Abstract
Considering titanium dioxide nanoparticles (TiO2 NPs) role in plant growth and especially in plant tolerance against abiotic stress, a greenhouse experiment was carried out to evaluate TiO2 NPs effects (0, 50, 100 and 200 mg L−1) on agronomic traits of Moldavian balm (Dracocephalum moldavica L.) plants grown under different salinity levels (0, 50 and 100 mM NaCl). Results demonstrated that all agronomic traits were negatively affected under all salinity levels but application of 100 mg L−1 TiO2 NPs mitigated these negative effects. TiO2 NPs application on Moldavian balm grown under salt stress conditions improved all agronomic traits and increased antioxidant enzyme activity compared with plants grown under salinity without TiO2 NP treatment. The application of TiO2 NPs significantly lowered H2O2 concentration. In addition, highest essential oil content (1.19%) was obtained in 100 mg L−1 TiO2 NP-treated plants under control conditions. Comprehensive GC/MS analysis of essential oils showed that geranial, z-citral, geranyl acetate and geraniol were the dominant essential oil components. The highest amounts for geranial, geraniol and z-citral were obtained in 100 mg L−1 TiO2 NP-treated plants under control conditions. In conclusion, application of 100 mg L−1 TiO2 NPs could significantly ameliorate the salinity effects in Moldavian balm.
Subject terms: Plant sciences, Materials science, Nanoscience and technology
Introduction
Moldavian balm (Dracocephalum moldavica L.), a perennial herb of the Lamiaceae family and native to central Asia, naturalized in central and eastern Europe and is cultivated around the world as a medicinal plant. Essential oils and extracts of Moldavian balm have been traditionally used as a painkiller for kidney complaints, toothache and colds. In addition, it has antimicrobial activities1, antirheumatic, antitumor, antimutagenic, antioxidant and antiseptic properties2. Aerial parts of Moldavian balm are important sources of monoterpene glycosides, trypanocidal terpenoids, rosmarinic acid and flavonoids3.
Salinity stress is considered as one of the main environmental factors limiting plant distribution in their natural habitats4. Soil salinity affects about 800 million hectares of arable land worldwide. Salinity stress causes major problems regarding plant growth, development and productivity, especially in arid and semi-arid regions of the world5 manifested as changes in morphological, physiological and biochemical characteristics of plants, ion toxicity (Na+ and Cl−), nutritional disorders and osmotic stress. These negative impacts significantly decrease plant yield under salinity stress conditions6. The tolerance mechanisms of plants to salinity stress are different in terms of osmotic regulation, CO2 assimilation, toxic ion uptake, ion compartmentation and/or exclusion, chlorophyll content, chlorophyll fluorescence, reactive oxygen species (ROS) generation, antioxidant defenses and photosynthetic electron transport4,7. Several studies have recently focused on new strategies to deal with salinity in order to minimize its negative effects8,9.
Nanotechnology is the study and application of small-sized materials (1–100 nm), a specific quality that makes these tiny entities unique. Thus, application of nanoparticles is one of the new strategies to improve growth and plant performance under salinity stress10. Titanium dioxide (TiO2) nanoparticles (NPs) lead to various profound effects on morphological, physiological and biochemical properties of some plant species. Lei et al.11 reported that the application of TiO2 NPs improved rubisco and antioxidant enzymes activities, photosynthetic rate and chlorophyll formation that subsequently caused enhanced crop yield. Latef et al.12 reported positive effect of TiO2 NPs on enhancement of plant growth, antioxidant enzyme activities, soluble sugars, amino acids and proline content in addition to a reduction in H2O2 and MDA contents in broad bean plants under saline conditions. Khan13 reported mitigation of salt stress by TiO2 NP application in tomato by improving agronomic traits, leaf chlorophyll content, phenolics and antioxidant capacity, antioxidant enzyme activities and yield. TiO2 could be considered as a stimulant for plants that activates different defense mechanisms involved in plant tolerance against various abiotic stress factors11. These effects might vary under different environmental conditions or in diverse plant species and based on the applied concentrations14,15. Similar to other NPs, the size, shape and concentration of TiO2 NPs have very important roles in their application. On the contrary, several reports have presented the negative and toxic effects of high concentration of TiO2 in plants that varied between plant tissues, growth stages and plant species based on concentrations and properties of nanoparticles14,16. Therefore, concentration, size, method of treatment application, uptake by plants, properties, reactivity and translocation of NPs into different tissues could determine NP interference with various metabolic activities that lead to toxic impacts17,18. Furthermore, surface area of NPs, their reactive nature and tendency to aggregate are other possible reasons for their toxicological effects19. High concentration of TiO2 NPs mainly results in the elevated production of oxygen reactive species (ROS), followed by chlorophyll degradation and cellular toxicity20. In addition, cell wall and plasma membrane damage due due to high NP concentration result in NP interaction with various cellular process18,21. In fact, the toxic effect of TiO2 NPs is dose- and time- dependent and putative mechanisms leading to toxicity are oxidative stress through ROS over-production, cell wall damage and lipid peroxidation. TiO2 NP toxicity also depends on species, particle size and exposure condition21. The toxic effects of TiO2 NPs have been reported in barley14, tobacco21, onion22, wheat23 and spinach24 plants. The discovery of their widespread uses in agriculture and plant science is still under debate16. Thus, the present study tried to investigate beneficial and toxicological impacts of different concentrations of TiO2 NPs in nutrient solution on key morphophysiological and biochemical characteristics as well as essential oil profile in Moldavian balm, an aromatic and medicinal plant, grown under salinity stress conditions. In addition, the uptake and aggregation of TiO2 NPs in the plant root was investigated by epifluorescence microscopy.
Materials and Methods
Preparation of TiO2 NPs
TiO2 NPs were synthesized according to the protocol previously reported25. Briefly, desired amount of titanium isopropoxide was hydrolyzed and stirred vigorously at 4 °C to produce white precipitate of TiO(OH)2. The obtained precipitate was washed three times with distilled water and dissolved in nitric acid to obtain clear and homogeneous titanyl nitrate [TiO(NO3)2] solution. For the synthesis of TiO2, titanyl nitrate and urea solution with 1:1 molar ratio was kept in a 250 mL beaker and put into a muffle furnace at 400 °C. After 2 h, the solid product was collected as TiO2 NPs and stored in vacuum oven until usage.
Chemicals and Instruments
All chemicals and solvents were purchased from Merck and Sigma-Aldrich (Germany) and used without further purification. A Win-Bomem spectrometer, version 3.04 Galactic Industries Corporation over the range of 400–4000 cm−1 was used to obtain Fourier transform infrared (FT-IR) spectra. The synthesized TiO2 NPs were coated with a thin layer of gold and visualized using a scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDX) instrument, VWGA3 TESCAN (20.0 KV). For recording the Transmission electron microscopy (TEM) images, so-called TiO2 NPs were dispersed in distilled water and used a Zeiss EM-90 operating at 80 kV tension. Wide angle X-ray diffraction (XRD) profiles of TiO2 NPs were collected by using a Bruker D8 Advance diffractometer with wavelength, λ = 0.154059 nm (Cu Kα) at 30 keV.
Epifluorescence microscopy
In order to study the uptake of TiO2 NPs, epifluorescence microscopy was employed in the treated plants. Plant materials were stained with 0.1% auramine O solution in water for 10 min. Samples were observed using an Olympus BX51 (Olympus optical Co., Ltd. Tokyo, Japan). Fluorescence microscope was equipped with the catadioptric lenses UMP lan FL-BDP and the BXRFA (Olympus optical Co., Ltd. Tokyo, Japan) fluorescence illuminator26,27. Image (z-stack) acquisition was performed using an Evolution MP cooled CCD (Media Cybernetics, USA) high-resolution digital camera. For this purpose, series of consecutive images from different focal planes of the sample were taken and then superimposed automatically to improve the depth of focus using ImageJ 1.41 software (http://rsbweb.nih.gov/ij/) in accordance with Dadpour et al.28. Outputs from the z-stack acquisitions were trimmed and saved as TIFF-format images.
Experimental site, plant materials and Tio2 NPs treatments
The study was conducted at the research greenhouse of Department of Horticultural Sciences, University of Maragheh, Maragheh, East Azerbaijan Province, Iran (longitude 46°16′E, latitude 37°23′N, altitude 1485 m) as a factorial experiment in a completely randomized design (CRD). The experiment consisted of twelve treatments (each with three independent biological replications), three levels of salinity ((0, 50 and 100 mM NaCl) and four levels of TiO2 NPs (0, 50, 100 and 200 mg L−1). The seeds of Moldavian balm (Dracocephalum moldavica L.) were purchased from Pakanbazr Company, Isfahan, Iran. Regarding seed preparation, surface sterilization of the seeds was done with 1% (w/v) sodium hypochlorite (NaOCl) for 5 min, then washed three times with distilled water and finally soaked in distilled water for 10 min. The seeds were wetted with tap water and let to germinate for a week. Then, in each pot, eight plants were hydroponically grown in growth medium containing cocopite and perlite (2:1 ratio). Plants were irrigated daily with quarter-strength Hoagland solution with some modification29. After three weeks, salinity stress was imposed (eight-leaf stage), applied daily (in combination with quarter-strength Hoagland solution) and continued up to plant harvest (prolonged stress ≈ two months after applying salt stress) for the establishment of salinity effects on plant agronomic parameters. TiO2 NPs were added three times (three continuous days) to quarter-strength Hoagland solution two weeks after salinity stress application. Control plants were irrigated daily with quarter-strength Hoagland solution until harvest and treated with 0 mM NaCl and 0 mg L−1 TiO2 NPs.
