Abstract
Aim: Probucol is a controversial drug to inhibit ATP-binding cassette transporter A1 (ABCA1) and to exhibit some positive clinical effects such as regression of xanthomas. It reportedly rescues female infertility in scavenger receptor BI-deficient mice. Here, we investigated the effect of probucol on propagation in HDL-deficient mice as alternative models for impaired HDL-mediated cholesterol delivery.
Methods: Propagation of ABCA1-deficient (Abca1−/−) mice and lecithin: cholesterol acyltransferase (LCAT)-deficient (Lcat−/−) mice were quantitatively observed under the probucol treatment.
Results: Abca1−/− and Lcat−/− mice appear with negligible plasma HDL concentration. Upon backcrossing Abc1+/− with the Abc1−/− mice and cross-breeding between Abc1+/− mice, the numbers of Abc1−/− weaned pups were reduced to 54.7% and to 57.1% from those expected by Mendelian genetics, respectively. Similarly, Lcat-/-weaned pups decreased to 67.7% and to 35.9% but only in the male. Probucol severely reduced plasma HDL-cholesterol to 5% in the wild-type mice, but showed no effects on their propagation. Probucol corrected the deflections of the genotype distribution in the weaned pups recovery in the LCAT-deficient mice propagation but not in the ABCA1-deficient mice while plasma HDL was kept negligible. Probucol had no effect on cholesterol content in the steroidogenic organs of the HDL-deficient mice, while it somewhat increased plasma corticosterone and expression of adrenal cortex HMG-CoA reductase, StAR, cytochrome P450scc, and VKORC1 indicating increase in the synthesis of cholesterol and steroid hormones and in vitamin K turn-over. However, no evident mechanistic background was indicated.
Conclusions: Probucol corrected deflection of genotype distribution in propagation of the LCAT-deficient mice but not the ABCA1-deficient mice at the weaning stage, apparently not through normalization of hypoalphalipo-proteinemia.
Keywords: Probucol, HDL, ABCA1, LCAT, Mouse propagation
See editorial vol. 27: 4–5
Introduction
Cholesterol is an essential component of animal cell membrane and a precursor of bioactive lipid molecules such as bile acids, steroid hormones, and vitamin D. Somatic cells synthesize cholesterol for their needs but dietary cholesterol is also incorporated into cholesterol homeostasis. Liver plays a central role in cholesterol metabolism in the vertebrate for its biosynthesis, handling dietary cholesterol, and its catabolism. Extracellular transport of cholesterol between the liver and other organs is carried by plasma lipoproteins, for distribution from the liver to peripheral tissues and catabolic transport from somatic cells to the liver for conversion to bile acids. The former pathway is mainly by apolipoprotein B-containing lipoproteins such as very-low and low density lipoprotein, and the latter is conveyed by high density lipoproteins (HDL). However, the two pathways are not clearly separated and lipid transport by lipoproteins is rather kinetically regulated. While primates, including humans use apoB-lipoproteins for both pathways, some rodents depend much on HDL even for the delivery of cholesterol to steroidogenic organs1–4). Deficiency of HDL may cause more fundamental problems in these animals than in apoB-dependent animals.
Typical impairment of HDL metabolism is caused by silencing the gene of ATP binging cassette transporter A1 (ABCA1) (Abca1−/−) or lecithin: cholesterol acyl transferase (LCAT) (Lcat−/−), both to show negligible plasma HDL concentration. Infertility has been reported in the ABCA1-null mice where the primary source of HDL biogenesis is abolished5). However, no report has been found for fertility or reproduction problem in the LCAT-null mice, which lack acyl-esterification of cholesterol on HDL and accordingly maturation of HDL to spherical particles6). Probucol, a strong inhibitor of ABCA1 and HDL biogenesis7, 8), severely reduces plasma HDL in mice9), but causes no abnormal reproduction in our preliminary observation. On the other hand, genetic deficient mice in a selective receptor for uptake of HDL-cholesteryl ester, scavenger receptor BI (SR-BI)10), exhibit increase of large abnormal HDL and female infertility11, 12). Interestingly, Krieger and his colleagues reported that probucol rescued this infertility13). They hypothesized that impaired delivery of cholesterol to steroidgenic organs may be a part of the mechanism13), but probucol unlikely reverses this problem because its “normalizing” lipoprotein profile in the SR-B1-deficient mice is due to inhibition of ABCA1 and not because of improvement in the cholesterol delivery to the cells. It is still puzzling how HDL metabolism is related to reproduction/propagation in rodents. Thus, we investigated the problem in HDL-deficient animals such as ABCA1-null and LCAT-null mice and examined the effects of probucol.
Methods
Animals
C57BL/6 mice were purchased locally for the wild-type control. Lcat−/− mice were generously provided from Dr Edward Rubin (Lawrence Berkeley National Laboratory, USA)6) and backcrossed to C57BL/6 for 8 generations. Abca1−/− mice were purchased from Jackson Laboratory (Bar Harbor, USA) and also backcrossed to C57BL/6 14, 15). The animals were maintained at the facility of Nagoya City University Graduate School of Medical Sciences. Light and dark cycle has been kept between 8 am to 8 pm at a temperature of 25°C. Female mice were fed with the indicated chow during gestation and lactation based on standard MF chow (Oriental yeast LTD, Japan) listed in Supplementary Table 1. Probucol was a gift from Dai-ichi Sankyo Co. Ltd and standard MF chow containing the drug was prepared at CLEA Japan (Tokyo, Japan) and purchased through Chubu Kagaku Shizai Co, Ltd. (Nagoya, Japan). The experimental protocol was approved by the experimental animal welfare committee of Nagoya City University (approval number H18-34, H19-17, H20-16). For the determination of ABCA1 genotype, primer set of 5′-TGG GAA CTC CTG CTA AAA T-3′and 5′-CCA TGT GGT GTG TAG ACA-3′ for ABCA1 wild-type allele, 5′-TTT CTC ATA GGG TTG GTC A-3′ and 5′-TGC AAT CCA TCT TGT TCA AT-3′ for ABCA1-null allele were used. For LCAT genotype, a set of primers, 5′-TGA ACT CAG TAA CCA CAC ACG GCC TG-3′ for LCAT for wild-type allele, 5′-AAC GAG ATC AGC AGC CTC TGT TCC AC-3′ for LCAT-null allele and 5′-GTC CTC TGT CTT ACG GTA GCA CAT CC-3′ for common reverse primer was used. Sry gene was detected to identify the mouse gender by using 5′-ATC CCA GCA TGC AAA ATA CAG-3′ and 5′-CTG GTG GTG GTT ATG GAA CTG-3′ as primer pairs. To test the fertility, virgin females were placed with males in a genetic combination indicated and numbers of litters and pups were counted at their weaning stage that is generally considered as 3-week old during the mating period. On average, mating was continued for 3 months. Genotype of the pups were determined as described above.
