Abstract
Background and Purpose
In patients with pulmonary hypertension (PH) associated with lung disease and/or hypoxia (Group III), decreased pulmonary vascular tone and tissue hypoxia is therapeutically beneficial. PGE2 and PGI2 induce potent relaxation of human bronchi from non‐PH (control) patients via EP4 and IP receptors, respectively. However, the effects of PGE2/PGI2 and their mimetics on human bronchi from PH patients are unknown. Here, we have compared relaxant effects of several PGI2–mimetics approved for treating PH Group I with several PGE2–mimetics, in bronchial preparations derived from PH Group III and control patients.
Experimental Approach
Relaxation of bronchial muscle was assessed in samples isolated from control and PH Group III patients. Expression of prostanoid receptors was analysed by western blot and real‐time PCR, and endogenous PGE2, PGI2, and cAMP levels were determined by ELISA.
Key Results
Maximal relaxations induced by different EP4 receptor agonists (PGE2, L‐902688, and ONO‐AE1‐329) were decreased in human bronchi from PH patients, compared with controls. However, maximal relaxations produced by PGI2–mimetics (iloprost, treprostinil, and beraprost) were similar for both groups of patients. Both EP4 and IP receptor protein and mRNA expressions were significantly lower in human bronchi from PH patients. cAMP levels significantly correlated with PGI2 but not with PGE2 levels.
Conclusion and Implications
The PGI2–mimetics retained maximal bronchodilation in PH Group III patients, whereas bronchodilation induced by EP4 receptor agonists was decreased. Restoration of EP4 receptor expression in airways of PH Group III patients with respiratory diseases could bring additional therapeutic benefit.
What is already known
Inhaled PGI2 mimetics are major vasodilators used in the treatment of pulmonary hypertension (PH).
What does this study add
Relaxation to PGE2 mimetics is impaired in isolated bronchi from PH Group III patients.
Relaxation to PGI2 mimetics is retained in these isolated bronchi.
What is the clinical significance
Impairment of PGE2‐induced bronchodilation via EP4 receptors may be involved in PH Group III pathogenesis.
Abbreviations
- 6MWD
6‐min walk distance
- FEV
forced expiratory volume
- FVC
forced vital capacity
- mPAP
mean pulmonary arterial pressure
- PH
pulmonary hypertension
1. INTRODUCTION
Pulmonary hypertension (PH) has recently been defined as mean pulmonary arterial pressure (mPAP) higher than 20 mmHg and is associated with a high rate of mortality (Simonneau et al., 2019). According to classification established by the World Health Organization (Galie et al., 2016), PH Group III has a high prevalence rate and is associated with chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, emphysema, or bronchial dilatation dysfunction (Hoeper, McLaughlin, Dalaan, Satoh, & Galie, 2016). These patients are mostly hypoxic, and the administration of supplemental oxygen is an important standardized step in their treatment (Fein, Zaidi, & Sulica, 2016).
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1915 (prostacyclin) and its mimetics (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1895, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5820, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5852, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=summary&ligandId=1967) are known to be effective treatment options for Group I pulmonary arterial hypertension (PAH) patients, through their vasodilatory and antiproliferative properties in pulmonary vessels (Clapp & Gurung, 2015; Hill et al., 2015; Pluchart, Khouri, Blaise, Roustit, & Cracowski, 2017). In addition, some studies with PH Group III patients demonstrate that PGI2–mimetics improve their 6‐min walk distance (6MWD), dyspnoea, and mPAP (Bourge et al., 2013; Hoeper et al., 2016; Olschewski et al., 1999; Shimizu, Imanishi, Takano, & Miwa, 2011). The results of such studies and others support the proposal that vasorelaxant therapy in Group III patients with severe PH could be beneficial (Harari, Elia, & Humbert, 2018; Olschewski et al., 1999; Reichenberger et al., 2007).