Agronomic parameters
Plant agronomic traits including plant height, shoot and leaf fresh and dry weights and leaf number were recorded at the harvest stage. For this purpose, five plants from each treatment were randomly sampled to measure the above traits. For fresh and dry weights, five samples were individually weighed for fresh weight and then kept in the oven (70 °C, 72 h) for dry weight measurements.
Chlorophyll a, b and carotenoid content
Chlorophyll (Chl) and carotenoids amounts were achieved by extracting 0.2 g of fresh leaves in 0.5 mL acetone (3% v/v). After centrifuging (10000 rpm, 10 min) and obtaining the supernatant, absorption was recorded at 645 nm (Chl b), 663 nm (Chl a) and 470 nm (carotenoids) by UV-Vis spectrophotometry (UV-1800 Shimadzu, Japan). The youngest and fully expended leaves (from growing point) were used for measurements. Photosynthetic pigment contents (Chl a, b and carotenoids) were calculated from the following equations as described by Sharma et al.30:
Note: V = Solution volume of the filtrate, A = Light absorption in wavelengths 663, 645 and 470 nm and W = Sample fresh weight (g).
Chlorophyll fluorescence
Chlorophyll fluorescence parameters (Fv/Fm, Fv/Fo and Y(ll)) were measured using dual-pam-100 chlorophyll fluorometer (Heinz Walz, Effeltrich, Germany) after adaption of D. moldavica in the dark for 20 min31.
Hydrogen peroxide (H2O2) content
H2O2 content of Moldavian balm leaves was measured according to Sinha et al.32. Briefly, fresh leaves (0.2 g) were homogenized with 5 mL trichloroacetic acid (0.1% w/v) in an ice bath and then centrifuged (12000 rpm, 15 min). At that time, 0.5 mL of the supernatant was added to 0.5 mL potassium phosphate buffer (pH 6.8, 10 mM) and 1 mL potassium iodide (KI) (1 M). Finally, the absorbance of the mixture was recorded at 390 nm. H2O2 (µmol g−1 FW) content was estimated by standard calibration curve previously made by various H2O2 concentrations.
Antioxidant enzyme activity assays
Young and fully expanded leaves were collected to assay antioxidant enzymes activities. For this purpose, samples were collected in an ice bucket and brought to the laboratory. All steps of enzyme extraction were carried out at 4 °C as follows: 0.5 g of the homogenized leaves were extracted with potassium phosphate buffer (pH 6.8, 10 mM) containing 1% polyvinylpyrrolidone (PVP) using magnetic stirrer for 10 min. The homogenate was centrifuged (6000 rpm, 20 min) and the supernatant was used for the assay of catalase (CAT), ascorbate peroxidase (APX), superoxide dismutase (SOD) and guaiacol peroxidase (GP) enzyme activities.
In order to determine CAT activity, the mixture of 0.5 mL potassium phosphate buffer, 4.5 mL H2O2 (3%) and 50 µL crude enzyme extract in a quartz cuvette was assayed using a UV-Vis spectrophotometer (UV-1800 Shimadzu, Japan) at 240 nm for 120 s33.
SOD activity was measured based on the method described by Sun et al.34 with slight modifications. The reaction mixture consisted of 2.5 mL potassium phosphate buffer, 0.2 mL methionine (0.2 M), 0.1 mL EDTA (3 mM), nitro blue tetrazolium (NBT), 1 mL distilled water, 0.1 mL NaCa3 (1.5 M), 0.1 mL riboflavin and 50 µL enzyme extract illuminating in glass tubes. The unilluminated mixtures were used as blanks. Test tubes were exposed to light by immersing in a beaker 2/3 filled with clean water, maintained at 27 °C. The increase in absorbance due to formazan formation was recorded at 560 nm. One unit of SOD was defined as the amount of enzyme that inhibited the rate of nitro blue tetrazolium reduction by 50%.
Considering APX activity35, the reaction mixture consisted of 250 µL potassium phosphate buffer, 250 µL ascorbate (1 mM), 250 µL EDTA (0.4 mM), 190 µL distilled water, 250 µL H2O2 (10 mM) and 0.5 mL enzyme extract. The changes in absorbance of samples at 290 nm, demonstrating enzymatic activity, were recorded and the extinction coefficient was considered as 2.8 cm−1 mmol−1.
The assay mixture for the estimation of GP activity comprised of 1 mL potassium phosphate buffer, 250 µL EDTA, 1 mL guaiacol (5 mM), 1 mL H2O2 (15 mM) and 50 µL enzyme extract. The rate of change in absorbance at 470 nm was determined according to Tang and Newton36.
Essential oil extraction and profiling
The essential oils were extracted from 50 g air-dried powdered aerial parts of plants by the hydro-distillation technique and heated by heating jacket at 100 °C for 2 h in an all-glass Clevenger type apparatus, according to procedures outlined in the European pharmacopeia. The collected crude essential oils were dried over anhydrous sodium sulfate and then stored in sealed glass vials. Obtained samples were evaluated for their essential oil components by GC/MS instrument (Agilent 6890 N GC and Agilent 5973 mass selective detector operating in the EI mode, USA)37.
Statistical analysis
All obtained data analysis performed by SAS software and the means of each treatment were analyzed by Duncan’s multiple range test at the 95% level of probability (SAS Institute Inc., ver. 9.1, Cary, NC, USA).
Results and Discussion
Characterization of TiO2 NPs
FTIR spectrum was used for the chemical elucidation of the synthesized TiO2 NPs. The existence of unresolved stretching vibrations of Ti-O-Ti could be assigned as broad band in the region of 400–900 cm−1 (Fig. 1). In addition, two bands at 1620 and 3427 cm−1 were related to bending and stretching vibrations of O-H groups38.
Figure 1.
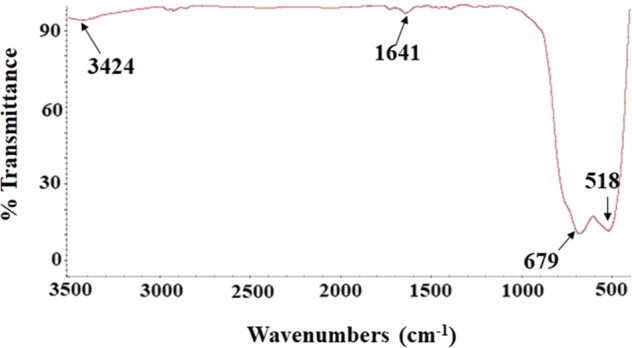
FTIR spectrum of TiO2 NPs.
X-ray diffraction (XRD) pattern of TiO2 NPs was investigated to study the structure and phase formation of the sample. According to Fig. 2, a well-crystallized anatase profile was observed for TiO2 NPs, in good agreement with the JCPDS data (JCPDS data file No. 21–1272).
Figure 2.
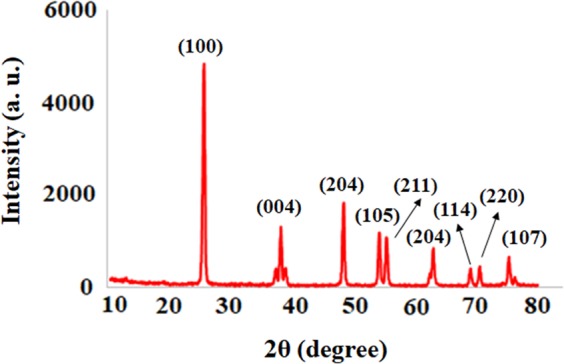
X-ray diffraction (XRD) pattern for TiO2 NPs.
Surface, size and the particle morphology of TiO2 NPs were imaged by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Fig. 3a,b). Based on the SEM image, spherical-like shapes with particle diameter of 70–90 nm could be seen for the synthesized TiO2 NPs, while particle size was determined as 20–30 nm according to TEM. The difference in the size of nanoparticles obtained by SEM and TEM techniques may be related to the loss of stability of nanoparticles during the freezing-drying process as well as due to particle aggregation phenomena.
Figure 3.
(a) SEM and (b) TEM images of TiO2 NPs.
Epifluorescence microscopy
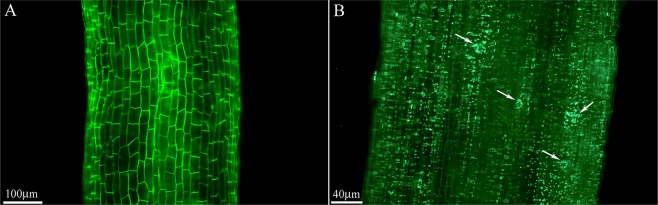
Epifluorescence microscopy confirmed the uptake of different concentration of TiO2 NPs into Moldavian balm (Dracocephalum moldavica L.) root tissue (Fig. 4).
Figure 4.
Epifluorescence microscopic images of D. moldavica L roots in 0 mg L−1 (A) and 200 mg L−1 (B) of TiO2 suspensions grown under control conditions.