Supplementary Table 1. Contents of mouse chow.
| MF standard chow | per 100 g chow |
|---|---|
| Water | 7.9 g |
| Protein | 23.1 g |
| Lipid | 5.1 g |
| Ash | 5.8 g |
| Fiber | 2.8 g |
| Nitrogen free extract | 55.3 g |
| Calories | 359 kcal |
| Vitamin A | 1283 IU |
| Vitamin D3 | 137 IU |
| Vitamin E | 9.1 mg |
| Vitamin K3 | 0.04 mg |
| Vitamin B1 | 2.05 mg |
| Vitamin B2 | 1.1 mg |
| Vitamin B6 | 0.87 mg |
| Vitamin B12 | 5.5 µg |
| Ca++ | 1.07 g |
| P | 0.83 g |
| Mg++ | 0.24 g |
| Na+ | 0.19 g |
| K+ | 0.9 g |
Plasma Lipoprotein Profile, ApoA-I Level, Corticosterone, and Glucose Concentration
Mouse blood was taken from retroorbital venous plexus under anesthesia and 5 µL of 0.5 M EDTA was mixed to 500 µL blood. Plasma obtained by centrifugation was stored at −80°C until analyzed. Lipoprotein profile was measured by high performance liquid chromatography using a TSK-Lipopropak XL column (4.6 mm I.D. × 300 mm) with an on-line enzymatic colorimetric detection system for cholesterol and triglyceride16, 17) (Skylight Biotech, Inc., Japan). ApoA-I concentration was determined by a mouse apoA-I ELISAPRO kit (anti-mouse HDL 38-biotin and anti-mouse HDL 94 rat monoclonal antibodies) with an acidic pretreatment system (Mabtech AB, Sweden).
Other Methods
Unpaired t test was performed for comparing litter size per single mating and two-tailed p values were obtained. Chi-squared analysis was applied to examine genotype distribution profile of weaned pups.
Results
Effect of Probucol Chow on the Litter Size at Weaning of Pups
The average litter size of weaned pups from one delivery is shown in Table 1 in the normal and low HDL mice models fed with the chow indicated in Supplementary Table 1. Over all litter size was significantly smaller with the ABCA1 and LCAT mutant mice at hetero-hetero mating and female hetero-male homo backcross mating (female Abca1−/− mice are infertile5)). Natural mating between Abca1+/− parents reproduced significantly reduced the number of pups to 60% of the wild type even with high hydrophobic vitamins containing CMF breeding chow (data not shown). On the other hand, feeding 0.2% probucol-containing chow that induces 95% reduction in plasma HDL in wild-type mice7) caused no significant reduction in the total litter size of offspring in the wild type, and no further significant decrease in the ABCA1 and LCAT mutant mice.
Table 1. Litter size and Genotype of weaned pups of low HDL mice: Single and double asterisks indicate reduction of the litter size from Wild x Wild under standard chow as p < 0.05 and p < 0.01, respectively. Underlined Chi-squared p indicates reduction from the expected number by Mendelian genetics by p < 0.05.
| Genotype of parents |
n (mating pair) |
Litter size | Male % | Chi-squared p |
|||
|---|---|---|---|---|---|---|---|
| Gender ratio | Homozygote |
||||||
| Female | Male | ||||||
| MF standard chow | Wild x Wild | 5 | 10.0 ± 1.4 | 47.6 | 0.827 | ||
| Abca1+/− (f) x Abca1−/−(m) | 11 | 6.6 ± 2.6* | 37 | 0.059 | 0.078 | < 0.001 | |
| Abca1+/−x Abca1+/− | 29 | 6.2 ± 2.3** | 53.3 | 0.748 | 0.018 | 0.0014 | |
| Lcat+/− (f) x Lcat−/−(m) | 40 | 5.6 ± 2.3** | 43.3 | 0.352 | 0.715 | 0.033 | |
| Lcat+/−x Lcat+/− | 15 | 7.5 ± 1.6** | 46 | 0.249 | 0.920 | 0.024 | |
| 0.2% probucol | Wild x Wild | 5 | 8.6 ± 1.1 | 53.5 | 0.647 | ||
| Abca1+/−x Abca1+/− | 27 | 6.3 ± 1.5* | 44.2 | 0.122 | 0.010 | 0.022 | |
| Lcat+/−x Lcat+/− | 23 | 6.9 ± 2.4** | 51.3 | 0.656 | 0.839 | 0.846 | |
Deviation of Genotype Distribution among the Weaned Pups from Mendelian Inheritance
Genotypes and gender of the offspring were determined at the weaning period of 3-week old for the cross-breeding of the mutant mice under the MF standard chow. Mating of the Abca1+/− female with Abca1−/− male yielded the overall ratio of the heterozygotes to the homozygotes of 1: 0.56, yielding less homozygotes than that expected (CHISQ p = 0.0036) and the reduction was significant in both male and female (Fig. 1A, Table 1). Mating between the Abca1+/−, the genotype ratio in the littermates of wild type, heterozygotes and homozygotes was 1: 2.2: 0.57 in total, showing reduction in homozygotes from the expected Mendelian genetic distribution (CHISQ p = 0.001) and the reduction was significant in both male and female (Fig. 1B, Table 1). In the case of LCAT deficiency, natural mating of the Lcat+/− female and Lcat−/− male mice yielded less homozygote pups only in male at the weaning stage (1: 0.66, CHISQ p = 0.033, Fig. 1C, Table 1). Mating between the heterozygotes, significant reduction of the homozygote was also unique in male pups (1:1.8:1 in female and 1:1.8:0.3 in male, CHISQ p = 0.92, p = 0.024, respectively) (Fig. 1D, Table 1). Thus, the reduction of the litter size in the genetic HDL-deficient mice is attributed to decrease of Abca1−/− male and female or decrease of Lcat−/− male pups.
Fig. 1.
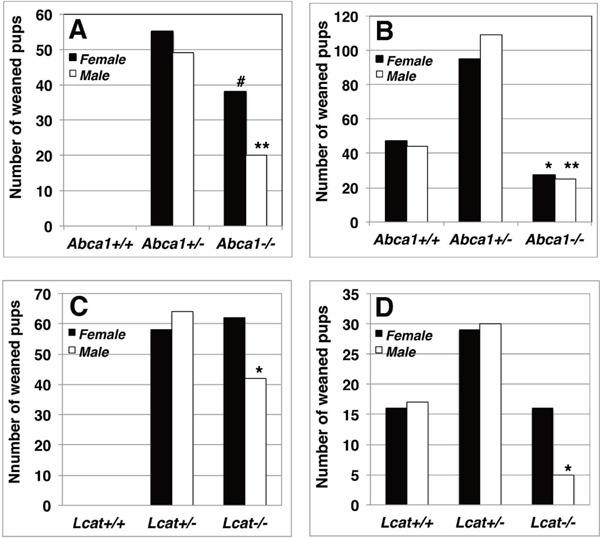
Genotype distribution profile of the offspring at the weaned stage by mating HDL-deficient mutant mice under feeding of standard MF breeding chow
A. Abca1+/− female were crossed to Abca1−/− male. B. Natural mating of Abca1+/− parents. C. Natural mating between Lcat+/− female and Lcat−/− male. D. Mating between Lcat+/− mice. Numbers and genders of the offspring were recorded at 3 weeks old. Solid columns indicate female and open columns indicate male. Single and double asterisks indicate deflection from Mendelian distribution of total number of pups by p < 0.05 and 0.01, respectively, by Chi-square analysis. # indicates deflection by p = 0.059 and p = 0.013 from Mendelian distribution of total number of pups and that standardized for the heterozygote, respectively.
