Background and Purpose
We set out to investigate the effects of electroacupuncture (EAP) and moxibustion on pain states mediated by a range of acid‐ and ATP‐sensitive nociceptors in acute and inflammatory rodent pain models.
Experimental Approach
We injected PBS (pH 6.0 or 4.0) or α,β‐methylene ATP into the paw of rats or mice to cause thermal hypersensitivity, which was quantified by the paw withdrawal latency (PWL). Inflammatory pain was induced by the injection of complete Freund's adjuvant (CFA) into the rat paw.
Key Results
EAP and moxibustion counteracted the decrease of PWL mediated by acid‐sensing ion channel 3 (ASIC3) and transient receptor potential vanilloid 1 (TRPV1) channels in response to pH 6.0 PBS, but not that mediated by TRPV1 channels only, initiated by the injection of pH 4.0 PBS. Similarly, EAP and moxibustion prevented the purinergically induced pain which was caused by stimulation of P2X3 (P2X2/3) and P2X7 receptors. The effect of CFA was also relieved by EAP and moxibustion.
Conclusions and Implications
During acute thermal pain and CFA‐induced inflammatory pain, ASIC3/TRPV1 channels and P2X3/P2X7 receptors are activated by protons and exogenous α,β‐methylene ATP or endogenously released ATP respectively. Low‐threshold acute acidic pain mediated by the activation of ASIC3/TRPV1 channels was reversed by EAP/moxibustion, while high‐threshold acidic pain that is mediated exclusively by the activation of TRPV1 channels was not. ASIC3 and P2X3 receptors appear to interact with each other in responding to both protons and ATP, by forming an ASIC3/P2X3 “cognate receptor”, sensitive to EAP/moxibustion.
What is already known
Acupuncture family procedures cause analgesia via stimulation of certain points of the skin.
What this study adds
Electroacupuncture and moxibustion counteract slight acidic pain mediated by ASIC3 and TRPV1 channels in rodents.
Electroacupuncture and moxibustion alleviate purinergically induced pain mediated by ATP‐sensitive P2 receptors (P2X3 and P2X7).
What is the clinical significance
Based on its identified mode of action, acupuncture efficiently relieves subacute inflammatory pain.
Abbreviations
- ASIC3
acid‐sensing ion channel 3
- Bz‐ATP
dibenzoyl‐ATP
- CFA
complete Freund's adjuvant
- EAP
electroacupuncture
- PWL
paw withdrawal latency
- TRPV1
transient receptor potential vanilloid 1
- α,β‐meATP
α,β‐methylene ATP
1. INTRODUCTION
Local acidosis has been suggested to play a major role in pain and hyperalgesia (Reeh & Steen, 1996). On the one hand, protons activate pain‐sensing receptors (nociceptors) located at primary afferent fibres in peripheral tissues transmitting information to the spinal cord and eventually to higher brain centres (Burnstock, 2009b; Y. Dai, 2016; Deval & Lingueglia, 2015). These pain‐sensing receptors represent mainly the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=507 member of the transient receptor potential (TRP) protein superfamily (Jardin et al., 2017; Moran & Szallasi, 2018) as well as the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=684&familyId=118&familyType=IC) and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=686 members of the ASIC family (Y. R. Cheng, Jiang, & Chen, 2018; Wemmie, Taugher, & Kreple, 2013). On the other hand, protons may modulate the function of various receptors responding to compounds released by the damaged tissue (histamine, 5‐HT, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1713; Burnstock, 2009b; Burnstock, 2013; Jardin et al., 2017). For example, ATP leaves the cell interior through the injured cell membrane and activates at lower concentrations the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=480 (and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=479/3) and at higher concentrations the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=484 members of the P2X family of receptors (Burnstock, 2009b; Wirkner, Sperlagh, & Illes, 2007).
ASICs belonging to the epithelial sodium channel/degenerin superfamily (Deval & Lingueglia, 2015; Grunder & Chen, 2010) exhibit high structural similarity to P2X receptors (Baconguis, Hattori, & Gouaux, 2013; Kellenberger & Grutter, 2015). Although the amino acid composition of the two receptor‐channel types is different, their trimeric structure and ion conductive pathways are similar. Recently, it has been suggested that ASIC3 and P2X3 subunits do not form a heteromeric channel but tightly associate with each other to constitute a protein complex, mediating unidirectional inhibition (Stephan et al., 2018).
Acupuncture family procedures have been practiced as a subdiscipline of Traditional Chinese Medicine for the past 3,000 to 4,000 years; they are based on the stimulation of certain points of the skin (acupoints) and the underlying subcutaneous tissue via mechanical or thermal (moxibustion) stimulation (Campbell, 2006; Tang, Yin, Rubini, & Illes, 2016). Increasing evidence from both basic and clinical research on acupuncture indicates that pain is particularly sensitive to this therapeutic manoeuvre (Vickers & Linde, 2014; Zhang, Lao, Ren, & Berman, 2014). Analgesia appears to be due to the release of opioid peptides from peripheral immunocytes during inflammation but also from opioidergic neurons in the brain and spinal cord (Pomeranz & Chiu, 1976; Zhang et al., 2014). More recently, it was suggested that the local release of ATP and probably its enzymic degradation to https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2844 is responsible for acupuncture‐induced analgesia (Burnstock, 2009a; Goldman et al., 2010; Tang et al., 2016).
Because clinical studies on acupuncture‐induced analgesia have often yielded conflicting results (Colquhoun & Novella, 2013; Madsen, Gotzsche, & Hrobjartsson, 2009), it is of eminent importance to rely on experiments carried out on laboratory animals and to evaluate the data with stringent statistical methods including comparison with a sufficient number of control animals (Tang et al., 2016; Tang, Yin, Liu, Rubini, & Illes, 2018). For example, systematic investigations on pain mediated by acid‐sensing nociceptors in conjunction with the application of acupuncture are largely missing.
The aim of the present work was threefold: (a) to clarify whether electroacupuncture (EAP)/moxibustion equally interfere with acidic and purinergic pain; (b) to find out whether low‐ and high‐threshold acidic pain is differentially modulated by EAP/moxibustion; and (c) to investigate whether there is an interaction between ASIC3/ P2X3 receptors in vivo as was suggested to occur on the basis of in vitro experiments (Stephan et al., 2018). We report that during acute acidic pain and complete Freund's adjuvant (CFA)‐induced inflammatory pain, ASIC3/TRPV1 channels and P2X3/P2X7 receptors are activated by endogenously released protons and ATP respectively. Furthermore, low‐threshold acute acidic pain mediated by the activation of ASIC3/TRPV1 channels was prevented by EAP/moxibustion, while high‐threshold acidic pain mediated exclusively by the activation of TRPV1 channels was not. ASIC3 and P2X3 receptors appeared to interact with each other in response to both protons and ATP, by forming an ASIC3/P2X3 “cognate” receptor, sensitive to EAP/moxibustion. Inflammatory pain was also sensitive to these acupuncture family procedures.
2. METHODS
2.1. Animals
All animal care and experimental procedures complied with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Animal Ethics Committee of Chengdu University of Traditional Chinese Medicine. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. The experiments were performed on 429 adult male Sprague–Dawley rats (180–220 g of weight), TRPV1 knockout (Trpv1tm1Jul; RRID:MGI:4417977; KO; n = 36) mice, and their wild‐type (WT; n = 29) controls (18–22 g; Jackson Laboratory, Bar Harbor, ME, USA). P2X7 receptor KO mice (P2rx7tm1.2Jde; MGI:6203042; n = 7) and their WT controls (n = 7; 17–22 g each) were a generous gift of Dr Jan M. Deussing (Max Planck Institute of Psychiatry, Munich, Germany). We established colonies of TRPV1 and P2X7 KO as well as WT mice in our animal house. Only male rats and mice were used for experiments in order to exclude any sex‐dependent variability. Animals were 7–8 weeks of age, were housed 3–6 per cage, and experienced at least 1‐week acclimation to our laboratory animal observation room in a natural light/dark cycle at 22–24°C with free access to water and food.
2.2. Drug application protocols
All drugs were applied by the intra‐plantar (i.pl.) route into the left hind paw of rats and mice in volumes of 100 and 20 μl respectively. Drugs, except in experiments with CFA, were injected immediately after the baseline testing of the paw withdrawal latency (PWL) at the −30‐min time point (see, e.g., Figure 1). Then, the PWL was measured in 30‐min intervals, for an additional 120 min.
Figure 1.