In the plants treated with high concentration of TiO2 (200 mg L−1), the presence of NP aggregates was indicated by fluorescent light spots inside the root (Fig. 4B). No spots were observed in control (0 mg L−1 TiO2) plants, as expected (Fig. 4A). Only a few studies exist on subcellular localization of TiO2 in plants. Present results demonstrated that the high concentration of TiO2 NPs increased their aggregations in the plant root. TiO2 was actively taken up in Spirodela polyrrhiza roots and aggregated in the plant cells at toxic concentration39 (See Supporting Information Fig. S1). Both studies demonstrated the entry of TiO2 NPs in the roots by markedly shiny spots at high inside the roots, representing aggregation. Similar to our observations, fluorescence microscopy imaging techniques were used to indicate the entrance of magnetic NPs into soybean plant tissues as shown in previous reports26,39. From an application point of view, various parameters such as size, concentration and aggregation of NPs are the most important issues in agriculture, playing important roles in determining reactivity, toxicity, fate, transport and risk in the environment40.
Assessments of agronomic parameters
Plant agronomic parameters was significantly influenced by application of TiO2 NPs, salt stress and their interactions (Table 1).
Table 1.
Effect of different concentrations of TiO2 NPs on key agronomic parameters of D. moldavica L. plants under salinity stress.
| Salt stress × TiO2 (interaction effect) | Plant height (cm) | Shoot FW (g) | Shoot DW (g) | Leaf number | Leaf FW (g) | Leaf DW (g) | |
|---|---|---|---|---|---|---|---|
| Traits | |||||||
| 0 mM NaCl | 0 mg L−1 TiO2 | 44.33b | 15.44c | 4.72b | 84.00c | 4.21c | 2.39c |
| 50 mg L−1 TiO2 | 45.00b | 16.24b | 5.10a | 95.33b | 4.72b | 2.81b | |
| 100 mg L−1 TiO2 | 62.33a | 17.49a | 4.69b | 101.33a | 5.34a | 3.15a | |
| 200 mg L−1 TiO2 | 40.00de | 14.53d | 3.83c | 71.33d | 3.70d | 2.33c | |
| 50 mM NaCl | 0 mg L−1 TiO2 | 33.66hi | 8.10h | 2.11f | 44.33g | 2.26f | 0.69h–g |
| 50 mg L−1 TiO2 | 36.66gf | 8.87g | 2.43e | 42.00g | 3.57d | 1.55d | |
| 100 mg L−1 TiO2 | 43.00bc | 10.48e | 2.75d | 57.33e | 3.74d | 1.58d | |
| 200 mg L−1 TiO2 | 41.66cd | 9.66ef | 2.66d | 51.66f | 3.11e | 0.58hg | |
| 100 mM NaCl | 0 mg L−1 TiO2 | 30.00j | 7.82h | 0.63i | 37.33h | 2.27f | 0.54h |
| 50 mg L−1 TiO2 | 32.00ji | 7.65h | 1.07h | 40.66gh | 1.88g | 0.72fg | |
| 100 mg L−1 TiO2 | 35.00gh | 8.89g | 1.95g | 41.00gh | 3.19e | 0.76f | |
| 200 mg L−1 TiO2 | 38.66ef | 9.74f | 2.17f | 44.00g | 3.63d | 1.06e | |
*Different letters indicate significant differences at 5% level of confidence according to Duncan’s test.
Results demonstrated that the maximum plant height (≈62.33 cm) was observed in 100 mg L−1 TiO2-treated plants under control conditions. On the contrary, the lowest height was achieved in 100 mM NaCl without TiO2 treatment. Regarding shoot fresh weight, the maximum and minimum values were recorded in 100 mg L−1 TiO2-treated plants under no salinity and 50 mg L−1 TiO2 under 100 mM salinity conditions, respectively. In the case of shoot dry weight, 50 mg L−1 TiO2 NPs under control conditions demonstrated the highest value, whereas 100 mM NaCl resulted in the lowest value. Application of 100 mg L−1 TiO2 under no salinity conditions caused maximum leaf number (≈101.33), while 100 mM NaCl with no TiO2 application had the lowest (≈37.33). Current results also showed that the highest and lowest amounts of leaf FW were achieved in plants treated with 100 mg L−1 TiO2 without salinity and 50 mg L−1 TiO2 under 100 mM salinity conditions, respectively. Furthermore, plants treated with 100 mg L−1 TiO2 NPs without salinity stress had the highest leaf DW (≈3.15 g), as expected considering their FW. Lowest DW values were recorded in 100 mM NaCl-treated plants. In total, plants treated with 100 mg L−1 TiO2 displayed optimal performance for most agronomic traits, whereas worst-performing plants were the ones grown under severe salinity stress (100 mM NaCl). It is worth stating that TiO2 application, especially in low and medium concentrations, also improved agronomic parameters under control conditions, thus rendering them as potential growth promoters. Contrarily, plants treated with 200 mg L−1 TiO2 showed significant decrease in their agronomic attributes, indicative of toxicity effects. In addition, application of TiO2 NPs showed positive effects on the agronomic traits under salinity conditions and significantly ameliorated the stressor’s negative effects. In detail, approximately all TiO2 concentrations could reverse the negative effects of salinity stress by improving the agronomic parameters examined under different salinity levels; 100 mg L−1 TiO2 under 50 mM NaCl and 200 mg L−1 TiO2 under 100 mM NaCl achieved optimal performance in this regard.
Salt stress (NaCl) reduces plant growth due to its negative effect on photosynthesis rate, cell division and elongation, changes in enzymatic activity (subsequently affects protein synthesis), decrease in carbohydrates and growth hormone levels and disruption of biological and metabolic activities that finally could lead to growth inhibition8. Thus, plant height commonly decreases by increase in NaCl levels due to its destructive effects. Aziz et al.41 previously reported a reduction in plant height by increasing salinity levels. Considering the result of TiO2 application, all concentrations and 100 mg L−1 in particular, caused a significant increase in plant height, demonstrating that the application of TiO2 NPs ameliorated the negative effects of salinity. The observed decrease in leaf number and FW in moderate and high salinity levels is attributed to a reduction in cell expansion due to low turgor controlled by cellular water uptake and cell-wall extension42. In addition, Kapoor and Pande43 concluded that leaf numbers decreased under salinity conditions due to a reduction in branches per plant as a result of decrease in nutrient concentrations. Decline in fresh and dry weights, leaf numbers and abscissions under salinity stress were previously reported44. However, the positive effects of TiO2 NPs were observed in leaf numbers as well as fresh and dry weights in the current study. In this regard, Rahneshan et al.45 reported that TiO2 application enhanced absorption rate of macro- and micro-nutrients, improved plant growth characteristics (e.g., plant height, leaf number) and reduced negative effects of salinity by affecting photosynthesis and absorption of essential elements.
Photosynthetic pigments
Application of TiO2 NPs had significant effects on photosynthesis pigments (Table 2).
Table 2.
Effect of different concentrations of TiO2 NPs on photosynthesis pigments of D. moldavica L. plants under salinity stress.
| Salt stress × TiO2 (interaction effect) | Chlorophyll a (mg g−1 FW) | Chlorophyll b (mg g−1 FW) | Carotenoids (mg g−1 FW) | |
|---|---|---|---|---|
| 0 mM NaCl | 0 mg L−1 TiO2 | 3.19d | 2.18b | 0.65bc |
| 50 mg L−1 TiO2 | 4.47c | 1.77c | 0.73ab | |
| 100 mg L−1 TiO2 | 5.38a | 2.66a | 0.81a | |
| 200 mg L−1 TiO2 | 3.47c | 1.37c | 0.46df | |
| 50 mM NaCl | 0 mg L−1 TiO2 | 2.4fg | 0.69fh | 0.52ce |
| 50 mg L−1 TiO2 | 2.62f | 0.81efg | 0.53cd | |
| 100 mg L−1 TiO2 | 2.53f | 1.37d | 0.62bc | |
| 200 mg L−1 TiO2 | 2.26g | 0.58h | 0.27g | |
| 100 mM NaCl | 0 mg L−1 TiO2 | 1.46j | 0.86ef | 0.22 g |
| 50 mg L−1 TiO2 | 1.99h | 0.93e | 0.21g | |
| 100 mg L−1 TiO2 | 2.91e | 0.68fgh | 0.35eg | |
| 200 mg L−1 TiO2 | 1.77i | 0.6gh | 0.31fg | |
*Different letters indicate significant differences at 5% level of confidence according to Duncan’s test.
The highest contents of chl a, b and carotenoids were observed in 100 mg L−1 TiO2 without salt stress. Furthermore, salinity stress decreased pigment content, but application of 100 mg L−1 TiO2 increased chl a, b and carotenoid contents under both salinity levels. 200 mg L−1 TiO2 led to significantly lower pigment contents compared with lower TiO2 concentrations similar to salt-stressed samples, suggesting toxicity. The observed decrease in photosynthesis pigment content under salt stress conditions could be attributed to reduced biosynthesis or more likely increased breakdown due to ROS damage of the pigments in cells, functional disorders observed during stomatal movement and instability of the pigment protein complex under salinity stress. Salinity stress is known to result in pigment breakdown due to accumulation of toxic ions in chloroplasts and ROS-induced oxidative stress in plants45. In addition, Hernandez et al.46 stated that pigment reduction in NaCl-sensitive plants happened due to changes in number and size of chloroplasts, starch content, disorganized chloroplast membranes, loss of envelope and disorganization of grana and thylakoids.