Respiratory function is one of the key parameters in PH patients and, therefore, agents that induce bronchorelaxation and reduce hypoxia may provide greater benefit for PH Group III patients (Harari et al., 2018). In addition, if agents are delivered through the inhaled route, as with iloprost or treprostinil, then bronchodilation could enhance both drug and oxygen delivery to the pulmonary vessels and blood circulation, while avoiding untoward ventilation/perfusion mismatch potential (Bourge et al., 2013; Pluchart et al., 2017).
The dilatory effects of PGI2 and its mimetics are mostly mediated via stimulation of the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=345 in human pulmonary vessels and bronchi (Haye‐Legrand et al., 1987; Norel et al., 1999). However, if we consider the other prostanoid receptors involved in relaxation, iloprost can also bind somewhat to http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=343, and treprostinil potently can bind to https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=341, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=338, and somewhat to EP4 receptors, which are the preferred receptors for http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1883 (EP1–4) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1881 (DP1; Abramovitz et al., 2000; Whittle, Silverstein, Mottola, & Clapp, 2012). These affinities exhibited by some PGI2–mimetics for other PG receptors could have beneficial effects as PGE2, and in particular EP4 receptor agonists, are known to induce bronchorelaxation in humans (Benyahia et al., 2012; Buckley et al., 2011; Safholm et al., 2015). Furthermore, PGE2, via the EP4 receptor, inhibited proliferation and migration of human airway smooth muscle cells and played an anti‐inflammatory role in lungs (Aso et al., 2013; Birrell et al., 2015; Mori et al., 2011). However, these protective effects of EP4 receptor agonists were shown only in healthy subjects, and it is not known whether these functions will be similar in PH Group III patients.
Clinically, the efficacy of PH treatments is evaluated by decreased dyspnoea and improved capacity to perform physical effort (6MWD). From this perspective, bronchial reactivity could also enhance cardiorespiratory performance, most likely in PH Group III patients. Given the complexity of adaptation to physical effort and exertion, when these prostanoids are investigated in the context of PH, it is important to understand how these agonists differentially affect airway reactivity and which prostanoid receptors are involved. Therefore, the aim of our study was to assess these complex interactions using bronchial preparations derived from control or PH Group III patients.
2. METHODS
2.1. Human pulmonary bronchial preparations
Human bronchial preparations were collected in Bichat Hospital (Paris) after obtaining patients' informed consent with Ethics Committee approval from INSERM and AP‐HP (CEERB du GHU Nord) Institutional Review Board (no. IRB00006477). These investigations conform to the principles outlined in the Declaration of Helsinki. Control bronchi preparations were obtained from patients (25 male and 14 female) who underwent surgery mostly for lung carcinoma while PH bronchial preparations were obtained from patients (13 male and 9 female) who had undergone surgery for lung transplantation. Categories of patients are PH due to lung diseases and/or hypoxia (Group III of PH classification, Galie et al., 2016) with detailed characteristics presented in Table S1 and control patient characteristics presented in Table S2. PH lungs used in our study were from patients having catheter‐measured mPAP ≥20 mmHg. Bronchi were carefully removed from the macroscopically normal regions of the lungs. All preparations were used within 1–12 hr of surgery.
2.2. Organ bath and isometric measurements
Human bronchial specimens derived from control and PH patients (3‐ to 6‐mm internal diameter) were cut as rings and set up in 10‐ml organ baths containing Tyrode's solution (concentration mM): NaCl 139.2, KCl 2.7, CaCl2 1.8, MgCl2 0.49, NaHCO3 11.9, NaH2PO4 0.4, glucose 5.5, gassed with 5% CO2 and 95% O2 at 37°C and pH 7.4. Each ring was initially stretched to an optimal load (~1–2 g). Following equilibration (90 min), the preparations were precontracted with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1204 (50 μM) in the presence of the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=269 inhibitor (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1909; 1.7 μM) or http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=346/https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=339 (CRTH2) receptor antagonist (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1911; 1 μM, when PGE2 was used as a relaxant agonist). These agents were used to avoid any physiological effects induced by the release of endogenous prostanoids and/or activation of the TxA2 receptor (TP) by PGE2. When the response reached a plateau, a cumulative concentration of EP receptor agonists (PGE2 [EP1–4 receptors], https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1933 [EP4 receptors ], https://www.guidetopharmacology.org/GRAC/DatabaseSearchForward?searchString=L-902688+&searchCategories=all&species=none&type=all&comments=includeComments&order=rank&submit=Search+Database [EP4 receptors ], or https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1932 [EP2 receptors]) or PGI2–mimetics (iloprost, treprostinil, beraprost, or MRE‐269) was added to the baths (1 nM to 10 μM).
