Abstract
Aim: Lifetime risk (LTR) is a measure of disease burden, which presents the probability of occurrence of a specific disease in the remaining lifetime of a group of people for a given index age. This measure is useful for presenting the risk dynamics of a disease at the population level, which constitutes important public health information toward prevention. To date, there have been no studies investigating the LTR for coronary heart diseases (CHDs) in relation to hypercholesterolemia in Asian populations. Therefore, we estimated the LTR of CHDs according to serum low-density lipoprotein cholesterol (LDL-C).
Methods: The participants included in this study were 2,559 men and 2,848 women, enrolled in the Suita Cohort Study of urban residents followed up from 1989 to 2007 for a total of 69,823 person-years. We estimated the sex- and index-age-specific LTR for the first CHD event among participants with or without hypercholesterolemia (LDL-C ≥ 160 mg/dL), accounting for the competing risk for mortality.
Results: For men with hypercholesterolemia, the LTR was 47.2% (95% confidence interval [CI]: 29.3–65.1%) and 44.5% (95% CI: 21.4–68.5%) for those aged 45 and 75, respectively. The LTRs of women with hypercholesterolemia were also higher than of those without hypercholesterolemia. However, their LTRs were lower for all index ages compared to men. These results did not differ for hypercholesterolemia defined by non-high-density lipoprotein cholesterol.
Conclusions: The presence of hypercholesterolemia increases the LTR for CHDs in the Japanese population, especially in men. This estimate can be used in preventive knowledge translation efforts at the population level.
Keywords: Lifetime risk, Coronary heart diseases, Hypercholesterolemia, Low-density lipoprotein cholesterol, Non-high-density lipoprotein cholesterol
See editorial vol. 27: 11–12
Introduction
The incidence of and mortality resulting from coronary heart diseases (CHDs) in Japan are lower than those reported in western countries1–4). However, owing to changes in the lifestyles and worsening of the cardiovascular risk factors, including hypercholesterolemia, in several studies, an increasing trend of CHDs has been reported in Japan5–8). Additionally, the burden of CHDs is expected to be much larger because of the increasing aging population in Japan9). Therefore, prevention of CHDs in Japan is imperative for public health programs. One of the major modifiable risk factors that can be targeted for the reduction of the CHD risk is serum lipid profile or hypercholesterolemia.
The positive association between hypercholesterolemia and CHDs in the general Japanese population has already been described in detail in previous publications9–11), and it was found in these studies that elevated serum total cholesterol (TC) significantly increased the rate of death due to CHDs. These findings were incorporated in the 10-year CHD death risk charts that were used in the Japan Atherosclerosis Society (JAS) guidelines12–14). However, younger people showed an extremely lower absolute risk compared to the absolute risk of older people by using these risk chart which predict CHD death risk with relatively shorter span. The estimation of lifetime risk (LTR), a risk projection method that accounts for the longer residual life span, provides a perspective that can complement the shorter duration risk estimation with long-term risk estimates for a better understanding of the disease burden15, 16). Moreover, the effect of hypercholesterolemia on the LTR of CHDs for any Asian population has not been reported.
Aim
The aim of this study was to estimate the influence of hypercholesterolemia on short- and intermediate- term risk as well as the LTR for CHDs based upon an urban cohort in Japan.
Methods
Study Sample
The Suita Cohort Study, which was established in 1989, is a cohort study for cardiovascular diseases in the city of Suita, Osaka Prefecture, Japan. The participants of this study were randomly selected from a population aged between 30 and 79. Residents were stratified by sex and age (10-year increments), and the overall participation rate was 53.2%. The details of this study have been described in detail in previous publications17–20). Overall, 6,483 men and women participated in the baseline survey at the National Cardiovascular Center between September 1989 and March 1994. For this analysis, participants with the following issues were excluded: lack of baseline information or loss to follow-up (n = 602), nonfasting blood sampling (n = 280), taking lipid-lowering drugs (n = 120), having a history of CHD (n = 42), and missing information on serum lipid profile (n = 32). In total, 2,559 men and 2,848 women were included in this study (Fig. 1).
Fig. 1.
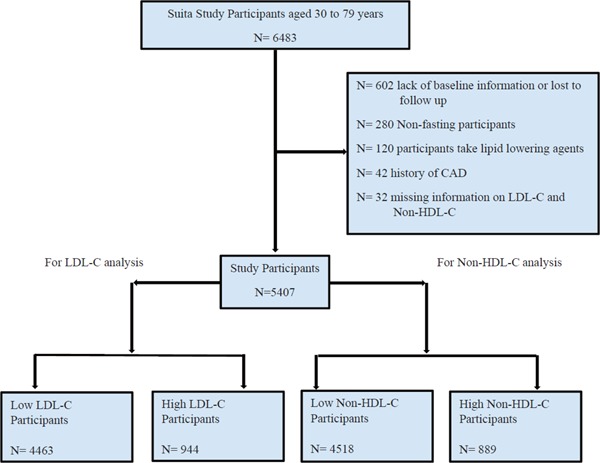
Flow chart of the cohort creation
CHD: coronary heart disease; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol.
The Institutional Review Board of the National Cardiovascular Center approved this cohort study. Informed consent was obtained from all participants at the baseline examination, and the collected data were anonymized.