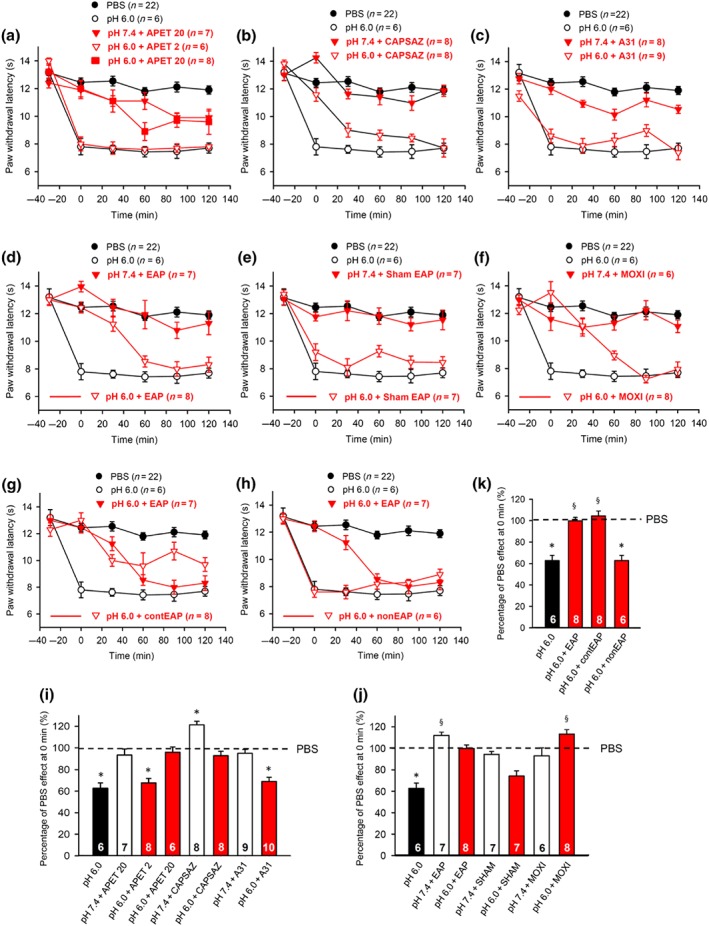
Modulation by pharmacological antagonists and acupuncture family procedures of acute thermal hypersensitivity caused by local acidification to pH 6.0. At −30 min, pH 7.4 or pH 6.0 PBS alone, APETx2 (20 μM), capsazepine (10 μM), or A‐317491 (300 nM), all dissolved in pH 7.4 or pH 6.0 PBS were injected to the left hind paw of rats; in another series of experiments, EAP or moxibustion, both for 30 min, was delivered to the Zusanli acupoint (ST36) of rats at the ipsilateral or contralateral side, again after injecting pH 7.4 or pH 6.0 PBS. For sham EAP, the needle was positioned as for EAP, but without electrical stimulation. The PWL was determined at −30 min and every 30 min afterwards, six times in total, until the 120‐min time point. This experimental protocol was used in Figures 1, 2, 3, 4, 5, 6. One hundred and eleven rats were used to generate the data present in this figure (a–f). The time‐dependent effects of APETx2 (a), capsazepine (b), A‐317491 (c), EAP (d), sham acupuncture (e), moxibustion (f), contralateral EAP (g), and EAP at a non‐acupoint (h) are shown on the acidification‐induced decrease of PWL. For comparison, the change in PWL measured after injection of pH 7.4 PBS injection was plotted at each time point. Mean ± SEM determined on the number of animals in brackets. (i–k) Effects of pH 6.0 PBS, APETx2, capsazepine, and A‐317491 (i), or EAP, contralateral EAP, sham EAP, and moxibustion (j), or contralateral EAP, and EAP at a non‐acupoint (k) determined in pH 6.0 PBS and expressed as a percentage of the normal PBS (pH 7.4; 100%) effect at 0 min. As controls, the effects of pharmacological antagonists and acupuncture‐family treatments were also measured, when applied together with normal PBS (pH 7.4) (i,j). Mean ± SEM of the indicated number of experiments. APET, APETx2; CAPSAZ, capsazepine; A31, A‐317491; EAP, electro‐acupuncture; SHAM, sham EAP; MOXI, moxibustion; contEAP, contralateral EAP; nonEAP, EAP applied to a non‐acupoint. * P < .05, significantly different from the mean PWL measured at 0 min after PBS (pH 7.4) injection. § P < .05, significantly different from the effect of pH 6.0 PBS injection; Kruskal–Wallis one‐way ANOVA on ranks (H = 50,872, i; H = 45,893, j; H = 29,398, k) followed by Dunn's test
In experiments with inflammatory pain, the PWL was determined and immediately afterwards, a CFA cell suspension (100 μl) was injected into the left hind paw of rats. Subsequently, the animals were returned to their cages for 24 hr. Then, at the −30‐min time point, the PWL was redetermined, various pharmacological antagonists were injected, or EAP/moxibustion was applied for 30 min (see Figure 7). Once again, thereafter, the PWL was measured in 30‐min intervals for 120 min in total.
Figure 7.
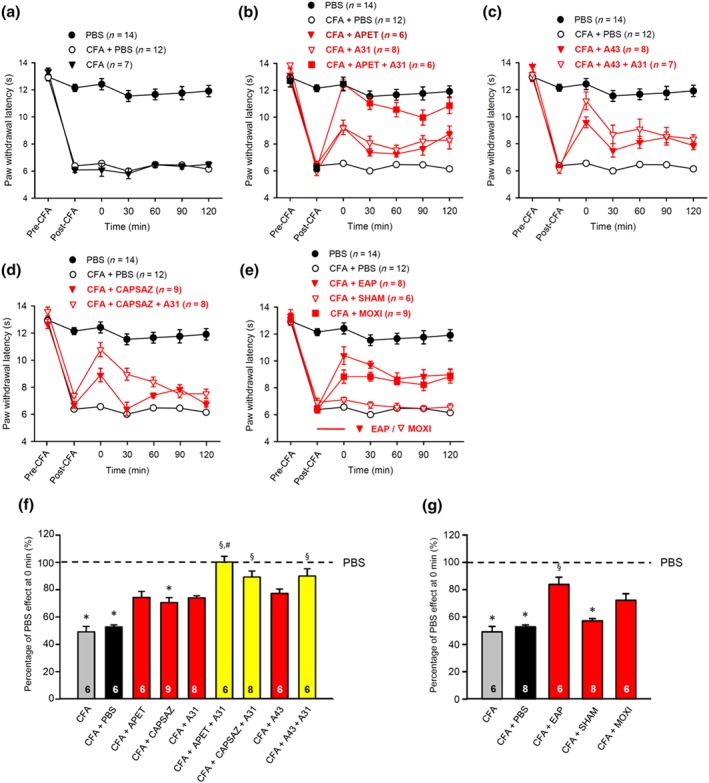
At −30 min (post‐CFA), pharmacological antagonists were applied, or EAP, sham EAP, or moxibustion were delivered for 30 min to the Zusanli acupoint (ST36) of rats at the ipsilateral side. Then, the PWL was determined every 30 min, six times in total until 120 min. Eighty rats were used to generate the data presented below. (a–e) CFA caused a similar decrease in PWL either with or without the intra‐plantar injection of PBS (a). APETx2 (20 μM) and A‐317491 (300 nM) were applied separately or in combination (b). A‐438079 (300 nM) was applied alone or in combination with A‐317491 (300 nM) (c). Capsazepine was applied alone or in combination with A‐317491 (300 nM) (d). EAP, sham EAP, or moxibustion were delivered separately (e). For comparison, the change in PWL measured after injection of pH 7.4 PBS was plotted at each time point. Mean ± SEM determined on the bracketed number of animals. (f,g) Effects of CFA alone, or in combination with APETx2, capsazepine, A‐317491, APETx2 plus A‐317491, capsazepine plus A‐317491, A‐438079, A‐438079 plus A‐317491 (f), EAP, sham EAP and moxibustion (g), and expressed as a percentage of the normal PBS (pH 7.4; 100%) effect at 0 min. CFA, complete Freund's adjuvant. Mean ± SEM of the indicated number of experiments. * P < .05, significantly different from the mean PWL measured after PBS (pH 7.4) injection before CFA treatment of rats (pre‐CFA). § P < .05, significantly different from the effect of PBS (pH 7.4) injection at 0 min after CFA treatment of rats (post‐CFA); Kruskal–Wallis ANOVA on ranks (H = 67.636, f; H = 43.672, g) followed by Holm–Sidak test
2.3. EAP and moxibustion
EAP and moxibustion were administered at roughly the same time of the day (10:00 a.m. to 12:00 a.m.) to awake animals, immobilized by two Velcro brand hooks and loop fasteners as well as additional tapes fixed to a wooden block for the duration of EAP and moxibustion only. Although immobilization is certainly a stressful stimulus, sham acupuncture applied to the same acupoint but without electrical stimulation or EAP delivered to a non‐acupoint, both did not modify the baseline PWL value when compared with the PWL values measured in all other experiments (compare Figure 1e,h with, e.g., Figure 1a–d).
EAP was delivered to the Zusanli acupoint (ST36; located at the left knee, about 2 mm for mice or 6 mm for rats below the fibular head). An electrical current of 1 mA for rats or 0.5 mA for mice and a frequency of 15 Hz was delivered for 30 min, by an “acupoint nerve electrostimulator” (HANS‐200, Nanjing Jisheng Medical Technology Co., Jiangsu, China). EAP was applied through stainless steel needles (2.5 cm long, 0.25 mm diameter; Hwato‐Med. Co., Jiangsu, China), introduced 5–8 mm deep (rats) or 2–3 mm deep (mice) below the skin at ST36 unilaterally. As controls, a sham EAP group of rats was treated with the same procedure, but without electrical stimulation. In another group of rats, a non‐acupoint was stimulated about 3 cm distal from the ST36 acupoint towards the tail and opposite to the knee joint (Torres‐Rosas et al., 2014). This non‐acupoint is neither referred to in the acupoint map of rodents nor is it close to any major nerve.
Moxibustion was applied for 30 min at a distance of approximately 1 cm from the skin to ST36 unilaterally, onto the left limb of the animals using moxa sticks (Nanyang Han‐yi moxa LLC., Henan, China) with a diameter of 5 mm and a length of 120 mm.
2.4. Measurement of thermal PWL
Responses to thermal laser stimulation were determined at the plantar surface of the left hind paw by using a “Thermal Stimuli Instrument” (PL‐200, Techman Software Co., Chengdu, China). The instrument was preheated for 30 min before use. The intensity of the light beam was adjusted to 30% (10% for mice) and the cut‐off time was set to 20 s (in TRPV1 KO mice to 30 s). In spite of the prolongation of the baseline PWL in TRPV1 KO mice, there was no evident tissue damage caused by the thermal stimulus. All measurements were made in an air‐conditioned room (22–24°C). Withdrawal, shaking, or licking of the hind paw was considered as responses to thermal stimulation. Animals were allowed to get accustomed to a transparent plastic enclosure (210 mm × 210 mm × 160 mm) on the top of the vitreous experimental platform (800 mm × 400 mm × 165 mm) for 30–40 min for three consecutive days each, before the beginning of the test. The baseline PWL was determined only once. Then, the PWL of rats and mice was measured three times (each time with 5‐min intervals), every 30 min, including the following six time points: baseline (−30), 0, 30, 60, 90, and 120 min.
All experimenters were blinded to the experimental treatments. Rats and mice were randomly assigned to one of the experimental groups or to the control group. The number of animals in the control group was higher than that in the respective experimental groups. We increased the number of animals in the control group (pH 7.4, PBS) to 22 by performing measurements on three groups of 6–8 animals each (approximately time matching with the experimental groups). The mean PWL values in the three groups at the 0‐min time points were 12.3 ± 0.4 s (n = 8), 12.8 ± 0.8 s (n = 6), and 12.4 ± 0.5 s (n = 8) respectively. As there was no statistically significant difference between these values (P > .05; F = 0.236; Kruskal–Wallis ANOVA on ranks), they were pooled for the final calculations. In another control group (CFA, pH 7.4, PBS), two groups of 6–8 animals each were used for measurements and the PWL values obtained at different times were again pooled (12.9 ± 0.4 s, n = 6; 12.1 ± 0.6 s, n = 8; P > .05; Mann–Whitney rank sum test). Otherwise, sample size estimation for the different experimental groups was not conducted; they consisted of five to 10 animals throughout. Rats or mice were used only once for drug/acupuncture procedure experiments.
