patient.
The likelihood of each outcome was assessed for patients colonized with VRE, patients in a room contaminated with VRE, and for patients for which neither condition was verified.The odds ratios were adjusted for gender, age, race, Charlson’s comorbidity score, and PSMS in multivariable analysis. Separate analyses were performed for patients/rooms contaminated at baseline and for patients/rooms contaminated later, at follow-up visits.
Statistical Analysis
Microbiological data were analyzed using SAS software (SAS Institute, Cary, NC). Only patients receiving at least 2 screening visits were included in the analyses. Patient colonization and environmental contamination were assessed at each visit. Odds ratio of occurrence of our target health outcomes in colonized and noncolonized patients, and in patients residing in contaminated and in noncontaminated rooms, were evaluated using univariable and multivariable logistic regression analysis adjusting for gender, age, race, Charlson’s comorbidity score, and PSMS. Because there is a higher number of observation opportunities for patients with longer stay, and to account for colonization weight from patients with persistent colonization over several visits, analyses were performed using each of 2 alternative approaches: (1) single patient data (patient-level data) and (2) next-visit data (visit-level data, which may include multiple visits for each patient). For visit-level data analysis, generalized estimating equation (GEE) [15] was used, to adjust for potential self-correlation between different visits in the same patient. In addition, we performed a sensitivity analysis by flagging patients and rooms contaminated at multiple sites. Finally, odds ratios of new patient colonization in contaminated versus noncontaminated rooms, and vice versa, were also calculated on a subset of patients with no diagnosed infections and no antibiotic use at baseline.
To assess the epidemiological significance of pulsotype findings between patient and room isolates, we estimated the background expected probabilities of recovering strains with the same pulsotype due to chance only, based on the distribution and frequency of isolation of each individual pulsotype at each facility, according to the following calculation: a priori probability , where n is the number of unique pulsotypes isolated in the facility, and x is the proportion of isolates belonging to the specific pulsotype.
RESULTS
Four hundred sixty-three patients consented to be screened and had at least 1 follow-up screening and were therefore included in the analysis (Figure 1). Median age (76 in colonized, 77 in noncolonized patients), race (57% and 60% white, respectively), Charlson’s comorbidity index (median of 2, range 0–6 and 0–7, respectively), and PSMS score (median 15, range 8–30 and median 14, range 6–29, respectively) were similar between patients colonized at baseline and noncolonized patients. Female sex was more common in noncolonized patients (64% vs 51% in colonized patients), regardless of room contamination status.
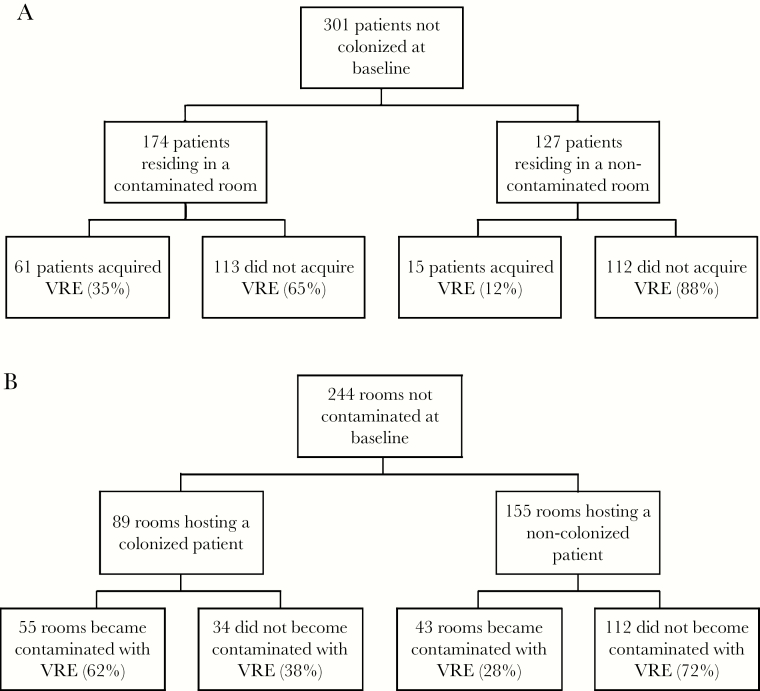
Figure 1.