Baseline Examination
Blood samples were collected from the study participants after fasting for at least 10 h. These samples were immediately centrifuged after sampling, and routine blood examinations were performed, including investigation of serum TC, high-density lipoprotein cholesterol (HDL-C), triglyceride, and glucose levels. Low-density lipoprotein cholesterol (LDL-C) was estimated using the Friedewald formula, and serum LDL-C ≥ 160 mg/dL (4.14 mmol/L) was defined as high LDL-C according to the NCEP/ATP-III criteria21). Non-HDL-C was calculated by subtracting HDL-C from TC, and high serum non-HDL-C ≥ 190 mg/dL (4.91 mmol/L) was defined as high non-HDL-C. Blood pressure was measured in triplicate by well-trained physicians using a standard mercury sphygmomanometer. Measurements were performed on the subjects' right arms after 5 min of rest in the seated position. The average of the second and the third measurements recorded more than 1 min apart was used22). Hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg and/or the use of antihypertensive agents. Diabetes was defined as fasting serum glucose ≥ 126 mg/dL (7.0 mmol/L) and/or the use of antidiabetic agents.
Endpoint Determination
The details of the endpoint ascertainment for the Suita Cohort Study have been reported elsewhere23–25). The first step of the survey for CHDs involved checking the health status of all the participants by repeated clinical visits every two years and thereafter by yearly questionnaires sent by mail or conducted over the telephone. The second step involved reviewing the inhospital medical records of the participants who were suspected to have new-onset CHDs or those who died because of any CHD. Reviews were performed by registered hospital physicians or research physicians who were blinded to the baseline information. Definite and probable acute myocardial infarction were defined according to the criteria of the Monitoring Trends and Determinants in Cardiovascular Disease (MONICA) project26–28). Comprehensive systematic searches of death certificates were also performed in order to complete the surveillance for acute myocardial infarction. In addition to acute myocardial infarction, the criteria for diagnosing a CHD included sudden cardiac death within 24 h after the onset of acute symptoms and CHD followed by coronary bypass or angiography19).
Statistical Analysis
For the estimation of LTR, age (in years) was used as time scale and follow-up began at each patient's baseline age. Participants who were below 40 years of age at the beginning of the study period were entered into the study sample when they became 40 years old. The age categories started at the age of 40 years, and the highest age category was ≥ 90 years. The likelihood of death from a particular cause at a given time is simply the product of the overall survival until that time. The follow-up ended at CHD occurrence, death, or on December 31, 2007, depending upon which event occurred first. We estimated the cumulative CHD incidence conditional on survival to ages 45, 55, 65, and 75 years, accounting for the competing risks of death15, 16, 29–32). We then utilized the competing risk adjustment to avoid risk inflation introduced in standard survival methods that do not fully account for individuals who died during follow-up because of other competing causes. A double-decrement approach was applied to take both the CHD occurrence and the all-cause mortality into consideration when formulating the population at risk for each index age. Sex-specific 10-, 20-, 30-, and 40-year risks and the LTR for CHD by LDL-C or non-HDL-C categories were estimated for CHD-free participants at different index ages. Estimates were calculated using a modified survival analysis technique, as previously reported33, 34). We also performed a sensitivity analysis with high LDL-C defined as ≥ 140 mg/dL. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Table 1 shows the characteristics of the participants stratified by LDL-C levels at baseline. At baseline, 12.5% of the men and 22.0% of the women had high LDL-C, whereas 14.1% of the men and 18.6% of the women had high non-HDL-C. During the 69,823 person-years of follow-up, 124 men and 57 women were diagnosed with CHD. Table 2 displays the 10-, 20-, 30-, and 40-year risks as well as the LTR of CHDs based on the LDL-C categories for men and women of various index ages. The person-year of follow-up, incidence of CHD, and mortality due to CHD across the age groups are shown in Table 2 for the LDL-C categories and in Table 3 for the non-HDL-C categories.
Table 1. Characteristics of study participants.
| LDL-C categories |
|||
|---|---|---|---|
| Variables | Low (LDL-C < 160) | High (LDL-C ≥ 160) | |
| Men | Number of subjects | 2240 | 319 |
| Age, years (s.d) | 55.7 (13.4) | 56.6 (12.1) | |
| LDL-C, mg/dL (s.d) | 115.8 (26.7) | 178.2 (17.2) | |
| HDL-C, mg/dL (s.d) | 49.4 (13.5) | 46.6 (10.6) | |
| Non-HDL-C, mg/dL (s.d) | 144.1 (29.1) | 206.8 (20.4) | |
| BMI, kgm−2 (s.d) | 22.7 (2.9) | 23.5 (2.8) | |
| Waist circumference, cm (s.d) | 82.4 (8.1) | 84.0 (7.8) | |
| Systolic blood pressure, mmHg (s.d) | 128.2 (20.9) | 129.6 (20.0) | |
| Diastolic blood pressure, mmHg (s.d) | 79.0 (12.2) | 80.3 (11.1) | |
| Hypertension, n (%) | 723 (32.3) | 117 (36.7) | |
| Diabetes, n (%) | 130 (5.8) | 21 (6.6) | |
| Smoking, n (%) | |||
| Never smoker | 420 (18.8) | 63 (19.8) | |
| Current smoker | 1124 (50.2) | 154 (48.3) | |
| Ex-smoker | 664 (29.6) | 97 (30.4) | |
| Unknown | 32 (1.4) | 5 (1.6) | |
| Drinking, n (%) | |||
| Never drinker | 453 (20.2) | 83 (26.0) | |
| Current drinker | 1671 (74.6) | 216 (67.7) | |
| Ex-drinker | 86 (3.8) | 16 (5.0) | |
| Unknown | 30 (1.3) | 4 (1.3) | |
| Women | Number of subjects | 2223 | 625 |
| Age, years (s.d) | 52.5 (13.1) | 58.7 (10.0) | |
| LDL-C, mg/dL (s.d) | 120.9 (23.7) | 183.9 (22.8) | |
| HDL-C, mg/dL (s.d) | 57.4 (13.7) | 53.9 (12.1) | |
| Non-HDL-C, mg/dL (s.d) | 141.2 (27.8) | 208.8 (28.1) | |
| BMI, kgm−2 (s.d) | 22.0 (3.2) | 23.1 (3.3) | |
| Waist circumference, cm (s.d) | 76.5 (10.1) | 80.4 (10.1) | |
| Systolic blood pressure, mmHg (s.d) | 122.4 (21.5) | 130.7 (21.0) | |
| Diastolic blood pressure, mmHg (s.d) | 74.2 (11.8) | 78.5 (11.6) | |
| Hypertension, n (%) | 511 (23.0) | 238 (38.1) | |
| Diabetes, n (%) | 69 (3.1) | 30 (4.8) | |
| Smoking, n (%) | |||
| Never smoker | 1826 (82.1) | 505 (80.8) | |
| Current smoker | 269 (12.1) | 70 (11.2) | |
| Ex-smoker | 80 (3.6) | 23 (3.7) | |
| Unknown | 48 (2.2) | 27 (4.3) | |
| Drinking, n (%) | |||
| Never drinker | 1389 (62.5) | 428 (68.5) | |
| Current drinker | 748 (33.7) | 166 (26.6) | |
| Ex-drinker | 41 (1.8) | 8 (1.3) | |
| Unknown | 45 (2.0) | 23 (3.7) | |
LDL-C: low-density lipoprotein cholesterol, s.d: standard deviation, HDL-C: high-density lipoprotein cholesterol, BMI: body mass index
Table 2. Person-years of follow-up and number of events (CHD and Death) by sex and LDL-C Categories.