Normalization of the Genotype Deflection by Probucol
A strong ABCA1 inhibitor probucol caused only slight non-significant decrease in the reproduction litter size without gender ratio change at the mating between wild-type mice (Table 1). No apparent litter size change was observed by probucol in the genetic HDL-deficient mice (Table 1). Genotype distribution in the offspring by mating Abca1+/− vs Abca1+/− was not influenced by feeding probucol-containing chaw showing significant decrease in Abca1−/− genotype either male or female (Fig. 2A, Table 1). On the other hand, probucol feeding increased genotype Lcat−/− in male weaned pups from mating of the Lcat+/− parents to cancel the significant decrease in the male Lcat−/−(Fig. 2B, Table 1).
Fig. 2.
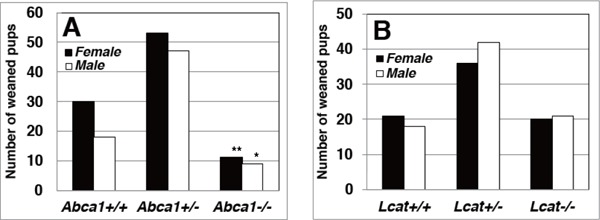
Genotype distribution profile of the offspring at the weaned stage by mating HDL-deficient mutant mice under feeding of standard MF chow containing 0.2% probucol
A. Mating between Abca1+/− parent mice. B. Mating between Lcat+/− pairs. Numbers and genders of the offspring were recorded at 3 weeks old. Solid columns indicate female and open columns indicate male.
Analyses of Plasma Lipoprotein and Related Parameters in the HDL-Deficient Mice
Lipoprotein profile and glucose level in plasma were analyzed for the HDL-deficient mice. Plasma lipoprotein was analyzed by high performance liquid chromatography (HPLC) for wild-type mice, Abca1−/− mice and Lcat−/− mice under standard chow or 2 week-feeding of 0.5% probucol chow. Total cholesterol in HDL fraction was not detectable in Abca1−/− and Lcat−/− mice at conventional measurement sensitivity (Fig. 3A, C, E). Increase of sensitivity demonstrated trace amount of triacylglycerol-rich HDL particles in Abca1−/− mice plasma, with a diameter of 10.1 nm based on calibration for retention time. Lcat−/− mice also have minuscule amount of HDL peak of diameter of 14.9 nm, larger than that found in Abca1−/− mice. Probucol reduced mouse HDL by 95% in wild-type mice being consistent with our previous finding (Fig. 3B)7). Probucol slightly increased HDL particle size while reducing it plasma level both in Abca1−/− mice and Lcat−/− mice (Fig. 3D, F). Plasma apoA-I concentration was also decreased by probucol in the wildtype animals (Table 2).
Fig. 3.
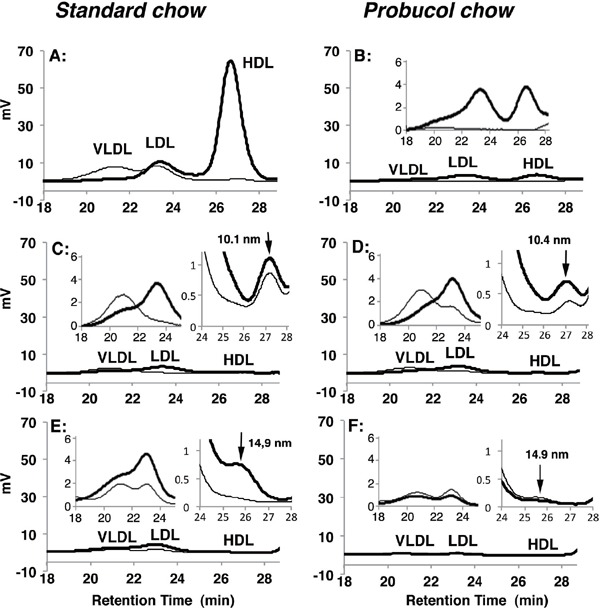
Plasma lipoprotein profile analyzed by HPLC using a tandem TSK gel lipopropak XL (4.6 mm I.D. × 300 mm) equipped with on-line enzymatic coloring reagent system to monitor cholesterol (thick lines) and triglyceride (thin lines) (Skylight Biotech Inc, Japan)
A. Wild type mice under standard chow. B. Wild type mice fed with 0.5% probucol-containing chow. C. Abca1−/− mice under standard chow. D. Abca1−/− mice fed with 0.5% probucol chow. E. Lcat−/− mice under standard chow. F. Lcat−/− mice fed with 0.5% probucol chow. Bold solid lines indicate total cholesterol, and thin sold lines indicate triacylgrycerol. Insets to each panel show VLDL/LDL fraction and HDL fraction in expanded/magnified scale. Arrow in insets show a lipoprotein peak in HDL fraction.
Table 2. Plasma apoA-I concentration (mg/dL).
| Standard chow | Probucol chow | |
|---|---|---|
| Wild type | 153.8 ± 146.2 | 54.5 ± 31.9* |
| ABCA1-null | 6.5 ± 6.8 | 6.3 ± 3.6 |
| LCAT-null | 14.4 ± 17.3 | 4.6 ± 4.2 |
Mouse plasma apoA-I concentration in wild type and genetic low HDL mice, with and without 0.2% probucol-containing chow, determined by mouse apoA-I ELISA Pro kit (MABTECH, Sweden). Asterisk indicates statistical significance in the decrease from standard chow (p < 0.05).
Other Parameters under Probucol Treatment in the HDL-Deficient Mice
Plasma corticosterone level was low in the genetic HDL-deficient mice and somewhat increased by probucol in the Abca1−/− mice and the Lcat−/− mice. However, it decreased in the wild-type mice by probucol to the similar level to the HDL-deficient mice (Supplementary Fig. 1). Cholesterol contents in the steroidgenic organs, such as adrenal cortex and ovaries, decreased in the low HDL mice, including the probucol-treated normal mice, but probucol had no apparent further effect in the genetic HDL-deficient mice (Supplementary Fig. 1 and Table 3). On the other hand, expression of the enzymes such as HMG-CoA reductase, StAR, and cytochrome P450scc (CYP11a1) indicates increased cholesterol turn-over by probucol (Supplementary Fig. 2). VKORC1 was also found increased by probucol, which may indicate accelerated vitamin K turn-over (Supplementary Fig. 3).
Supplementary Fig. 1.
