See article vol. 27: 47–59
Gender Differences in Atherosclerotic Cardiovascular Disease (ASCVD)
There are substantial sex/gender differences in the prevalence and burden of ASCVD1). George et al. evaluated the age and sex distribution of 60,155 incidences in men and 54,704 in women representing the initial presentation of a wide range of 12 major cardiovascular diseases, showing that myocardial infarction, but not unstable and stable angina, predominantly developed in men aged 30–39, 40–49, 50–59, and 60–69 years1). The prevalence of traditional ASCVD risk factors and their differential impact in men and women, as well as the emerging and nontraditional risk factors, may contribute to the gender differences in ASCVD incidence2). However, understanding the mechanisms leading to different outcomes in men and women remains to be elucidated. Given the evidence that experimental studies consistently demonstrate that estrogen has beneficial physiological effects on the vascular endothelium at cellular and molecular levels, it is imperative to clarify the precise mechanism of how the depletion of estrogen causes vascular derangement in women after menopause3).
Vascular Senescence, SIRT1, and ASCVD
Aging-induced functional and structural changes of vasculatures contribute to the pathogenesis of a wide range of age-related diseases, including ASCVD. Cellular senescence is a fundamental aging process wherein cells, including vascular endothelial and smooth muscle cells (vascular senescence4)), permanently withdraw from the cell cycle in response to a range of endogenous and exogenous stressors and undergo distinctive phenotypic alterations, including profound pro-inflammatory secretome changes5).
Activation of SIRT1, a homolog of the yeast lifespan regulator Sir2, was reported to prolong the lifespan of rodents, whereas overexpression of SIRT1 in vascular smooth muscle (VSMC) or vascular endothelial cells was reported to suppress senescence and extend the survival of these cells6). It has been suggested that SIRT1 is protective for vascular senescence through the activation of endothelial nitric oxide synthase (eNOS), inhibition of reactive oxygen species (ROS), inflammation, and DNA damage7). Donato et al. indicated that reductions in SIRT-1 and eNOS activities play essential roles in vascular endothelial dysfunction with aging in both mice and human8).
Underlying Mechanisms of Gender Difference in ASCVD: Estrogen, SIRT1, and eNOS
Although protective roles of SIRT1 against vascular senescence and atherosclerosis have been reported, gender differences in the roles of SIRT1 in these conditions remain unknown. In this issue, Sasaki et al. elegantly indicated the roles of estrogen in SIRT1-associated vascular senescence and atherosclerosis9). First, the authors demonstrated that vascular senescence and the formation of atherosclerotic plaque were facilitated in ovariectomized ApoE knockout (ApoE-KO) mice, concomitantly with a decrease in vascular SIRT1 expression. Second, administration of 17β-estradiol (E2) restored SIRT1 expression and vascular senescence as well as development of atherosclerosis in the mice, which was cancelled by a specific SIRT1 and SIRT2 inhibitor (sirtinols). Third, selective estrogen receptor modulator (SERM)/estrogen receptor agonist-antagonist (ERAA) mimicked the beneficial effects of E2 by inducing anti-senescence and anti-atherosclerosis through SIRT1 activation. Collectively, the authors concluded that SIRT1 plays a crucial role in estrogen in protecting arteries from senescence and atherosclerosis.
Among the seven members of the mammal sirtuin family, from SIRT1 to SIRT7, each has different tissue specificity, localization of subcellular activity, and target molecules6). It is well known that Sirt1 activates eNOS and genetically engineered mouse models demonstrated that Sirt1 exerts atheroprotective effects by activating eNOS or by diminishing NFkB activity in endothelial cells and macrophages6). Sirt3, as well as Sirt4 and Sirt5, is located in the mitochondria, and the protective effects of caloric restriction on cellular senescence depend on the presence of Sirt3, which increases the ratio of glutathione to glutathione disulfide. Mitochondrial Sirt3 drives the TCA (tricarboxylic acid) cycle, ß-oxidation, and oxidative phosphorylation, thus maintaining metabolic homeostasis and preventing the development of risk factors associated with the metabolic syndrome. Moreover, Sirt3 also carries out deacetylation and consecutive activation of superoxide dismutase 2 (SOD), mediating antioxidative protection and improving endothelial dysfunction.
Sasaki et al. found that administration of 17β-estradiol (E2) or SERM/ERAA restored SIRT1 expression but did not change SIRT3 expression in their protective role for vascular senescence and development of atherosclerosis. Therefore, it is considered that the actions of 17β-estradiol (E2) or SERM/ERAA can be exerted primarily by interactions of eNOS and SIRT1. Estrogens employ their physiological effects by binding with three estrogen receptors, ERα, ERβ, and an orphan G-protein-coupled estrogen receptor, which differentially modulate gene expression and the protein levels in a tissue-specific manner3). It has been reported that ERα expression modulated by estrogen in endothelial cells is linked to eNOS activation (phosphorylated-eNOS-Ser1177)3).
