Abstract
For a better understanding terpenoid volatile production in Camellia sinensis, global terpenoid synthase gene (TPS) transcription analysis was conducted based on transcriptomic data combined with terpenoid metabolic profiling under different abiotic stress conditions. Totally 80 TPS-like genes were identified. Twenty-three CsTPS genes possessed a complete coding sequence and most likely were functional. The remaining 57 in the currently available database lack essential gene structure or full-length transcripts. Distinct tempo-spatial expression patterns of CsTPS genes were found in tea plants. 17 genes were substantially expressed in all the tested organs with a few exceptions. The other 17 were predominantly expressed in leaves whereas additional eight were primarily expressed in flowers. Under the treatments of cold acclimation, salt and polyethylene glycol, CsTPS67, -69 and -71 were all suppressed and the inhibited expression of many others were found in multiple stress treatments. However, methyl jasmonate resulted in the enhanced expression of the majority of CsTPS genes. These transcription data were largely validated using qPCR. Moreover, volatile terpenoid profiling with leaves, flowers and stress-treated plants revealed a general association between the abundances of mono- and sesqui-terpenoids and some CsTPS genes. These results provide vital information for future studies on CsTPS regulation of terpenoid biosynthesis.
Subject terms: Plant stress responses, Secondary metabolism
Introduction
Plant terpenoids (isoprene-C5, monoterpenes-C10, sesquiterpenes-C15, diterpenes-C20, and polyterpenoids-C5xn) possess diverse functions in plant growth and development1–7. They play significant ecological roles in the interactions between plants and stress conditions. Generally, terpenoid molecules smaller than diterpenoids are volatile and well known for their airborne signaling function, particularly against herbivore attack8,9. High volatility of monoterpenes and sesquiterpenes enhances the flavor and aroma of crop products10 such as tea, which is a popular beverage well known for its fragrance and aroma11. Tea volatile terpenoids not only are defense components against insects12 or high solar radiation13, but are also essential odorants of tea products with a direct influence on flavor and quality14–16. Aroma from volatile terpenoids is one of the main sensory properties affecting tea flavor quality17. For instance, monoterpene alcohols such as linalool and geraniol, two of the most abundant and odor active terpenoids in tea15, impart pleasant floral scent to green tea and black tea17.
Terpene synthases possess a characteristic catalytic function that generates multiple terpenoid products with one substrate18, thus collectively contributing to numerous and different structures of plant terpenoids in addition to other modifying enzymes such as uridine diphosphate (UDP)-glucosyl transferases19,20 and P450s21. TPSs are responsible for converting the precursors of geranyl diphosphate (GPP), isoprenyl diphosphate (IPP), farnesyl diphosphate (FPP) and geranylgeranyl diphosphate (GGPP) into a multitude of cyclic and acyclic monoterpenes, sesquiterpenes and diterpenes by different pathways, respectively (Fig. 1)18,22. In general, the TPS family is characterized by two large domains defined in the PFAM (Protein families) database (pfam.xfam.org/): PF01397 corresponds to the N-terminal region and PF03936 corresponds to the C-terminal metal cofactor binding domain23. In addition, the expected gene size and organization comply with seven exons for TPS-a, TPS-b, and TPS-g and between 13 and 15 exons for TPS-e/TPS-f and TPS-c24,25. TPSs also contain structural features such as the conserved ‘DDXXD’ and ‘NSE’ motifs24.
Figure 1.
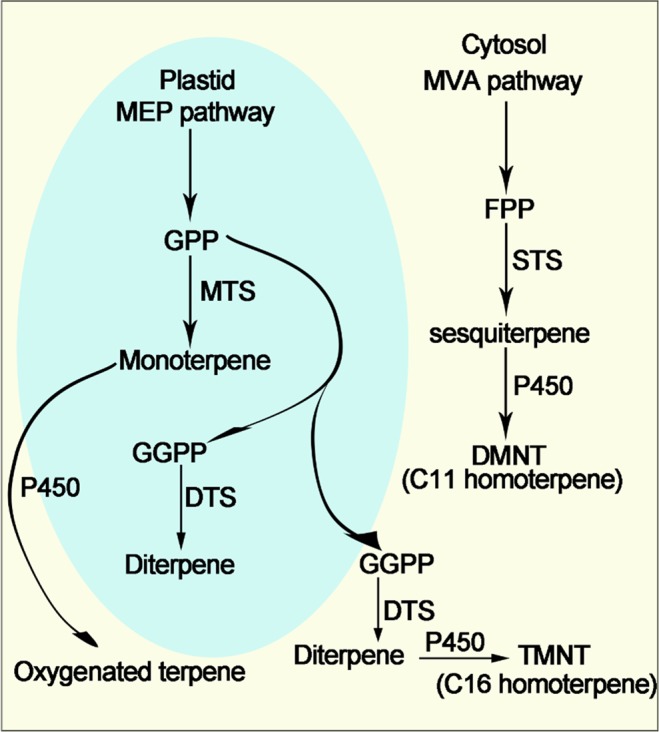
The pathway of terpene synthase gene responsible for the formation of terpenoids in planta. MTS, monoterpene synthase; STS, sesquiterpene synthase; DTS, diterpene synthase.
The majority of sequenced plant genomes that have been analyzed contain different sizes of TPS families with 30 to 100 members, which probably evolved through duplication of genes followed by functional divergence24. The plant TPSs are divided into six subfamilies named from TPS-a to TPS-f based on their amino acid sequence relatedness26 therein TPS-a, TPS-b, and TPS-g are angiosperm-specific clades while TPS-d are gymnosperm-specific clade. TPS-h is specific to the spike moss and TPS-e and -f are proposed to combined together into the group of TPS-e/f24. So far the TPS gene family members have been characterized in many plant species including Arabidopsis thaliana22,24, Sorghum bicolor L.27, grape (Vitis vinifera)23, tomato (Solanum lycopersicum)28, apple (Malus domestica)25, poplar (Populus trichocarpa)29, Eucalyptus species30 and carrot (Daucus carota L.)31.
Compared to other plants, the tea plant is a perennial woody plant species belonging to the Theaceae family with a characteristic secondary metabolite profile containing approximately 100 types of different volatile terpenoids32. To date, only few tea TPS genes have been identified. A striking study presents CsLIS/NES, which generates two splicing forms and results in cytosolic nerolidol synthase and plastidial linalool synthase, consequently producing (E)-nerolidol and linalool in planta33. Another tea TPS recently reported is CsNES, which is only responsible for the formation of nerolidol, notably contributing to flavor and aroma of oolong tea34. However, a comprehensive study about tea TPS genes has not been reported yet.
Recently, genome databases from the tea cultivar “Yun-Kang 10” of Camellia sinensis var. assamica (CSA)35 and “Shu-Cha Zao” of C. sinensis var. sinensis (CSS)36 have been released. In the present study, eighty CsTPS-like genes were identified. Their phylogeny, structure, and expression patterns were comprehensively evaluated, with a special focus on their expression patterns under abiotic stress. Our findings provide a foundation for further exploration of tea TPS genes from other lineages with the aim of improving our understanding of the biosynthesis of terpenoids.
Results
Identification of TPS gene members in tea genomes
To retrieve tea TPS genes from recently publicized tea genome databases, the PF01397 and PF03936 domains, representing respectively N-terminal and C-terminal domains of TPS, were used. Those genes contained one or two domains of PF01397 and PF03936 were retrieved as tea TPS candidate genes from CSS and CSA genomes, meanwhile manual curation and validation of these TPS gene candidates were performed using each candidate genes as query to do BLASTP against the database at the National Center for Biotechnology Information (NCBI). A total of 80 and 60 TPS-like genes were found in CSS “Shu-Cha-Zao” (Supplementary Table S2) and CSA “Yunkang10” genomes (Supplementary Table S3), respectively. TPS genes from two tea genomes were found highly conserved in cDNA sequence with identity ranging from 77% to 100% but with varying sequence coverages. No TPS genes with identical sequences were found from the two genomes. Compared to the corresponding TPS homologues in CSS “Shu-Cha-Zao”, eight TPS genes from CSA “Yunkang 10” (highlighted in Supplementary Table S3) were found containing similar protein sequence length (90–110%), high identity at both cDNA and protein levels (>90%). However, 31 and 13 TPS genes respectively from CSA “Yunkang10” and “Shu-Cha-Zao” had incomplete protein sequences with less than 200 amino acid residues, all lacking either Pfam domain PF01397 or PF03936 (Supplementary Tables S2,S3). For further analysis, TPS gene models from the CSS genome assembly was employed.