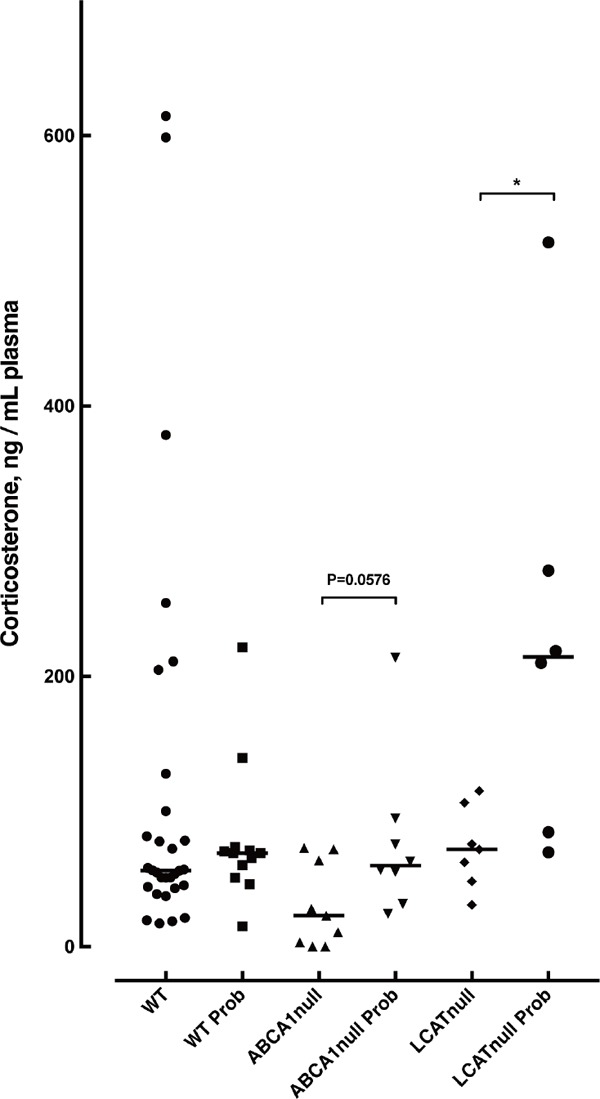
Plasma corticosterone levels in mice
Wild type, Abca1−/− mice and Lcat−/− mice were treated with standard chow or 0.2% probucol chow for 2 weeks. Mouse plasma was collected retroobitally. Asterisk indicates statistical significance (p < 0.05).
Supplementary Fig. 2.
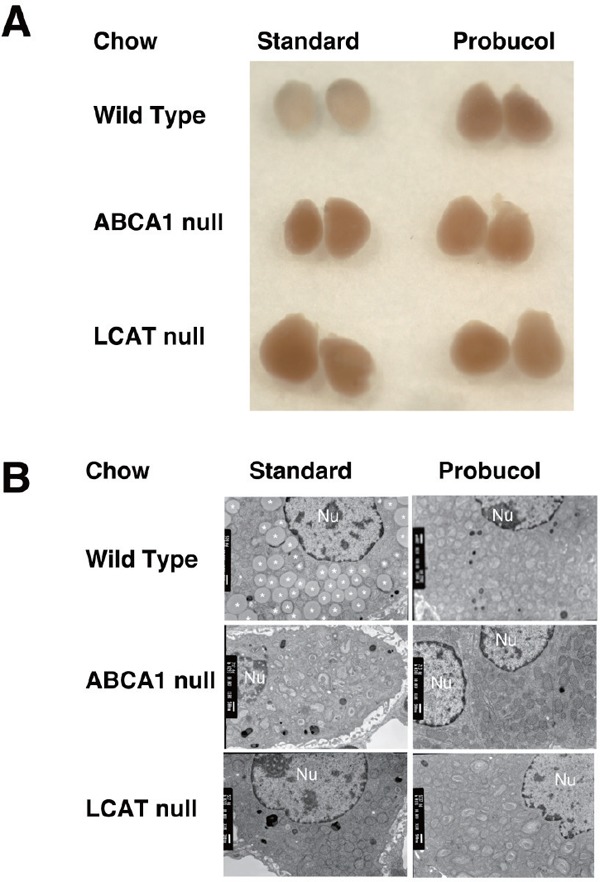
Adrenal glands of the low-HDL model mice
A. Outlook of the isolated organs. Adrenal glands were isolated from wild type, Abca1t−/−, and Lcat−/− mice, after feeding standard chow-fed or 0.5% probucol chow for 2 weeks. B. Transmitting electron microscopy of the adrenal cortex cells, from the animals fed standard chow or probucol-containing chow for 2 weeks. Nu, indicate nucleolus.
Supplementary Fig. 3.
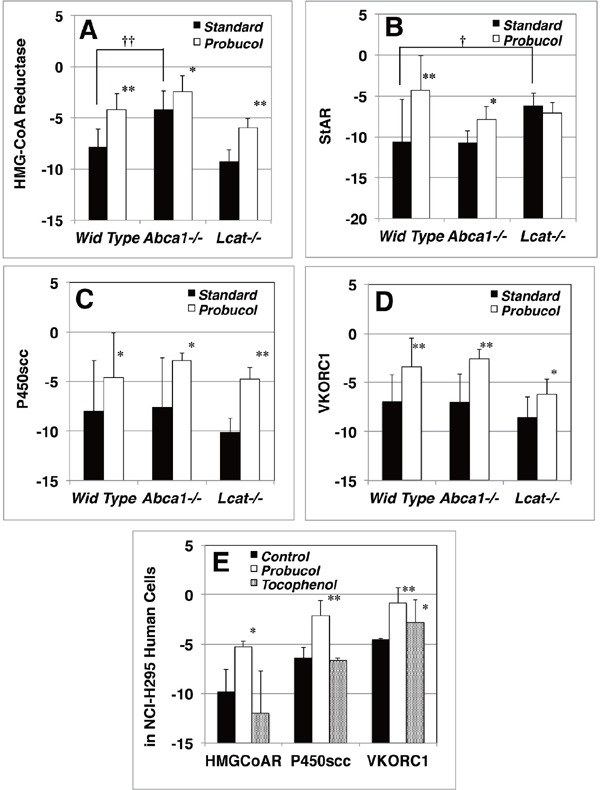
Expression of lipid-related genes measured as mRNA
Quantitative RT-PCR analysis was performed using mice adrenal glands mRNA converted to first strand cDNA as the template cDNA for HMG-CoA reductase, StAR, cytochrome P450scc, and VKORC1. A–D. Gene expression in the adrenal cortex of mice standard chow-fed (solid column) and 0.2% probucol chow-fed (open column), by using a reference gene of glyceroaldehyde-3-phosphate dehydrogenase. *, P < 0.05; **, P < 0.01, for standard chow vs probucol chow; †, P < 0.05; ††, P < 0.01 against wild type. E. Gene Expression in NCI-H295 adrenocortical cells. Cells were incubated in MEMα/F12 + 1% ITS + 0.5% BSA for 18 h to deplete cholesterol from medium. Probucol or α-Tocopherol conjugated BSA was supplemented to the medium for 48 h. Gene expressions were determined by real time RT-PCR to a reference gene β-actin. Solid column, control-BSA; open column, probucol-BSA; dotted column, α-Tocopherol-BSA. *, P < 0.05; **, P < 0.01 against control. The data are expressed as ΔCq values.