2.5. Data analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). All data were expressed as means ± SEM of n observations, where n means the number of animals per group. SigmaPlot 13.0 was used for statistical evaluation. We tested for and found that, when using parametric tests, all sampled distributions satisfied the normality and equal variances criteria. Multiple comparisons between data were performed in case of their normal distribution by one‐way ANOVA followed by the Holm–Sidak test or repeated measures ANOVA on ranks followed by the Tukey's test. Multiple comparisons between data were performed in case of their non‐normal distribution by Kruskal–Wallis ANOVA on ranks, followed by the Dunn's test. Two‐way ANOVA followed by the Dunn's or Tukey's test was performed to compare data obtained in KO and WT mice in Figures 3c,e and 6f as well as to compare data obtained at the time points 0 and 60 min in Figure 2d,e. Post hoc tests were run only if F or H achieved P < .05. Two data sets were compared by the parametric Student's t‐test or the non‐parametric Mann–Whitney rank sum test, as appropriate. A probability level of .05 or less was considered to be statistically significant.
Figure 3.
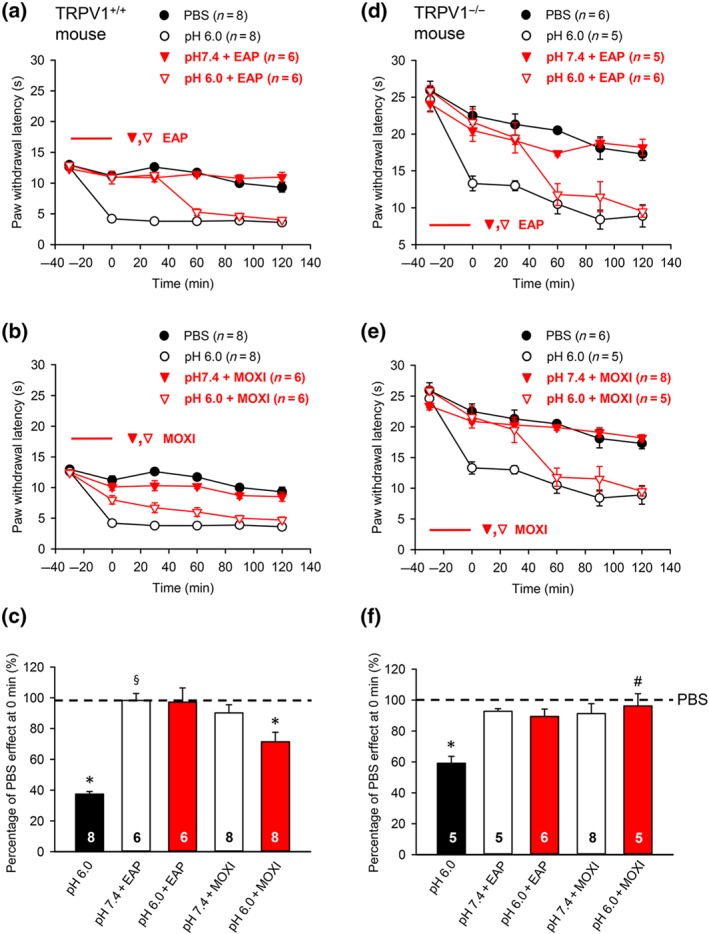
Modulation by pharmacological antagonists and acupuncture family procedures of acute thermal hypersensitivity caused by local acidification to pH 6.0 in TRPV1−/− KO and TRPV1+/+ wild‐type (WT) mice. At −30 min, pH 7.4 or pH 6.0 PBS was injected to the left hind paw of either type of mice; in another series of experiments, EAP or moxibustion, both for 30 min, was delivered to the Zusanli acupoint (ST36) at the ipsilateral side to wild‐type and KO animals. PWL measurement was as in Figure 1. The time‐dependent effect of pH 6.0 PBS alone, or together with EAP, or moxibustion are shown in the acidification‐induced decrease of PWL in TRPV1+/+ (n = 36; a,b) or TRPV1−/− (n = 29; d,e) mice. For comparison, the change in PWL measured after injection of pH 7.4 PBS was plotted at each time point. Mean ± SEM determined on the bracketed number of animals. (c,f) Effects of pH 6.0 PBS alone or together with EAP, or moxibustion expressed as a percentage of the normal PBS (pH 7.4; 100%) effect at 0 min in TRPV1+/+ (c) or TRPV1−/− (f) mice. Mean ± SEM of the indicated number of experiments. The abbreviations are the same as in Figure 1. * P < .05; statistically significant difference from the mean PWL measured after PBS (pH 7.4) injection at 0 min in TRPV1+/+ (c) and TRPV1−/−(f) mice respectively. § P < .05, significantly different from the effect of pH 6.0 PBS injection at 0 min in TRPV1−/−(f) mice; Kruskal–Wallis one‐way ANOVA on ranks (H = 27.273, c; H = 18,085, f) followed by Dunn's test. # P < .05,significantly different from effect of pH 6.0 PBS in TRPV1+/+ mice; two‐way ANOVA (Ftreatment = 23.140, Fgenotype × treatment = 3.554) followed by Dunn's test
Figure 6.
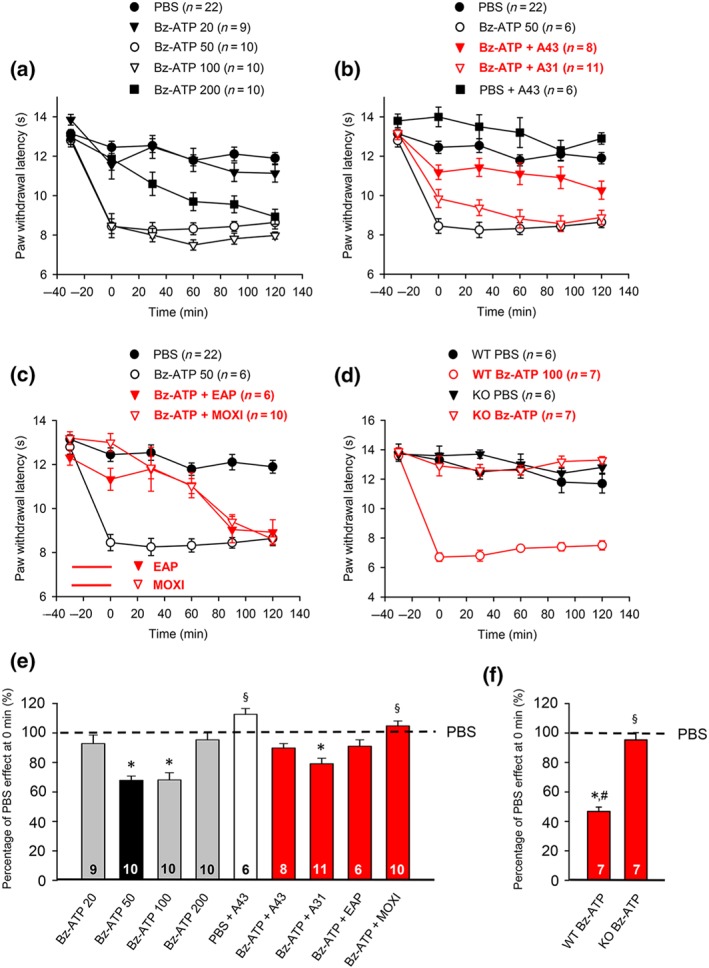
Modulation by pharmacological antagonists and acupuncture family procedures of acute thermal hypersensitivity caused by local injection of Bz‐ATP. At −30 min, Bz‐ATP (20, 50, 100, and 200 nM) was injected into the left hind paw of rats. In another series of experiments, Bz‐ATP (50 nM) was injected together with A‐438079 (300 nM) or A‐317491 (300 nM) at the −30 min time point, or EAP/moxibustion, both for 30 min, were delivered to the Zusanli acupoint (ST36) of rats at the ipsilateral side, after injecting Bz‐ATP (50 nM). PWL measurement was as in Figure 1. (a–c) Eighty rats were used to generate the data present below. The time‐dependent effects of the Bz‐ATP doses indicated are shown on the PWL (a); the effects of Bz‐ATP (50 nM) applied together with A‐438079 or A‐317491 (b), and EAP or moxibustion for 30 min (c), are also displayed. In addition, Bz‐ATP (100 nM) or PBS was injected to the Zusanli acupoint of P2X7−/− (KO) and P2X7+/+ wild‐type (WT) mice. Bz‐ATP decreased the PWL of the WT group of animals only (d). For comparison, the change in PWL measured after injection of pH 7.4 PBS was plotted at each time point. Mean ± SEM determined on the bracketed number of animals. (e) Effects of pH 7.4 PBS and Bz‐ATP (50 nM) injected alone or together with A‐438079, A‐317491, EAP, and moxibustion expressed as a percentage of the normal PBS (pH 7.4; 100%) effect at 0 min. (f) Effects Bz‐ATP (100 nM) in P2X7R KO and WT mice paws, respectively, expressed as a percentage of the normal PBS (pH 7.4; 100%) effect at 0 min. Bz‐ATP, dibenzoyl‐ATP; A43, A‐438079. (e) * P < .05, significantly different from the mean PWL measured after PBS (pH 7.4) injection at 0 min. § P < .05, significantly different from the mean PWL measured after Bz‐ATP (50 nM) injection at 0 min; Kruskal–Wallis one‐way ANOVA on ranks (H = 60.151) followed by Dunn's test. (f) * P < .05, significantly different from the mean PWL measured after Bz‐ATP (100 nM) injection at 0 min in WT mice; Kruskal–Wallis one‐way ANOVA (H = 18.965) followed by Dunn's test. § P < .05, significantly different from the effect of Bz‐ATP (100 nM) in P2X+/+ mice; two‐way ANOVA (Ftreatment = 99.955, Fgenotype × treatment = 70.190) followed by Tukey's test. # P < .05, significantly different from the effect of Bz‐ATP (50 nM) measured in rats; Student's t‐test
Figure 2.