For the pharmacological studies, control preparations were incubated in the presence or absence of one of the following prostanoid receptor antagonists: RO3244019 (AGN‐230933) or https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1969 (IP), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=summary&ligandId=1953 (EP4/TP receptors), L‐877499 (DP1 receptors), BAY‐u3405 (TP receptors), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1921 or https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1924 (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=340 receptors), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=summary&ligandId=5844, or https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5822 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=342). Following the incubation period, a precontraction was induced with histamine (50 μM), and when the contraction reached a plateau, cumulative concentrations of iloprost and treprostinil were added to the baths.
2.3. Measurement of the expression of prostanoid receptors by western blot analysis
The experimental detail provided conforms with British Journal of Pharmacology Guidelines (Alexander et al., 2018). Human bronchial preparations were homogenized under liquid nitrogen using a porcelain mortar. The homogenates were resuspended in RIPA solution containing Tris–HCl buffer (in mM: Tris, 50; NaCl, 150; EDTA, 5; Triton X‐100, 1%; sodium deoxycholate, 1%; SDS, 0.1%) at 4°C (1 ml per 100 mg of tissue) with a protease inhibitor cocktail. The homogenates were centrifuged at 4,000 g for 20 min, at 4°C. The supernatants were assayed for protein content using a bicinchoninic acid protein assay kit; 50 μg of protein were loaded on a 13% SDS‐PAGE. Proteins were transferred to nitrocellulose membranes which were subsequently blocked for 1 hr in Tris‐buffered saline 0.1% Tween 20, 5% nonfat dry milk. Membranes were then incubated overnight at 4°C with an anti‐EP2 receptor antibody (polyclonal, 1/200), an anti‐DP1 receptor antibody (polyclonal 1/200), an anti‐ EP4 receptor antibody (polyclonal, 1/200), or an anti‐IP receptor antibody (polyclonal, 1/500). After overnight incubation, the membranes were washed and then incubated with appropriate peroxidase‐conjugated secondary antibody (1/10,000). Bands were visualized using the ECL plus luminescence system. For quantification, the film was scanned (GS‐800 Calibrated densitometer), and the integrated OD of the bands was estimated with Scion Image software® (RRID:SCR_008673) and normalized to α‐actin. The homogenates of rat brain and pulmonary artery smooth muscle cells samples were used as standards for the IP receptor in our western blot experiments.
2.4. Real‐time PCR analysis of prostanoid receptors mRNA expression
Tissue samples (50–100 mg) were placed into a safe lock tubes containing two beads (tungsten carbide beads, 3 mm). The preparations were lysed in 1 ml of Qiazol® lysis reagent by using the TissueLyser Adapter Set 2 × 24 and ground for 4 min at 30 Hz. Addition of 300 μl of chloroform followed by a centrifugation (15 min, 16,000 g, 4°C) separated the solution with an aqueous phase containing RNA. Total RNA was extracted using the RNeasy Plus Mini kit according to the manufacturer's instructions. The quantity of RNA was measured using a spectrophotometer (NanoDrop 2000c; Thermo Scientific; Waltham, MA, USA). The preparations were reverse transcribed using Maxima First Strand cDNA Synthesis Kits according to the manufacturer's standard protocol. Real‐time PCRs were performed in the CFX96 (Bio‐Rad CFX Manage; CA, USA) device with the iQ SYBR Green Supermix Kit, according to the manufacturer's standard protocol, using specific primers (Table S3). To determine the relative accumulation of the prostanoid receptor transcripts in human bronchi from control or PH patients, the threshold cycle (CT) values of each transcript were normalized by subtracting the corresponding CT values obtained from the GAPDH control used as the internal standard (ΔCT). The difference in expression of the target genes (EP4, EP2, and IP receptors) was analysed using the formula: 2−ΔΔCT, where ΔΔCT = (CTprostanoid receptors − CTGAPDH) − (mean CTprostanoid receptors − mean CTGAPDH).