Discussion
Roles of plasma HDL in propagation in mice was investigated using models of genetic HDL-deficiency by silencing the genes for ABCA1 or LCAT and by feeding a strong ABCA1 inhibitor probucol that reportedly rescued female infertility in the SRB1-deficient mice13). The yield of the homozygote weaned pups decreased both in the second filial generation of the hybrid mating and in the backcross propagation, deflecting from the expected profile in Mendelian genetics. Interestingly, the decrease was only in males in the LCAT-deficient model. On the other hand, probucol severely decreased plasma HDL but had no effects on the litter size in propagation. This compound normalized the deflection of the genotype distribution in the second filial generation in the LCAT-deficient model.
Lipoprotein profiles and some lipid-related metabolic parameters were examined in these animals; however, nothing was identified to relate to the specific genotype deflection in propagation of HDL-deficient mice or its normalization by probucol. Lipoprotein profiles were largely consistent with what is known for these types of HDL-deficiency. ABCA1 deficiency lacks generation of new HDL so that basically no HDL is found in plasma, including its very nascent particle, preβ HDL. A trace amount of TG-rich particle was found in the HDL fraction potentially generated by lipolysis of VLDL18). LCAT deficiency, a defect of HDL maturation by cholesterol acyl-esterification, is characterized by the accumulation of preβ-HDL, so that a trace amount of HDL-like particle with relatively lager diameter is consistent with such fraction19). Probucol as a strong inhibitor of ABCA1 induced the phenotype of HDL metabolism similar to ABCA1 deficiency9). Cholesterol content in steroidogenic adrenal glands and ovaries decreased in all the types of these low HDL models, indicating that HDL is its major source for these organs in mice.
The effect of probucol is puzzling. This compound has been known for many “beneficial” actions20) despite ABCA1 inhibition and subsequent severe reduction of HDL21), including regression of skin and tendinous xanthomas22) and the prevention of atherosclerotic events23–26), a part of which may be due to strong antioxidative nature of this drug23). Vitamin E, α-tocopherol, is known to improve reproduction rate in experimental animals, so that the antioxidative nature of probucol may partially be responsible for the effect presented.
Deficiency of SR-BI, the cellular-uptake mediator of HDL-cholesteryl ester in rodents10), also causes cholesterol depletion the steroidogenic organs in mice like the HDL-deficient models despite of marked increase of plasma HDL27). This model exhibited impairment of female fertility12), and probucol reportedly normalized this infertility13). The authors discussed this effect in relation to decrease in abnormal large HDL by probucol, which however unlikely supports the current findings that peculiar deflection of propagation profile in HDL-deficient mice is normalized by probucol without significant change in lipoprotein profile.
Probucol apparently increased transcription of the enzymes related to synthesis of cholesterol and steroid hormones, HMG-CoA reductase, StAR, and cytochrome P450scc (CYP11a1, CYP11A1) and this seems reflected in the level of plasma corticosteroid. However, it is not evident whether these effects are related to the recovery of the deflection of genotype distribution in the weaned pups. Another interesting finding is the increase by probucol of the expression of VKORC1, the rate-limiting factor in the vitamin K cycle and the target subunit of anticoagulant warfarin. Thus, it is conceivable that probucol may enhance the effect of vitamin K. If the cause of low reproduction of the HDL-deficient mice is anything unknown but related to bleeding tendency, this effect may rescue such a problem. It should be noted that idiopathic type of vitamin K deficiency afflicts two times higher in male infant (587 male /304 female)28) suggesting suppression of vitamin K cycle or activation by androgen as the gender difference disappeared by castration or by supplement of estrogen in rat29). This is still very preliminary speculation, requiring more profound validation.
The findings reported here are largely empirical and descriptive. The parameter evaluated was the number of weaned pops that survived through many steps involved before this stage such as fertilization, implantation, embryo growth, delivery process, and postnatal nutrition. Each step should be investigated for the cause of infertility and the effect of probucol. It is still totally puzzling what probucol does in the propagation of the HDL-deficient mice. However, this compound may be useful to have high yield of the homozygous HDL-deficient mice of certain genotypes. Further investigation is required to elucidate the background mechanism for this effect of probucol.
Clinical relevance of the present findings is uncertain. A recent case report indicated oligoasthenoteratozoospermia and male infertility in a Tangier patient probably due to decrease in testosterone production30). On the other hand, SR-B1 deficiency may not generally cause female infertility in humans31). No report has been found about reproduction problem in LCAT-deficient patients. It may not be easy to extract quantitative conclusion from these relatively-rare human genetic diseases cohort.
Acknowledgments
Authors are grateful to Mr. Takumi Kishida and Mr. Yasutaka Maekawa, medical students in Nagoya City University Graduate School of Medical Sciences, for their excellent technical assistance. Authors also appreciate Dr. Shigetoshi Tomimoto, MD.PhD, a former graduate student in Nagoya City University for his supports in animal handlings and breeding during the time. Authors would like to show gratitude to Mr. Hiroshi Takase, Core lab staff of Nagoya City University Graduate School of Medical Sciences for his outstanding technical support on electron microscopies.
Funding Sources
This project was supported by Grant-in-Aid from Ministry of Education, Culture, Science and Technology of Japan (MEXT) (12557095, 13770651, 14370340, 1559052, 23591338, 15K08615, 15K15349), MEXT-supported Program for Strategic Founding of Research in Private Universities (S1201007), Takahashi Industrial and Economic Research Foundation (08-00303038), Nagoya City University Grant-in-Aid for Encouragement of Scientists 2018 and by Health, Labour and Welfare Sciences Research Grant for Research on Rare and Intractable Diseases.
Clinical Interest and Disclosures
No author has conflict of interest to be disclosed.
Supplementary Materials
Changes by Probucol in the Parameters other than Lipoprotein and the Related Factors
Materials and Methods
Plasma Corticosterone Concentration
Plasma corticosterone level was measured by Corticoterone ELISA Kit (Assaypro LLC, USA), quantitative competitive enzyme immunoassay system with biotinylated corticosterone as a competitor. Plasma glucose level was determined by Glucose CII-test WAKO kit (Wako Pure Chemical Industries, Ltd., Japan).
Measurement of Cholesterol Content in Adrenal Grand, Ovary and Liver
For determination of cholesterol content in the organs, adrenal glands, ovaries and liver were harvested after perfusion with PBS containing 5 mM EDTA from the left ventricle under euthanasia. Mouse organs were dissected and homogenized by micro homogenizer physcotoron NS-310E (NITION) in PBS containing 0.5 mM EDTA, 1% proteinase inhibitor cocktail (Sigma-Aldrich). Protein concentration was measured by using BCA protein assay reagent using bovine serum albumin (BSA) or bovine gamma globulin as a standard. Lipid was extracted with 4 volume of chloroform: methanol (2:1), and total and unesterified cholesterol were measured by enzymatic assay reagents (Kyowa Medics, Japan).