Flow diagram illustrating the likelihood of future patient colonization with vancomycin-resistant enterococci (VRE) according to room contamination status (A) and likelihood of future room contamination with VRE according to resident colonization status (B).
Patient rooms were contaminated with VRE in 46% of the visits, with an average number of 2.6 positive swabs per room (range 1–10). Patients were colonized in 33% of the visits, with an average number of 1.6 positive swabs per patient (range 1–5). Three hundred one subjects were not colonized with VRE at the initial visit and were therefore at risk of new acquisition. Previous patient colonization with VRE was a risk factor for new room contamination (P < .01), and previous environmental VRE contamination was a risk factor for new acquisitions among patients (P < .01) (Tables 1 and 2). Specifically, 35% of patients residing in a contaminated room acquired VRE during follow-up, compared with 12% of residents of noncontaminated rooms. Sixty-two percent of rooms hosting a colonized patient became contaminated, compared with 28% of rooms hosting a noncolonized patient (Figure 1).
Table 1.
Odds Ratio (OR) of future patient colonization with VRE when residing in a contaminated versus a noncontaminated room. OR were calculated for every patient room (all patients), and for each visit (including when patients had multiple visits) (all visits).
| Patients/visits | Univariable OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|
| All patients | 4.03 (2.16–7.51) | 3.75 (1.98–7.11) |
| Patients with no infections, no antibiotic use* | 3.36 (1.33–8.48) | 3.19 (1.17–8.67) |
| All visits | 2.64 (1.64–4.24) | 2.58 (1.62–4.12) |
| Visits in patients with no infections, no antibiotic use* | 1.97 (0.94–4.12) | 2.06 (0.97–4.40) |
| Patients in rooms with multiple contaminated sites** | ||
| All visits | 3.11 (2.11–4.58) | 3.09 (2.10–4.54) |
| Visits in patients with no infections, no antibiotic use* | 2.79 (1.17–6.68) | 2.84 (1.33–6.09) |
*For both patient-level and visit-level analyses, OR were calculated for all patients and also for a subset of patients with no infections and no antibiotic use, in order to account for potential changes in sampling sensitivity.
** To perform a sensitivity analysis, OR were also calculated for rooms that were contaminated at two or more sites.
Abbreviations: CI: Confidence Interval. VRE: Vancomycin-resistant enterococcus.
Table 2.
Odds Ratio (OR) of future room contamination with VRE in patients colonized with VRE versus noncolonized patients. OR were calculated for every patient (all patients), and for each visit (including when patients had multiple visits) (all visits).
| Patients/visits | Univariable OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|
| All patient rooms | 4.21 (2.42–7.33) | 3.99 (2.23–7.16) |
| Rooms of patients with no infections, no antibiotic use* | 4.77 (2.05–11.10) | 5.02 (2.02–12.51) |
| All visits | 2.64 (1.60–4.35) | 2.88 (1.71–4.86) |
| Visits in rooms of patients with no infections, no antibiotic use* | 3.21 (1.51–6.81) | 3.87 (1.74–8.59) |
| Rooms of patients with multiple colonized body sites** | ||
| All visits | 3.20 (1.78–5.74) | 3.36 (1.82–6.22) |
| Visits in patients with no infections, no antibiotic use* | 3.34 (1.42–7.88) | 4.92 (2.06–11.78) |
| Rooms of patients with no hand contamination | ||
| All visits | 3.70 (1.59–4.62) | 3.09 (1.7–5.40) |
| Visits in patients with no infections, no antibiotic use* | 3.50 (1.53–8.01) | 4.72 (2.04–10.95) |
*For both patient-level and visit-level analyses, OR were calculated for all patients and also for a subset of patients with no infections and no antibiotic use, in order to account for potential changes in sampling sensitivity.
** To perform a sensitivity analysis, OR were also calculated for patients who were colonized at two or more sites.
Abbreviations: CI: Confidence Interval. VRE: Vancomycin-resistant enterococcus.
It is notable that similar results were obtained when patients with infection or antibiotic use at admission were excluded from the analysis (Tables 1 and 2). The percentage of new VRE acquisitions was 27% and 10%, respectively, for such patients when residing in contaminated and noncontaminated rooms. New contaminations in rooms were 62% and 25% from colonized and noncolonized patients, respectively.