2.5. Measurements of cAMP, PGI2, and PGE2
The endogenous levels of cAMP (after acetylation), 6‐keto‐PGF1α (a stable metabolite of PGI2), and PGE2 were measured in human bronchial homogenate supernatants using an enzyme immunoassay kit according to the manufacturer's instructions. The cAMP and PGs concentrations were expressed as pmol·mg−1 or ng·μg−1 of protein concentrations calculated in these supernatants, respectively. Technical replicates were used to ensure the reliability of single values.
2.6. Measurement of functional respiratory tests in patients from the TRIUMPH study
These unpublished data from the pivotal Phase III study of inhaled treprostinil in PH Group I patients (Benza et al., 2011; McLaughlin et al., 2010) were obtained from United Therapeutics Corporation. The methods used in the TRIUMPH‐1 study have been described (Benza et al., 2011; McLaughlin et al., 2010). According to this, eligible patients were between the ages of 18 and 75 years with a confirmed diagnosis of idiopathic or familial PAH or PAH associated with collagen vascular disease, human immunodeficiency virus infection, or anorexigen use. Patients were New York Heart Association functional class III or IV with a baseline 6MWD between 200 and 450 m and were receiving bosentan 125 mg daily or any prescribed dose of sildenafil, 20 mg tid, for at least 3 months before study entry.
2.7. Protocol (data on file, courtesy of United Therapeutics Corporation)
At baseline (Visit 1), patients had been assessed to verify that they meet entrance criteria and then underwent a physical exam including a review of PH signs and symptoms and a review of medical history and concomitant medications, chest X‐ray, pulmonary function tests (forced expiratory volume [FEV] and forced vital capacity [FVC]), The Minnesota Living With Heart Failure Questionnaire, and vital signs. Patients who had been included in this study provided blood samples, and women of childbearing potential also provided a urine sample for pregnancy testing. Patients had performed a trough (predosing) 6MWD test and had their Borg dyspnoea score noted. Visit 4 had consisted of dosing at the centre, measurement of vital signs, and a peak 6MWD with monitoring. Within 24–72 hr after Visit 4, patients returned (Visit 5) to complete the final study procedures, which included physical examination, including a review of PH signs and symptoms, vital signs, pulmonary function tests, The Minnesota Living With Heart Failure Questionnaire, a trough 6MWD test, New York Heart Association classification, Borg dyspnoea scoring, and chest X‐ray, and provided blood samples.
2.8. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). For all experiments, the number of observations (group size) is provided in the figure legends, with a minimum of five independent observations performed in patient samples. Statistical analysis was undertaken only for studies where each group size was at least n = 5. The declared group size is the number of independent values, and that statistical analysis was done using these independent values. ELISA measurements were performed in duplicate and an average taken in each sample to calculate the final mean data. The pharmacological protocol was randomly assigned and predetermined before mounting the bronchial preparations in each organ bath. Experimental blinding was not used for this study as there was one core experimenter responsible for each of the protocols described, where individuals also performed the subsequent analysis. In order to limit experimental bias, analysis was not routinely performed until experimental data set was complete.