Morphological Observation of the Adrenal Glands
For harvesting organs, anesthetized mice were perfused with phosphate buffered saline (PBS) containing 5 mM EDTA. Adrenal grands were photographed with Canon EOS-digital camera equipped with EF100 mm f/2.8L Macro IS USM microlens. For transmission electron microscopy observation, the mice adrenals were transferred into ice cold 5% Glutaraldehyde and 2% formaldehyde in 0.1 M PBS, pH 7.4, for the primary fixation immediately after decapitation. The organs were fixed further with 2% OsO4 in 0.1 M PBS, pH 7.4, washed with ethanol dehydrated and embedded. The thin sections were stained with uranyl acetate and Sato's modified lead solution for density contrast1). The cellular morphologies were observed by a transmission electron microscopy, JEM- 1011J (JEOL Ltd., Japan).
Gene Expression Analysis
cDNA was synthesized from 1 ng total RNA using Superscript® III reverse transcriptase kit (Invitrogen, Carlsbad, CA). For qPCR analysis, primers were designed using the NCBI/Primer-blast (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) based on EST sequence data and selected primer set covering the exon-exon junctions (In-Silico PCR, http://genome.ucsc.edu/cgi-bin/hgPcr?command=start), for hydroxylmethylglutaryl coenzyme A (HMG-CoA) reductase, steroidogenic acute regulators protein (StAR), cytochrome P450scc (CYP11a1, CYP11A1), and vitamin K epoxide reductase complex 1 (VKORC1). Supplemental Table 2 lists the primer sequences. qPCR reactions contained 227 ng of cDNA, 83 nM of each primer and 6 µL of SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) in a total volume of 12 µL. All the reactions were performed in triplicate on an Applied Biosystems StepOne Real-Time PCR System. Relative mRNA levels were calculated by the comparative threshold cycle method using β-actin as the internal control.
Supplementary Table 2. Primer sequences used for real-time RT-PCR Analysis.
| Primer | Sequence (5′-3′) Forward | Sequence (5′-3′) Reverse |
|---|---|---|
| β-actin (mouse/human) | AAGAGAGGCATCCTCACCCT | CTGTGTTGGCGTACAGGTCT |
| CYP11A1 | ACGCTCAGTCCTGGTCAAAG | TACTGGTGATAGGCGACCCA |
| CYP11a1 | GCGTCGATACTCTTCTCATGC | AAGGAAAAGCGGAATAGGTCA |
| GAPDH (mouse) | GCCAGCCTCGTCCCGTAGACA | ACCCGTTTGGCTCCACCCTTC |
| HMG-CoA R (human) | GCCCTCAGTTCCAACTCACA | GCCAGAGGGAAACACTTGGT |
| HMG-CoA R (mouse) | GATCGAAGGACGAGGAAAGAC | CACATCACCAGTTTCCAGCTT |
| VKORC1 (human) | CAGGACAGCATCCTCAATCA | CGGCTCACGTTGATAGCATA |
| VKORC1 (mouse) | TTTGGTTGCCTGTTCTACACC | TCTAGGAACCCACACACTTGG |
Probucol Incorporation into NCI-H295
Probucol was introduced into NCI-H295 cells, a generous gift from Professor Takeshi Yamazaki, Hiroshima University, by using probucol-conjugated BSA. α-Tocopherol acetate (Wako, Japan) was incorporated by the same manner. The NCI-H295 cells were cultured in MEM alpha / F12 culture medium with 1% ITS and 2% NuSerum® (BD Co, Japan). Twenty-four hours prior to the lipoprotein treatment, cell medium was changed to the same medium but replacing 2% NuSerum to 0.2% BSA to deplete cholesterol in the medium. In some dishes, BSA was replaced by probucol or α-tocopherol conjugated BSA in the medium. The cells were collected and lipids and total RNA were extracted after 48 hours of incubation at 37°C 5% CO2.
Results
Plasma Corticosterone Concentration
Plasma corticesterone concentration was examined for the 6 groups of mice (Supplementary Fig. 1). As the plasma was collected under mild natural stresses environment, corticosterone was found as high as over 600 ng/mL in some of wild type mice under the standard chow, while those probucol-fed showed up to 200 ng/mL (119.2 ± 154 vs 79.44 ± 52.9, P = 0.3915). Abca1−/− mice and Lcat−/− mice showed 30.39 ± 30.98 ng/mL and 73.02 ± 30.02 ng/mL, respectively. Though it is somewhat decreased, steroid hormone synthesis in the basal condition seems to be maintained even in the HDL deficient mice. ABCA-null mice under 0.2% probucol chow showed somewhat increased corticosterone level (76.99 ± 59.71, two-tailed t-test vs. standard chow fed ABCA1-null mice, P = 0.0576). Interestingly, some of the probucol-fed LCAT-null mice showed increased corticosterone level as high as the wild type resulting in significant increase overall (230.4 ± 163.9, two-tailed t-test vs. standard chow fed LCAT-null mice, P = 0.029). No significant difference was observed in plasma glucose levels by probucol chow challenge (WT 103.2 ± 52.3 vs 93.43 ± 30.7, ABCA1-null 93.14 ± 27.42 vs 118.4 ± 39, LCAT-null 100.24 ± 21.7 vs 121.2 ± 38.5 mg glucose/dL).
Analyses of Cholesterol Homeostasis in the Steroidogenic Organs and Cells
We further examined adrenal glands for cholesterol homeostasis. Adrenal glands of Abca1−/− and Lcat−/− mice both appeared reddish-blown and enlarged compared to whitish outlook of the wild type glands (Supplementary Fig. 2A, left lane). LCAT-null mice showed adrenomegaly. Probucol challenge on wild type mice for two weeks modified the color to reddish-blown and the size enlarged, similarly to other low HDL mice. The color and outlook of the adrenal gland still appeared with reddish color under the probucol treatment despite somewhat increase of corticosterone level in Abca1−/− and Lcat−/− mice. Supplementary Fig. 2B indicates typical images of adrenal cortex cells by transmission electron microscopy. Abundant lipid droplets observed in wild type mice cells were vanished in the cortex cells of any low-HDL mice and the probucol treated mice. Supplementary Table 2 summarizes cholesterol contents in the adrenal glands, the ovaries and the liver. Probucol feeding significantly reduced cholesteryl ester in the adrenal glands and ovary in the wild type mice (Supplementary Fig. 2B). Cholesterol content in the adrenal glands also significantly decreased in Abca1−/− and Lcat−/− mice. Probucol chow challenge brought no apparent change in these steroidogenic organs in Abca1−/− and in Lcat−/− mice.