| Age Group (Years) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30–44 |
45–54 |
55–64 |
65–74 |
75–100 |
|||||||||||
| PY | CHD | Death | PY | CHD | Death | PY | CHD | Death | PY | CHD | Death | PY | CHD | Death | |
| Men | |||||||||||||||
| Low LDL-C | 7,616 | 3 | 10 | 6,142 | 10 | 23 | 7,356 | 29 | 116 | 5,203 | 39 | 217 | 1,307 | 15 | 100 |
| High LDL-C | 850 | 2 | 0 | 960 | 4 | 3 | 1,315 | 10 | 12 | 834 | 7 | 21 | 134 | 5 | 11 |
| Women | |||||||||||||||
| Low LDL-C | 10,302 | 1 | 8 | 7,055 | 4 | 15 | 6,952 | 8 | 49 | 4,297 | 14 | 79 | 1,093 | 7 | 42 |
| High LDL-C | 756 | 0 | 2 | 2,257 | 2 | 7 | 3,112 | 8 | 15 | 2,012 | 11 | 31 | 277 | 2 | 13 |
LDL-C: low-density lipoprotein cholesterol, CHD: coronary heart disease, PY: person-years
Serum LDL-C < 160 mg/dL was defined as “Low LDL-C”.
Serum LDL-C ≥ 160 mg/dL was defined as “High LDL-C”.
Table 3. Person-years of follow-up and number of events (CHD and Death) by sex and non-HDL-C Categories.
| Age Group (Years) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30–44 |
45–54 |
55–64 |
65–74 |
75–100 |
|||||||||||
| PY | CHD | Death | PY | CHD | Death | PY | CHD | Death | PY | CHD | Death | PY | CHD | Death | |
| Men | |||||||||||||||
| Low non-HDL-C | 7,363 | 1 | 10 | 6,018 | 10 | 22 | 7,224 | 26 | 111 | 5,259 | 35 | 219 | 1,304 | 15 | 96 |
| High non-HDL-C | 1,103 | 4 | 0 | 1,084 | 4 | 4 | 1,447 | 13 | 17 | 778 | 11 | 19 | 137 | 5 | 15 |
| Women | |||||||||||||||
| Low non-HDL-C | 10,607 | 1 | 8 | 7,551 | 4 | 17 | 7,316 | 7 | 55 | 4,487 | 15 | 81 | 1,111 | 8 | 40 |
| High non-HDL-C | 452 | 0 | 2 | 1,761 | 2 | 5 | 2,748 | 9 | 9 | 1,822 | 10 | 29 | 259 | 1 | 15 |
HDL-C: high-density lipoprotein cholesterol, CHD: coronary heart disease, PY: person-years
Serum non-HDL-C < 190 mg/dL was defined as “Low non-HDL-C”.
Serum non-HDL-C ≥ 190 mg/dL was defined as “High non-HDL-C”.
The estimated LTR of CHDs, accounting for the competing risk of death, at 45 years of age was 13.7% (95% confidence interval [CI]: 10.9–16.5%) for men with low LDL-C and 47.2% (95% CI: 29.3–56.1%) for men with high LDL-C (Table 4). For women at the index age of 45 years, the estimated LTR of CHDs for low and high LDL-C was 7.1% (95% CI: 4.4–9.8%) and 10.2% (95% CI: 5.6–14.8%) for low and high LDL-C, respectively. For both sexes, the estimated intermediate-term risks (10, 20, 30, and 40 years) increased across time. However, the estimated LTRs in men were lower with higher index ages, whereas in women, they were nearly constant regardless of the index age.