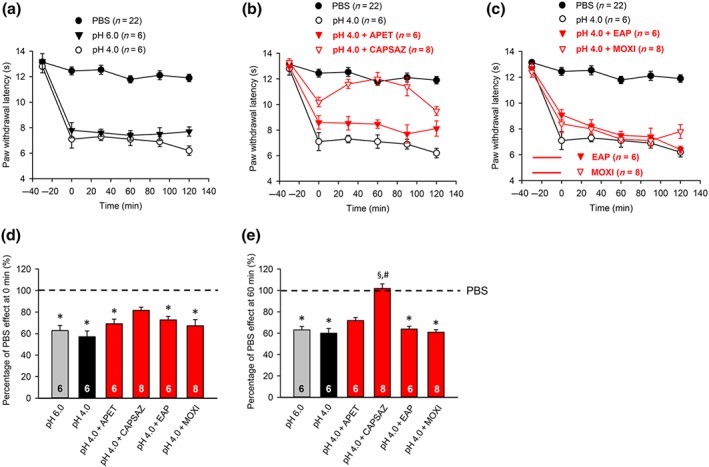
Modulation by pharmacological antagonists and acupuncture family procedures of acute thermal hypersensitivity caused by local acidification to pH 4.0. At −30 min, pH 4.0 PBS alone, or alternatively capsazepine (10 μM), or APETx2 (20 μM) dissolved in pH 4.0 PBS, was injected to the left hind paw of rats; in another series of experiments, EAP or moxibustion, both for 30 min, was delivered to the Zusanli acupoint (ST36) of rats at the ipsilateral side, again after injecting pH 4.0 PBS. PWL measurement was as in Figure 1. Thirty four rats were used to generate the data presented below. (a–c) The time‐dependent effects of pH 6.0 and pH 4.0 PBS are shown alone (a), as well as the effects of pH 4.0 PBS together with APETx2 (20 μM), capsazepine (10 μM) (b), EAP, or moxibustion (c) on the acidification‐induced decrease of PWL. For comparison, the change in PWL measured after injection of pH 7.4 PBS injection was plotted at each time point. Mean ± SEM determined on the bracketed number of animals. (d,e) Effects of pH 4.0 PBS, and APETx2 (20 μM), capsazepine (10 μM), EAP, or moxibustion determined in pH 4.0 PBS, expressed as a percentage of the normal PBS (pH 7.4; 100%) effect at 0 min (d) or 60 min (e). Mean ± SEM of the indicated number of experiments. * P < .05, significantly different from the mean PWL measured after PBS (pH 7.4) injection at 0 min (d) and at 60 min respectively. § P < .05, significantly different from the effect of pH 4.0 PBS injection at 60 min; Kruskal–Wallis one‐way ANOVA on ranks (D,H = 50,290; E,H = 50,223) followed by Dunn's test. # P < .05, significantly different from the effect of pH 4.0 PBS injection at 0 min (d) or 60 min (e); two‐way ANOVA (Ftreatment = 18.784, Ftime × treatment = 3.615) followed by Dunn's test
2.6. Materials
The drugs used were the following: 5‐[[[(3‐phenoxyphenyl) methyl][(1S)‐1,2,3,4‐tetrahydro‐1‐naphthalenyl]amino]carbonyl]‐1,2,4‐benzenetricarboxylic acid sodium salt hydrate (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4115), 3‐[[5‐(2,3‐dichlorophenyl)‐1H‐tetrazol‐1‐yl]methyl]pyridine hydrochloride (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4118), amiloride hydrochloride, 2′(3′)‐O‐(4‐benzoylbenzoyl)adenosine‐5′‐triphosphate tri(triethylammonium) salt (Bz‐ATP), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2461, CFA cell suspension (CFA), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4093; Sigma‐Aldrich; Saint Louis, MO, USA), and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4135 (Tocris Bioscience; Abingdon, UK). Ugr9‐1 was obtained by the production of a recombinant analogue in Escherichia coli (Osmakov et al., 2013). Stock solutions of all drugs (A317491, 1 mM; A438079, 10 mM; APETx2, 1 mM; Bz‐ATP, 10 mM; α,β‐meATP, 1 mM; Ugr9‐1, 1 mM) were prepared in distilled water, with the exception of capsazepine (1 mM) and amiloride (10 mM) which were diluted in DMSO (Biofroxx, Einhausen, Germany). Further dilutions were made in PBS (Solarbio, Beijing, China), the pH of which was set to the required values (7.4, 6.0, or 4.0) by adding 0.1‐N HCl, or in addition 0.1‐N NaOH, if required.
2.7. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Peters et al., 2017; Alexander, Striessnig et al., 2017).
3. RESULTS
3.1. Effects of pharmacological antagonists, EAP, and moxibustion on acidification‐induced pain in rats
In all experiments, the PWL of rats was measured with a thermal laser stimulator as described in Section 2. The application of a PBS with a slightly alkaline pH (7.4; termed “normal”) into the left hind paw caused no change in PWL from the first measurement at −30 min to the second and third measurements at 0 and 30 min. However, a statistically significant, although minor, decline from the fourth measurement at 60 min onwards, was observed in comparison with the first measurement (Figure 1a–h; P < .05; one‐way repeated measures ANOVA; Chi‐square = 18.953; followed by the Tukey test). In an attempt to exclude any time‐dependent variability, we decided to compare all drug effects with their time‐matched controls.
In contrast to the effect of PBS at a normal pH, injection of a moderately acidic PBS (pH, 6.0) to the left hind paw at −30 min caused a pronounced shortening of the PWL as measured at 0 min (Figure 1a–h). It has to be mentioned that the pH does not remain stable at the site of injection over time. A constant dilution of the low pH PBS is expected to occur. Nonetheless, it is reassuring that the effect of the PBS injection is absolutely stable throughout the experiment, and therefore, this may not interfere with the reliability of the conclusions.
The effect of pH 6.0 PBS was abolished by the ASIC3‐selective marine toxin APETx2 (20 μM; Figure 1a) and the selective TRPV1 channel antagonist capsazepine (10 μM; Figure 1b) at 0 min but not by the selective P2X3 receptor antagonist A‐317491 (300 nM; Figure 1c). By contrast, the same dose of A‐317491 abolished the effect of α,β‐meATP (200 nM) in another series of experiments carried out according to a similar application protocol (Figure 4b). It is also noteworthy that APETx2 at a lower dose of 2 μM did not alter the effect of the acidic PBS, whereas at a higher dose of 20 μM, it caused a complete blockade (Figure 1a,i). Therefore, we used in all subsequent experiments 20 μM of APETx2.
Figure 4.
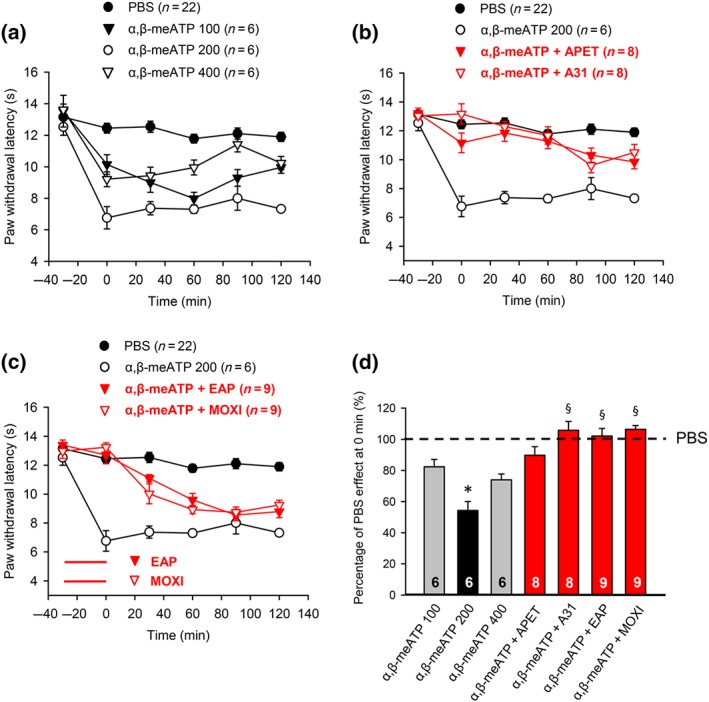
Modulation by pharmacological antagonists and acupuncture family procedures of acute thermal hypersensitivity caused by local injection of α,β‐meATP. At −30 min, α,β‐meATP (100, 200, and 400 nM) was injected into the left hind paw of rats; α,β‐meATP (200 nM) was also co‐injected with APETx2 (20 μM) or A‐317491 (300 nM). In another series of experiments, EAP or moxibustion, both for 30 min, was delivered to the Zusanli acupoint (ST36) of rats at the ipsilateral side, immediately after injecting α,β‐meATP (200 nM). PWL measurement was as in Figure 1. Fifty two rats were used to generate the data presented below. (a–c) The time‐dependent effects of α,β‐meATP (100, 200, and 400 nM) alone are shown on the PWL (a); the effects of α,β‐meATP (200 nM) applied together with APETx2 (20 μM) or A‐317491 (300 nM) (b), and EAP or moxibustion for 30 min (c), are also displayed. For comparison, the change in PWL measured after injection of pH 7.4 PBS was plotted at each time point. Mean ± SEM determined on the bracketed number of animals. (d) Effects of pH 7.4 PBS and α,β‐meATP (200 nM) injected alone or together with APETx2 (20 μM), A‐317491 (300 nM), EAP, and moxibustion expressed as a percentage of the normal PBS (pH 7.4; 100%) effect at 0 min. The effects of α,β‐meATP (100–400 nM) are also shown. Mean ± SEM of the indicated number of experiments. * P < .05, significantly different from the mean PWL measured after PBS (pH 7.4) injection at 0 min. § P < .05, significantly different from the effect of α,β‐meATP (200 nM) injection at 0 min; Kruskal–Wallis one‐way ANOVA on ranks (H = 38.308) followed by Dunn's test
Inspection of the graphs shows that the antagonism by APETx2 (20 μM) was of longer duration than that caused by capsazepine (10 μM; compare Figure 1a and b). Interestingly, APETx2 (20 μM) significantly depressed the effect of the pH 7.4 PBS at the 90‐min and 120‐min sampling points (P < .05; Student's t‐test each). Presently, we have no explanation for this depression; however, at the 0‐min time point relevant for the evaluation of the data, we did not observe any effect of APETx2 (20 μM).
Further, EAP (Figure 1d) and moxibustion (Figure 1f) applied for 30 min each at the −30‐min time point counteracted the decrease of PWL produced by the injection of acidic PBS. The effects of EAP and moxibustion had approximately identical durations (compare Figure 1d with Figure 1f). When an acupuncture needle was introduced into the left hind paw of rats, without electrical stimulation (“sham EAP”), the PWL continued to decrease in response to local acidification (Figure 1e). Moreover, acupuncture applied to ST36 at the contralateral hind paw also interfered with the acidification‐induced pain (Figure 1g), while acupuncture delivered to a non‐acupoint had no effect at all on this pain quality (Figure 1h).