Analysis of single-visit data confirmed that patients residing in contaminated rooms were more likely to become colonized (Tables 1 and 2), and the opposite was also true. A similar pattern was observed when noncolonized patients and noncontaminated rooms were compared with patients/rooms with multiple sites colonization/contamination. Furthermore, because hands colonization may be transient, we also assessed next visit environmental contamination for patients colonized at body sites other than the hands: the results were similar.
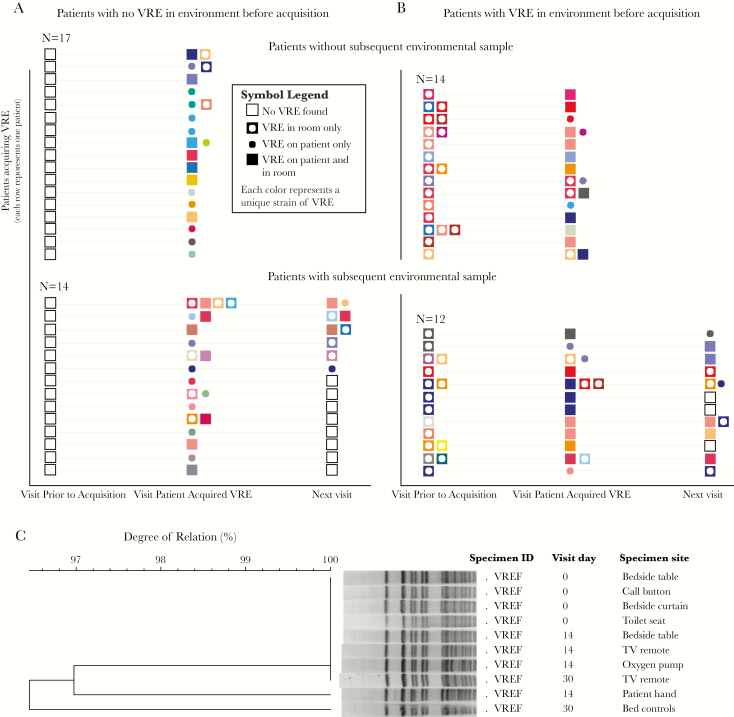
Molecular typing on 57 patients with new VRE acquisition during follow-up and for which all previously and subsequently isolated strains were available showed that, of 26 cases in which a VRE was present in the environment at the previous visit, 15 (58%) belonged to the same pulsotype (Figure 2) and thus were likely transmitted to the patient from their own contaminated room (either directly or through an indirect interaction). Moreover, VRE strains were often isolated from the environment at the visit after patient acquisition and belonged to the same pulsotype in 78% of the cases. Such percentages are higher than what would be expected by chance based on the distribution and number of unique patient and environmental pulsotypes found at each facility (14%).
Figure 2.
New acquisitions of vancomycin-resistant enterococci (VRE). (A and B) Longitudinal scheme of VRE new acquisition events and events of environmental shedding by the patient. Every row represents a patient, and each symbol color represent a separate pulsotype. (C) Example of an Enterococcus faecium strain found on several environmental sites at the first sampling visit (visit 0). The patient later became colonized as evidenced by the positive hand sample at the next visit. The strain persisted in the environment for at least 2 more weeks (day 30 sampling).
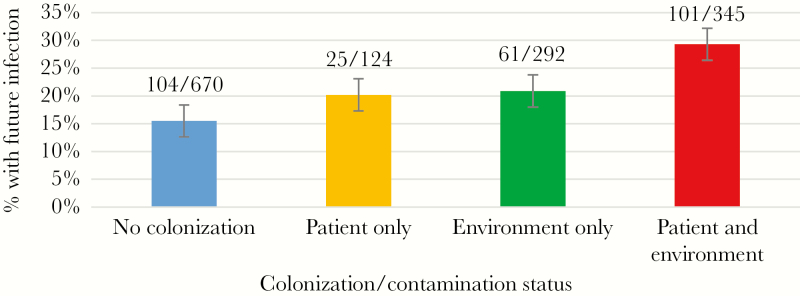
On multivariable analysis, new VRE environmental contamination was followed by a longer than planned stay in the facility (more than 90 days) and a higher likelihood of requiring transfer to a hospital (Table 3). The odds ratio for such outcomes were similar to the ones observed when new patient acquisition was considered. In addition to new acquisition during follow-up, baseline environmental contamination also was associated with higher hospitalization rates but not with longer stay. Both environmental contamination and patient colonization, at baseline and at later visits, were associated with either of the 2 outcomes to come to fruition (failure to be discharged home within 90 days). On univariable analysis, there was an association between patient VRE colonization, or environmental VRE contamination, and the incidence of future infections (P = .001) of any kind (Figure 3). However, multivariable analysis did not establish a statistically significant degree of correlation when other potential risk factors were included in the model, and thus a causative relationship could not be established at this time. It is interesting to note that the percentage of future infections was very similar after patient-only and environmental-only contamination, compared with noncolonized patients residing in noncolonized rooms. In addition, the percentage increased further in patients who were colonized and were also residing in contaminated rooms.