Table 4. Age and sex-specific 10-, 20-, 30- and 40-year and lifetime risk estimates for coronary heart disease based on low-density lipoprotein cholesterol level (adjusted for the competing risk of death).
| Sex | Index age (yrs) | Short and intermediate term risk (Yrs) | LDL-C Categories |
|
|---|---|---|---|---|
| Low LDL-C | High LDL-C | |||
| Men | 45 | 10 | 0.5 (0.0–1.0) | 3.7 (0.0–7.9) |
| 20 | 2.8 (1.6–4.0) | 8.5 (3.1–14.0) | ||
| 30 | 6.0 (4.4–7.6) | 14.2 (8.0–20.5) | ||
| 40 | 10.6 (8.4–12.7) | 20.2 (12.9–27.5) | ||
| LTR | 13.7 (10.9–16.5) | 47.2 (29.3–65.1) | ||
| 55 | 10 | 2.4 (1.3–3.4) | 5.1 (1.1–9.2) | |
| 20 | 5.6 (4.1–7.2) | 11.2 (5.8–16.6) | ||
| 30 | 10.3 (8.2–12.4) | 17.6 (10.8–24.4) | ||
| LTR | 13.5 (10.6–16.3) | 40.4 (27.5–65.2) | ||
| 65 | 10 | 3.6 (2.3–4.8) | 6.6 (2.4–10.8) | |
| 20 | 8.6 (6.6–10.7) | 13.5 (7.1–19.8) | ||
| LTR | 12.0 (9.2–14.9) | 44.7 (24.5–64.9) | ||
| 75 | 10 | 6.4 (4.4–8.5) | 8.1 (2.2–14.1) | |
| LTR | 10.7 (7.4–14.0) | 45.0 (21.4–68.5) | ||
| Women | 45 | 10 | 0.5 (0.0–1.0) | 0.0 (0.0–0.0) |
| 20 | 0.6 (0.1–1.2) | 0.7 (0.0–1.6) | ||
| 30 | 1.4 (0.6–2.3) | 3.9 (1.8–6.0) | ||
| 40 | 4.7 (3.0–6.4) | 8.9 (5.0–12.8) | ||
| LTR | 7.1 (4.4–9.8) | 10.2 (5.6–14.8) | ||
| 55 | 10 | 0.1 (0.0–0.4) | 0.7 (0.0–1.6) | |
| 20 | 1.0 (0.3–1.7) | 4.0 (1.9–6.1) | ||
| 30 | 4.3 (2.6–6.0) | 9.2 (5.2–13.2) | ||
| LTR | 6.7 (4.0–9.4) | 10.5 (5.8–15.2) | ||
| 65 | 10 | 0.9 (0.2–1.6) | 3.5 (1.4–5.5) | |
| 20 | 4.3 (2.6–6.0) | 8.9 (4.8–13.0) | ||
| LTR | 6.8 (4.1–9.6) | 10.3 (5.4–15.2) | ||
| 75 | 10 | 3.7 (2.0–5.4) | 5.9 (2.0–9.9) | |
| LTR | 6.4 (3.5–9.3) | 7.5 (2.6–12.4) | ||
LDL-C: low-density lipoprotein cholesterol, LTR: lifetime risk
Serum LDL-C < 160 mg/dL was defined as “Low LDL-C”.
Serum LDL-C ≥ 160 mg/dL was defined as “High LDL-C”.
Table 5 shows the 10-, 20-, 30-, and 40-year risks as well as the LTR of CHDs based on the non-HDL-C categories for men and women of various index ages; these results were similar to those for the LDL-C categories.
Table 5. Age and sex-specific 10-, 20-, 30- and 40-year and lifetime risk estimates for coronary heart disease based on Non-high-density lipoprotein cholesterol level (adjusted for the competing risk of death).
| Sex | Index age (yrs) | Short and intermediate term risk (Yrs) | Non-HDL-C Categories |
|
|---|---|---|---|---|
| Low Non-HDL-C | High Non-HLDL-C | |||
| Men | 45 | 10 | 0.2 (0.0–0.5) | 5.1 (0.7–9.5) |
| 20 | 2.5 (1.4–3.7) | 9.2 (3.9–14.4) | ||
| 30 | 5.2 (3.7–6.8) | 17.3 (11.0–23.6) | ||
| 40 | 9.5 (7.5–11.6) | 25.2 (17.6–32.9) | ||
| LTR | 12.7 (9.9–15.4) | 41.45 (25.0–57.7) | ||
| 55 | 10 | 2.4 (1.3–3.5) | 4.3 (0.9–7.7) | |
| 20 | 5.2 (3.7–6.7) | 13.1 (7.7–18.5) | ||
| 30 | 9.5 (7.5–11.6) | 21.7 (14.4–28.9) | ||
| LTR | 12.7 (9.9–15.6) | 39.9 (21.7–56.4) | ||
| 65 | 10 | 3.0 (1.8–4.2) | 9.5 (4.8–14.3) | |
| 20 | 7.7 (5.7–9.7) | 18.8 (11.5–26.1) | ||
| LTR | 11.2 (8.3–14.0) | 37.7 (19.1–56.3) | ||
| 75 | 10 | 5.9 (3.9–7.9) | 11.6 (4.3–19.0) | |
| LTR | 10.2 (7.0–13.5) | 35.3 (12.5–58.2) | ||
| Women | 45 | 10 | 0.5 (0.0–0.9) | 0.0 (0.0–0.0) |
| 20 | 0.6 (0.1–1.1) | 0.8 (0.0–1.9) | ||
| 30 | 1.3 (0.5–2.0) | 4.7 (2.3–7.2) | ||
| 40 | 4.9 (3.1–6.6) | 9.1 (5.0–13.1) | ||
| LTR | 7.2 (4.5–9.9) | 10.5 (5.6–15.3) | ||
| 55 | 10 | 0.1 (0.0–0.4) | 0.8 (0.0–2.0) | |
| 20 | 0.8 (0.2–1.4) | 4.9 (2.4–7.4) | ||
| 30 | 4.5 (2.7–6.2) | 9.4 (5.2–13.6) | ||
| LTR | 6.9 (4.2–9.6) | 10.9 (5.9–15.9) | ||
| 65 | 10 | 0.7 (0.1–1.3) | 4.3 (1.9–6.6) | |
| 20 | 4.5 (2.7–6.3) | 8.9 (4.7–13.2) | ||
| LTR | 7.0 (4.2–9.8) | 10.5 (5.4–15.6) | ||
| 75 | 10 | 4.1 (2.3–5.9) | 5.2 (1.2–9.2) | |
| LTR | 6.8 (3.8–9.7) | 6.9 (1.8–12.0) | ||
HDL-C: high-density lipoprotein cholesterol, LTR: lifetime risk
Serum non-HDL-C < 190 mg/dL was defined as “Low non-HDL-C”.