For a better comparability of the drug‐ and treatment‐induced changes in PWL, they were expressed at 0 min as percentage values relative to the normal PBS effect at 0 min (designated as 100% and indicated by a broken line; Figure 1i–k). The bar graphs shown confirm the conclusions made on the basis of Figure 1a–h, showing also that normal (pH 7.4) PBS in combination with APETx2 (20 μM) and A317491 (300 nM) at the doses applied (Figure 1i), as well as EAP, sham EAP, and moxibustion alone (Figure 1j), in the absence of an acidic PBS injection, did not alter the percentage drug or treatment effects. Capsazepine (10 μM) prolonged the effect of normal PBS probably because it antagonized the activation of TRPV1 by its endogenous agonists (Figure 1i). Whereas stimulation of St36 at the contralateral paw inhibited the thermal hypersensitivity induced by acidic pain, stimulation of a non‐acupoint with the standard electrical parameters failed to modify this pain quality (Figure 1k). We decided to calculate the inhibition of the acidic PBS (α,β‐meATP and Bz‐ATP)‐induced sensitivity increase by various drugs/treatments 30 min after drug application or after the start of EAP/moxibustion, because this inhibition was in almost all cases maximal at 0 min, with no change or a time‐dependent decrease afterwards.
Then, we investigated the effects of the used drugs and treatments in identical doses as previously, against a stronger local acidification by a PBS solution of pH 4.0 (Figure 2a). A decrease of the pH from 6.0 to 4.0 did not cause a further increase in PWL (Figure 2a). Whereas capsazepine (10 μM) antagonized the effect of a strongly acidic PBS, and this effect even increased with time, neither APETx2 (20 μM) nor EAP, or moxibustion caused any change (Figure 2b,c); the calculation of the percentage effects of these drugs and treatments at 0 min (Figure 2d) and 60 min (Figure 2e) confirmed our findings. The two bar charts also show that capsazepine at 0 min caused only partial inhibition of the strong acidification‐induced effect, while at 60 min, the inhibition was complete. By contrast, the effects of all other treatments than those with capsazepine remained stable with time.
3.2. Effects of pharmacological antagonists, EAP, and moxibustion on acidification‐induced pain in WT and TRPV1 receptor‐deleted mice
In view of the failure of EAP and moxibustion to counteract the decrease of PWL by local acidification to pH 4.0, but not pH 6.0, and the antagonism by APETx2 of the latter but not the former effect, we decided to compare the modulation by EAP and moxibustion of the pH 6.0‐induced decrease in PWL of WT and TRPV1‐deficient mice (Figure 3). The initial (pretreatment) PWL value of the knockout mice (Figure 3d) was higher than the corresponding value of the WT animals (Figure 3a; P < .05; Student's t‐test). This difference was expected, because TRPV1 is one of the cation channels which respond to heat perceived by cutaneous, sensory nerve terminals. From the evaluation of the percentage changes at 0 min of the normal PWL in the two types of animals, it could be deduced that the effects of EAP were similar, although the inhibition by pH 6.0 PBS of the PWL appeared to be somewhat more marked in the WT than in the knockout mice, although this change did not reach the level of statistical significance (Figure 3c,e). By contrast, moxibustion had more effect in the knockout than in the WT mice (Figure 3c,e). Eventually, we tentatively suggest that in the knockout mice, ASIC3 took over the function of TRPV1 channels in sensing cutaneous tissue acidification; however, the participation of acid‐sensing ion channels other than ASICs (voltage‐sensitive tetrodotoxin‐resistant Na+ channels; https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=585; Nakamura & Jang, 2015) or proton‐sensing GPCRs (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=113; S. P. Dai et al., 2017) cannot be excluded either.
3.3. Effects of pharmacological antagonists, EAP, and moxibustion on P2X3 and P2X7 receptor‐mediated pain in rats
After having carried out measurements on rats and mice by injection of acidic PBS, we turned our attention to acute pain hypersensitivity induced by the P2X1,3 receptor agonist α,β‐meATP (Figure 4). α,β‐meATP (100 and 200 nM) caused a dose‐dependent decrease of PWL (Figure 4a). A further increase of the α,β‐meATP dose to 400 nM failed to induce more inhibition; therefore, we used in the subsequent experiments 200 nM of the purinergic agonist. APETx2 (20 μM), A‐317491 (300 nM), EAP, and moxibustion caused an equal inhibition of the α,β‐meATP (200 nM) effect, although the pharmacological antagonists caused a longer lasting normalization of the PWL than the AP family procedures (compare Figure 4b with Figure 4c). It was an unexpected finding that not only the selective P2X3 receptor blocker A‐317491 but also the ASIC3 blocker APETx2 was inhibitory (see also Figure 5). All findings could be confirmed by considering the percentage changes at 0 min of the normal PWL, as documented in Figure 4d. The effect of 200‐nM α,β‐meATP (Figure 4d) calculated on a percentage basis did not differ from that of pH 6.0 or pH 4.0 (Figure 2d; P > .05; one‐way ANOVA; F = 1.744), as determined in previous experiments.
Figure 5.
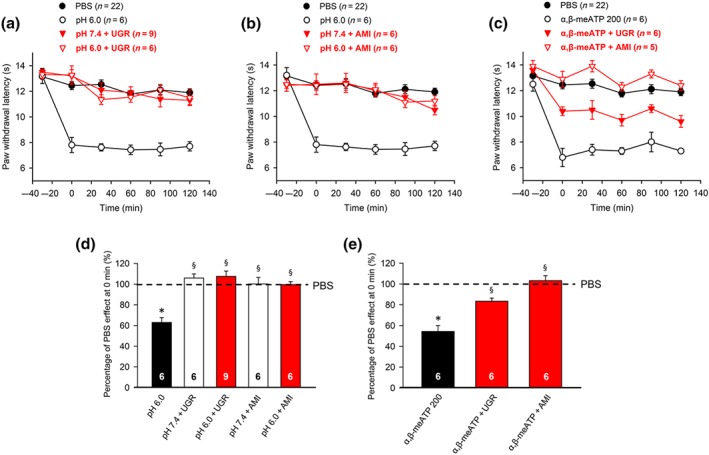
Modulation by selective (Ugr9‐1) or non‐selective (amiloride) ASIC3 antagonists of acute thermal hypersensitivity caused by local acidification to pH 6.0 or injection of α,β‐meATP (200 nM) into the left hind paw of rats at the −30 min time point. PWL measurement was as in Figure 1. Eighty rats as well as seven KO and seven WT mice were used to generate the data presented below. (a,b) The time‐dependent effects of pH 6.0 PBS alone, or together with Ugr9‐1 (20 μM; a) or amiloride (10 μM; b), are shown. For comparison, the change in PWL measured after injection of pH 7.4 PBS was also plotted. (c) The time‐dependent effect of α,β‐meATP (200 nM) alone, or together with Ugr9‐1 (20 μM) or amiloride (10 μM), is shown. Mean ± SEM determined on the bracketed number of animals. Effects of pH 6.0 PBS alone or together with Ugr9‐1 or amiloride expressed as a percentage of the normal PBS (pH 7.4; 100%) effect at 0 min (d). The missing effects of the two antagonistic drugs dissolved in pH 7.4 PBS are also plotted. Mean ± SEM of the indicated number of experiments. Effect of α,β‐meATP (200 nM) alone or together with Ugr9‐1 or amiloride expressed as a percentage of the normal PBS (pH 7.4; 100%) effect at 0 min (d). Mean ± SEM of the indicated number of experiments. AMI, amiloride. * P < .05, significantly different from the mean PWL measured after PBS (pH 7.4) injection at 0 min in b or d respectively. § P < .05, significantly different from the effect of pH 6.0 PBS injection at 0 min in d or e; Kruskal–Wallis one‐way ANOVA on ranks (H = 21.886; d) and (H = 27.726; e), followed by Holm–Sidak test
In view of the blockade of the pH 6.0 as well as the α,β‐meATP‐induced thermal hypersensitivity by APETx2 (20 μM), we decided to investigate whether this is due to a failure of this specific marine toxin to reliably differentiate between ASIC3 and P2X3 receptors or whether an ASIC3/P2X3 cognate receptor appears to operate at the cutaneous nerve terminals (Stephan et al., 2018). We considered this latter possibility highly likely because not only APETx2 (20 μM) but also another selective ASIC3 antagonist Ugr9‐1 (20 μM; Andreev et al., 2018) and the general https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=122/https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=685 antagonist amiloride (10 μM; Boscardin, Alijevic, Hummler, Frateschi, & Kellenberger, 2016) inhibited the thermal hypersensitivity caused by both acidic pH and purinergic stimulation (Figure 5a–e). It is noteworthy that neither UGr9‐1 nor amiloride altered the PWL when injected into the paw dissolved in pH 7.4 PBS (Figure 5a,b).
The prototypic P2X7 receptor agonist dibenzoyl‐ATP (Bz‐ATP) depressed the PWL with very steep dose–response dependence (Figure 6). Bz‐ATP at 20 nM had no effect, while at 50‐nM Bz‐ATP, the inhibition was already maximal (Figure 6a). When the dose of Bz‐ATP was increased from 50 to 100 nM, no further reduction of the PWL occurred. Moreover, Bz‐ATP at 200 nM had less effect than Bz‐ATP at 100 nM, indicating a bell‐shaped dose–response relationship. Therefore, we used Bz‐ATP at 50 nM in rats for all consecutive measurements. Experiments with the selective antagonists A‐317491 (P2X3 receptors; 300 nM) and A‐438079 (P2X7 receptors; 300 nM) showed that only antagonism at P2X7 receptors, but not at P2X3 receptors, blocked the reduction of PWL by Bz‐ATP (Figure 6b). In addition, A‐438079 alone facilitated the PWL at the 0‐min time point, probably by antagonizing a tonic inhibition of the P2X7 receptors by endogenous ATP (Figure 6b,e). Eventually, both EAP and moxibustion inhibited the Bz‐ATP (50 nM)‐induced inhibition with equal potency and comparable time course (Figure 6c). All findings could be confirmed by calculating the percentage changes at 0 min of the normal PWL, as shown in Figure 6e. By inspecting these data, it was surprising that, although Bz‐ATP was effective at a lower concentration (50 nM) than α,β‐meATP (200 nM), the maximum percentage inhibitory effect at 0 min was less when P2X7 receptors were activated by Bz‐ATP, compared with activation of P2X3 receptors by α,β‐meATP (compare Fig 4d with Figure 6e; P < .05; Student's t‐test). Further, the dose–response curves of Bz‐ATP and α,β‐meATP both exhibited a bell‐shaped pattern (compare Figure 6a with Figure 4a).