Table 3.
Multivariable Analysis of Body and Environmental VRE Contamination at Baseline as Risk Factors for Increased Length of Stay, Hospitalization, and Failure to Be Discharged Home Within 90 Days
| Colonization Status | Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Occurrence | Site | Length of Stay >90 Days | Hospitalization | Failure to Be Discharged Home | ||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | ||
| At baseline | Body | 2.34 | 1.08–5.07 | .031 | 2.33 | 1.35–4.01 | .002 | 2.03 | 1.23–3.34 | .005 |
| Environment | 1.09 | 0.51–2.33 | .83 | 2.93 | 1.66–5.17 | .001 | 2.08 | 1.25–3.45 | .004 | |
| Any site | 1.3 | 0.59–2.86 | .82 | 2.64 | 1.45–4.81 | .001 | 1.87 | 1.11–3.17 | .019 | |
| During follow-up | Body | 4.36 | 1.86–10.2 | .001 | 2.42 | 1.39–4.22 | .002 | 2.56 | 1.49–4.39 | .001 |
| Environment | 4.61 | 1.92–11.0 | .003 | 2.80 | 1.52–5.15 | .001 | 3.01 | 1.68–5.42 | .001 | |
| Any site | 4.36 | 1.75–10.9 | .006 | 2.20 | 1.17–4.16 | .014 | 2.73 | 1.45–5.13 | .001 |
Abbreviations: CI, confidence interval; OR, odds ratio; VRE, vancomycin-resistant enterococci.
Figure 3.
Risk of future infection of any kind based on present colonization status at current visit. First future infection only. Numerators represent visits with a future infection, and denominators represent total number of visits.
DISCUSSION
In the present report, we establish and quantify the relationship between PAC patient VRE colonization and environmental contamination over time, and we investigate how they are reflected on several future health outcomes in the postacute care setting. Very little is known about the dynamics of VRE transfer between patients and their environment in hospitals [16, 17], and even less is known in PAC settings. To our knowledge, this is the first study analyzing changes and relation of patient and environmental contamination status over the duration of stay in PACs, and it is the first study to report that room contamination may be indicative of future health outcomes to the same degree as patient colonization.
Despite the rise of VRE in PACs [2–5], and evidence that colonization pressure is a key risk factor for new VRE acquisitions [16], patient colonization status is seldom known, and optimal body sampling may be challenging to obtain in postacute care. If confirmed by other observations on different facilities, our findings may introduce the case for incorporating longitudinal environmental screening as appropriate in VRE surveillance protocols in facilities with significant prevalence of this pathogen [16, 17]. Such an approach may prove especially useful in settings in which previous information on other risk factors is incomplete, as is often the case in nonhospital environments such as PACs. For example, in the present study, we observed that environmental contamination information was as useful as, and complementary to, body colonization information in flagging patients who may develop new infections. Further research is warranted to investigate the clinical importance of environmental contamination [18], because its role in infection and other health outcomes is an unclear and underinvestigated aspect of clinical epidemiology in all healthcare settings [19], and especially in PACs.