Chlorophyll fluorescence
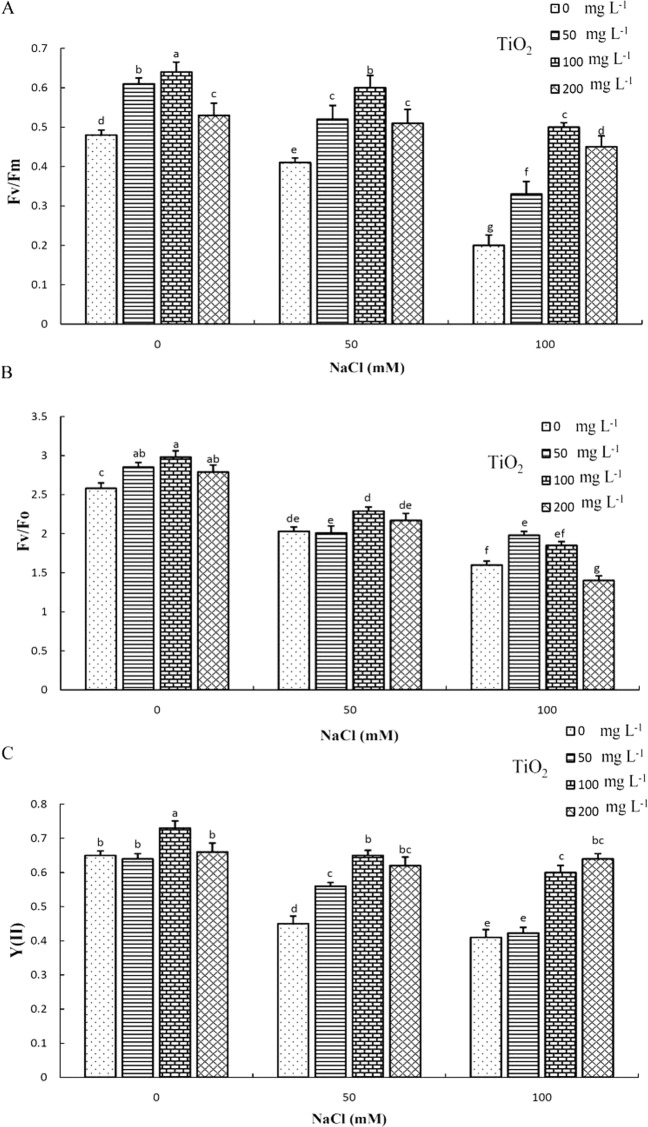
Moderate and high salinity levels significantly decreased chlorophyll fluorescence parameters including Fv/Fm (a ratio that indicates about the quantum efficiency of photosystem II: maximal quantum yield of PSII), Fv/Fo (a parameter that accounts for the simultaneous variations in Fm and Fo in determinations of the maximum quantum yield of PS II: Efficiency of the water-splitting complex on the donor side of PSII) and Y(II) (the complementary quantum yields of PS II). TiO2 NP application ameliorated the salt-induced drop in chlorophyll fluorescence parameters. Specifically, all TiO2 NP treatments increased Fv/Fm values under both control and stress conditions with highest values recorded following100 mg L−1 TiO2 NP application under control conditions (Fig. 5A). Furthermore, all TiO2 NP concentrations increased Fv/Fo with the highest value being recorded at 100 mg L−1 under control conditions, while NP pre-treatment ameliorated decreases recorded in this parameter under salt stress conditions (Fig. 5B). Similar findings were observed for Y (II) parameter, where NP application increased Y (II) under control conditions and reversed decreases observed in salt-stressed plants (Fig. 5C).
Figure 5.
Effect of different concentrations of TiO2 NPs on chlorophyll florescence Fv/Fm (A), Fv/Fo (B), and Y(II) (C) of D. moldavica L. under salinity stress. Different letters indicate significantly different values at p < 0.05.
The significant decrease in these parameters was likely due to the dissipation of a major proportion of light energy as heat under salt stress47. Similar reduction in chlorophyll fluorescence parameters under salt stress was previously reported in maize48, as well as in sorghum49. Increase in the examined chlorophyll fluorescence parameters after TiO2 NP application could be attributed to enhancement in light energy of PSI absorbed by chloroplast membrane to be transferred to PSII, promotion of light energy conversion to electron energy and electron transport and acceleration of water photolysis and oxygen evolution50. In addition, Rubisco enzyme activity increased after TiO2 NP application due to increase in the expression of its mRNA51. Rubisco enzyme plays an important role in photosynthesis and optimal expression of this enzyme improves chlorophyll fluorescence parameters, while also increasing absorption of carbon dioxide in plants52. Overall, current findings suggest that TiO2 NPs potentially ameliorated the negative effects of salinity stress through the improvement in chlorophyll fluorescence parameters and by maximizing PSII efficiency46.
Assessment of biochemical traits
Evaluation of hydrogen peroxide (H2O2) content
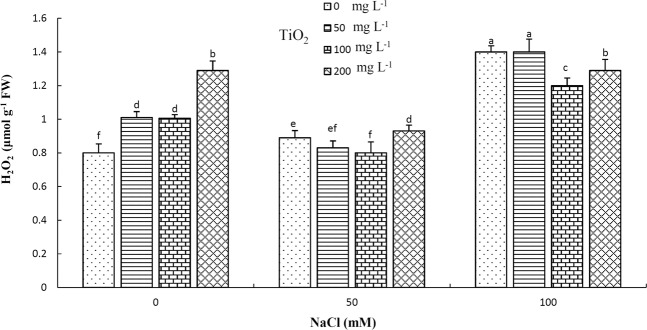
The highest (≈1.4 μmol g−1 FW) H2O2 content was observed in 100 mM NaCl-treated plants without TiO2 application, whereas control plants had the lowest content, along with plants treated with 50 and 100 mg L−1 TiO2 under 50 mM NaCl (Fig. 6).
Figure 6.
Effect of different concentrations of TiO2 NPs on H2O2 concentration of D. moldavica L. under salinity stress. Different letters indicate significantly different values at p < 0.05.
In addition, application of 100 mg L−1 TiO2 in plants growing under moderate and high salinity stress as well as in 50 mg L−1 TiO2-treated plants under moderate NaCl stress decreased H2O2 content in leaf tissues compared with plants subjected to similar stress conditions without any TiO2 treatments. H2O2, produced in various vital processes of different organs cells, is highly toxic for cells and causes oxidative stress at high concentrations53, as well as damages to biological membranes via their peroxidation. Thus, the mentioned TiO2 treatments could amplify plant performance under saline conditions likely by decreasing oxidative stress and lessening membrane damage. Superoxide dismutase (SOD), as the primary ROS scavenger localizing in chloroplasts, mitochondria, peroxisomes and cytosol, catalyzes the disproportion of two O2·− radicals to H2O2 and O254. Moreover, H2O2 is scavenged by ascorbate-peroxidase (APX) in ascorbate-glutathione cycle and through guaiacol peroxidase (GP) and catalase (CAT) in cytoplasm and divided into water and oxygen55. The increased activity of the mentioned enzymes by TiO2 treatments in the present study might be another reason for the observed decrease in H2O2 values under salinity stress compared with control conditions. Although all TiO2 treatments increased H2O2 values and the high concentration of TiO2 might be considered as toxic, these increases were lower than those under salinity stress, demonstrating lower negative effects of NP treatments even at high concentration compared with salinity. Moreover, considering the positive impact of TiO2 towards lowering H2O2 content under salinity, NP treatments could be considered as beneficial for removing undesirable effects of salinity.
Evaluation of antioxidant enzymes
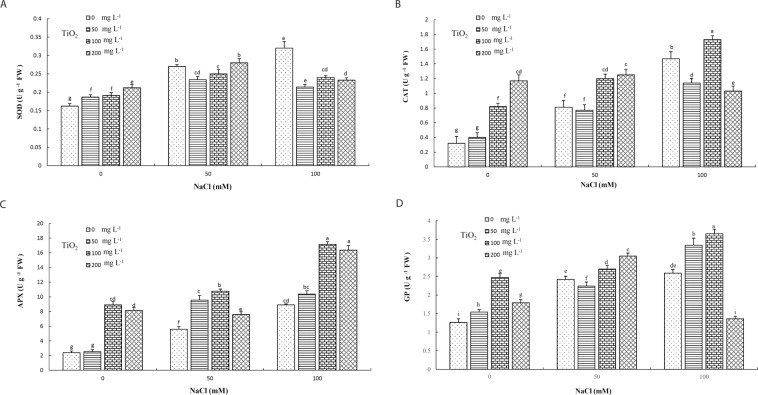
Application of TiO2 NPs, salt stress and their interactions significantly affected superoxide dismutase (SOD) activity. The maximum and minimum activities were recorded in 100 mM NaCl-treated plants under no TiO2 application and control samples, respectively. SOD activity of leaf tissues under moderate and high salinity stresses increased significantly compared with controls. Amongst treatments, the highest activity was observed in 200 mg L−1 TiO2 under 50 mM NaCl, while the lowest was observed in 50 mg L−1 TiO2 under no salinity and 200 mg L−1 TiO2 under 100 mM NaCl (Fig. 7A).
Figure 7.