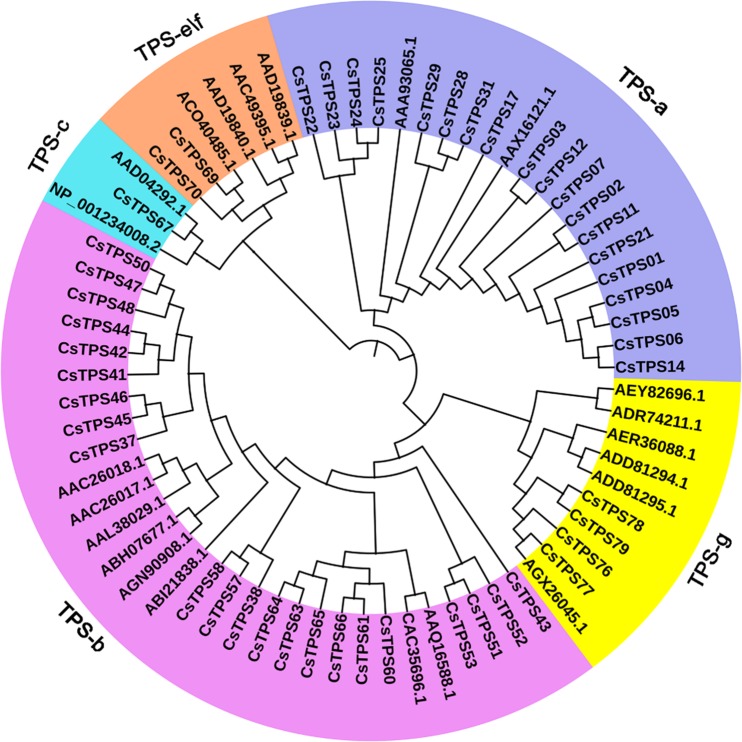
Phylogenetic analysis was performed using 48 CsTPSs (the remaining CsTPS genes were too short for meaningful alignment) and another 22 documented TPS from different plant species, indicating that tea TPS genes belongs to six subfamilies from TPS-a to TPS-g, but without TPS-d based on their protein sequences (Fig. 2). The TPS-a gene family in tea was the most expanded, with 36 genes, approximately 45% of the total TPS genes identified. This is in accordance with other plant species, including grape, Arabidopsis, and rice24. TPS-b gene subfamily as the second largest, included 30, about 37.5% of the total tea TPS genes. For the remaining TPS subgroups, only one gene encoding copalyl diphosphate synthase representing the TPS-c subgroup, eight representing the TPS-e/f subgroup, and five genes representing the TPS-g subgroups, respectively, were also identified (Supplementary Table S2). We designated these gene models of CSS genome as TPS1 through TPS80 according to the order of their subfamilies.
Figure 2.
Phylogenetic analysis of tea CsTPS genes based on their predicted protein sequences. TPS, terpene synthases. The maximum likelihood algorithm tree was generated from an alignment of 70 TPS proteins, comprising 48 CsTPSs (the remaining TPS genes were too short or no common sites for meaningful alignment) and another 43 documented TPS from different plant species.
Putatively functional TPS genes
For assessment of the tea TPS gene functions, full-length transcriptome sequencing data37,38 were employed to verify the putative functional TPS genes obtained from tea genome. Sequence similarity comparison between the full-length transcripts of TPS and genome assembly were conducted using BLAST with a threshold E-value < 1e-5 and identity >98%. After series of BLAST using the nucleotide sequence and manual evaluation, 80 tea TPS genes divided into three types: putative functional TPS genes with full coding sequences and complete structures (23 members); full-length coding TPS genes with disordered structure (9 members); and partial TPS genes (48 members), respectively (Supplementary Table S4). The first type of tea TPS genes had 23 members and all had an uncompromised open reading frame in either transcriptomic or genomic data (Table 1). Thus, they were most likely functional. CsTPS57, CsTPS76, and CsTPS78 have been proved as active ocimene synthase, bifunctional linalool/nerolidol synthases and nerolidol synthase, respectively33,34,39. Another 20 TPS genes possessed full-length coding sequences either revealed by transcriptome data or genome assembly. These genes also possessed the intron-exon structure, i.e. seven exons for TPS-a, TPS-b, and TPS-g and 13 to 15 exons for TPS-e/f and TPS-c. Moreover, these genes contain the specific protein features of TPS gene family, such as the ‘DDXXD’ and ‘NSE/DTE’ motifs (Supplementary Table S4), which are important for metal dependent ionization of the prenyl diphosphate substrate in the C-terminal domain23,40,41. Out of these 20 TPS genes, 5, 11, and 2 were annotated as monoterpene, sesquiterpene and diterpene synthase genes in addition to 3 bifunctional TPS genes (mono- and sesqui-terpene synthase genes) (Table 1).
Table 1.
Potential functional TPS genes in tea genome.
| Name | Genome ID | Related transcript ID | Amino acid No. | Predicted functional |
|---|---|---|---|---|
| CsTPS01 | TEA032539.1 | CssPBTrans029702/PB.8683.1 | 567a | Germacrene D synthase |
| CsTPS02 | TEA029356.1 | CssPBTrans041118/PB.24310.30 | 537a | Germacrene D synthase |
| CsTPS03 | TEA014184.1 | CssPBTrans068400/PB.13101.1 | 569a | Germacrene D synthase |
| CsTPS04 | TEA031969.1 | PB.31351.2 | 572c | Germacrene D synthase |
| CsTPS05 | TEA031966.1 | PB.31351.8 | 522c | Germacrene D synthase |
| CsTPS21 | TEA029348.1 | CssPBTrans020115 | 567c | Germacrene D synthase |
| CsTPS22 | TEA012463.1 | PB.22660.1 | 547a | Germacrene D synthase |
| CsTPS23 | TEA023168.1 | CssPBTrans024950 | 540a | Germacrene D synthase |
| CsTPS25 | TEA010551.1 | CssPBTrans026737/PB.1889.1 | 552c | Germacrene D synthase |
| CsTPS29 | TEA024081.1 | None | 541a | Germacrene D synthase |
| CsTPS42 | TEA002963.1 | CssPBTrans058925 | 568a | Myrcene synthase |
| CsTPS43 | TEA014987.1 | 607a | Myrcene synthase | |
| CsTPS45 | TEA030379.1 | CssPBTrans048789 | 575c | Myrcene synthase |
| CsTPS47 | TEA022294.1 | PB.21690.2 | 595c | Myrcene synthase |
| CsTPS51 | TEA033306.1 | PB.25018.1 | 528a | α-Farnesene synthase |
| CsTPS57 | TEA004606.1 | CssPBTrans046146/PB.522.1 | 585b | β-ocimene synthase |
| CsTPS63 | TEA031457.1 | PB.3574.1 | 549c | Tricyclene synthase |
| CsTPS70 | TEA024176.1 | CssPBTrans055745 | 781c | ent-kaurene synthase |
| CsTPS73 | TEA019347.1 | 801a | (E,E)-geranyllinalool synthase | |
| CsTPS76 | TEA007191.1 | PB.20489.1 | 575b | (3 S)-linalool/ (E)-nerolidol synthase |
| CsTPS77 | TEA004822.1 | PB.4391.1 | 557a | (3 S)-linalool/ (E)-nerolidol synthase |
| CsTPS78 | TEA019472.1 | PB.5304.3 | 546b | (3 S)-linalool/ (E)-nerolidol synthase |
| CsTPS79 | TEA004657.1 | PB.19331.4 | 598a | (3 S)-linalool/ (E)-nerolidol synthase |
The functions of the second type of 9 TPS genes were uncertain because full-length transcripts were not found or with low identity between the two sequence sources (the full-length transcriptomic data and genome assembly). In addition, their gene structures in genome assembly exhibited alterations in different extents compared to common structures of functional TPS genes, likely resulting in non-function or malfunction. For instance, CsTPS24, -37, -41, -67 had one or more retrotransposon segment inserts, leading to disturbed gene structures. In particular, CsTPS67 encoding a copalyl diphosphate synthase (CPS), harboring three retrotransposons with a total of 13,296 bp in length in the first intron (Supplementary Fig. S1), probably leading to failure of gene transcription. Even though it was unknown whether its transcription could occur regularly, Although the active domain ‘DXDD’ still present in its protein structure, 89 amino acids sequences in N-terminal is lost compared to CPS genes of other plant species identified. The TPS genes of this group were annotated as mono-, sesqui-, and di-terpene synthase genes (4, 4, and 2, respectively) according to manual BLASTP homologue searches.