Serum non-HDL-C ≥ 190 mg/dL was defined as “High non-HDL-C”.
The results of the sensitivity analysis with high LDL-C defined as ≥ 140 mg/dL showed that the estimated LTRs for CHDs, in both men and women, were smaller than the ones where high LDL-C was defined as ≥ 160 mg/dL. The estimated LTRs of men with high LDL-C were 28.0% (95% CI: 16.5–39.6%) at the index age of 45 years and 22.8% (95% CI: 7.7–37.9%) at the index age of 75 years. In women with high LDL-C, the estimated LTRs were 8.1% (95% CI: 5.1–11.2%) at the index age of 45 years and 5.8% (95% CI: 2.7–8.9%) at the index age of 75 years.
Discussion
In this urban-community-based cohort in Japan, we observed that the LTR of CHDs was affected by hypercholesterolemia in middle-aged men and women. Individuals with low LDL-C or non-HDL-C had a lower LTR of CHDs than those with high LDL-C or non-HDL-C. For all index ages, the LTR of CHDs was higher in men than in women. This is the first study describing the relationship between hypercholesterolemia and LTR of CHDs in Asian populations. There is currently no report comparing directly the incident of CHDs between Japan and other countries, including Asian countries, using the same definition of CHDs. However, Steg et al. showed that the one-year event rates of nonfatal myocardial infarction were almost the same between Japan and Asia in the Reduction of Atherothrombosis for Continued Health Registry (REACH registry)35). We think our results may be useful to not only Japan, but also other Asian countries.
We previously reported on the LTR of CHDs from the Suita Cohort Study among individuals with and without hypertension and with and without diabetes mellitus36, 37). At 45 years of age, the competing-risk-adjusted LTR of CHDs was 14.1% and 27.0% for normotensive and hypertensive men, respectively; 6.2% and 14.9% for normotensive and hypertensive women, respectively; 21.1% and 16.7% for men with and without diabetes, respectively; and 14.2% and 9.2% for women with and without diabetes, respectively. When compared to other risk factors, the estimated LTRs of CHDs were very high in men (approximately 45%) and low in women (approximately 11%) with hypercholesterolemia. In most Japanese community-based cohort studies, hypercholesterolemia is strongly related to CHDs but weakly related to stroke, as shown in the JAS guidelines14). On the other hand, hypertension and diabetes are related to stroke as well as CHDs14, 38, 39). Diabetes is also associated with cancer40). In general, the LTR for a targeted disease is calculated by the accumulation of cases among a population, excluding deaths due to other reasons, and this adjustment is known to be the one of competing risk, and this approach is essential for estimating the LTR16, 31, 32). Therefore, the LTR of a disease due to a risk factor, which can be associated with various other diseases, will be influenced by the exposure (the risk factor of interest) and outcome (disease of interest) relationship. Actually, if high LDL-C is defined by serum LDL-C ≥ 140 mg/dL, the estimated LTR of high LDL-C for CHDs in men will be 22.8%, which is smaller than the one defined by serum LDL-C ≥ 160 mg/dL (45.0%). This discrepancy, to some extent, can be attributed to the difference between the numbers of deaths in the age group 75–100 with high LDL-C defined by ≥ 140 mg/dL and ones with high LDL-C defined by ≥ 160 mg/dL. The number of CHDs in the high LDL-C category was almost similar (seven events defined by ≥ 140 mg/dL and five events defined by ≥ 160 mg/dL), but the number of deaths in the high LDL-C category defined by ≥ 140 mg/dL was observed much more than the one defined by 160 mg/dL (29 events versus 11 events, respectively).
There is very limited evidence regarding the LTR of CHDs in relation to hypercholesterolemia. In one previous study based upon the Framingham Heart Study (FHS)41), it was reported that, at 40 years of age, the LTRs of CHDs were 57.3% and 32.9% for men and women with high TC (serum TC ≥ 240 mg/dL, which is thought to be equivalent to LDL-C ≥ 160 mg/dL and non-HDL-C ≥ 190 mg/dL), respectively. The estimated LTR for men was very similar to that reported in our study; however, in women, the estimated LTR in the FHS was much larger than that reported in our study. In Japan, the influence of hypercholesterolemia on CHDs has been reported to be quite different across men and women. The results from National Integrated Project for Prospective Observation of Non-communicable Disease And its Trends in the Aged, 1980 (NIPPON DATA80)9, 42) showed that the risk of CHDs linearly increased over TC 200 mg/dL in men; however, there was no association below TC 260 mg/dL in women. Therefore, the difference of LTR between men and women may be larger in Japan than in western countries.