Our study has strengths and limitations. Among the strengths are collection of multiple samples, the acquisition of clinical information matching the microbiological data, and the longitudinal nature of patient sampling. In particular, sampling over multiple successive visits coupled with molecular typing enables (1) tracking the progression from patient to environmental contamination and vice versa in specific patients and their rooms and (2) quantifying the likelihood of these events in the patient population. This sampling strategy is time-intensive, and thus it has rarely been used in the PAC setting. Point prevalence studies have been more commonly carried over, and they have provided a much needed picture of the broad scope and clinical relevance of VRE epidemiology in PACs and long-term care facilities from different geographic areas [1]. Nevertheless, more longitudinal studies will be required (1) to obtain a complete picture of clinically relevant patient outcomes stemming from VRE colonization and contamination and (2) to understand the best strategies to prevent them.
An inherent limitation of our study lies in the sensitivity of our patient and environmental sampling protocol. It is inevitable that a small number of events that we consider new acquisitions may in fact be the mere reflection of a previous false-negative screen [20]. To limit this phenomenon to a minimum, we performed multiple sampling at each visit. In addition, samples known to have a low burden of VRE (hands, environmental) were enriched before isolation and identification.
Due to unavailability of Clinical Microbiology Laboratory results regarding the causative agent of each specific infection, no distinction between VRE-related and non-VRE-related infections was possible, and our outcome analysis was limited to infections of any kind. A poorer general health status that was not captured by our measurements may be the underlying cause of worse health outcomes in patients colonized with VRE. This limitation may be overcome by further studies that (1) include a large number of participants whose health and functional status are tested against multiple comorbidity and function assessment protocols, to test a large range of confounders, and (2) include availability of clinical microbiology results demonstrating the causative agent of each reported infection.
An additional limitation of our study is that our patient population may not be a perfect representation of the general PAC population, because participants in our study were residents of just 6 facilities in a single geographic area. We hope that more postacute and long-term care institutions may be willing and able to perform longitudinal screening for VRE and/or other MDROs, to establish the clinical impact of patient and environmental contamination on patient health outcomes.
Longitudinal screening for MDRO colonization has been advocated in healthcare settings ranging from intensive care units [21] to postacute care. In the latter setting, we have previously reported same-visit correlation between patient and environmental VRE contamination [11]. The findings of the present study suggest that patient and environmental contamination dynamics, and their consequences in terms of health outcomes, can be uncovered if longitudinal screening is used.
It is important to note that persistence of colonizing organisms may extend beyond the time a patient is residing in PAC. In addition, some unfavorable patient and/or population health outcomes may take longer to come to fruition. A study following patients only during their stay in a single institution may thus fail to provide a complete assessment of the true impact of MDROs. Many institutions are moving towards creating health network systems that follow patients with continuity during their care transitions and use a shared clinical data recording platform. This will enable studies with longer follow-up times and the ability to answer a series of critical questions in clinical epidemiology of infections. Furthermore, there will be the opportunity (1) to establish a system-wide infection prevention leadership that is capable of obtaining a comprehensive view of epidemiological challenges in the network and (2) to organize effective solutions [22].
CONCLUSIONS
In conclusion, we assessed whether environmental VRE contamination may help identify patients at risk for adverse health outcomes in postacute care such as increased length of stay and requiring hospitalization, and our data point to this hypothesis as a target worthy of further investigation. In addition, we describe its close links to patient colonization, which bears public health consequences beyond the patient stay at the facility, because a proportion of patients may remain VRE carriers and/or contribute to VRE dissemination in their future encounters within all care settings and in the community after discharge. Although an association between VRE colonization and contamination with infections of any kind was observed, it was not possible to establish a causal relationship. Further studies using a larger number of clinical and microbiological data observations are urgently needed (1) to clarify the interaction of VRE contamination and other predictors of adverse outcomes in postacute care and (2) to clarify transmission patterns and mechanisms.
Acknowledgments
We thank the patients and their families who participated in this study.
Financial support. This publication presents independent research funded by the National Institutes of Health (RO1 AG041780 and K24 AG050685; to L. M.) and the University of Michigan Claude D. Pepper Older Americans Independence Center (MICHR PESC F039569; to M. C.).