Effect of different concentrations of TiO2 NPs on SOD (A), CAT (B), APX (C) and GP (D) enzyme activity of D. moldavica L. under salinity stress. Different letters indicate significantly different values at p < 0.05.
In regard with catalase (CAT), enzymatic activity in leaf tissues under 50 and 100 mM NaCl increased significantly compared with control. Therefore, a positive regulation of CAT activity by salt concentration was observed; increasing NaCl levels resulted in increasing CAT activity, similar to SOD. The highest activity among treatments was achieved in 100 mg L−1 TiO2 under 100 mM NaCl, while the lowest was in 50 mg L−1 TiO2-treated plants under no salt stress. Considering CAT activity, TiO2 at 100 mg L−1 concentration generally increased enzymatic activity under both stress conditions compared with those plants at similar conditions without receiving any TiO2 treatment (Fig. 7B).
The highest and lowest ascorbate peroxidase (APX) activities were observed in 100 and 200 mg L−1 TiO2-treated plants under 100 mM NaCl and the control and 50 mg L−1 TiO2 under no salinity stress, respectively. Similar to SOD and CAT, increasing salinity levels lead to increasing APX activity, under no TiO2 treatment. TiO2 treatments increased APX activity under both non-stress and stress conditions, with these increases being higher than non-treated plants at the same conditions (Fig. 7C).
Maximum guaiacol peroxidase (GP) activity was observed in 100 mg L−1 TiO2 under 100 mM NaCl. In this regard, minimum activity was noticed in the control and 200 mg L−1 TiO2-treated samples under 100 mM NaCl. GP activity in leaf tissues under moderate and high salinity stress levels increased significantly compared with control samples. In fact, increase in salinity level increased GP activity. As well, TiO2 treatments increased the activity in which TiO2-treated plants had higher activity that non-treated ones under both non-stress and stress conditions (Fig. 7D).
In total, the activity of GP, APX, CAT and SOD significantly increased under both moderate and high salinity levels. In addition, TiO2 treatments at 100 and 200 mg L−1 concentrations increased antioxidant enzyme activities under control conditions. A similar increasing trend was observed in 50 and 100 mg L−1 TiO2-treated plants under both salinity levels for the above-mentioned enzymes. It is noteworthy that, although the applied salt stress increased enzymatic activities, highest levels were observed in 100 mg L−1-treated plants for CAT, APX and GP. SOD enzyme was an interesting exception as its activity was enhanced by TiO2 application under control conditions, whereas SOD activity decreased significantly in TiO2-treated plants under moderate and severe salt stress compared with plants without any TiO2 application. Additionally, the high concentration of TiO2 (200 mg L−1) applied in plants, under both salinity levels, showed lowest antioxidant enzymatic activity levels overall in comparison with plants treated with 50 and 100 mg L−1 TiO2 which could be correlated with toxicity phenomena.
Overall, it could be concluded that 100 mg L−1 TiO2 application under moderate and high salt stress induces antioxidant enzyme activities, thus contributing in the effective protection of plants from salinity. This is likely through the detoxification of ROS, which is known to over accumulate in saline environments56. ROS compounds are generated by normal cellular activities (e.g., fatty acids β-oxidation), photorespiration and biotic or abiotic stress conditions. ROS elimination is mainly achieved by antioxidant mechanisms such as antioxidant enzymes (e.g., SOD, CAT, APX)57. SOD is the key enzyme for neutralizing ROS as the first line of defense mechanism against oxidative stress. Enhancement in SOD activity is tightlylinked with increased protection against negative effects of stress factors58. CAT, another important antioxidant enzyme, scavenges H2O2 by converting it to water in peroxisomes and neutralizes its deleterious damages59. APX activity, yet another key antioxidant enzyme, eliminates H2O2 activity and modulates its steady-state level in various subcellular compartments of plants60. Moreover, high levels of intercellular H2O2 are known to induce cytosolic APX activity under salinity stress61. Thus, APX plays an important role in the collection and decomposition of H2O2 during stress62. GP acts as an electron transmitter to H2O2, in an attempt to detoxify cells under stress conditions by converting H2O2 into water63. Previous studies reported considerable induction of enzymatic antioxidants under salinity stress, thus preventing ROS-related damage (e.g. Filippou et al.7). Our findings are in agreement with Weisany et al.56, who noted that CAT and APX enzymatic activities in soybean increased under salinity stress due to oxidative reactions caused by higher levels of H2O2. Regarding the enhancement in antioxidant enzyme activities of the plants treated with TiO2 NPs under salt stress, positive interactions might take place which likely provide better signaling towards the activation of these defense enzymes. Moreover, the observed increases in SOD, CAT, APX and GP enzymatic activities under salinity in the present study might be related with the high intercellular H2O2 levels in Moldavian balm leaf tissues. Likewise, enhancement in SOD, CAT, APX and GP activities was observed in plants treated with TiO2 NPs. In addition, the lowest H2O2 content was observed in 100 mg L−1 TiO2-treated plant. Therefore, it could be concluded that the lowest H2O2 content recorded after 100 mg L−1 TiO2 application was closely related to the significantly increased activities of CAT, APX and GP in the same samples. ROS detoxification after TiO2 NP application might be due to stabilized composition of cells and improved physical properties of cell membranes. Lei et al.11 reported that application of TiO2 NPs under drought stress increased antioxidant enzyme activities in plants due to a reduction in lipid peroxidation and improvement in membrane integrity.
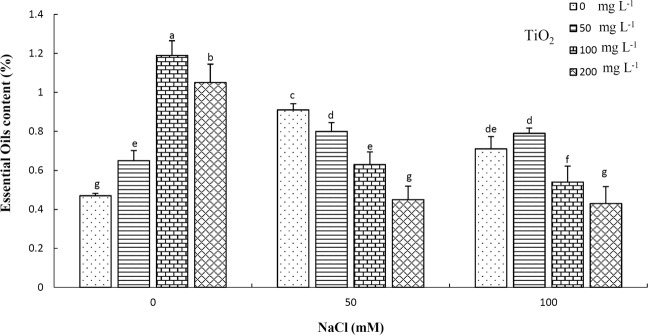
Essential oil content and composition
Essential oil content was significantly affected by salinity, TiO2 and their interactions. The highest essential oil content (≈1.19%) was recorded in 100 mg L−1 TiO2-treated plants under no salinity stress. The lowest (≈0.43%) contents were observed in 200 mg L−1 TiO2 under 50 and 100 mM NaCl, as well as in control samples (Fig. 8). Salinity stress positively affected essential oil content. Generally, both salinity levels increased essential oil content, but maximal yield was achieved by 50 mM NaCl. Similarly, TiO2 NP application had a positive impact in essential oil content, significantly increasing it under control conditions with optimal content recorded following 100 mg L−1 TiO2 NP application. However, under salinity conditions, TiO2 treatments had no considerable impact on this component compared with non-treated plants under stress.
Figure 8.
Effect of different concentrations of TiO2 NPs in essential oil content (%) of D. moldavica L. under salinity stress. Different letters indicate significantly different values at p < 0.05.
The essential oil composition of D. moldavica L. under different salt stresses and TiO2 NPs applications is shown in Table 3. Base on the results, 29 constituents were identified by GC/MS analysis. Main components were geranial, z-citral, geranyl acetate and geraniol. 50 mM NaCl caused significant decrease in geranial and z-citral as well as minor decrease in geraniol, while it significantly increased geranyl acetate concentration. However, 100 mM NaCl had a different effect, as geranial concentration was not affected, geraniol and geranyl acetate content showed increase, while z-citral decreased compared with control samples.
Table 3.
Effect of different concentrations of TiO2 NPs on essential oil composition of D. moldavica L.