The guidelines for CHD prevention adopted the estimated 10-year risk for CHD onset or death based upon the findings of cohort studies with long-term follow-up periods, such as the NIPPON DATA80 12), the Suita Study19), and the FHS43). However, the estimated 10-year risks were very low in the younger population, particularly in women, so it is difficult to motivate such individuals to make lifestyle improvements for the prevention of CHDs. On the other hand, LTR is an indicator that can be easily interpreted. For example, based upon our results, the probability of new CHD onset for a 45-year-old man with hypercholesterolemia is 47.2% in his remaining life. Therefore, LTR can be useful in promoting health-oriented motivation for the prevention of atherosclerotic cardiovascular diseases (ASCVDs) through lifestyle modification and/or medication. The 2013 American College of Cardiology/American Heart Association (ACC/AHA) Guidelines on the Assessment of Cardiovascular Risk showed the LTR of ASCVDs for a population aged 20–59, who were free from ASCVD and were not at a high short-term risk44). However, there are no applications for the Asian population, including Japan, so we must establish guidelines for the prevention of ASCVDs utilizing LTR.
The major strength of our study is the uniqueness of the study cohort. This is an urban-population-based cohort in Japan. Moreover, the prospective ascertainment of the endpoints using standardized and previously validated clinical diagnostic criteria contributed to the accurate confirmation of outcomes including CHD and deaths23–25).
There are a few limitations that need to be kept in mind while utilizing the results of this study. First, we had a small number of CHD events as outcomes. Because of this small number of CHD events, we could not perform more detailed analyses (e.g., using three or more categories for LDL-C or non-HDL-C or combinations of other major risk factors) for the determination of LTRs of CHDs. The small number of CHD events also affected the LTR estimates. The CIs of the estimates for the high LDL-C and high non-HDL-C are quite wide and are reflective of less stable estimates even when taking a conservative approach. We acknowledge the need for further studies based upon a pooled analysis of the larger cohorts in the future. Second, the Suita Cohort is based on an urban population, so the estimates in this study may not represent the overall Japanese population, thus impacting the external validity of our study. However, there is a high urbanization rate in Japan, and most of the changes in lifestyle factors associated with the risk for CHDs are thought to be due to urbanization2, 3, 45, 46). Therefore, we believe that our findings of high LTRs of CHDs in an urban population are representative of a large proportion of the Japanese population.
Conclusions
In conclusion, the presence of hypercholesterolemia increases the LTR for CHDs in the Japanese population, especially in men. These estimates can be used for preventive knowledge translation efforts at the population level.
Acknowledgments
We thank the members of the Suita City Health Center, the Suita Medical Association, and all of the researchers and staff of the Department of Preventive Cardiology for performing the medical examinations and follow-ups. We also thank Satsuki-Junyukai and the volunteers involved in the administration of the Suita Cohort Study.
Conflict of Interest
The authors have no conflicts of interest.
Financial Support
This study was supported by the Intramural Research Fund (27-4-3) for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center, Comprehensive Research on Cardiovascular and Lifestyle Related Diseases: H29-Junkankitou [Seishuu]-Ippan-003) and a Grant-in-Aid for Young Scientists B 17K15834 from the Japan Society for the Promotion of Science
References
- 1). Uemura K, Pisa Z. Trends in cardiovascular disease mortality in industrialized countries since 1950. World Health Stat Q, 1987; 41: 155-178 [PubMed] [Google Scholar]
- 2). Ueshima H. Trends in Asia. In: Marmot M.G, Elliott P. (eds), Coronary Heart Disease Epidemiology: From Aetiology to Public Health, Oxford University Press, New York, USA, 2005; 102-113 [Google Scholar]
- 3). Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N, Kita Y, Watanabe M, Kadota A, Okuda N, Kadowaki T, Nakamura Y, Okamura T. Cardiovascular disease and risk factors in asia: a selected review. Circulation, 2008; 118: 2702-2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Ueshima H. Explanation for the Japanese paradox: prevention of increase in coronary heart disease and reduction in stroke. J Atheroscler Thromb, 2007; 14: 278-286 [DOI] [PubMed] [Google Scholar]
- 5). Rumana N, Kita Y, Turin TC, Murakami Y, Sugihara H, Morita Y, Tomioka N, Okayama A, Nakamura Y, Abbott RD, Ueshima H. Trend of increase in the incidence of acute myocardial infarction in a Japanese population: Takashima AMI registry, 1990–2001. Am J Epidemiol, 2008; 167: 1358-1364 [DOI] [PubMed] [Google Scholar]
- 6). Kitamura A, Iso H, Iida M, Naito Y, Sato S, Jacobs DR, Nakamura M, Shimamoto T, Komachi Y. Trends in the incidence of coronary heart disease and stroke and the prevalence of cardiovascular risk factors among Japanese men from 1963 to 1994. Am J Med, 2002; 112: 104-109 [DOI] [PubMed] [Google Scholar]