Supplementary Fig. 3 shows expression of the genes related to cholesterol homeostasis and steroido-genesis in the adrenal cortex. Expression of HMG-CoA reductase gene significantly increased in Abca1−/− mice under the standard chow but not in Lcat−/− mice (Supplementary Fig. 3A). Interestingly, Probucol chow feeding significantly increased expression of this gene in the wild type adrenal cortex. Furthermore, probucol increased this gene expression in both Abca1−/− and Lcat−/− mice. It also increased expression of StAR which transfers cholesterol into the inner leaflet of mitochondria in the adrenal cortex of wild type and Abca1−/− mice. In Lcat−/− mice, StAR expression significantly increased compared to standard chow-fed wild type mice regardless of probucol challenge (Supplementary Fig. 3B). Interestingly, probucol significantly increased expression of cytochrome P450scc (CYP11a1), the rate-limiting enzyme for steroid hormone synthesis, in Abca1−/− and Lcat−/− mice (Supplementary Fig. 3C). These results conjecture that probucol challenge stimulates cholesterol de novo synthesis for steroid hormone generation in the restrained cholesteryl ester storage in adrenal cortex cells in low-HDL mice. Furthermore, probucol increased VKORC1, a key enzyme for vitamin K cycle to regulate turnover of active vitamin K, in the adrenal cortex of the all genotypes (Supplementary Fig. 3D) which avoids life threatening vitamin K dependent bleeding of pups.
Further analysis was carried out by using adrenal cortex cell line cells, NCI-H295, human adrenal carcinoma for the effects of probucol and α-tocopherol as an antioxidant. The cells were incubated with BSA conjugated-probucol or BSA conjugated-α-tocopherol. Expression of HMG-CoA reductase and cytochrome P450scc (CYP11A1) significantly increased by probucol (Supplementary Fig. 3D) being consistent with the findings in the probucol-fed mice (Supplementary Fig. 3A, C). VKORC1 expression also significantly increased by probucol treatment, implicating efficient vitamin K cycle in this condition (Supplementary Fig. 3D). α-Tocopherol laden NCIH295 cells express VKORCI significantly higher than the control, indicating that α-tocopherol may not be involved in cholesterol and steroid synthesis but possibly maintaining vitamin K level (Supplementary Fig. 3E).
Supplementary Table 3. Cholesterol contents in steroidogenic organs in low HDL mice (mg/g protein).
| Organ | Wild Type |
ABCA1-null |
LCAT-nul |
|||
|---|---|---|---|---|---|---|
| Standard | Probucol | Standard | Probucol | Standard | Probucol | |
| Adrenal glands | ||||||
| FC | 52.3 ± 16.2 | 39.7 ± 9.18 | 33.8 ± 4.26 | 26.8 ± 5.82 | 35.07 ± 14.7 | 28.2 ± 12.8 |
| CE | 616 ± 219 | 193 ± 134** | 14.6 ± 6.35†† | 14.8 ± 1.69 | 18.4 ± 7.14† | 34.4 ± 29.9 |
| Ovary | ||||||
| FC | 32.3 ± 3.85 | 31.7 ± 3.45 | 38.0 ± 3.61 | 35.9 ± 5.08 | 33.3 ± 3.15 | 34.4 ± 0.46 |
| CE | 148.2 ± 39.1 | 59.2 ± 21.2** | 64.5 ± 2.57† | 99.4 ± 42.2 | 28.8 ± 9.24†† | 20.4 ± 2.49† |
| Liver | ||||||
| FC | 6.40 ± 0.94 | 7.19 ± 1.07 | 6.29 ± 0.80 | 6.31 ± 0.12 | 6.74 ± 0.54 | 7.15 ± 0.42 |
| CE | 3.75 ± 0.53 | 3.60 ± 0.89 | 3.19 ± 1.58 | 3.28 ± 0.40 | 4.45 ± 1.07 | 2.16 ± 0.62† |
p < 0.01 vs standard chow,
p < 0.05 vs wild type,
p < 0.01 vs wild type.
FC, free (unesterified) cholesterol; CE, cholesteryl ester
References
- 1). Gwynne JT, Hess B: The role of high density lipoproteins in rat adrenal cholesterol metabolism and steroidogenesis. J Biol Chem, 1980; 255: 10875-10883 [PubMed] [Google Scholar]
- 2). Higashijima M, Kato K, Nawata H, Ibayashi H: Studies on lipoprotein and adrenal steroidogenesis: Ii. Utilization of low density lipoprotein- and high density lipoprotein-cholesterol for steroid production in functioning human adrenocortical adenoma cells in culture. Endocrinol Jpn, 1987; 34: 647-657 [DOI] [PubMed] [Google Scholar]
- 3). Bochem AE, Holleboom AG, Romijn JA, Hoekstra M, Dallinga-Thie GM, Motazacker MM, Hovingh GK, Kuivenhoven JA, Stroes ES: High density lipoprotein as a source of cholesterol for adrenal steroidogenesis: A study in individuals with low plasma hdl-c. J Lipid Res, 2013; 54: 1698-1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Bochem AE, Holleboom AG, Romijn JA, Hoekstra M, Dallinga GM, Motazacker MM, Hovingh GK, Kuivenhoven JA, Stroes ES: Adrenal function in females with low plasma hdl-c due to mutations in abca1 and lcat. PLoS One, 2014; 9: e90967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Christiansen-Weber TA, Voland JR, Wu Y, Ngo K, Roland BL, Nguyen S, Peterson PA, Fung-Leung WP: Functional loss of abca1 in mice causes severe placental malformation, aberrant lipid distribution, and kidney glomerulonephritis as well as high-density lipoprotein cholesterol deficiency. Am J Pathol, 2000; 157: 1017-1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Ng DS, Francone OL, Forte TM, Zhang J, Haghpassand M, Rubin EM: Disruption of the murine lecithin: Cholesterol acyltransferase gene causes impairment of adrenal lipid delivery and up-regulation of scavenger receptor class b type i. J Biol Chem, 1997; 272: 15777-15781 [DOI] [PubMed] [Google Scholar]
- 7). Tsujita M, Yokoyama S: Selective inhibition of free apolipoprotein-mediated cellular lipid efflux by probucol. Biochemistry, 1996; 35: 13011-13020 [DOI] [PubMed] [Google Scholar]
- 8). Wu CA, Tsujita M, Hayashi M, Yokoyama S: Probucol inactivates abca1 in the plasma membrane with respect to its mediation of apolipoprotein binding and high density lipoprotein assembly and to its proteolytic degradation. J Biol Chem, 2004; 279: 30168-30174 [DOI] [PubMed] [Google Scholar]
- 9). Tsujita M, Tomimoto S, Okumura-Noji K, Okazaki M, Yokoyama S: Apolipoprotein-mediated cellular cholesterol/phospholipid efflux and plasma high density lipoprotein level in mice. Biochim Biophys Acta, 2000; 1485: 199-213 [DOI] [PubMed] [Google Scholar]
- 10). Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M: Identification of scavenger receptor sr-bi as a high density lipoprotein receptor. Science, 1996; 271: 518-520 [DOI] [PubMed] [Google Scholar]