| Compounds | RI | 0 mg L−1 TiO2 | 50 mg L−1 TiO2 | 100 mg L−1 TiO2 | 200 mg L−1 TiO2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 mM NaCl | 50 mM NaCl | 100 mM NaCl | 0 mM NaCl | 50 mM NaCl | 100 mM NaCl | 0 mM NaCl | 50 mM NaCl | 100 mM NaCl | 0 mM NaCl | 50 mM NaCl | 100 mM NaCl | |||
| 1 | Camphene | 946 | — | — | — | 0.19 | — | 0.19 | 0.22 | 0.21 | 0.22 | 0.2 | — | — |
| 2 | Sabinene | 969 | — | — | 0.35 | 0.35 | 2.07 | 0.37 | 0.6 | 0.35 | 0.35 | 0.31 | 0.2 | 0.43 |
| 3 | 1,8-Cineole | 1026 | 0.26 | 0.73 | 0.73 | 0.15 | — | 1.28 | 0.23 | 0.35 | 0.82 | 0.2 | 0.44 | 0.65 |
| 4 | Fenchone | 1083 | 0.05 | — | — | 0.05 | — | 0.14 | 0.06 | 0.07 | — | 0.06 | — | — |
| 5 | Linalool | 1095 | 0.47 | 0.49 | 0.5 | 0.36 | — | 0.67 | 0.59 | 0.53 | 0.71 | 0.5 | 0.44 | 0.62 |
| 6 | Pinocarveol | 1135 | 0.18 | 0.19 | — | 0.19 | — | 0.26 | 0.16 | 0. 2 | 0.22 | 0.19 | — | — |
| 7 | Camphor | 1141 | 0.87 | 0.59 | 0.59 | 0.78 | — | 0.74 | 1.03 | 0.88 | 0.88 | 0.85 | 0.59 | 0.78 |
| 9 | Borneol | 1165 | — | — | — | 0.09 | — | — | 0.07 | 0.07 | — | 0.08 | — | — |
| 10 | Menthol | 1167 | 1.31 | 1.01 | 1.01 | 1.19 | 1.12 | 1.4 | 1.57 | 1.21 | 1.46 | 1.31 | 0.99 | 1.36 |
| 11 | beta fenchyl alcohol | 1180 | 0.16 | — | — | 0.66 | — | 1.04 | 0.2 | 0.38 | 0.41 | 0.29 | — | — |
| 12 | Myrtenol | 1194 | 0.09 | 1.2 | 0.25 | 0.08 | 4.36 | 0.8 | 0.07 | 0.84 | 2.37 | 0.07 | — | — |
| 13 | n-Dodecane | 1200 | — | — | — | 0.06 | — | 0.14 | 0.06 | 0.06 | — | 0.05 | — | — |
| 14 | Nerol | 1227 | 0.26 | 0.34 | 0.42 | 0.23 | — | 0.55 | 0.35 | 0.32 | 0.35 | 0.23 | 0.28 | 0.36 |
| 15 | Z-citral | 1238 | 25.26 | 18.53 | 23.62 | 26.71 | 18.13 | 21.96 | 26.98 | 22.13 | 20/1 | 23.5 | 20.7 | 23.2 |
| 16 | Geraniol | 1252 | 5.33 | 4.4 | 6.72 | 4.37 | 5.66 | 5.28 | 7.51 | 5.84 | 5.64 | 4.94 | 6.4 | 6.21 |
| 17 | Geranial (E-citral) | 1267 | 41.88 | 36.77 | 41.91 | 44.05 | 32.98 | 39.1 | 43.97 | 39.85 | 40.65 | 40.56 | 41.72 | 38.76 |
| 18 | Carvacrol | 1298 | — | — | — | 0.03 | — | — | 0.1 | — | — | 0.1 | — | — |
| 19 | Methyl geranate | 1322 | 0.24 | 0.23 | 0.27 | 0.26 | — | 0.21 | 0.21 | 1.21 | 1.21 | 0.19 | 0.24 | 0.28 |
| 20 | Neryl acetate | 1361 | 0.57 | 0.92 | 0.86 | 0.56 | — | 0.78 | 0.47 | 0.75 | 0.68 | 0.64 | 0.88 | 0.86 |
| 21 | Geranyl acetate | 1381 | 18.96 | 22.01 | 19.99 | 15.09 | 15.91 | 16.92 | 11.17 | 18.65 | 16.5 | 19.27 | 23 | 21.1 |
| 22 | n-Tetradecane | 1400 | 0.12 | 0.15 | — | 0.12 | — | 0.11 | 0.15 | 0.2 | — | 0.1 | — | 0.28 |
| 23 | (E)-β -caryophylene | 1417 | 0.12 | 0.78 | — | 0.13 | 1.35 | 0.21 | 0.14 | 0.23 | — | 0.22 | — | — |
| 24 | Germacrene D | 1484 | 0.06 | 1.35 | — | 0.4 | 2.39 | 0.4 | 0.45 | 0.97 | 0.69 | 0.09 | 0.5 | 0.91 |
| 25 | β-selinene | 1489 | 0.13 | 0.49 | — | 0.12 | — | 0.1 | 0.15 | 0.18 | 0.18 | 0.1 | 0.21 | — |
| 26 | Spathulenol | 1577 | 0.15 | 0.27 | — | 0.14 | — | 0.14 | 0.16 | — | — | 0.19 | 0.24 | — |
| 27 | Caryophyllene oxide | 1582 | 0.05 | — | — | 0.07 | — | — | — | — | — | 0.06 | — | — |
| 28 | β-eudesmol | 1649 | 0.09 | 2.05 | 0.31 | 0.1 | 0.23 | 0.37 | 0.11 | 0.12 | — | 0.12 | 0.25 | — |
| 29 | Bisabolol oxide | 1656 | — | — | — | — | — | — | — | 0.08 | — | 0.07 | — | — |
Under prolonged salinity stress. RI values represent retention indices determined on GC/MS capillary column.
TiO2 application at 50 and 100 mg L−1 concentrations under control conditions enhanced geranial and z-citral content, while 100 mg L−1 increased geraniol content. Contrarily, these TiO2 treatments under both salinity levels decreased geranial and z-citral content with the highest decrease being recorded at 50 mg L−1 TiO2 application under 50 mM NaCl stress. Moreover, geranyl acetate was significantly decreased at the above-mentioned TiO2 treatments under both stress and non-stress conditions with the exception of 100 mg L−1 TiO2 under 50 mM NaCl. 200 mg L−1 TiO2 treatment demonstrated no difference in geranial, z-citral, and geraniol values under non-stress condition. In addition, this treatment caused a decrease in z-citral and increase in geraniol content under both salinity levels. Geranial showed no significant difference under 50 mM salinity, while it lowered under 100 mM NaCl. Moreover, geranyl acetate content increased significantly following 200 mg L−1 TiO2 treatment under both control and stress conditions with the highest increase being recorded at 50 mM NaCl application. Furthermore, in spite of considerable enhancement in myrtenol and germacrene D contents by 50 mM NaCl without TiO2 application, their highest values were observed in 50 mg L−1 TiO2 under 50 mM NaCl stress. Regarding nerol content, although salinity increased its content (increasing NaCl concentrations leading to increasing nerol content), the highest value was observed in 50 mg L−1 TiO2-treated plants under 100 mM NaCl stress. Nerol content was also increased following 100 mg L−1 TiO2 under both control and stress conditions as well as following 200 mg L−1 TiO2 under 100 mM NaCl.
Essential oils of Moldavian balm, as an important aromatic and medicinal plant, have various application in the pharmaceutical industry. Considering the importance of its essential oils, any treatment with positive effects on its essential oil content and dominant constituents could be of great value to growers. The positive effect of TiO2 NPs was previously reported in Salvia officinalis essential oil content and constituents55. Taking into account these factors, the current study examined the effect on Moldavian balm under normal and salt stress conditions. Present results revealed that the essential oil content increased under both NaCl levels, in agreement with Khalid and Teixeira de Silva64 and Neffati et al.65. However, a similar trend was not recorded for individual components of the essential oil profile, since the dominant constituents mostly decreased following salt stress particularly 50 mM NaCl. This decrease might be attributed to an impairment in photosynthesis, changes in metabolic systems and increase in osmotic pressure, which might then decrease nutrients and water uptake. Salinity stress has been previously shown to modify essential oil production and profile41. Current results demonstrated that salinity stress altered the content of specific essential oil components in Dracocephalum moldavica L. plants, in agreement with Khalid and Teixeira de Silva64 and Neffati et al.65 who attributed such changes to the regulation of the activity of essential oil biosynthetic enzymes following salt stress imposition.
TiO2 application caused a remarkable increase in essential oil content under control conditions with maximum content being observed at 100 mg L−1 concentration. Results were in accordance to those reported by Ahmad et al.66, who demonstrated that TiO2 NP application increased essential oil content in Mentha piperita L. Furthermore, Lafmejani et al.67 reported that Fe NPs foliar application increased essential oil content in M. piperita plants. Such an increase in essential oil content could be potentially explained by the observed increase in growth, photosynthesis, expression of secondary metabolite enzymes and size and distribution of oil glands as special sites for biosynthesizing essential oils following NP application66. In line with the increase in essential oil content, 100 mg L−1 TiO2 NP application increased main components of essential oil profile. This could be the result of increased expression of specific biosynthetic enzymes involved in the production of components and availability of substrates, in line with previous findings by Ahmad et al.66 and Lafmejani et al.67. The actual mechanism by which NP application modulates plant secondary metabolites is not yet fully elucidated. Recently, coordinated phytochemical and genomic studies confirmed that NPs might act as elicitors for secondary metabolite production in plants by inducing different cellular signal transduction pathways (e.g., mitogen-activated protein kinases, calcium flux and ROS metabolism). Accordingly, the observed changes in the above-mentioned pathways might lead to alterations in gene expression levels and metabolic enzyme activation that could alter secondary metabolite production68.
Conclusion
Nanotechnology is a highly promising novel approach that has great potential for application towards plant protection against different stress conditions. TiO2, recently developed nanoparticles with profound effects in plant morphological, physiological and biochemical properties, could improve overall plant performance. Its application in Moldavian balm plants demonstrated these positive effects under moderate and severe salinity stress as enhanced agronomic traits under both control and stress conditions. TiO2 NP application additionally lowered H2O2 content and increased antioxidant enzyme activities), thus ameliorating oxidative damage and demonstrating positive effects in plants under both conditions. Importantly, enhancement in essential oil content by TiO2 treatments demonstrated another positive impact of TiO2 NPs with implications in the potential for commercial application Interestingly, application of high concentration of TiO2 (200 mg L−1) showed toxic symptoms in specific parameters, likely linked with NP aggregation in high concentrations which lead to increased ROS content. Consequently, TiO2 might act as an inducer of secondary metabolite production (such as essential oils) and trigger for the activation of the enzymatic defense system, ultimately enhancing plant performance under control and stress conditions and thus acting as a promising stress protecting and growth promoting molecule.