An additional argument for the stimulation of P2X7 rather than P2X3 receptors by Bz‐ATP in its relevant dose was provided by experiments using P2X7 receptor KO (P2X7R−/−) animals (Figure 6d,f). It was clear that Bz‐ATP (100 nM) had a marked effect in the WT (P2X7R+/+) mice, while it had no effect at all in the P2X7R−/− mice. In contrast to the classic P2X7R−/− mice (Glaxo and Pfizer), which are not a complete null allele, because certain functional splice variants evade inactivation, in the humanized P2X7R−/− mouse used by us, this receptor is completely inactivated (Metzger et al., 2017). Bz‐ATP (100 nM) was used in mice, which caused a considerably larger inhibition in the WT animals than the maximum effective dose of 50 nM in rats.
3.4. Effects of pharmacological antagonists, EAP, and moxibustion on CFA‐induced inflammatory pain in rats
In the above experiments, we found that EAP and moxibustion reversed the thermal hypersensitivity due to the injection of acidic PBS into the rat or mouse paw in an acute experimental setting. This effect was suggested to be due to a preferential modification of ASIC3‐mediated nociception at pH 6.0 but not at pH 4.0. It is generally believed that elevated proton concentrations can be registered in inflamed tissue and that inflammatory pain triggers the release of autacoids such as bradykinin, PGs, interleukins, and growth factors (Julius & Basbaum, 2001). Accordingly, in the present experiments, 24 hr after CFA (100 μl) injection into the left hind paw of rats, inflammatory hypersensitivity developed against radiant thermal pain.
In Figure 7, we present a summary of the experimental data generated on CFA‐treated rats. Figure 7a shows that the injection of PBS, 24 hr after applying CFA, did not change the extent and time course of the thermal hypersensitivity. The intra‐plantar application of APETx2 (20 μM) or A‐317491 (300 nM) partly reversed the decrease of PWL by CFA, whereas the combination of these antagonists caused complete blockade (Figure 7b). The injection of the selective P2X7 receptor antagonist A‐438079 (300 nM) also counteracted the effect of CFA; the combined blockade of P2X7 and P2X3 receptors by A‐438079 (300 nM) and A‐317491 (300 nM) caused a still larger effect (Figure 7c). The co‐application of capsazepine to block TRPV1 and A‐317491 to block P2X3 receptors caused also stronger inhibition, than the application of the two agonists alone (Figure 7b,d). Further, both EAP and moxibustion increased the fall in PWL, while sham EAP did not modify it (Figure 7e).
These observations were confirmed by calculating the percentage changes of the normal PWL at 0 min, as documented in Figure 7f,g. The occupation of ASIC3 and P2X3 receptors by the co‐application of APETx2 with A‐317491, the blockade of both TRPV1 channels and P2X3 receptors by capsazepine plus A317491, and the occupation of P2X7 and P2X3 receptors by the co‐application of A‐438079 with A‐317491, all eliminated the CFA‐induced inflammatory pain (Figure 7f). EAP and moxibustion, although probably to a lesser extent than the pharmacological antagonists, also inhibited this pain modality (Figure 7g). It became also evident that sham EAP on the ipsilateral side did not alter the effect of CFA alone. Hence, it could be concluded that inflammatory pain under our experimental conditions is mediated by a number of pH‐ and ATP‐sensitive nociceptors and that EAP/moxibustion most likely induced analgesia by reducing pain mediated by ASIC3 (and probably TRPV1 channels), as well as P2X3 and P2X7 receptors. The abolition of the CFA‐induced pain by combining A‐317491 with APETx2, capsazepine, or A‐438079 suggests that the stimulation of the pH‐ and ATP‐sensitive nociceptors involved caused a supra‐maximal effect.
4. DISCUSSION
There appears to be a direct link between tissue acidosis and the development of pain. Acute cutaneous pain is accompanied by a rapid drop of tissue pH and injection of acidic saline subcutaneously or intramuscularly results in the development of localized pain both in rodents and human subjects (Karczewski et al., 2010; Ugawa et al., 2002). It has been repeatedly shown that acid‐sensitive homomeric ASIC channels (ASIC1a and ASIC3; Deval & Lingueglia, 2015; Grunder & Chen, 2010), TRP channels (TRPV1 and TRPA1; Caterina et al., 1997; Y. Dai, 2016), homomeric P2X3 and P2X7 receptors, and heteromeric P2X2/3 receptors (North, 2002; Wirkner et al., 2007) mediate pain (Burnstock, 2009b; Y. Dai, 2016; Deval & Lingueglia, 2015).
P2X receptors respond to the injurious release of ATP from the intracellular into the extracellular space (Burnstock, 2009b; Burnstock, 2013; Wirkner et al., 2007). Whereas the effect of low concentrations of ATP is decreased at homomeric P2X3 receptors, in an acidic milieu (Stoop, Surprenant, & North, 1997; Virginio, Church, North, & Surprenant, 1997), that of high ATP concentrations are potentiated by a low pH (Gerevich et al., 2007). A direct comparison of the pH‐induced modulation of P2X receptor sensitivities showed that shifting the pH from 8.3 to 6.3, the current amplitudes evoked by ATP in dorsal root ganglia (DRG) neurons became smaller at homomeric P2X1, P2X3, P2X4, and P2X7 receptors (Flittiger, Klapperstuck, Schmalzing, & Markwardt, 2010; Virginio et al., 1997), but at the same time, they were potentiated at homomeric P2X2 receptors (North, 2002; North & Surprenant, 2000; Stoop et al., 1997). Because P2X2 and P2X3 subunits may assemble into heteromeric P2X2/3 receptors, this receptor type acquires significance in mediating acidic pain during co‐stimulation with ATP and protons (Hausmann et al., 2012; Kowalski et al., 2015).
As already pointed out, it is quite clear that acupuncture relieves painful conditions, but the reported experimental evidence is rather incomplete, and systematic investigations are missing. It has been concluded that moderate accumulation of protons (>pH 6.0) may gate preferentially ASIC3, while a greater accumulation of protons (< pH 6.0) appears to gate mostly TRPV1 channels (Deval et al., 2010). Thus, ASIC3 and TRPV1 channels have probably complementary roles in the acid sensitivity of sensory neurons. In fact, the distribution of both types of channels is significantly different, with less than 50% overlap in rat DRGs (Molliver et al., 2005; Ugawa, Ueda, Yamamura, & Shimada, 2005). In accordance with this assumption, we found that the injection of a pH 6.0 PBS into the rat paw decreased the PWL by primarily stimulating ASIC3, because a more marked and longer lasting inhibition of this decrease was achieved with APETx2 than with capsazepine. On the other hand, the reduction of PWL induced by the injection of pH 4.0 PBS primarily stimulated TRPV1 channels, because capsazepine but not APETx2 blocked the thermal hypersensitivity. EAP and moxibustion delivered to the ST36 acupoint were able to relieve pain caused by the weakly acidic but not the strongly acidic PBS.
In the rat CFA model of inflammatory pain, APETx2, a selective ASIC3 antagonist, significantly reduced both thermal and mechanical hypersensitivity (Deval et al., 2008). It was concluded that amiloride‐sensitive channels including ASICs play a role in the development of inflammatory pain (Karczewski et al., 2010; Kellenberger & Schild, 2002). We had to use a rather high dose of APETx2 throughout our experiments, in excess of those used previously for relieving acid‐induced inflammatory pain in the rodent paw (2.2 μM, rats; 1 μM, mice; Karczewski et al., 2010; Yen, Hsieh, Hsu, & Lin, 2017). However, these authors tested tactile hypersensitivity with von Frey filaments after the repeated injection of acidic solution into the gastrocnemius muscle, whereas we injected acidic PBS subcutaneously into the paw and measured thermal hypersensitivity. The repeated intramuscular injection of acidic medium with a pH of 4.0 causes inflammatory fibromyalgia and has been reported to be sensitive to EAP stimulation at ST36 acupoint but also to treatment with opioid and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=18 agonists (Yen et al., 2017). The injection of APETx2 into the paw of WT mice or experiments in ASIC3‐deficient mice also counteracted the inflammatory pain. Similarly, both miRNAs directed against ASIC3 (Walder, Gautam, Wilson, Benson, & Sluka, 2011) and EAP at ST36 (Chen et al., 2011) reduced mechanical hypersensitivity as measured in the mouse paw after carrageenan‐induced paw and/or muscle inflammation.
Our experiments showed for the injection of weakly acidic PBS (pH, 6.0) to the hind paw of TRPV1 WT mice that the percentage change in thermal hyperalgesia was eliminated by EAP and reduced, but not abolished, by moxibustion. By contrast, a similar procedure carried out in TRPV1‐deficient mice led to the complete blockade of the percentage change in thermal hypersensitivity by both EAP and moxibustion. We suggest that the deletion of the TRPV1 gene led to a compensatory increase of ASIC3 sensitivity, which resulted in a strengthening of the ASIC3‐dependent effect of EAP/moxibustion. In accordance with these results, EAP applied to ST36 abolished the CFA‐induced decrease in PWL of TRPV1−/− mice (Liao, Hsieh, Huang, & Lin, 2017).
In our inflammatory pain model, we found only a moderate decrease of the thermal paw hypersensitivity after the application of APETx2 or capsazepine. As mentioned above, ASIC3 and TRPV1 channels appear to work together in a complementary manner to perceive pain. Experimental fibromyalgia or inflammatory pain induced by CFA injection into the rodent paw up‐regulated the expression of both channel types in lumbar DRGs and the spinal cord dorsal horn, as shown by immunohistochemistry and western blotting techniques (Chen et al., 2011; Fang, Du, Liang, & Fang, 2013; Liao et al., 2017; Yang, Hsieh, & Lin, 2017). EAP applied to ST36 prevented not only nociception but also the increase of the respective channel proteins.
In contrast to the relatively restricted evidence for the effect of EAP on pain caused by acidification, there is a wealth of data available about the effect of EAP on pain caused by purinergic mechanisms (see Tang et al., 2018; Tang et al., 2016). It has been suggested that mechanical acupuncture and also EAP release ATP from (sub)cutaneous keratinocytes and mast cells and that either ATP itself or its enzymic degradation product, adenosine, is responsible for the ensuing analgesic effect (Burnstock, 2009a; Goldman et al., 2010). Whereas A1 receptors mediated the acupuncture‐induced analgesia (Goldman et al., 2010; Hurt & Zylka, 2012), we suggest that P2X3, P2X2/3, and P2X7 receptors might also mediate the comparable effect of EAP in our current experiments. It is noteworthy that Goldman et al. (2010) used manual acupuncture by turning around the needles inserted to ST36 every 5 min for 30 min, whereas we used EAP with continuous electrical stimulation of the same acupoint also for 30 min. Thus, our stimulation procedure was more powerful than the manual acupuncture, and therefore, these P2X receptors may come into play in addition to the A1 receptors involved.