Acquisition and processing of the physiological data (contraction/relaxation) were performed with the IOX software® (EMKA, Paris, France). The effects induced by the different agonists were expressed in grams or normalized (%) with respect to an initial reference contraction (histamine, 50 μM) measured just before the addition of the lowest concentration of the vasorelaxant agonist. This allowed for comparison of agonist responses independent of size of bronchial specimens or contraction. The values are positive for contractions and negative for relaxations. Where possible, a four‐parameter logistic equation of the form
was fitted to data obtained from each organ bath protocols to provide estimates of the maximal relaxation (Emax) of the EP or IP receptor ligands [A], the half‐maximum effective concentration values (EC50), and Hill slope (nH) parameters. All results were analysed using SigmaPlot® (RRID:SCR_003210) for Windows (Systat software, Inc, Richmond, CA, USA, 12.0 version). The pEC50 values (potency) were calculated as the negative log of EC50 values. All data are means ± SEM derived from (n) independent patients, and statistical analyses on the curves, on Emax and pEC50 values, on mRNA expression, and on the optical density of the band were performed using two‐ or one‐way ANOVA followed by Student–Newman–Keuls test or Student's t test with a confidence level of 95%. Post hoc tests were carried out only if F was significant and there was no variance in homogeneity. Pearson's correlations were performed, correlation coefficients (r) were calculated, and P values less than .05 were considered statistically significant. SigmaStat® (RRID:SCR_010285) statistical software (SYSTAT, Richmond, CA, USA) was used.
2.9. Materials
Iloprost, beraprost, treprostinil, PGE2, MRE‐269, BAY‐u3405, CAY10441, SC‐51322, GW627368, anti‐EP4 (RRID:AB_327850), anti‐EP2 (RRID:AB_327848), and anti‐DP1 (RRID:AB_10078133) antibodies and ELISA kits (PGE2, 6‐keto‐PGF1α, and cAMP) were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). The IP receptor antibody was made as previously described (Falcetti et al., 2010). Treprostinil was obtained from United Therapeutics Corporation (Silver Spring, MD, USA). ONO‐AE1‐329, ONO‐AE1‐259, and ONO‐8713 were gifts from Ono Pharmaceutical Co., Ltd. (Chūō‐ku, Osaka, Japan); L‐902688, L‐877499, and L‐826266 were gifts from Merck (Kirkland, Quebec, Canada). RO3244019 (AGN‐230933) was a gift from Allergan (Irvine, CA, USA); ECL plus luminescence system and nitrocellulose membranes were purchased from Amersham Biosciences (Glattbrugg, Switzerland). Protease inhibitor cocktail, chloroform, indomethacin, and primers were purchased from Sigma‐Aldrich (St. Louis, MO, USA). RNeasy Plus Mini kit and Qiazol lysis reagent were obtained from Qiagen (Valencia, CA, USA). Bicinchoninic acid protein assay kit and Maxima First Strand cDNA Synthesis Kits were purchased from Thermo (Rockford, IL, USA). iQ SYBR Green Supermix Kit was from Bio‐Rad (https://en.wikipedia.org/wiki/Hercules,_California, USA). The peroxidase‐conjugated secondary antibody was from Jackson (West Chester, PA, USA). DG‐041 was a gift from deCODE Genetics (Reykjavik, Iceland). All compounds were dissolved in ethanol, DMSO, or Tyrode's solution to give a stock solution of 10 mM.
2.10. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
3. RESULTS
The mean age of the patients was 62 ± 02 (range: 37–81, n = 39) for control patients and 54 ± 03 (range: 19–66, n = 22) for PH patients. The haemodynamic (clinical) data for control and PH patients are detailed in Tables S1 and S2. There is no significant difference in histamine‐induced precontractions in human bronchial preparations derived from control compared with values from PH patients (1.54 ± 0.10 g [n = 32] in control and 1.62 ± 0.14 g [n = 16] in PH patients).
3.1. Bronchodilation induced by EP2/4 receptor agonists (control or PH preparations)
PGE2 and the two EP4 receptor selective agonists (L‐902688 and ONO‐AE1‐329) induced potent and concentration‐dependent relaxations in precontracted human bronchial preparations from control patients. However, these relaxations were significantly decreased in the human bronchial preparations derived from PH patients (Figure 1a–c). In addition, the potency (pEC50) to PGE2 was significantly lower in human bronchial preparation from PH patients compared with control patients (Table 1). On the other hand, the selective EP2 receptor agonist ONO‐AE1‐259 induced no or little relaxation with the highest concentrations (≥1 μM) from control and PH patients (Figure 1d), and no difference was observed between control and PH curves for this agonist (Table 1).