Supplementary information
Acknowledgements
We are thankful to Professor Mojtaba Amini (Department of Chemistry, University of Maragheh, Maragheh, Iran) for kind assistance in nanoparticle synthesis. The authors are thankful from Central Laboratory of University of Maragheh, Iran, for their kind supports to this research project. This work was partially supported by JSPS (Japan Society for the Promotion of Science) KAKENHI (18H04787 and 18H04844 to S.K.), and by the MEXT Supported Program for the Strategic Research Foundation at Private Universities from the Ministry of Education, Culture, Sports, Science & Technology of Japan (Grant No. S1511023 to S.K.).
Author contributions
G.G. and S.K. designed the experimental setup. A.M., G.G. and S.P. performed greenhouse experiments, biochemical and essential oil analyses. A.A. synthesized nanomaterial, M.D. analyzed florescence microscopic images. G.G., V.F. and S.P. analyzed data and results, while G.G., S.P., V.F., A.A. and S.K. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gholamreza Gohari, Email: gohari.gh@maragheh.ac.ir.
Ali Akbari, Email: akbari.a@umsu.ac.ir.
Supplementary information
is available for this paper at 10.1038/s41598-020-57794-1.
References
- 1.Carović-StanKo K, PeteK M, Martina G. Medicinal plants of the family Lamiaceaea functional foods – a Review. Czech. J. Food Sci. 2016;34:377. doi: 10.17221/504/2015-CJFS. [DOI] [Google Scholar]
- 2.Aprotosoaie AC, Mihai CT, Vochita G. Antigenotoxic and antioxidant activities of a polyphenolic extract from European Dracocephalum moldavica L. Ind. Crop Prod. 2016;79:248–257. doi: 10.1016/j.indcrop.2015.11.004. [DOI] [Google Scholar]
- 3.Fattahi M, Nazeri V, Torras-Claveria L. Identification and quantification of leaf surface flavonoids in wild-growing populations of Dracocephalum kotschyi by LC–DAD–ESI-MS. Food Chem. 2013;141:139–46. doi: 10.1016/j.foodchem.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Tang X, Mu X, Shao H. Global plant-responding mechanisms to salt stress: physiological and molecular levels and implications in biotechnology. Crit. Rev. biotechnol. 2015;35:425–437. doi: 10.3109/07388551.2014.889080. [DOI] [PubMed] [Google Scholar]
- 5.Munns R, Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant. Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 6.Parihar P, Singh S, Singh R. Effect of salinity stress on plants and its tolerance strategies: a review. Environ. Sci. Pollut. Res. 2015;22:4056–4075. doi: 10.1007/s11356-014-3739-1. [DOI] [PubMed] [Google Scholar]
- 7.Filippou P, Bouchagier P, Skotti E, Fotopoulos V. Proline and reactive oxygen/nitrogen species biosynthesis is involved in the tolerant response of the invasive plant species Ailanthus altissima to drought and salinity. Environ. Exper. Bot. 2014;97:1–10. doi: 10.1016/j.envexpbot.2013.09.010. [DOI] [Google Scholar]
- 8.Liang W, Ma X, Wan P, Liu L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018;495(1):286–291. doi: 10.1016/j.bbrc.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 9.Gohari, G. et al. Interaction between hydrogen peroxide and sodium nitroprusside following chemical priming of Ocimum basilicum L. against salt stress. Physiol. Plant. 10.1111/ppl.13020 (2019). [DOI] [PubMed]
- 10.Duhan JS, et al. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017;15:11–23. doi: 10.1016/j.btre.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei Z, Mingyu S, Xiao W. Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol. Trace. Elem. Res. 2008;121:69–79. doi: 10.1007/s12011-007-8028-0. [DOI] [PubMed] [Google Scholar]
- 12.Latef A, et al. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land. Degrad. Dev. 2018;29:1065–1073. doi: 10.1002/ldr.2780. [DOI] [Google Scholar]
- 13.Khan MN. Nano-titanium Dioxide (Nano-TiO2) mitigates NaCl stress by enhancing antioxidative enzymes and accumulation of compatible solutes in tomato (Lycopersicon esculentum Mill.) J. Plant. Sci. 2016;11:1–11. doi: 10.3923/jps.2016.1.11. [DOI] [Google Scholar]
- 14.Feizi H, Moghaddam PR, Shahtahmassebi N, Fotovat A. Impact of bulk and nanosized titanium dioxide (TiO2) on wheat seed germination and seedling growth. Biol. Trace. Elem. Res. 2012;146:101–106. doi: 10.1007/s12011-011-9222-7. [DOI] [PubMed] [Google Scholar]
- 15.Tan W, Peralta-Videa JR, Gardea-Torresdey JL. Interaction of titanium dioxide nanoparticles with soil components and plants: current knowledge and future research needs–a critical review. Environ. Sci. Nano. 2018;5:257–278. doi: 10.1039/C7EN00985B. [DOI] [Google Scholar]
- 16.Nair R, et al. Nanoparticulate material delivery to plants. Plant. Sci. 2010;179:154–163. doi: 10.1016/j.plantsci.2010.04.012. [DOI] [Google Scholar]
- 17.Mattiello A, et al. Evidence of phytotoxicity and genotoxicity in Hordeum vulgare L. exposed to CeO2 and TiO2 nanoparticles. Front. Plant Sci. 2015;6:1043–1050. doi: 10.3389/fpls.2015.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rastogi A, et al. Impact of metal and metal oxide nanoparticles on plant: a critical review. Front. Chem. 2017;78:1–16. doi: 10.3389/fchem.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi K, Kubota H, Hojo R, Miyagawa M. Effective dispersal of titanium dioxide nanoparticles for toxicity testing. J Toxic Sci. 2019;44:515–521. doi: 10.2131/jts.44.515. [DOI] [PubMed] [Google Scholar]
- 20.Rico CM, Peralta-Videa JR, Gardea-Torresdey JL. Chemistry, biochemistry of nanoparticles, and their role in antioxidant defense system in plants. Nanotechnol. Plant. Sci. 2015;2:1–17. [Google Scholar]
- 21.Hou J, et al. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019;75:40–53. doi: 10.1016/j.jes.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Filho S, et al. Genotoxicity of titanium dioxide nanoparticles and triggering of defense mechanisms in Allium cepa. Genet. Mol. Biol. 2019;42:1–12. doi: 10.1590/1678-4685-gmb-2017-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva S, et al. Antioxidant mechanisms to counteract TiO2-nanoparticles toxicity in wheat leaves and roots are organ dependent. J. Hazard. Material. 2019;380:1–10. doi: 10.1016/j.jhazmat.2019.120889. [DOI] [PubMed] [Google Scholar]
- 24.Fenoglio I, Greco G, Livraghi S, Fubini B. Non-UV-induced radical reactions at the surface of TiO2 nanoparticles that may trigger toxic responses. Chem. Eur. J. 2009;15:4614–4621. doi: 10.1002/chem.200802542. [DOI] [PubMed] [Google Scholar]
- 25.Nagaveni K, Hegde MS, Ravishankar N, Subbanna GN, Madras G. Synthesis and structure of nanocrystalline TiO2 with lower band gap showing high photocatalytic activity. Langmuir. 2004;20:2900–2907. doi: 10.1021/la035777v. [DOI] [PubMed] [Google Scholar]
- 26.Ghafariyan MH, Malakouti MJ, Dadpour MR. Effects of magnetite nanoparticles on soybean chlorophyll. Environ. Sci. Technol. 2013;47:10645–10652. doi: 10.1021/es402249b. [DOI] [PubMed] [Google Scholar]
- 27.Tarrahi R, Movafeghi A, Khataee A, Rezanejad F, Gohari G. Evaluating the Toxic Impacts of Cadmium Selenide Nanoparticles on the Aquatic Plant Lemna minor. Molecules. 2019;24(3):410. doi: 10.3390/molecules24030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dadpour MR, Grigorian W, Nazemieh A, Valizadeh M. Application of epi-illumination light microscopy for study of floral ontogeny in fruit trees. Int. J. Bot. 2008;4:49–55. doi: 10.3923/ijb.2008.49.55. [DOI] [Google Scholar]
- 29.Hoagland, D. R. & Arnon, D. I. ‘The water culture method for growing plants without soil’. Calif. Agric. Exper. Stn. Circ. 337 (1950).