We demonstrated that the acute pain due to the effect of the P2X1 and P2X3 selective agonist α,β‐meATP was ameliorated by the selective P2X3 receptor antagonist A‐317491. Inflammatory pain caused by CFA injection was also diminished by P2X3 receptor blockade and EAP/moxibustion, apparently because of the inflammation‐dependent release of ATP and the subsequent activation of P2X3 and P2X2/3 receptors (present study). In agreement with these findings, the involvement of P2X3 receptors in neuropathic pain and also the beneficial effect of EAP in this pain state has been repeatedly confirmed (R. D. Cheng et al., 2013; Wang et al., 2014; Zhou et al., 2018).
Convincing experimental evidence has been published for the participation of P2X7 receptors in neuropathic (Xu, Chen, Zheng, & Wang, 2016) and neck incision‐induced pain states (Gao et al., 2017), but not in inflammatory pain. In the present study, we demonstrated that acute thermal hypersensitivity induced by intra‐plantar injection of the preferential P2X1,3,7 receptor agonist Bz‐ATP was due to the stimulation of P2X7 rather than P2X1 or P2X3 receptors, because the highly selective P2X7 receptor antagonist A‐438079 caused a complete inhibition. Accordingly, the combined blockade of P2X3 and P2X7 receptors by A‐317491 and A‐438079 almost abolished the CFA‐induced inflammatory pain. Further, the co‐application of A‐317491 with APETx2 or capsazepine also strongly inhibited the effect of CFA. In consequence, the injurious release of ATP may activate P2X3 and P2X7 receptors, whereas the simultaneous release of protons activates ASIC3 and TRPV1 channels. EAP and to a lesser extent, moxibustion, might alleviate inflammatory pain arising through the stimulation of ASIC3/(TRPV1) and P2X3/P2X7 receptors.
In conclusion, EAP/moxibustion abolished the low pH‐ and ATP‐induced pain both under acute and subchronic inflammatory conditions. Although this does not necessarily imply that acid and purine‐sensitive receptors are involved in this effect, the limited number of channels/receptors mediating these pain states suggests that EAP/moxibustion interfered with the signalling pathways of ASIC3/P2X3 and P2X7 receptors.
AUTHOR CONTRIBUTIONS
P.R., Y.T., and P.I. participated in research design. Y.Z. and L.H. conducted the experiments. S.K. contributed new reagents or analytic tools. Y.Z., L.H., and P.I. performed data analysis. S.K., P.R., Y.T., and P.I. wrote or contributed to the writing of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/abs/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/abs/10.1111/bph.14208, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
ACKNOWLEDGEMENTS
We are most grateful to Dr Jan M. Deussing for the supply of P2X7R‐KO mice and their WT controls as well as for many helpful discussions. Our work was made possible by a generous grant (“The Project First‐Class Disciplines Development”; CZYHW1901) of the Chengdu University of Traditional Chinese Medicine to Y.T. and P.I. in order to build up “The International Collaborative Center for Purinergic Signaling” and grants of the Sichuan Provincial Administration of Foreign Affairs to support the stays of P.I. and P.R. in Chengdu (SZD201731 and SZD201846). Financial support from the National Natural Science Foundation of China (Grants 81774437, 81173320, and 81373735) and Sichuan Science and Technology Program (Grant 2019YFH0108) are also gratefully acknowledged.
Zhang Y, Huang L, Kozlov SA, Rubini P, Tang Y, Illes P. Acupuncture alleviates acid‐ and purine‐induced pain in rodents. Br J Pharmacol. 2020;177:77–92. 10.1111/bph.14847
REFERENCES
- Andreev, Y. A. , Osmakov, D. I. , Koshelev, S. G. , Maleeva, E. E. , Logashina, Y. A. , Palikov, V. A. , … Kozlov, S. (2018). Analgesic activity of acid‐sensing ion channel 3 (ASIC3) inhibitors: Sea anemones peptides Ugr9‐1 and APETx2 versus low molecular weight compounds. Marine Drugs, 16 10.3390/md16120500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174, S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Striessnig, J. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. British Journal of Pharmacology, 174, S160–S194. 10.1111/bph.13884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baconguis, I. , Hattori, M. , & Gouaux, E. (2013). Unanticipated parallels in architecture and mechanism between ATP‐gated P2X receptors and acid sensing ion channels. Current Opinion in Structural Biology, 23, 277–284. 10.1016/j.sbi.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscardin, E. , Alijevic, O. , Hummler, E. , Frateschi, S. , & Kellenberger, S. (2016). The function and regulation of acid‐sensing ion channels (ASICs) and the epithelial Na+ channel (ENaC): IUPHAR review 19. British Journal of Pharmacology, 173, 2671–2701. 10.1111/bph.13533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock, G. (2009a). Acupuncture: A novel hypothesis for the involvement of purinergic signalling. Medical Hypotheses, 73, 470–472. 10.1016/j.mehy.2009.05.031 [DOI] [PubMed] [Google Scholar]
- Burnstock, G. (2009b). Purinergic mechanosensory transduction and visceral pain. Molecular Pain, 5, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock, G. (2013). Purinergic mechanisms and pain—An update. European Journal of Pharmacology, 716, 24–40. 10.1016/j.ejphar.2013.01.078 [DOI] [PubMed] [Google Scholar]
- Campbell, A. (2006). Point specificity of acupuncture in the light of recent clinical and imaging studies. Acupuncture in Medicine, 24, 118–122. 10.1136/aim.24.3.118 [DOI] [PubMed] [Google Scholar]
- Caterina, M. J. , Schumacher, M. A. , Tominaga, M. , Rosen, T. A. , Levine, J. D. , & Julius, D. (1997). The capsaicin receptor: A heat‐activated ion channel in the pain pathway. Nature, 389, 816–824. 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- Chen, W. H. , Hsieh, C. L. , Huang, C. P. , Lin, T. J. , Tzen, J. T. , Ho, T. Y. , & Lin, Y. W. (2011). Acid‐sensing ion channel 3 mediates peripheral anti‐hyperalgesia effects of acupuncture in mice inflammatory pain. Journal of Biomedical Science, 18, 82 10.1186/1423-0127-18-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, R. D. , Tu, W. Z. , Wang, W. S. , Zou, E. M. , Cao, F. , Cheng, B. , … Jiang, S. H. (2013). Effect of electroacupuncture on the pathomorphology of the sciatic nerve and the sensitization of P2X3 receptors in the dorsal root ganglion in rats with chronic constrictive injury. Chinese Journal of Integrative Medicine, 19, 374–379. 10.1007/s11655-013-1447-1 [DOI] [PubMed] [Google Scholar]
- Cheng, Y. R. , Jiang, B. Y. , & Chen, C. C. (2018). Acid‐sensing ion channels: Dual function proteins for chemo‐sensing and mechano‐sensing. Journal of Biomedical Science, 25, 46 10.1186/s12929-018-0448-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun, D. , & Novella, S. P. (2013). Acupuncture is theatrical placebo. Anesthesia and Analgesia, 116, 1360–1363. 10.1213/ANE.0b013e31828f2d5e [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Glembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, S. P. , Huang, Y. H. , Chang, C. J. , Huang, Y. F. , Hsieh, W. S. , Tabata, Y. , … Sun, W. H. (2017). TDAG8 involved in initiating inflammatory hyperalgesia and establishing hyperalgesic priming in mice. Scientific Reports, 7, 41415 10.1038/srep41415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, Y. (2016). TRPs and pain. Seminars in Immunopathology, 38, 277–291. 10.1007/s00281-015-0526-0 [DOI] [PubMed] [Google Scholar]
- Deval, E. , Gasull, X. , Noel, J. , Salinas, M. , Baron, A. , Diochot, S. , & Lingueglia, E. (2010). Acid‐sensing ion channels (ASICs): Pharmacology and implication in pain. Pharmacology & Therapeutics, 128, 549–558. 10.1016/j.pharmthera.2010.08.006 [DOI] [PubMed] [Google Scholar]
- Deval, E. , & Lingueglia, E. (2015). Acid‐sensing ion channels and nociception in the peripheral and central nervous systems. Neuropharmacology, 94, 49–57. 10.1016/j.neuropharm.2015.02.009 [DOI] [PubMed] [Google Scholar]
- Deval, E. , Noel, J. , Lay, N. , Alloui, A. , Diochot, S. , Friend, V. , … Lingueglia, E. (2008). ASIC3, a sensor of acidic and primary inflammatory pain. The EMBO Journal, 27, 3047–3055. 10.1038/emboj.2008.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, J. Q. , Du, J. Y. , Liang, Y. , & Fang, J. F. (2013). Intervention of electroacupuncture on spinal p38 MAPK/ATF‐2/VR‐1 pathway in treating inflammatory pain induced by CFA in rats. Molecular Pain, 9, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flittiger, B. , Klapperstuck, M. , Schmalzing, G. , & Markwardt, F. (2010). Effects of protons on macroscopic and single‐channel currents mediated by the human P2X7 receptor. Biochimica et Biophysica Acta, 1798, 947–957. 10.1016/j.bbamem.2010.01.023 [DOI] [PubMed] [Google Scholar]
- Gao, Y. H. , Li, C. W. , Wang, J. Y. , Tan, L. H. , Duanmu, C. L. , Jing, X. H. , … Liu, J. L. (2017). Effect of electroacupuncture on the cervicospinal P2X7 receptor/fractalkine/CX3CR1 signaling pathway in a rat neck‐incision pain model. Purinergic Signalling, 13, 215–225. 10.1007/s11302-016-9552-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerevich, Z. , Zadori, Z. S. , Koles, L. , Kopp, L. , Milius, D. , Wirkner, K. , … Illes, P. (2007). Dual effect of acid pH on purinergic P2X3 receptors depends on the histidine 206 residue. The Journal of Biological Chemistry, 282, 33949–33957. 10.1074/jbc.M705840200 [DOI] [PubMed] [Google Scholar]