- 7). Takii T, Yasuda S, Takahashi J, Ito K, Shiba N, Shirato K, Shimokawa H, MIYAGI-AMI Study Investigators Trends in acute myocardial infarction incidence and mortality over 30 years in Japan: report from the Miyagi-AMI Registry Study. Circ J, 2010; 74: 93-100 [DOI] [PubMed] [Google Scholar]
- 8). Nishiyama S, Watanabe T, Arimoto T, Takahashi H, Shishido T, Miyashita T, Miyamoto T, Nitobe J, Shibata Y, Konta T, Kawata S, Kato T, Fukao A, Kubota I. Trends in coronary risk factors among patients with acute myocardial infarction over the last decade: the Yamagata AMI Registry. J Atheroscler Thromb, 2010; 17: 989-998 [DOI] [PubMed] [Google Scholar]
- 9). Sugiyama D, Okamura T, Watanabe M, Higashiyama A, Okuda N, Nakamura Y, Hozawa A, Kita Y, Kadota A, Murakami Y, Miyamatsu N, Ohkubo T, Hayakawa T, Miyamoto Y, Miura K, Okayama A, Ueshima H, NIPPON DATA 80/90 Research Group Risk of hypercholesterolemia for cardiovascular disease and the population attributable fraction in a 24-year Japanese cohort study. J Atheroscler Thromb, 2015; 22: 95-107 [DOI] [PubMed] [Google Scholar]
- 10). Tanabe N, Iso H, Okada K, Nakamura Y, Harada A, Ohashi Y, Ando T, Ueshima H, Japan Arteriosclerosis Longitudinal Study Group Serum total and non-high-density lipoprotein cholesterol and the risk prediction of cardiovascular events -the JALS-ECC-. Circ J, 2010; 74: 1346-1356 [DOI] [PubMed] [Google Scholar]
- 11). Nagasawa SY, Okamura T, Iso H, Tamakoshi A, Yamada M, Watanabe M, Murakami Y, Miura K, Ueshima H; Evidence for Cardiovascular Prevention from Observational Cohorts in Japan (EPOCH-JAPAN) Research Group. J Am Heart Assoc, 2012; 1: e001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). NIPPON DATA80 Research Group Risk assessment chart for death from cardiovascular disease based on a 19-year follow-up study of a Japanese representative population. Circ J, 2006; 70: 1249-1255 [DOI] [PubMed] [Google Scholar]
- 13). Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K, Japan Atherosclerosis Society Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version. J Atheroscler Thromb, 2013; 20: 517-523 [DOI] [PubMed] [Google Scholar]
- 14). Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S; Committee for Epidemiology and Clinical Management of Atherosclerosis. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Turin TC, Hemmelgarn BR. Long-term risk projection and its application to nephrology research. J Nephrol, 2012; 25(4): 441-449 [DOI] [PubMed] [Google Scholar]
- 16). Satoh M, Ohkubo T, Asayama K, Murakami Y, Sugiyama D, Yamada M, Saitoh S, Sakata K, Irie F, Sairenchi T, Ishikawa S, Kiyama M, Ohnishi H, Miura K, Imai Y, Ueshima H, Okamura T, on behalf of the EPOCH-JAPAN Research Group Lifetime Risk of Stroke and Coronary Heart Disease Deaths According to Blood Pressure Level EPOCH-JAPAN (Evidence for Cardiovascular Prevention From Observational Cohorts in Japan). Hypertension, 2019; 73: 52-59 [DOI] [PubMed] [Google Scholar]
- 17). Tsukinoki R, Okamura T, Watanabe M, Kokubo Y, Higashiyama A, Nishimura K, Takegami M, Murakami Y, Okayama A, Miyamoto Y. Blood pressure, low-density lipoprotein cholesterol and incidences of coronary artery disease and ischemic stroke in Japanese: the Suita study. Am J Hypertens, 2014; 27: 1362-1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Arai H, Kokubo Y, Watanabe M, Sawamura T, Ito Y, Minagawa A, Okamura T, Miyamato Y. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: the Suita study. J Atheroscler Thromb, 2013; 20: 195-203 [DOI] [PubMed] [Google Scholar]
- 19). Nishimura K, Okamura T, Watanabe M, Nakai M, Takegami M, Higashiyama A, Kokubo Y, Okayama A, Miyamoto Y. Predicting coronary heart disease using risk factor categories for a Japanese urban population, and comparison with the Framingham risk score: the Suita study. J Atheroscler Thromb, 2014; 21: 784-798 [DOI] [PubMed] [Google Scholar]
- 20). Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Ono Y, Nishimura K, Okayama A, Miyamoto Y. A revised definition of the metabolic syndrome predicts coronary artery disease and ischemic stroke after adjusting for low density lipoprotein cholesterol in a 13-year cohort study of Japanese: the Suita study. Atherosclerosis, 2011; 217: 201-206 [DOI] [PubMed] [Google Scholar]
- 21). Expert Panel on Detection Evaluation, and Treatment of High Blood Cholesterol in Adults, Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA, 2001; 285: 2486-2497 [DOI] [PubMed] [Google Scholar]
- 22). Kokubo Y, Kamide K, Okamura T, Watanabe M, Higashiyama A, Kawanishi K, Okayama A, Kawano Y. Impact of high-normal blood pressure on the risk of cardiovascular disease in a Japanese urban cohort: the Suita study. Hypertension, 2008; 52: 652-659 [DOI] [PubMed] [Google Scholar]
- 23). Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Miyamoto Y, Yoshimasa Y, Okayama A. Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort study: The Suita study. Atherosclerosis, 2009; 203: 587-592 [DOI] [PubMed] [Google Scholar]
- 24). Higashiyama A, Okamura T, Ono Y, Watanabe M, Kokubo Y, Okayama A. Risk of smoking and metabolic syndrome for incidence of cardiovascular disease--comparison of relative contribution in urban Japanese population: the Suita study. Circ J, 2009; 73: 2258-2263 [DOI] [PubMed] [Google Scholar]