- 30.Sharma DK, Andersen SB, Ottosen CO, Rosenqvist E. Phenotyping of wheat cultivars for heat tolerance using chlorophyll a fluorescence. Funct. Plant Biol. 2012;39(11):936–947. doi: 10.1071/FP12100. [DOI] [PubMed] [Google Scholar]
- 31.Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. et Biophys. Acta. 1989;99:87–92. doi: 10.1016/S0304-4165(89)80016-9. [DOI] [Google Scholar]
- 32.Sinha S, Saxena R, Singh S. Chromium induced lipid peroxidation in the plants of Pistia stratiotes L.: role of antioxidants and antioxidant enzymes. Chemosphere. 2005;58:595–604. doi: 10.1016/j.chemosphere.2004.08.071. [DOI] [PubMed] [Google Scholar]
- 33.Ezhilmathi K, Singh VP, Arora A, Sairam RK. Effect of 5-sulfosalicylic acid on antioxidant activity in relation to vase life of Gladiolus cut flowers. Plant Growth Regul. 2007;51:99–106. doi: 10.1007/s10725-006-9142-2. [DOI] [Google Scholar]
- 34.Sun Y, et al. Comparative phytochemical profiles and antioxidant enzyme activity analyses of the southern highbush blueberry (Vaccinium corymbosum) at different developmental stages. Molecules. 2018;23(9):2209–2218. doi: 10.3390/molecules23092209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sairam RK, Rao KV, Srivastava GC. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant. Sci. 2002;163:1037–1046. doi: 10.1016/S0168-9452(02)00278-9. [DOI] [Google Scholar]
- 36.Tang W, Newton RJ. Peroxidase and catalase activities are involved in direct adventitious shoot formation induced by thidiazuron in eastern white pine (Pinus strobes L.) zygotic embryos. Plant Physiol. Biochem. 2005;43:760–769. doi: 10.1016/j.plaphy.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Hussain AI, Anwar F, Sherazi ST, Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depend on seasonal variations. Food Chem. 2008;108(3):986–995. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Becheri A, Dürr M, Nostro PL, Baglioni P. Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV-absorbers. J. Nanopart. Res. 2008;10:679–689. doi: 10.1007/s11051-007-9318-3. [DOI] [Google Scholar]
- 39.Movafeghi A, et al. Effects of TiO2 nanoparticles on the aquatic plant Spirodela polyrrhiza. Evaluation of growth parameters, pigment contents and antioxidant enzyme activities. J. Environ. Sci. 2018;64:130–138. doi: 10.1016/j.jes.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 40.Prasad R, Bhattacharyya A, Nguyen QD. Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front. Microbiol. 2017;8:1014–1021. doi: 10.3389/fmicb.2017.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aziz EE, Al-Amier H, Craker LE. Influence of salt stress on growth and essential oil production in peppermint, pennyroyal, and apple mint. J. Herbs. Spesices Med. Plant. 2008;14:77–87. doi: 10.1080/10496470802341375. [DOI] [Google Scholar]
- 42.Acosta-Motos J, Ortuño M, Bernal-Vicente A. Plant responses to salt stress: adaptive mechanisms. Agronomy. 2017;7:18–25. doi: 10.3390/agronomy7010018. [DOI] [Google Scholar]
- 43.Kapoor N, Pande V. Effect of salt stress on growth parameters, moisture content, relative water content and photosynthetic pigments of fenugreek variety RMt-1. J. Plant Sci. 2015;10:210–221. doi: 10.3923/jps.2015.210.221. [DOI] [Google Scholar]
- 44.Alam, M. A., Juraimi, A. S., Rafii, M. Y. & Abdul Hamid, A. Effect of salinity on biomass yield and physiological and stem-root anatomical characteristics of purslane (Portulacaoleracea L.) accessions. Biomed. Res. Int. 1–15 (2015). [DOI] [PMC free article] [PubMed] [Retracted]
- 45.Rahneshan Z, Nasibi F, Moghadam AA. Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J. Plant. Interact. 2018;13:73–82. doi: 10.1080/17429145.2018.1424355. [DOI] [Google Scholar]
- 46.Hernández JA, Corpas FJ, Gómez M. Salt-induced oxidative stress mediated by activated oxygen species in pea leaf mitochondria. Physiol. Plant. 1993;89:103–110. doi: 10.1111/j.1399-3054.1993.tb01792.x. [DOI] [Google Scholar]
- 47.Yamane K, Kawasaki M, Taniguchi M, Miyake H. Correlation between chloroplast ultrastructure and chlorophyll fluorescence characteristics in the leaves of rice (Oryza sativa L.) grown under salinity. Plant Prod. Sci. 2008;11:139–145. doi: 10.1626/pps.11.139. [DOI] [Google Scholar]
- 48.Omoto E, Kawasaki M, Taniguchi M, Miyake H. Salinity induces granal development in bundle sheath chloroplasts of NADP-malic enzyme type C4 plants. Plant Prod. Sci. 2009;12:199–207. doi: 10.1626/pps.12.199. [DOI] [Google Scholar]
- 49.Netondo GW, Onyango JC, Beck E. Sorghum and salinity: II. Gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Sci. 2004;44:806. [Google Scholar]
- 50.Mingyu S, Fashui H, Chao L. Effects of nano-anatase TiO2 on absorption, distribution of light, and photoreduction activities of chloroplast membrane of spinach. Biol. Trace Elem. Res. 2007;118:120–130. doi: 10.1007/s12011-007-0006-z. [DOI] [PubMed] [Google Scholar]
- 51.Frazier TP, Burklew CE, Zhang B. Titanium dioxide nanoparticles affect the growth and microRNA expression of tobacco (Nicotiana tabacum) Funct. Integr. Genomics. 2014;14:75–83. doi: 10.1007/s10142-013-0341-4. [DOI] [PubMed] [Google Scholar]
- 52.Yamori W, Masumoto C, Fukayama H, Makino A. Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. The Plant J. 2012;71(6):871–880. doi: 10.1111/j.1365-313X.2012.05041.x. [DOI] [PubMed] [Google Scholar]
- 53.Molassiotis A, Fotopoulos V. Oxidative and nitrosative signaling in plants: two branches in the same tree? Plant Signaling and Behavior (Special Issue on Plant Abiotic. Stress) 2011;6:210–214. doi: 10.4161/psb.6.2.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Begara-Morales JC, et al. Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. J. Exp. Bot. 2014;65:527–538. doi: 10.1093/jxb/ert396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghorbanpour M. Major essential oil constituents, total phenolics and flavonoids content and antioxidant activity of Salvia officinalis plant in response to nano-titanium dioxide. Indian J. Plant Physiol. 2015;20(3):249–256. doi: 10.1007/s40502-015-0170-7. [DOI] [Google Scholar]
- 56.Weisany W, Sohrabi Y, Heidari G, Siosemardeh A, Ghassemi-Golezani K. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.) Plant. Omics. 2012;5:60–67. [Google Scholar]
- 57.Harinasut P, Poonsopa D, Roengmongkol K, Charoensataporn R. Salinity effects on antioxidant enzymes in mulberry cultivar. Sci. Asia. 2003;29:109–113. doi: 10.2306/scienceasia1513-1874.2003.29.109. [DOI] [Google Scholar]
- 58.Song F, Yang C, Liu X, Li G. Effect of salt stress on activity of superoxide dismutase (SOD) in Ulmus pumila L. J. For. Res. 2006;17(1):13–16. doi: 10.1007/s11676-006-0003-7. [DOI] [Google Scholar]
- 59.Esfandiari E, Gohari G. Response of ROS-scavenging systems to salinity stress in two different wheat (Triticum aestivum L.) cultivars. Not. Bot. Horti. Agrobot. Cluj. Napoca. 2017;45(1):287–291. doi: 10.15835/nbha45110682. [DOI] [Google Scholar]
- 60.Najami N, et al. Ascorbate peroxidase gene family in tomato: its identification and characterization. Mol. Gene. Genom. 2008;279:171–182. doi: 10.1007/s00438-007-0305-2. [DOI] [PubMed] [Google Scholar]
- 61.Lee DH, Kim YS, Lee CB. The inductive responses of the antioxidant enzymes by salt stress in the rice (Oryza sativa L.) J. Plant. Physiol. 2001;158:737–745. doi: 10.1078/0176-1617-00174. [DOI] [Google Scholar]
- 62.Gill SS, et al. Superoxide dismutase—mentor of abiotic stress tolerance in crop plants. Environ. Sci. Pollut. Res. 2015;22(14):10375–10394. doi: 10.1007/s11356-015-4532-5. [DOI] [PubMed] [Google Scholar]
- 63.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2004;9(10):490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 64.Khalid KA, Teixeira de Silva JA. Yield, essential oil and pigment content of Calendula officinalis L. flower heads cultivated under salt stress conditions. Sci. Hort. 2010;126(2):297–305. doi: 10.1016/j.scienta.2010.07.023. [DOI] [Google Scholar]
- 65.Neffati M, Sriti J, Hamdaoui G, Kchouk ME. Salinity impact on fruit yield, essential oil composition and antioxidant activities of Coriandrum sativum fruit extracts. Food Chem. 2011;124(1):221–225. doi: 10.1016/j.foodchem.2010.06.022. [DOI] [Google Scholar]
- 66.Ahmad B, Shabbir A, Jaleel H, Khan MM, Sadiq Y. Efficacy of titanium dioxide nanoparticles in modulating photosynthesis, peltate glandular trichomes and essential oil production and quality in Mentha piperita L. Curr. Plant Biol. 2018;13(1):6–15. doi: 10.1016/j.cpb.2018.04.002. [DOI] [Google Scholar]
- 67.Lafmejani ZN, Jafari AA, Moradi P, Moghadam AL. Impact of foliar application of iron-chelate and iron nano particles on some morpho-physiological traits and essential Oil composition of peppermint (Mentha piperita L.) J. Essent. Oil Bear. Pl. 2018;21(5):1374–1384. doi: 10.1080/0972060X.2018.1556122. [DOI] [Google Scholar]
- 68.Ebadollahi R, Jafarirad S, Kosari-Nasab M, Mahjouri S. Effect of Explant Source, Perlite Nanoparticles and TiO2/perlite Nanocomposites on Phytochemical Composition of Metabolites in Callus Cultures of Hypericum perforatum. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-49504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.