The third type of tea TPS genes (48 members) with incomplete sequences contained no expected gene structures (i.e. intron-exon structure, open reading frames), compared to other plant species. The sequences of these TPS genes could not be validated using full transcriptomic data or RNA-seq data. Thus, these type of the TPS genes might not be functional, even though some of their incomplete sequences could be resulted from imperfect sequencing technique or transposon insertions (Supplementary Table S5).
Tempo-spatial expression patterns of TPS genes in different tea organs
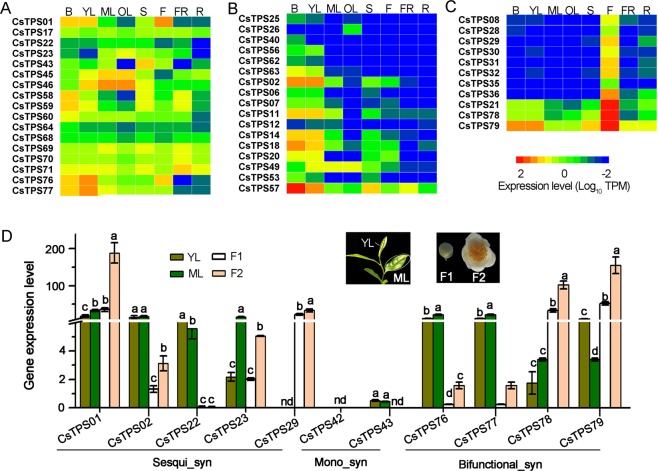
To learn the tempo-spatial expression patterns of tea TPS genes, transcriptome datasets from eight organs of tea plants (i.e. apical buds, young leaves, mature leaves, old leaves, stem, flowers, fruit and roots) (tpia.teaplant.org) were employed (Supplementary Table S6). Logarithm value (Log10) of transcripts per million (TPM) of each annotated tea TPS transcript was obtained. Results indicated that TPS genes had distinct tempo-spatial expression patterns (Fig. 3). Seventeen genes, particularly CsTPS17, -59, -69, -70, and -71, were substantially expressed in all the tested organs with a few exceptions such as CsTPS22, -23, -43, -58 and -76 with undetectable levels in one or two organs (Fig. 3A). On the contrary the majority of TPS genes that exhibited distinct tempo-spatial expression patterns. Six genes (CsTPS25, -26, -40, -56, -62, and -63) were solely and another 11 genes (TPS02, -06, -07, -11, -12, -14, -18, -20, -49, -53, and -57) were predominantly expressed in leaves (Fig. 3B); another eight (CsTPS08, -28 ~ -32, -35, and -36) and three (-21, -78, and -79) were almost exclusively and primarily expressed in flowers (Fig. 3C). In addition, eight genes (CsTPS09, -10, -13, -16, -27, -33, -65 and -73) had no detectable or trace transcript levels in all the tested organs, all of which belonged to the third group of putatively non-functional TPS genes except for CsTPS73 (Supplementary Fig. S2 and Table S5).
Figure 3.
Tempo-spatial expression patterns of CsTPS genes in tea apical buds (B), young leaves (YL), mature leaves (ML), old leaves (OL), immature stems (S), flowers (FL), young fruits (FR) and roots (R). (A), CsTPS genes substantially expressed in eight tested organs with a few exceptions; (B), CsTPS genes predominantly expressed in leaves; (C), CsTPS genes primarily expressed in flowers; Transcripts per million (TPM) were used to evaluate gene expression levels; (D) qRT-PCR validation of transcriptomic data of the CsTPS genes in red in young and mature leaves (YL and ML) and flowers at the two developmental stages (F1 and F2) relative to the level of CsTPS02 in F1. Mean levels (n ≥ 3) distinguished with different letters for each gene among different leaves and flowers are significantly different from each other (p < 0.05). Sesqui-syn, Mono-syn, and Bifunctional-syn represent sesquiterpenoid synthase, monoterpenoid synthase and bifunctional ternpenoid synthase, respectively.
Interestingly, 38 TPS genes expressed with substantial levels either in flowers or leaves were annotated as sesquiterpene synthases genes, while monoterpenoid synthase genes largely with low transcription levels were only seven (CsTPS40, -43, -45, -46, -49, -60, -62, and -63). These mono-TPS genes were restricted to be expressed in vegetative organs except for CsTPS43, -46 and -49 which also expressed in flowers.
qRT-PCR was performed to validate the expression patterns of CsTPS genes using some of putatively functional genes in Table 1. Significantly distinct transcript levels of CsTPS01, and -2 were found in young leaves (YL) compared to the corresponding levels in flowers using qPCR methods. Higher transcript levels of CsTPS22 and -23 were found in mature leaves (ML) than in both flower buds (F1) and open flowers (F2). Similarly, higher transcript levels of CsTPS43, -76, and -77 were also found in leaves than in flowers. However, for CsTPS29, -78, and -79, higher transcript levels were noted in flowers compared to those in leaves (Fig. 3D). It is interesting to note that CsTPS29 was exclusively expressed in flowers. All these quantitative analysis results obtained using qRT-PCR were well consistent with the transcriptomic data (Fig. 3A–C). Gene annotation indicates that CsTPS01 and -02 likely encoded sesquiterpene synthases and that CsTPS76-79 were annotated as difunctional CsTPS genes, among which CsTPS76 has been proved as linalool synthase and nerolidol synthase33.
Terpenoid profiling in tea leaves and flowers
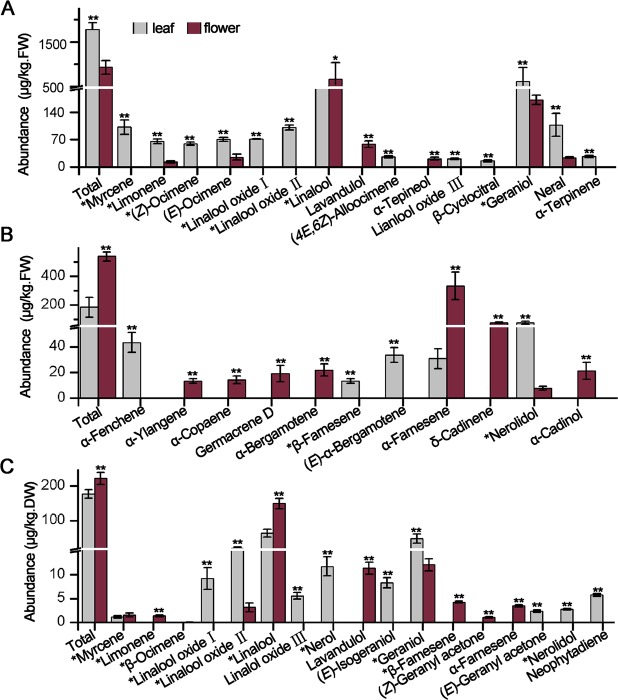
It was obvious that transcription regulation is crucial for terpenoid production in plants7,22. Intact tea leaves usually do not release any perceivable volatiles, but they contain significant amount of terpenoid volatile precursors, which contribute to the aroma formation of made teas11. On the contrary, tea flowers have a great potential to be utilized because of their abundant functional molecules such as saponins, polysaccharides, aromatic compounds and functional proteins42 and emit substantial volatiles, including terpenoid compounds16. Therefore, terpenoid profiling in tea leaves and flowers combined with CsTPS transcriptomic analysis may reveal the association between CsTPS gene expression and their chemical products, which could be helpful for further CsTPS gene functional characterization. In the present study, the abundance of the internal volatile terpenoids and emitted volatile terpenoids (collectively from free and hydrolyzed glycosides) from tea leaves and flowers were analyzed by GC-MS. In total, seventeen monoterpenes and eleven sesquiterpene compounds were identified from tea leaves and flowers (Fig. 4A,B). The total abundance of all monoterpenes extracted from flowers was lower than that from leaves and almost all the monoterpenes detected from leaves were more than their counterparts in flowers, except for linalool, lavandulol and α-terpineol.