SERMs/ERAAs have been used in the prevention and treatment of postmenopausal bone fractures related to osteoporosis or to breast cancer10). SERMs/ERAAs include antagonist (tamoxifen) and agonists (raloxifene, ospemifene, arzoxifene, lasofoxifene, toremifene, and conjugated estrogen/bazedoxifene)10). Sasaki et al. found that conjugated estrogen/bazedoxifene activates SIRT1 and prevents atherosclerotic plaque formation. Bazedoxifene is one of new third-generation SERMs/ERAAs and prevents bone loss in postmenopausal women at risk of osteoporosis through binding to both Erα and ERβ. It is suggested that SERMs/ERAAs have potential as alternative therapeutic agents for atherosclerosis. Future experimental studies and clinical evidence conducted to show links among estrogen (SERM/ERAA), SIRT1, and eNOS may give us a new preventive tool for ASCVD. Possible underlying mechanisms of gender differences in ASCVD including current discussion in estrogen, SIRT1, and eNOS are shown in Fig. 1.
Fig. 1.
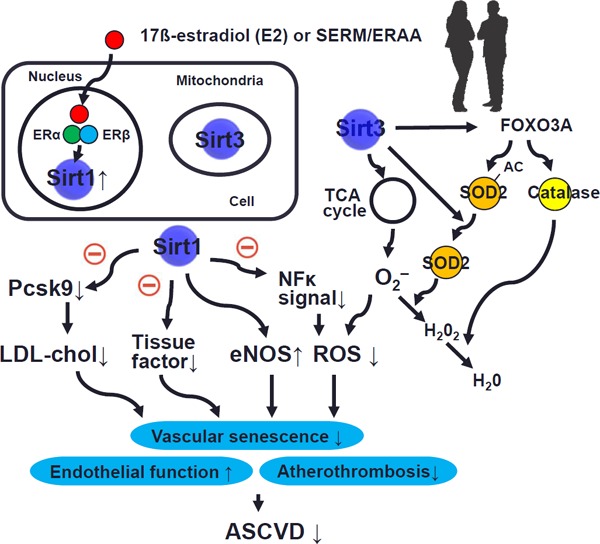
Possible underlying mechanisms of gender difference in ASCVD: estrogen, SIRT1, and eNOS
ASCVD: atherosclerotic cardiovascular disease; Sir1: sirtuin 1; Sir3: sirtuin 3; SERM: selective estrogen receptor modulator; ERAA: estrogen receptor agonist-antagonists; ERα: estrogen receptor α; ERβ: estrogen receptor β; Pcsk9: proprotein convertase subtilisin/kexin family 9; eNOS: endothelial nitric oxide synthase; ROS: reactive oxygen species; SOD: superoxide dismutase.
Conflicts of Interest
None.
References
- 1). George J, Rapsomaniki E, Pujades-Rodriguez M, Shah AD, Denaxas S, Herrett E, Smeeth L, Timmis A, Hemingway H: How does cardiovascular disease first present in women and men? Incidence of 12 cardiovascular diseases in a contemporary cohort of 1,937,360 people. Circulation, 2015; 132: 1320-1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE: Cardiovascular disease in women: Clinical per spectives. Circ Res, 2016; 118: 1273-1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Pabbidi MR, Kuppusamy M, Didion SP, Sanapureddy P, Reed JT, Sontakke SP: Sex differences in the vascular function and related mechanisms: Role of 17beta-estradiol. Am J Physiol Heart Circ Physiol, 2018; 315: H1499-h1518 [DOI] [PubMed] [Google Scholar]
- 4). Katsuumi G, Shimizu I, Yoshida Y, Minamino T: Vascular senescence in cardiovascular and metabolic diseases. Front Cardiovasc Med, 2018; 5: 18-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A: Mechanisms of vascular aging. Circ Res, 2018; 123: 849-867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Winnik S, Auwerx J, Sinclair DA, Matter CM: Protective effects of sirtuins in cardiovascular diseases: From bench to bedside. Eur Heart J, 2015; 36: 3404-3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer GRY: Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res, 2018; 114: 622-634 [DOI] [PubMed] [Google Scholar]
- 8). Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR: Sirt-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol, 2011; 589: 4545-4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Sasaki Y, Ikeda Y, Miyauchi T, Uchikado Y, Akasaki Y, Ohishi M: Estrogen-sirt1 axis plays a pivotal role in protecting arteries against menopause-induced senescence and atherosclerosis. J Atheroscler Thromb, 2020; 27: 47-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Hirsch HD, Shih E, Thacker HL: Eraas for menopause treatment: Welcome the ‘designer estrogens’. Cleve Clin J Med, 2017; 84: 463-470 [DOI] [PubMed] [Google Scholar]