Potential conflicts of interest. M. J. Z. has received funding from Genentech, Merck, Medimune, Medicines co., and Pfizer and is a consultant for Contrafect, all unrelated to the present study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McKinnell JA, Miller LG, Singh R, et al. et al. Prevalence of and factors associated with multidrug resistant organism (MDRO) colonization in 3 nursing homes. Infect Control Hosp Epidemiol 2016; 37:1485–8. [DOI] [PubMed] [Google Scholar]
- 2. Mody L, Foxman B, Bradley S, et al. Longitudinal assessment of multidrug-resistant organisms in newly admitted nursing facility patients: implications for an evolving population. Clin Infect Dis 2018; 67:837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benenson S, Cohen MJ, Block C, et al. ; JIRMI Group Vancomycin-resistant enterococci in long-term care facilities. Infect Control Hosp Epidemiol 2009; 30:786–9. [DOI] [PubMed] [Google Scholar]
- 4. Gouliouris T, Blane B, Brodrick HJ, et al. Comparison of two chromogenic media for the detection of vancomycin-resistant enterococcal carriage by nursing home residents. Diagn Microbiol Infect Dis 2016; 85:409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Buul LW, van der Steen JT, Veenhuizen RB, et al. Antibiotic use and resistance in long term care facilities. J Am Med Dir Assoc 2012; 13:568.e1–13. [DOI] [PubMed] [Google Scholar]
- 6. Ye Z, Mukamel DB, Huang SS, et al. Healthcare-associated pathogens and nursing home policies and practices: results from a national survey. Infect Control Hosp Epidemiol 2015; 36:759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol 1997; 18:622–7. [PubMed] [Google Scholar]
- 8. Dancer SJ. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet Infect Dis 2008; 8:101–13. [DOI] [PubMed] [Google Scholar]
- 9. Das I, Lambert P, Hill D, et al. Carbapenem-resistant Acinetobacter and role of curtains in an outbreak in intensive care units. J Hosp Infect 2002; 50:110–4. [DOI] [PubMed] [Google Scholar]
- 10. de Abreu PM, Farias PG, Paiva GS, et al. Persistence of microbial communities including Pseudomonas aeruginosa in a hospital environment: a potential health hazard. BMC Microbiol 2014; 14:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cassone M, Mantey J, Perri MB, et al. Environmental panels as a proxy for nursing facility patients with methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus colonization. Clin Infect Dis 2018; 67:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 13. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9:179–86. [PubMed] [Google Scholar]
- 14. Donabedian SM, Perri MB, Abdujamilova N, et al. Characterization of vancomycin-resistant Enterococcus faecium isolated from swine in three Michigan counties. J Clin Microbiol 2010; 48:4156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986; 42:121–30. [PubMed] [Google Scholar]
- 16. Zhou MJ, Li J, Salmasian H, et al. The local hospital milieu and healthcare-associated vancomycin-resistant Enterococcus acquisition. J Hosp Infect 2019; 101:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheah ALY, Cheng AC, Spelman D, et al. Mathematical modelling of vancomycin-resistant enterococci transmission during passive surveillance and active surveillance with contact isolation highlights the need to identify and address the source of acquisition. BMC Infect Dis 2018; 18:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Linder KA, Hecker MT, Kundrapu S, et al. Evaluation of patients’ skin, environmental surfaces, and urinary catheters as sources for transmission of urinary pathogens. Am J Infect Control 2014; 42:810–2. [DOI] [PubMed] [Google Scholar]
- 19. Beggs C, Knibbs LD, Johnson GR, Morawska L. Environmental contamination and hospital-acquired infection: factors that are easily overlooked. Indoor Air 2015; 25:462–74. [DOI] [PubMed] [Google Scholar]
- 20. Baden LR, Thiemke W, Skolnik A, et al. Prolonged colonization with vancomycin-resistant Enterococcus faecium in long-term care patients and the significance of “clearance”. Clin Infect Dis 2001; 33:1654–60. [DOI] [PubMed] [Google Scholar]
- 21. Linfield RY, Campeau S, Injean P, et al. Practical methods for effective vancomycin-resistant Enterococci (VRE) surveillance: experience in a liver transplant surgical intensive care unit. Infect Control Hosp Epidemiol 2018; 39:1178–82. [DOI] [PubMed] [Google Scholar]
- 22. Chen LF, Knelson LP, Gergen MF, et al. ; CDC Prevention Epicenters Program A prospective study of transmission of multidrug-resistant organisms (MDROs) between environmental sites and hospitalized patients-the TransFER study. Infect Control Hosp Epidemiol 2019; 40:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]