Figure 4.
The abundances of terpenoids in tea leaves and flowers. (A), internal monoterpenes; (B), internal sesquiterpenes; (C), emitted terpenoid compounds. Significant differences between leaves and flowers are indicated (**p ≤ 0.01, *p ≤ 0.05). All data are expressed as mean ± S.D. (n = 3). The compounds labeled with stars were identified and quantified with authentic standards and standard curves, respectively.
Linalool and its oxides were the most abundant among all the monoterpenoids, followed by geraniol, two accounting for 66% and 76% of the total monoterpenoid amounts in tea leaves and flowers, respectively (Fig. 4A). However, the total abundances of internal sesquiterpenoids and emitted terpenoids in flowers were higher than those in leaves (p < 0.01) (Fig. 4B,C). α-Farnesene was the most abundant sesquiterpene detected from flowers, contributing to approximately 70% of the total amount of sesquiterpenoids in tea flowers, while nerolidol is the most abundant sesquiterpene in leaves, contributing to 38% of total sesquiterpenoids abundance. CsTPS51-56, were annotated as putative α-farnesene synthase according to BLASTP results (Supplementary Table S2). Nevertheless, their transcription only found in leaves (Supplementary Fig. S2), indicated that abundant emission of α-farnesene in flower might be depended on other TPS genes, such as CsTPS28-32. Another possibility could not be excluded that the function of these CsTPS genes could be wrong. Furthermore, all sesquiterpenoids were mainly generated from flowers, rather than leaves, except for nerolidol (Fig. 4B). As above the gene expression patterns analysis, sesquiterpenoids were abundant in flowers than leaves mainly attributed to the higher accumulation of their transcripts in tea flowers.
On the contrary, compared to 17 and 11 sesqui- and mono-terpenoids respectively detected, reduced numbers of emitted monoterpenoids (11) and sesquiterpenoids (2) in addition to a diterpene (neophytadiene) were detected from leaves and flowers, probably attributing to some terpenoids that is not readily emitted or unstable (Fig. 4C). It is interesting to note that emitted linalool from homogenized flowers was significantly higher than that from homogenized leaves (p < 0.01) while the opposite was found for geraniol. Either organic extraction analysis or emitted terpenoids analysis, linalool and geraniol were no doubt as the most abundant monoterpenoids in tea plants. It was clear that CsTPS77-79 are responsible for the linalool biosynthesis. However, the key enzyme involved in geraniol biosynthesis in tea plants is still unknown.
TPS gene expression under abiotic stress conditions
Tea plants are often subjected to drought, salinity, cold and other stress conditions most likely they are generally grown in mountainous regions with shallow soils, thereby possibly affecting the production of terpenoids as responding to stress conditions7,22,33. Hence, transcriptome data were employed to investigate the expression alterations of tea TPS genes responding to the treatments of stress or stress signal molecule methyl jasmonate (MeJA).
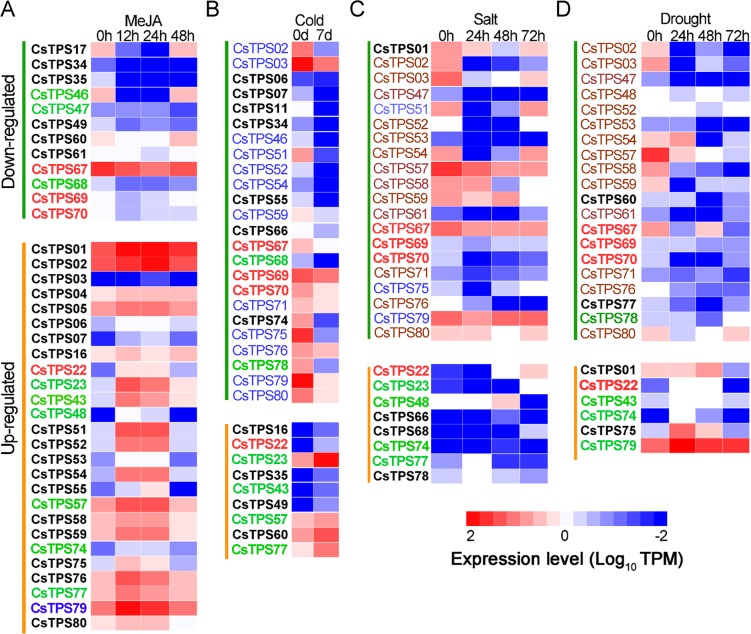
MeJA treatment differentially regulated CsTPS gene expression (Fig. 5). Compared to nontreated control, 12 hr after MeJA treatment, 12 CsTPS genes were suppressed in the range from 1.3- to 542.3-fold, the highest for CsTPS46 and the lowest for CsTPS61, with the average suppression of 59.0-fold (Fig. 5A). The suppression of gene expression was alleviated for 11 CsTPS genes 24 hr after the treatment, except for CsTPS17, whose expression was further suppressed from 45.0-fold (12 hr after the treatment) to 457.7-fold (24 hr after the treatment). The majority of the suppressed genes were recovered 48 hr after the treatment. On the contrary, 12 hr after the treatment 27 CsTPS genes were enhanced in the range from 1.2- to 94.2-fold, the highest for CsTPS48 and the lowest for CsTPS78, with the average enhancement of 13.2-fold (Fig. 5A). Time course study indicated enhanced expression of many CsTPS genes declined 24 hr after the treatment, except for CsTPS03, -07, -51, -52, -54, and -55, whose expression levels were further enhanced. Whereas the MeJA enhanced expression for almost all the CsTPS genes was recovered back to non-treated control levels 48 hr after the treatment.
Figure 5.
Transcriptomic responses of al the 80 CsTPS genes under different abiotic stress conditions. (A), Treated with 1 mM MeJA; (B), treated with cold acclimation (10 °C-4 °C for 7 d); (C) Treated with 200 mM NaCl; (D) treated with 25% PEG. Transcripts per million (TPM) were used to evaluate gene expression level. Genes in red and bold were either enhanced or suppressed across all the treatments; Genes in dark red were suppressed in both salt and PEG-induced drought treatments. Genes in green shared the same changes among two or three treatments either enhanced or suppressed.
Under cold acclimation (between 10 °C to 4 °C) for 7 days, more CsTPS genes (24) were suppressed than the number of enhanced genes (9) (Fig. 5B). compared to the non-acclimated controls, transcript suppression ranged from 1.4- to 32.3-fold with an average suppression 11.8-fold. The most severely suppressed was CsTPS78, followed by CsTPS11(28.4-fold) and CsTPS46 (26.4-fold). On the contrary, expression enhancement for another 9 CsTPS genes ranged from 1.3- to 37.8-fold with the average of 12.6-fold. The maximal enhancement occurred to CsTPS43, followed by CsTPS35 (23-fold) and CsTPS16 (19.5-fold) (Fig. 5B).