- Goldman, N. , Chen, M. , Fujita, T. , Xu, Q. , Peng, W. , Liu, W. , … Nedergaard, M. (2010). Adenosine A1 receptors mediate local anti‐nociceptive effects of acupuncture. Nature Neuroscience, 13, 883–888. 10.1038/nn.2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunder, S. , & Chen, X. (2010). Structure, function, and pharmacology of acid‐sensing ion channels (ASICs): Focus on ASIC1a. Int J Physiol Pathophysiol Pharmacol, 2, 73–94. [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann, R. , Bodnar, M. , Woltersdorf, R. , Wang, H. , Fuchs, M. , Messemer, N. , … Illes, P. (2012). ATP binding site mutagenesis reveals different subunit stoichiometry of functional P2X2/3 and P2X2/6 receptors. The Journal of Biological Chemistry, 287, 13930–13943. 10.1074/jbc.M112.345207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt, J. K. , & Zylka, M. J. (2012). PAPupuncture has localized and long‐lasting antinociceptive effects in mouse models of acute and chronic pain. Molecular Pain, 8, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardin, I. , Lopez, J. J. , Diez, R. , Sanchez‐Collado, J. , Cantonero, C. , Albarran, L. , … Rosado, J. A. (2017). TRPs in pain sensation. Frontiers in Physiology, 8, 392 10.3389/fphys.2017.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius, D. , & Basbaum, A. I. (2001). Molecular mechanisms of nociception. Nature, 413, 203–210. 10.1038/35093019 [DOI] [PubMed] [Google Scholar]
- Karczewski, J. , Spencer, R. H. , Garsky, V. M. , Liang, A. , Leitl, M. D. , Cato, M. J. , … Urban, M. O. (2010). Reversal of acid‐induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. British Journal of Pharmacology, 161, 950–960. 10.1111/j.1476-5381.2010.00918.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger, S. , & Grutter, T. (2015). Architectural and functional similarities between trimeric ATP‐gated P2X receptors and acid‐sensing ion channels. Journal of Molecular Biology, 427, 54–66. 10.1016/j.jmb.2014.06.004 [DOI] [PubMed] [Google Scholar]
- Kellenberger, S. , & Schild, L. (2002). Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiological Reviews, 82, 735–767. 10.1152/physrev.00007.2002 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski, M. , Hausmann, R. , Schmid, J. , Dopychai, A. , Stephan, G. , Tang, Y. , … Rubini, P. (2015). Flexible subunit stoichiometry of functional human P2X2/3 heteromeric receptors. Neuropharmacology, 99, 115–130. 10.1016/j.neuropharm.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Liao, H. Y. , Hsieh, C. L. , Huang, C. P. , & Lin, Y. W. (2017). Electroacupuncture attenuates induction of inflammatory pain by regulating opioid and adenosine pathways in mice. Scientific Reports, 7, 15679 10.1038/s41598-017-16031-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen, M. V. , Gotzsche, P. C. , & Hrobjartsson, A. (2009). Acupuncture treatment for pain: Systematic review of randomised clinical trials with acupuncture, placebo acupuncture, and no acupuncture groups. BMJ (Clinical Res Ed), 338, a3115 10.1136/bmj.a3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger, M. W. , Walser, S. M. , Aprile‐Garcia, F. , Dedic, N. , Chen, A. , Holsboer, F. , … Deussing, J. M. (2017). Genetically dissecting P2rx7 expression within the central nervous system using conditional humanized mice. Purinergic Signalling, 13, 153–170. 10.1007/s11302-016-9546-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver, D. C. , Immke, D. C. , Fierro, L. , Pare, M. , Rice, F. L. , & McCleskey, E. W. (2005). ASIC3, an acid‐sensing ion channel, is expressed in metaboreceptive sensory neurons. Molecular Pain, 1, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, M. M. , & Szallasi, A. (2018). Targeting nociceptive transient receptor potential channels to treat chronic pain: Current state of the field. British Journal of Pharmacology, 175, 2185–2203. 10.1111/bph.14044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, M. , & Jang, I. S. (2015). Acid modulation of tetrodotoxin‐resistant Na+ channels in rat nociceptive neurons. Neuropharmacology, 90, 82–89. 10.1016/j.neuropharm.2014.11.005 [DOI] [PubMed] [Google Scholar]
- North, R. A. (2002). Molecular physiology of P2X receptors. Physiological Reviews, 82, 1013–1067. 10.1152/physrev.00015.2002 [DOI] [PubMed] [Google Scholar]
- North, R. A. , & Surprenant, A. (2000). Pharmacology of cloned P2X receptors. Annual Review of Pharmacology and Toxicology, 40, 563–580. 10.1146/annurev.pharmtox.40.1.563 [DOI] [PubMed] [Google Scholar]
- Osmakov, D. I. , Kozlov, S. A. , Andreev, Y. A. , Koshelev, S. G. , Sanamyan, N. P. , Sanamyan, K. E. , … Grishin, E. V. (2013). Sea anemone peptide with uncommon β‐hairpin structure inhibits acid‐sensing ion channel 3 (ASIC3) and reveals analgesic activity. The Journal of Biological Chemistry, 288, 23116–23127. 10.1074/jbc.M113.485516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeranz, B. , & Chiu, D. (1976). Naloxone blockade of acupuncture analgesia: Endorphin implicated. Life Sciences, 19, 1757–1762. 10.1016/0024-3205(76)90084-9 [DOI] [PubMed] [Google Scholar]
- Reeh, P. W. , & Steen, K. H. (1996). Tissue acidosis in nociception and pain. Progr. Brain Research, 113, 143–151. 10.1016/S0079-6123(08)61085-7 [DOI] [PubMed] [Google Scholar]
- Stephan, G. , Huang, L. , Tang, Y. , Vilotti, S. , Fabbretti, E. , Yu, Y. , … Illes, P. (2018). The ASIC3/P2X3 cognate receptor is a pain‐relevant and ligand‐gated cationic channel. Nature Communications, 9, 1354 10.1038/s41467-018-03728-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop, R. , Surprenant, A. , & North, R. A. (1997). Different sensitivities to pH of ATP‐induced currents at four cloned P2X receptors. Journal of Neurophysiology, 78, 1837–1840. 10.1152/jn.1997.78.4.1837 [DOI] [PubMed] [Google Scholar]
- Tang, Y. , Yin, H. Y. , Liu, J. , Rubini, P. , & Illes, P. (2018). P2X receptors and acupuncture analgesia. Brain Research Bulletin, 151, 144–152. 10.1016/j.brainresbull.2018.10.015. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Tang, Y. , Yin, H. Y. , Rubini, P. , & Illes, P. (2016). Acupuncture‐induced analgesia: A neurobiological basis in purinergic signaling. The Neuroscientist, 22, 563–578. 10.1177/1073858416654453 [DOI] [PubMed] [Google Scholar]
- Torres‐Rosas, R. , Yehia, G. , Pena, G. , Mishra, P. , del Rocio Thompson‐Bonilla, M. , Moreno‐Eutimio, M. A. , … Ulloa, L. (2014). Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nature Medicine, 20, 291–295. 10.1038/nm.3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa, S. , Ueda, T. , Ishida, Y. , Nishigaki, M. , Shibata, Y. , & Shimada, S. (2002). Amiloride‐blockable acid‐sensing ion channels are leading acid sensors expressed in human nociceptors. The Journal of Clinical Investigation, 110, 1185–1190. 10.1172/JCI0215709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa, S. , Ueda, T. , Yamamura, H. , & Shimada, S. (2005). In situ hybridization evidence for the coexistence of ASIC and TRPV1 within rat single sensory neurons. Brain Research. Molecular Brain Research, 136, 125–133. 10.1016/j.molbrainres.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Vickers, A. J. , & Linde, K. (2014). Acupuncture for chronic pain. JAMA, 311, 955–956. 10.1001/jama.2013.285478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virginio, C. , Church, D. , North, R. A. , & Surprenant, A. (1997). Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology, 36, 1285–1294. 10.1016/S0028-3908(97)00141-X [DOI] [PubMed] [Google Scholar]
- Walder, R. Y. , Gautam, M. , Wilson, S. P. , Benson, C. J. , & Sluka, K. A. (2011). Selective targeting of ASIC3 using artificial miRNAs inhibits primary and secondary hyperalgesia after muscle inflammation. Pain, 152, 2348–2356. 10.1016/j.pain.2011.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. S. , Tu, W. Z. , Cheng, R. D. , He, R. , Ruan, L. H. , Zhang, L. , … Jiang, S. H. (2014). Electroacupuncture and A‐317491 depress the transmission of pain on primary afferent mediated by the P2X3 receptor in rats with chronic neuropathic pain states. Journal of Neuroscience Research, 92, 1703–1713. 10.1002/jnr.23451 [DOI] [PubMed] [Google Scholar]
- Wemmie, J. A. , Taugher, R. J. , & Kreple, C. J. (2013). Acid‐sensing ion channels in pain and disease. Nature Reviews Neuroscience, 14, 461–471. 10.1038/nrn3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirkner, K. , Sperlagh, B. , & Illes, P. (2007). P2X3 receptor involvement in pain states. Molecular Neurobiology, 36, 165–183. 10.1007/s12035-007-0033-y [DOI] [PubMed] [Google Scholar]
- Xu, J. , Chen, X. M. , Zheng, B. J. , & Wang, X. R. (2016). Electroacupuncture relieves nerve injury‐induced pain hypersensitivity via the inhibition of spinal P2X7 receptor‐positive microglia. Anesthesia and Analgesia, 122, 882–892. 10.1213/ANE.0000000000001097 [DOI] [PubMed] [Google Scholar]
- Yang, J. , Hsieh, C. L. , & Lin, Y. W. (2017). Role of transient receptor potential vanilloid 1 in electroacupuncture analgesia on chronic inflammatory pain in mice. BioMed Research International, 2017, 5068347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen, L. T. , Hsieh, C. L. , Hsu, H. C. , & Lin, Y. W. (2017). Targeting ASIC3 for relieving mice fibromyalgia pain: Roles of electroacupuncture, opioid, and adenosine. Scientific Reports, 7, 46663 10.1038/srep46663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, R. , Lao, L. , Ren, K. , & Berman, B. M. (2014). Mechanisms of acupuncture‐electroacupuncture on persistent pain. Anesthesiology, 120, 482–503. 10.1097/ALN.0000000000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. F. , Ying, X. M. , He, X. F. , Shou, S. Y. , Wei, J. J. , Tai, Z. X. , … Jiang, Y. L. (2018). Suppressing PKC‐dependent membrane P2X3 receptor upregulation in dorsal root ganglia mediated electroacupuncture analgesia in rat painful diabetic neuropathy. Purinergic Signalling, 14, 359–369. 10.1007/s11302-018-9617-4 [DOI] [PMC free article] [PubMed] [Google Scholar]