- 25). Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Ono Y, Miyamoto Y, Yoshimasa Y, Okayama A. Triglycerides and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort: the Suita study. Atherosclerosis, 2010; 209: 290-294 [DOI] [PubMed] [Google Scholar]
- 26). World Health Organization Document for meeting of MONICA PrincipalInvestigators. In: WHO , editors. Geneva, Switzerland: MONICA Project: Event Registration Data Component, MONICA Manual; Version 1.1. 1986; S-4: 9-11 [Google Scholar]
- 27). Ministry of Health and Welfare Study Project of Monitoring System for Cardiovascular Disease Commissioned by the Ministry of Health and Welfare: Manual for the Registry and Follow-Up of Ischemic Heart Disease, National Cardiovascular Center, Osaka, Japan, 1998 [Google Scholar]
- 28). Ministry of Health and Welfare Study Project of Monitoring System for Cardiovascular Disease Commissioned by the Ministry of Health and Welfare: Manual for the Registry and Follow-Up of Stroke, National Cardiovascular Center, Osaka, Japan, 1998 [Google Scholar]
- 29). Beiser A, D'Agostino RB, Sr, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer's disease in the Framingham study. The practical incidence estimators (pie) macro. Stat Med, 2000; 19: 1495-1522 [DOI] [PubMed] [Google Scholar]
- 30). Turin TC, Tonelli M, Manns BJ, Ahmed SB, Ravani P, James M, Hemmelgarn BR. Lifetime risk of ESRD. J Am Soc Nephrol, 2012; 23: 1569-1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Turin TC, Kokubo Y, Murakami Y, Higashiyama A, Rumana N, Watanabe M, Okamura T. Lifetime risk of stroke in Japan. Stroke, 2010; 41: 1552-1554 [DOI] [PubMed] [Google Scholar]
- 32). Turin TC, Kokubo Y, Murakami Y, Higashiyama A, Rumana N, Watanabe M, Okamura T. Lifetime risk of acute myocardial infarction in Japan. Circ Cardiovasc Qual Outcomes, 2010; 3: 701-703 [DOI] [PubMed] [Google Scholar]
- 33). Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D'Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men. JAMA, 2002; 287: 1003-1010 [DOI] [PubMed] [Google Scholar]
- 34). Driver JA, Djoussé L, Logroscino G, Gaziano JM, Kurth T. Incidence of cardiovascular disease and cancer in advanced age: prospective cohort study. BMJ, 2008; 337: a2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Steg PG, Bhatt DL, Wilson PW, D'Agostino R, Sr, Ohman EM, Röther J, Liau CS, Hirsch AT, Mas JL, Ikeda Y, Pencina MJ, Goto S, REACH Registry Investigators One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA, 2007; 297: 1197-1206 [DOI] [PubMed] [Google Scholar]
- 36). Turin TC, Okamura T, Afzal RA, Rumana N, Watanabe M, Higashiyama A, Nakao YM, Nakai, Takegami M, Nishimura K, Kokubo Y, Okayama A, Miyamoto Y. Impact of hypertension on the lifetime risk of coronary heart disease. Hypertens Res, 2016; 39: 548-551 [DOI] [PubMed] [Google Scholar]
- 37). Turin TC, Okamura T, Rumana N, Afzal AR, Watanabe M, Higashiyama A, Nakao YM, Nakai M, Takegami M, Nishimura K, Kokubo Y, Okayama A, Miyamoto Y. Diabetes and lifetime risk of coronary heart disease. Prim Care Diabetes, 2017; 11: 461-466 [DOI] [PubMed] [Google Scholar]
- 38). Fujiyoshi A, Ohkubo T, Miura K, Murakami Y, Nagasawa SY, Okamura T, Ueshima H, Observational Cohorts in Japan (EPOCH-JAPAN) Research Group Blood pressure categories and long-term risk of cardiovascular disease according to age group in Japanese men and women. Hypertens Res, 2012; 35: 947-953 [DOI] [PubMed] [Google Scholar]
- 39). Iso H, Imano H, Kitamura A, Sato S, Naito Y, Tanigawa T, Ohira T, Yamagishi K, Iida M, Shimamoto T. Type 2 diabetes and risk of non-embolic ischaemic stroke in Japanese men and women. Diabetologia, 2004; 47: 2137-2144 [DOI] [PubMed] [Google Scholar]
- 40). Sasazuki S, Charvat H, Hara A, Wakai K, Nagata C, Nakamura K, Tsuji I, Sugawara Y, Tamakoshi A, Matsuo K, Oze I, Mizoue T, Tanaka K, Inoue M, Tsugane S; Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Cancer Sci. Diabetes mellitus and cancer risk: pooled analysis of eight cohort studies in Japan, 2013; 104: 1499-1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Lloyd-Jones DM, Wilson PW, Larson MG, Leip E, Beiser A, D'Agostino RB, Cleeman JI, Levy D. Lifetime risk of coronary heart disease by cholesterol levels at selected ages. Arch Intern Med, 2003; 163: 1966-1972 [DOI] [PubMed] [Google Scholar]
- 42). Okamura T, Tanaka H, Miyamatsu N, Hayakawa T, Kadowaki T, Kita Y, Nakamura Y, Okayama A, Ueshima H, NIPPON DATA80 Research Group The relationship between serum total cholesterol and all-cause or causespecific mortality in a 17.3-year study of a Japanese cohort. Atherosclerosis, 2007; 190: 216-223 [DOI] [PubMed] [Google Scholar]
- 43). Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation, 1998; 97: 1837-1847 [DOI] [PubMed] [Google Scholar]
- 44). Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 2014; 129: S49-73 [DOI] [PubMed] [Google Scholar]
- 45). Okayama A, Ueshima H, Marmot M, Elliott P, Choudhury SR, Kita Y. Generational and regional differences in trends of mortality from ischemic heart disease in Japan from 1969 to 1992. Am J Epidemiol, 2001; 153: 1191-1198 [DOI] [PubMed] [Google Scholar]
- 46).https://www.estat.go.jp/statsearch/files?page=1&layout=d atalist&toukei=00200523&tstat=000000070001&cycle=0&tclass1=000001051218&stat_infid=000031589250&second2=1 https://www.estat.go.jp/statsearch/files?page=1&layout=d atalist&toukei=00200523&tstat=000000070001&cycle=0&tclass1=000001051218&stat_infid=000031589250&second2=1