Salinity stress primarily led to varying expression reduction of 20 CsTPS genes in the range from 1.4- to 47.6-fold 24 hr after the treatment. The most severely suppression was found for CsTPS02, followed by CsTPS54 (43.6-fold) and CsTPS51(36.2-fold). Time-course study revealed that the suppression was alleviated as the time proceeded after the treatment. Eight CsTPS genes were slightly salt stress induced. The most dramatically enhanced was CsTPS22 (11.6-fold), followed by CsTPS68 (9.2-fold), all occurred 72 hr after salinity treatment (Fig. 5C).
Furthermore, PEG treatment for a certain period to simulate drought treatment41 also resulted in the suppression of 20 TPS genes in the range from 1.3- to 324.7-fold, with the average of 36.8-fold. The most dramatically suppressed gene expression occurred to CsTPS03, followed by CsTPS59 (151.2-fold) and CsTPS02 (142.9-fold). Six genes were PEG enhanced with the most notably enhancement occurred to CsTPS74 (48.3-fold), followed by CsTPS75 (17.9-fold) 24 hr after the treatment (Fig. 5D).
It was interesting to note that across all the four different treatments three genes (CsTPS67, -69, and -70 in red and bold) were all suppressed while CsTPS22 (in red and bold) was enhanced (Fig. 5). Moreover, out of 20 CsTPS genes suppressed by salinity, 17 were also suppressed by PEG-induced drought (in dark red) and 15 were suppressed by cold acclimation (in blue). In addition, some genes (in green) shared the same suppression or induction alteration among two or three different treatments, suggesting that some genes responded to the abiotic stresses in the similar ways.
Further validation of the transcriptomic analysis result was conducted using qRT-PCR approach with some CsTPS genes and GS-MS quantification of volatile terpenoid abundance in tea leaves treated with MeJA or salinity (Fig. 6). Our qRT-PCR results confirmed the salinity suppression and MeJA induction of CsTPS genes (CsTPS03, -43, -51, -57, -76, -77, and -79) (p < 0.05), well consistent with transcriptomic data (Fig. 6A). Moreover, salinity stress resulted in a significant abundance reduction in linalool and its three oxides and nerol (p < 0.05). Geraniol was slightly decreased (p > 0.05) due to salinity treatment. Nevertheless, an increase (p < 0.05) in linalool, nerol, geraniol, and nerolidol was induced by MeJA (Fig. 6B). Neophytadiene, a putatively identified diterpene volatile, was also increased by both MeJA and salinity treatments. It was interesting to note that the amounts of linalool, geraniol and nerolidol in leaves were increased by 192%, 318% and 232%, respectively (Fig. 6B).
Figure 6.
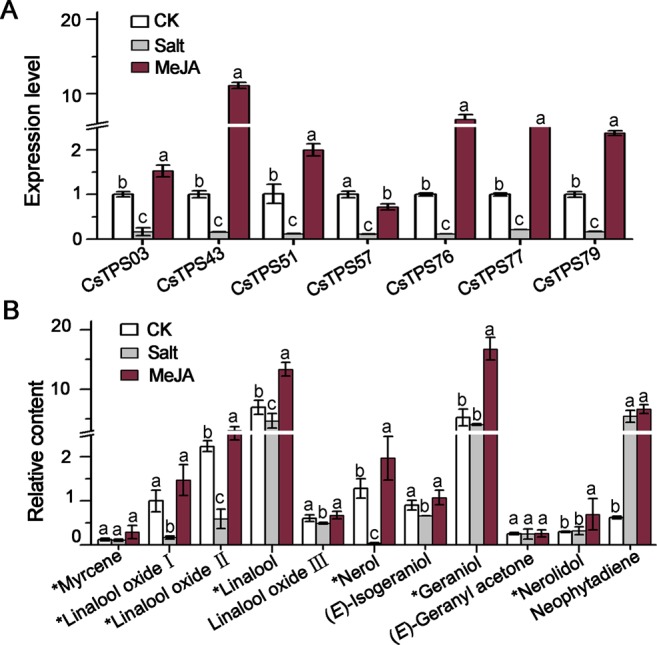
Expression pattern of TPS genes, and terpenoids production under different abiotic stresses. (A), gene expression patterns validated by qRT-PCR; (B), Stress induced changes in the abundance of emitted terpenoids. Means distinguished with different letters among non-treated control, MeJA and salt treatments are significantly different from each other (p < 0.05). All data are expressed as mean ± S. D. (n ≥ 3). The compounds labeled with stars were identified and quantified with authentic standards and standard curves, respectively.
Discussion
C. sinensis is an important economic crop widely grown in mountainous regions in South-Eastern Asian countries. Such a type of the geographic plantation often turns the tea plants under various and severe stress conditions such as drought, cold damage, herbivore attack. Tea plant volatile terpenoids play significant roles not only in resistance against stress conditions12,13, but also in tea beverage flavor formation11,14,15. Studies on tea plant TPS family genes and their responses to different stress conditions, are crucial for tea plant productivity and tea flavor improvement.
Plant TPS gene families are a medium sized group, although the numbers of TPS families from different plant species vary significantly24. For instance, in Arabidopsis 40 genomic AtTPS genes are found, but 32 are functional42; In grape (Vitis vinifera), there are 152 TPS-like genes and 62 are functional23; In tomato (Solanum lycopersicum) out of 44 TPSs, 29 are functional or potentially functional28. In apple, only 10 TPS genes out of 55 are functional25. In this study, different numbers of TPS genes were found in the recently released genomes of CSS “Shu-Cha-Zao” (80 genes) and CSA “Yunkang10” (60 genes), probably because of imperfect sequencing technology and genome assembly. Although the sequences of many TPS genes from both genomes were incomplete, similarity of orthologous TPS genes at nucleotide level between the two genomes was as high as 96%, slightly higher than 92%, the average similarity of orthologous genes at DNA levels between the two cultivars36, suggesting that the orthologous TPS genes likely had similar function in the two different cultivars, but with some distinct variations.
Among 80 CsTPS genes found in “ Shu-Cha-Zao” genome, three have been proved functional in previously published reports33,34,39 and 20 others were most likely functional due to their possession of expected full-length coding sequences and gene structures based on genome assemblies or/and full-length transcriptomic data. In addition to the three functional TPS genes, five, eleven and two were annotated as mono-, sesqui-, di-terpene synthase genes. The same function annotation of multiple TPS genes in the public databases was noted, probably because the functions of some genes were obtained from in vitro assays only. For examples, some terpene synthases were found bifunctional in vitro to produce both linalool and nerolidol, but actually monofunctional in planta43,44. It has been indicated in many cases that the function obtained in vitro are different from actual function in planta33.
For the remaining 57 CsTPS genes, their functions were uncertain due to their incomplete sequences or untypical gene structures obtained from the currently available genome databases, which requires improvement in its sequence precision. Full sequences of these genes should be obtained for their functional validation in vitro and in plants. Additionally, the possibility also could not be excluded that low number of predicted functional tea TPS genes perhaps attributes to gene duplication and simultaneous generation of many meaningless sequences in genome occurred over tea plant evolution36. Transposable elements (TEs), are the chief mechanistic drivers of genome evolution, representing at least 64% of the assembly (excluding undefined base Ns)36. It was found that 19 of tea TPS genes containing one or more transposable elements (TEs) within intron (Supplementary Table S5), likely leading to neofunctionalization of tea TPS genes. Further functional characterization is required to reveal their functions specifically in tea plants. In addition, transcriptomic data applied in this study from different groups indicated that CsTPS13, -27, -33, and -65 genes had undetectable transcript levels in all different tea organs nor under all the four tested stress treatments (Supplementary Fig S2 and S3), possibly suggesting that they might be silenced genes.