- 11). Christianson MS, Yates M: Scavenger receptor class b type 1 gene polymorphisms and female fertility. Curr Opin Endocrinol Diabetes Obes, 2012; 19: 115-120. 110.1097/MED.1090b1013e3283505771 [DOI] [PubMed] [Google Scholar]
- 12). Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, Krieger M: Influence of the high density lipoprotein receptor sr-bi on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci U S A, 1999; 96: 9322-9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Miettinen HE, Rayburn H, Krieger M: Abnormal lipoprotein metabolism and reversible female infertility in hdl receptor (sr-bi)-deficient mice. J Clin Invest, 2001; 108: 1717-1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Tsujita M, Wu CA, Abe-Dohmae S, Usui S, Okazaki M, Yokoyama S: On the hepatic mechanism of hdl assembly by the abca1/apoa-i pathway. J Lipid Res, 2005; 46: 154-162 [DOI] [PubMed] [Google Scholar]
- 15). Hossain MA, Tsujita M, Akita N, Kobayashi F, Yokoyama S: Cholesterol homeostasis in abca1/lcat double-deficient mouse. Biochim Biophys Acta, 2009; 1791: 1197-1205 [DOI] [PubMed] [Google Scholar]
- 16). Usui S, Hara Y, Hosaki S, Okazaki M: A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by hplc. J Lipid Res, 2002; 43: 805-814 [PubMed] [Google Scholar]
- 17). Toshima G, Iwama Y, Kimura F, Matsumoto Y, Miura M, Takahashi J, Yasuda H, Arai N, Mizutani H, Hata K, Usui S, Okazaki M: Liposearch®; analytical gp-hplc method for lipoprotein profiling and its applications. J Biol Macromol, 2013; 13: 21-32 [Google Scholar]
- 18). Pussinen PJ, Malle E, Metso J, Sattler W, Raynes JG, Jauhiainen M: Acute-phase hdl in phospholipid transfer protein (pltp)-mediated hdl conversion. Atherosclerosis, 2001; 155: 297-305 [DOI] [PubMed] [Google Scholar]
- 19). Roshan B, Ganda OP, Desilva R, Ganim RB, Ward E, Haessler SD, Polisecki EY, Asztalos BF, Schaefer EJ: Homozygous lecithin:Cholesterol acyltransferase (lcat) deficiency due to a new loss of function mutation and review of the literature. J Clin Lipidol, 2011; 5: 493-499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Yamashita S, Ruscica M, Macchi C, Corsini A, Matsuzawa Y, Sirtori CR: Cholesteryl ester transfer protein: An enigmatic pharmacology - antagonists and agonists. Atherosclerosis, 2018; 278: 286-298 [DOI] [PubMed] [Google Scholar]
- 21). Yokoyama S, Yamamoto A, Kurasawa T: A little more information about aggravation of probucol-induced hdlreduction by clofibrate. Atherosclerosis, 1988; 70: 179-181 [DOI] [PubMed] [Google Scholar]
- 22). Yamamoto A, Matsuzawa Y, Yokoyama S, Funahashi T, Yamamura T, B. K: Effects of probucol on xanthomata regression in familial hypercholesterolemia. Am J Cardiol, 1986; 57: 29H-35H [DOI] [PubMed] [Google Scholar]
- 23). Kita T, Nagano Y, Yokode M, Ishii K, Kume N, Ooshima A, Yoshida H, Kawai C: Probucol prevents the progression of atherosclerosis in watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A, 1987; 84: 5928-5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Braun A, Zhang S, Miettinen HE, Ebrahim S, Holm TM, Vasile E, Post MJ, Yoerger DM, Picard MH, Krieger JL, Andrews NC, Simons M, Krieger M: Probucol prevents early coronary heart disease and death in the high-density lipoprotein receptor sr-bi/apolipoprotein e double knockout mouse. Proc Natl Acad Sci U S A, 2003; 100: 7283-7288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Yamashita S, Hbujo H, Arai H, Harada-Shiba M, Matsui S, Fukushima M, Saito Y, Kita T, Matsuzawa Y: Longterm probucol treatment prevents secondary cardiovascular events: A cohort study of patients with heterozygous familial hypercholesterolemia in japan. J Atheroscler Thromb, 2008; 15: 292-303 [DOI] [PubMed] [Google Scholar]
- 26). Yamashita S, Masuda D, Ohama T, Arai H, Bujo H, Kagimura T, Kita T, Matsuzaki M, Saito Y, Fukushima M, Matsuzawa Y: Rationale and design of the prospective trial: Probucol trial for secondary prevention of atherosclerotic events in patients with prior coronary heart disease. J Atheroscler Thromb, 2016; 23: 746-756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M: A targeted mutation in the murine gene encoding the high density lipoprotein (hdl) receptor scavenger receptor class b type i reveals its key role in hdl metabolism. Proc Natl Acad Sci U S A, 1997; 94: 12610-12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Shirahata A: Vitamin k deficiency bleeding in newborn and young infant. The Japanese Journal of Pediatric Hematology, 2001; 22: 95-103 [Google Scholar]
- 29). Matschiner JT, Willingham AK: Influence of sex hormones on vitamin k deficiency and epoxidation of vitamin k in the rat. The Journal of Nutrition, 1974; 104: 660-665 [DOI] [PubMed] [Google Scholar]
- 30). Stocchi L, Giardina E, Varriale L, Sechi A, Vagnini A, Parri G, Valentini M, Capalbo M: Can tangier disease cause male infertility? A case report and an overview on genetic causes of male infertility and hormonal axis involved. Mol Genet Metab, 2018; 123: 43-49 [DOI] [PubMed] [Google Scholar]
- 31). Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, DerOhannessian S, Kontush A, Surendran P, Saleheen D, Trompet S, Jukema JW, De Craen A, Deloukas P, Sattar N, Ford I, Packard C, Majumder A, Alam DS, Di Angelantonio E, Abecasis G, Chowdhury R, Erdmann J, Nordestgaard BG, Nielsen SF, Tybjaerg-Hansen A, Schmidt RF, Kuulasmaa K, Liu DJ, Perola M, Blankenberg S, Salomaa V, Mannisto S, Amouyel P, Arveiler D, Ferrieres J, Muller-Nurasyid M, Ferrario M, Kee F, Willer CJ, Samani N, Schunkert H, Butterworth AS, Howson JM, Peloso GM, Stitziel NO, Danesh J, Kathiresan S, Rader DJ: Rare variant in scavenger receptor bi raises hdl cholesterol and increases risk of coronary heart disease. Science, 2016; 351: 1166-1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1). Hanaichi T, Sato T, Iwamoto T, Malavasi-Yamashiro J, Hoshino M, Mizuno N: A stable lead by modification of Sato's method. J Electron Microsc (Tokyo), 1986; 35: 304-306 [PubMed] [Google Scholar]