In the present study, tempo-spatial expression patterns of CsTPS genes was noted and validated using qPCR approach. Interestingly, CsTPS genes with high transcript levels in both flowers and leaves were all sesquiterpene synthase genes. It was noted that 17 sesquiterpene synthase genes were higher expressed in flowers than in leaves while limited monoterpene synthase genes maintained substantial transcript levels in tea leaves or any other tested organs, suggesting a strict regulation of terpenoid production. Moreover, transcriptomic data indicated that distinct tempo-spatial expression patterns of CsTPS genes could be significantly affected when the plants were subjected to different stress conditions, which were confirmed with qPCR data and volatile profiling results in this study. Many CsTPS genes such as CsTPS23, -25, -43, -51, -52, and -76 were significantly induced by MeJA treatment whereas the majority of tea TPS genes were suppressed by salinity, drought, and coldness. CsTPS76 is known being MeJA induced33 due to the presence of G-boxes in its promoter, which can interacts with MYC2 transcription factor of JA signalling pathway45 to deal with many stress conditions46. It is assumed that many of those MeJA induced CsTPS genes might contain cis-elements able to interact with JA signalling pathway. Our leaf chemical profiling data revealed a significant MeJA enhancement of multiple monoterpenoid volatiles such as linalool, geraniol and their derivatives as well as sesquiterpenoid nerolidol in tea leaves. Reduced production of many of the above-mentioned compounds in the leaves subjected to salinity were also noted. These chemical profiling results were well consistent with the transcription quantification data. Although many TPS genes, including those encoding mono- and sesqui-terpenoid synthases, were MeJA enhanced or salt suppressed, significant abundance changes were mainly detected in very limited numbers of the monoterpenoid compounds such as linalool, geraniol and their derivatives, rather than sesquiterpenoids except nerolidol. These were probably because of the following reasons: 1) detected terpenoids such as linalool and geraniol are likely abundant in fresh tea leaves15; 2) preference of SPME volatile collection to those small terpenoid molecules with high volatility. Moreover, it was noted that three CsTPS genes (-67, -69 and -70) were suppressed by all the four treatments and many more were suppressed by both salinity and PEG-induced drought, even by cold treatments. It is known that TPS genes are significantly involved in the resistance mechanism of plants under different abiotic stresses, thereby regulating terpenoid metabolism47,48. In addition, many stress conditions can activate the same signalling pathways in plants such as “reactive oxygen species”, “Mitogen-Activated Protein Kinase (MAPK) cascades”, “ABA/JA/Ethylene signalling pathways”49.
Previous study indicates that tea leaves contain abundant and diverse monoterpenoids but emit none of them unless damaged, while tea flowers possess sesquiterpenes and emit both sesquiterpene and monoterpenes33. In this study, significantly more abundant sesquiterpenoids were detected in flowers than in leaves and a quite number of monoterpenoid volatiles were detected from leaves, indicating distinct patterns of volatile terpenoid production and emission in tea plants, well consistent with distinct CsTPS expression patterns between leaves and flowers. This is also in agreement with the findings obtained in Arabidopsis vegetative and reproductive organs3,50. In this study, the finding that all the monoterpenes in leaves were more than their counterparts in flowers with a few exceptions was consistent with the high transcript levels of some monoterpene synthases in leaves, such as CsTPS37-50 and CsTPS 60-66 (Supplementary Fig. S2). Although a few monoterpene synthase genes maintained substantial transcript levels in tea leaves, diverse monoterpenoid volatiles were detected in tea leaves. This is not surprising because a terpenoid synthase usually can catalyze a single molecule into different products18,50. In this study, both leaves and flowers can release many volatile terpenoids, particularly linalool and geraniol, after tissue homogenization. This is partly because these compounds were present as internal free form, but also because they are accumulated as glycosidically bound forms11. Ohgami et al.19 demonstrated that geraniol and other volatiles produced in tea leaves can be sequentially glycosylated into β-primeverosides by two glycosyltransferases so that they can be stored in leaf tissues. For a better understanding the mechanisms of tea terpenoid production in tempo-spatial patterns, more efforts should be made to combine the studies with CsTPS gene expression manipulation with those on terpenoid profiling.
Materials and Methods
Plant materials and stress treatments
Six-year old plants of C. sinensis var. sinensis (CSS) cv. “Shu-Cha-Zao” grown at the experimental farm of Anhui Agricultural University in Hefei, China were used in this study. Leaves and flowers at different developmental stages were excised for gene expression and terpenoid analysis. For salinity and methyl jasmonate (MeJA) treatments, 12 one-year old potted plants of the same cultivar were placed in the green house (at 26 °C/22 °C, day/night and a 16 h photoperiod at 100 μmol photons m−2 s−1). For the salinity treatment, the same potted plants (12) were irrigated each with a 150 mM NaCl solution until it dripped out from the pot bottom and then the plants were left un-watered for the next 5 days. The control plants were irrigated in same way using tap water. Then leaves from the salt treatment were collected over a 72 h period and immediately frozen in liquid nitrogen and kept in −80 °C for future use. For the MeJA treatment, plants were sprayed with 1 mM MeJA in 0.05% DMSO until the solution dripped off the sprayed leaves. Control plants were sprayed with the same solution without MeJA. Leaves were collected up to 48 hours after the MeJA treatment, and then frozen immediately in liquid nitrogen and stored at −80 °C for further study.
Identification of tea TPS family members
The recently released tea genome assemblies of C. sinensis var. assamica (CSA) cv. “Yunkang10” and C. sinensis var. sinensis (CSS) cv. “Shu-Cha-Zao”35,36 were used in this study. Full length transcriptomic databases and RNA-seq datasets were retrieved from the Tea Plant Information Archive (http://tpia.teaplant.org/)37,38. PF01397 and PF03936 domain data which represent respectively the N-terminal and C-terminal domains of TPS from the Pfam database (http://pfam.xfam.org/)51 were used to identify the members of the tea TPS gene family. Manual annotation was also conducted based on the results of a BlastP against TPS genes from the GenBank of The National Center for Biotechnology Information (www.ncbi.nlm.nih.gov) and Swiss-Prot (www.uniprot.org/blast/).
Phylogenetic analysis
Multiple sequence alignments of TPS protein sequences in tea and their homologues from other plant species were conducted using ClustalX in MEGA 5.0 using default sets. The alignment was conducted using 70 TPS proteins, comprising 48 CsTPS and 43 documented TPS from different plant species (the remaining CsTPS genes were too short or did not possess any common sites for meaningful alignment). The obtained alignment was used as the input for the maximum likelihood algorithm in MEGA5.0 software to construct phylogenetic trees. Subfamilies are divided based on cluster analysis52.
Gene expression analysis
For gene expression analysis, transcriptomic data and their validation using quantitative real time polymerase chain reaction (qRT-PCR) were performed. In order to characterize tempo-spatial gene expression patterns in the tea plant, transcriptomic data from apical buds (tightly folded young leaves), young leaves (the first or second unfolded leaves of growing shoots), mature leaves (the fourth leaf with dark green color), old leaves (the leaves at shoot base), immature and unlignified stems, flowers, young fruits and tender roots and of tea plant under diverse biotic and abiotic stresses were retrieved from the Tea Plant Information Archive (TPIA) database37. An average of 11.8 Gb of clean RNA-seq data were generated electronically for each of the eight tissue types from published data37. For the treatments of salinity (200 mM NaCl) and drought (induced by 25% PEG, Polyethylene glycol), equal amounts of total RNA extracted from tea shoot tips collected at each time interval (0 h, 24 h, 48 h, 72 h) after stress or non-stressed treatment were pooled together for transcriptome analysis53. To study the effects of cold acclimation (10 °C ~ 4 °C for 7 days) and non-acclimation (25~20 °C, CK) on tea plant TPS gene expression, data were retrieved from TPIA database37. For MeJA treatments, publicized RNA-seq data resulted from shoot-tips (including folded and the first two unfolded young leaves) excised at four different time intervals (0, 12, 24, 28 hr) from 2000 tea plants after evenly sprayed with 1.1 mM MeJA solution54. Transcripts per million (TPM) of RNA molecules were used to evaluate expression level.
For qRT-PCR validation of the transcriptomic analysis results, total RNA was extracted from leaf and flower samples either at different developmental stages, or from the potted plants treated with NaCl or MeJA in this study using the RNA prep pure Plant Kit (TianGen Biotech., Ltd, Beijing, China). cDNA was synthesized using a PrimeScriptTM RT reagent kit with gDNA Eraser (TaKaRa, Tokyo, Japan). Quantification of tea TPS genes, which are most likely functional, was performed using gene specific primers (Supplementary Table S1) and the 18srRNA as an internal reference. qRT-PCR was performed using the BioRad CFX96 real-time PCR system as applied before33. The relative expression level of tea TPS genes was calculated using the 2−ΔΔCT method55. Each quantification had three biological replicates.
Collection of terpenoid volatiles
Organic solvent extractions were used to collect the internal volatiles that were not readily emitted but were stored in the tea samples according to Zeng et al.56. For extraction of terpenoid compounds in tea leaves or flowers, plant samples were homogenized in liquid nitrogen, and then a mixed pentane/ethanol solvent (1:1, v/v) was applied to the homogenate, followed by centrifugation at 10000 g for 10 min. One μl of 0.1% ethyl decanoate was added into the supernatant as an internal standard. Liquid samples were analyzed by gas chromatograph-mass spectroscopy (GC-MS) according to Liu et al.33. The solid phase microextraction (SPME) method was used to collect the released volatiles in the headspace of homogenized plant samples according to a previous report56. Collected volatiles were resulted from the release of free and glycosidically bound volatiles after hydrolysis. Tea samples collected from leaves and flowers and different abiotic stress treatments were freeze-dried under vacuum at −58 °C. The dried sample (0.2 g) was then placed into a glass sampling vial (20 mL) with the addition of 5 mL boiled water, then volatiles were collected using a solid phase microextraction (SPME) fiber (65 μm PDMS/DVB, Sigma-Aldrich, Shanghai, China) after placement in a 70 °C water-bath for 30 min. Ethyl decanoate (0.01%) was added to the samples as the internal standard. All of the volatile compounds absorbed onto the SPME fiber were desorbed in the GC-MS injector at 250 °C for 5 min and then immediately analyzed by GC-MS.
GC-MS analysis
To identify the volatile compounds, a gas chromatograph (Agilent 7697 A) and as mass spectrometer (Agilent 7890 A) outfitted with a DB-5 capillary column (30 m × 0.25 mm × 0.25μm; Agilent) were used in this study. Helium was used as the carrier gas. The initial GC oven temperature was at 50 °C for 1 min and increased to 100 °C at a rate of 10 °C/min (held for 1 min); then ramped up to 200 °C at a rate of 4 °C/min (held for 1 min), and then increased to 280 °C at 16 °C/min (held for 7 min). Chemicals were identified by comparing the retention time and mass spectrum either with those of authentic standards or the NIST database. Compounds were quantified based on calibration curves established using a series of diluted solutions prepared with authentic standards15 or based on the peak areas of the internal standard as relative content.
Statistical analysis
The SPSS statistical package (Version 19.0) was used to conduct one-way analysis of variance (ANOVA) among different samples over three replications. A Least-significant difference (LSD) test and Bonferroni test were applied to determine the significance of differences (p < 0.05, p < 0.01).
Supplementary information
Acknowledgements
We thank Drs Yan Hou, and Qiong Wu of the Anhui Agricultural University for academic advice. Our lab mates Dong-Wei Zhao and Meng-Xian Zhang offered their technical support to this study. The National Natural Science Foundation of China (Grant Number 31370687) to Shu Wei financially supported this work.
Author contributions
H.C.Z. conceived this study and conducted the experiments, analyzed data together with L.F.S., who also prepared the draft manuscript; X.K.Y. and Z.Y. helped to do the experiments and data analysis. S.W. provided critical suggestions and finalized the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Han-Chen Zhou and Lubobi Ferdinand Shamala.
Supplementary information
is available for this paper at 10.1038/s41598-020-57805-1.
References
- 1.Mosadegh H, et al. Applications of UV-B lighting to enhance phenolic accumulation of sweet basil. Sci. Hortic. (Amsterdam). 2018;229:107–116. doi: 10.1016/j.scienta.2017.10.043. [DOI] [Google Scholar]
- 2.Besser K, et al. Divergent egulation of terpenoid metabolism in the trichomes of wild and cultivated tomato species. Plant Physiol. 2009;149:499–514. doi: 10.1104/pp.108.126276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen F, et al. Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell. 2003;15:481–494. doi: 10.1105/tpc.007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee S, et al. Herbivore-induced and floral homoterpene volatiles are biosynthesized by a single P450 enzyme (CYP82G1) in Arabidopsis. Proc. Natl. Acad. Sci. 2010;107:21205–21210. doi: 10.1073/pnas.1009975107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li R, et al. PLANT IMMUNITY A terpenoid phytoalexin plays a role in basal defense of Nicotiana benthamiana. Sci. Rep. 2015;5:9682. doi: 10.1038/srep09682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwiczuk A., Skalicka-Woźniak K., Georgiev M.I. Pharmacognosy. 2017. Terpenoids; pp. 233–266. [Google Scholar]
- 7.Nagegowda DA. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010;584:2965–2973. doi: 10.1016/j.febslet.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 8.Schnee C, Ko TG, Gershenzon J. The maize gene terpene synthase 1 Encodes a sesquiterpene synthase catalyzing the formation of (E)-beta-farnesene, (E)-nerolidol, and (E,E)-farnesol after herbivore damage. Plant physiol. 2002;130:2049–2060. doi: 10.1104/pp.008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Davidovich-rikanati R, et al. Enrichment of tomato flavor by diversion of the early plastidial terpenoid pathway. Nat. Biotechnol. 2007;25:899–901. doi: 10.1038/nbt1312. [DOI] [PubMed] [Google Scholar]
- 11.Ho C, Zheng X, Li S. Tea aroma formation. Food Sci. Hum. Wellness. 2015;4:9–27. doi: 10.1016/j.fshw.2015.04.001. [DOI] [Google Scholar]
- 12.Dong F, et al. Herbivore-induced volatiles from tea (Camellia sinensis) plants and their involvement in intraplant communication and changes in endogenous nonvolatile metabolites. J. Agric. Food Chem. 2011;59:13131–13135. doi: 10.1021/jf203396a. [DOI] [PubMed] [Google Scholar]
- 13.Fu X, et al. Regulation of formation of volatile compounds of tea (Camellia sinensis) leaves by single light wavelength. Sci. Rep. 2015;5:16858. doi: 10.1038/srep16858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho J, et al. Chemical profiling and pene pxpression profiling during the panufacturing process of Taiwan Oolong tea “ Oriental Beauty”. Biosci. Biotechnol. Biochem. 2007;71:1476–1486. doi: 10.1271/bbb.60708. [DOI] [PubMed] [Google Scholar]
- 15.Han ZX, et al. Green tea flavour determinants and their changes over manufacturing processes. Food Chem. 2016;212:739–748. doi: 10.1016/j.foodchem.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 16.Joshi R, Gulati A. Biochemical attributes of tea flowers (Camellia sinensis) at different developmental stages in the Kangra region of India. Sci. Hortic. (Amsterdam). 2011;130:266–274. doi: 10.1016/j.scienta.2011.06.007. [DOI] [Google Scholar]
- 17.Yang Z, Baldermann S, Watanabe N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013;53:585–599. doi: 10.1016/j.foodres.2013.02.011. [DOI] [Google Scholar]
- 18.Degenhardt J, Köllner TG, Gershenzon J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry. 2009;70:1621–1637. doi: 10.1016/j.phytochem.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 19.Ohgami S, et al. Volatile glycosylation in tea plants: sequential glycosylations for the biosynthesis of aroma b-primeverosides are catalyzed by two Camellia sinensis glycosyltransferases. Plant Physiol. 2015;168:464–477. doi: 10.1104/pp.15.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bönisch F, et al. A UDP-glucose:monoterpenol glucosyltransferase adds to the chemical diversity of the grapevine metabolome. Plant Physiol. 2014;165:561–581. doi: 10.1104/pp.113.232470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caillieaudeaux R, et al. CYP76C1 (Cytochrome P450)-mediated linalool metabolism and the formation of volatile and soluble linalool oxides in Arabidopsis flowers: A strategy for defense against floral antagonists. Plant Cell. 2015;1:1–20. doi: 10.1105/tpc.15.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tholl D, Lee S. Terpene specialized metabolism in Arabidopsis thaliana. Arab. B. 2012;9:e0143. doi: 10.1199/tab.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin DM, et al. Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biol. 2010;10:226. doi: 10.1186/1471-2229-10-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen F, Tholl D, Bohlmann J, Pichersky E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011;66:212–229. doi: 10.1111/j.1365-313X.2011.04520.x. [DOI] [PubMed] [Google Scholar]
- 25.Nieuwenhuizen NJ, et al. Functional genomics reveals that a compact terpene synthase gene family can account for terpene volatile production in apple. Plant Physiol. 2013;161:787–804. doi: 10.1104/pp.112.208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bohlmann J, Meyer-Gauen G, Croteau R. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc. Natl. Acad. Sci. 1998;95:4126–33. doi: 10.1073/pnas.95.8.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paterson AH, et al. The Sorghum bicolor genome and the diversification of grasses. Nature. 2009;457:551–556. doi: 10.1038/nature07723. [DOI] [PubMed] [Google Scholar]
- 28.Falara V, et al. The tomato terpene synthase gene family. Plant Physiol. 2011;157:770–789. doi: 10.1104/pp.111.179648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irmisch S, Jiang Y, Chen F, Gershenzon J, Köllner TG. Terpene synthases and their contribution to herbivore-induced volatile emission in western balsam poplar (Populus trichocarpa) BMC Plant Biol. 2014;14:1–16. doi: 10.1186/s12870-014-0270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foley, W. J. et al. The Eucalyptus terpene synthase gene family. BMC Genomics16 (2015). [DOI] [PMC free article] [PubMed]
- 31.Keilwagen J, et al. The terpene synthase gene family of carrot (Daucus carota L.): identification of QTLs and candidate genes associated with terpenoid volatile compounds. Front. Plant Sci. 2017;8:1–18. doi: 10.3389/fpls.2017.01930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamanishi, T. Tea chemistry, aroma precursors, and flavour. in Global Advances in Tea Science (ed. Jain, N. K.) (Aravali Books International, 1999).
- 33.Liu GF, et al. Implementation of CsLIS/NES in linalool biosynthesis involves transcript splicing regulation in Camellia sinensis. Plant Cell Environ. 2018;41:176–186. doi: 10.1111/pce.13080. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, et al. Formation of (E)-nerolidol in tea (Camellia sinensis) leaves exposed to multiple stresses during tea manufacturing. Food Chem. 2017;231:78–86. doi: 10.1016/j.foodchem.2017.03.122. [DOI] [PubMed] [Google Scholar]
- 35.Xia EH, et al. The tea tree genome provides insights into tea flavor and independent evolution of caffeine biosynthesis. Melcular Plant. 2017;10:866–877. doi: 10.1016/j.molp.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Wei, C. et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc. Natl. Acad. Sci. 115, E4151–E4158 (2018). [DOI] [PMC free article] [PubMed]
- 37.Xia En‐Hua, Li Fang‐Dong, Tong Wei, Li Peng‐Hui, Wu Qiong, Zhao Hui‐Juan, Ge Ruo‐Heng, Li Ruo‐Pei, Li Ye‐Yun, Zhang Zheng‐Zhu, Wei Chao‐Ling, Wan Xiao‐Chun. Tea Plant Information Archive: a comprehensive genomics and bioinformatics platform for tea plant. Plant Biotechnology Journal. 2019;17(10):1938–1953. doi: 10.1111/pbi.13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiao D, et al. Comprehensive identification of the full-length transcripts and alternative splicing related to the secondary metabolism pathways in the tea plant (Camellia sinensis) Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Q, et al. Unraveling a crosstalk regulatory network of temporal aroma accumulation in tea plant (Camellia sinensis) leaves by integration of metabolomics and transcriptomics. Environ. Exp. Bot. 2018;149:81–94. doi: 10.1016/j.envexpbot.2018.02.005. [DOI] [Google Scholar]
- 40.Rynkiewicz MJ, Cane DE, Christianson DW. Structure of trichodiene synthase from Fusarium sporotrichioides provides mechanistic inferences on the terpene cyclization cascade. Proc. Natl. Acad. Sci. 2002;98:13543–13548. doi: 10.1073/pnas.231313098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whittington DA, et al. Bornyl diphosphate synthase: structure and strategy for carbocation manipulation by a terpenoid cyclase. Proc. Natl. Acad. Sci. 2002;99:15375–80. doi: 10.1073/pnas.232591099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, et al. Occurrence of functional molecules in the flowers of tea (Camellia sinensis) plants: Evidence for a second resource. Molecules. 2018;23:1–16. doi: 10.3390/molecules23040790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green SA, et al. Identification, functional characterization, and regulation of the enzyme responsible for floral (E)-nerolidol biosynthesis in kiwifruit (Actinidia chinensis) J. Exp. Bot. 2012;63:1951–1967. doi: 10.1093/jxb/err393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagegowda DA, et al. Two nearly identical terpene synthases catalyze the formation of nerolidol and linalool in snapdragon flowers. Plant J. 2008;55:224–239. doi: 10.1111/j.1365-313X.2008.03496.x. [DOI] [PubMed] [Google Scholar]
- 45.Hong G, Xue X, Mao Y, Wang L, Chen X. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell. 2012;24:2635–2648. doi: 10.1105/tpc.112.098749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spoel SH, Dong X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe. 2008;3:348–351. doi: 10.1016/j.chom.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Formentin E, et al. Transcriptome and cell physiological analyses in different rice cultivars provide new insights into adaptive and salinity stress responses. Front. Plant Sci. 2018;9:1–17. doi: 10.3389/fpls.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng L, Watanabe N, Yang Z. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. Nutr. 2018;0:1–14. doi: 10.1080/10408398.2018.1506907. [DOI] [PubMed] [Google Scholar]
- 49.Rejeb I, Pastor V, Mauch-Mani B. Plant responses to simultaneous biotic and abiotic stress: molecular mechanisms. Plants. 2014;3:458–475. doi: 10.3390/plants3040458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tholl D, et al. Two sesquiterpene synthases are responsible for the complex mixture of sesquiterpenes emitted from Arabidopsis flowers. Plant J. 2005;42:757–771. doi: 10.1111/j.1365-313X.2005.02417.x. [DOI] [PubMed] [Google Scholar]
- 51.Finn RD, et al. Pfam:clans,web tools and services. Nucleic Acids Res. 2006;34:D247–D251. doi: 10.1093/nar/gkj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aubourg S, Lecharny A, Bohlmann J. Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol. Genet. Genomics. 2002;267:730–745. doi: 10.1007/s00438-002-0709-y. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Q, Cai M, Yu X, Wang L, Guo C. Transcriptome dynamics of Camellia sinensis in response to continuous salinity and drought stress. Tree Genet. Genomes. 2017;13:78. doi: 10.1007/s11295-017-1161-9. [DOI] [Google Scholar]
- 54.Shi J, et al. Transcriptional responses and flavor volatiles biosynthesis in methyl jasmonate-treated tea leaves. BMC Plant Biol. 2015;15:233. doi: 10.1186/s12870-015-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat. Methods. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 56.Zeng L, et al. Does oolong tea (Camellia sinensis) made from a combination of leaf and stem smell more aromatic than leaf-only tea? Contribution of the stem to oolong tea aroma. Food Chem. 2017;237:488–498. doi: 10.1016/j.foodchem.2017.05.137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.