Figure 1.
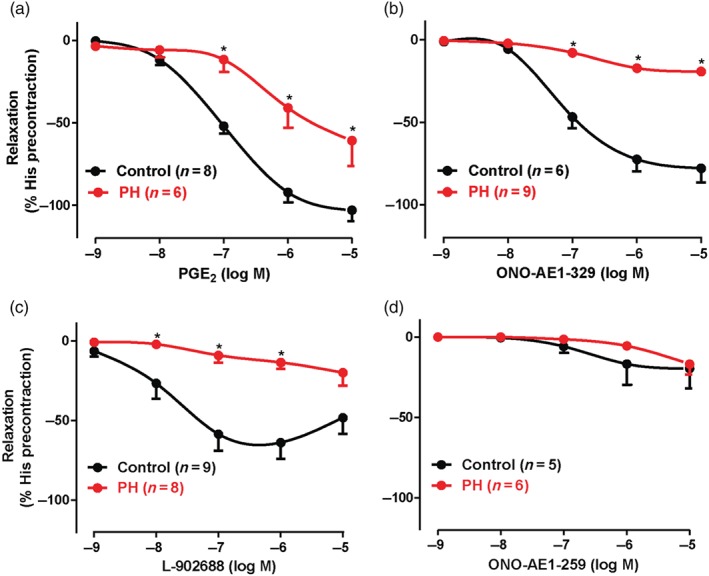
Relaxation induced by EP agonists in human bronchial preparations derived from control and pulmonary hypertensive (PH) Group III patients. Cumulative concentration–response curves induced by EP receptor agonists (PGE2 [EP2/4], ONO‐AE1‐329 [EP4], L‐902688 [EP4], and ONO‐AE1‐259 [EP2]). All rings were treated (30 min) with indomethacin (COX inhibitor, 1.7 μM) and BAY‐u3405 (TP receptor antagonist, 1 μM, when PGE2 concentration–response curve was performed). Responses are expressed as a percentage of precontraction induced by histamine (His, 50 μM). Values are means ± SEM; n indicates the number of patients. * P < .05, significantly different from control patients; two‐way ANOVA. See Table 1 for pEC50, Emax values, and relevant statistics.
Table 1.
Relaxation induced by EP receptor agonists in human bronchial preparations derived from control or pulmonary hypertensive (PH) Group III patients
| Control patients | PH patients | |||||
|---|---|---|---|---|---|---|
| Agonists | Emax (%) | pEC50 | n | Emax (%) | pEC50 | n |
| PGE2 | −105 ± 07 | 7.03 ± 0.12 | 8 | −69 ± 19* | 6.21 ± 0.19* | 6 |
| ONO‐AE1‐329 (EP4) | −79 ± 09 | 7.07 ± 0.11 | 6 | −19 ± 03* | NC | 9 |
| L‐902688 (EP4) | −63 ± 10 | 8.04 ± 0.22 | 9 | −20 ± 08* | NC | 8 |
| ONO‐AE1‐259 (EP2) | −19 ± 12 | NC | 5 | −16 ± 06 | NC | 6 |
Note. Human bronchial preparations were precontracted with histamine (50 μM; control: 1.56 ± 0.17 g; PH: 1.89 ± 0.16 g). The rings were incubated for 30 min with indomethacin (1.7 μM) and BAY u3405 (1 μM, when PGE2 concentration–response curve was performed). The maximal relaxations (Emax) and the pEC50 values are presented. The selectivity of receptor agonists is indicated in parentheses. Values represent means ± SEM and are derived from cumulative concentration–response curves induced by EP receptor agonists and from (n) different patients.
P < .05, significantly different from respective control values; one‐way ANOVA or Student's t test. NC, not calculable.
3.2. Bronchodilation induced by IP receptor agonists (control or PH preparations)
IP receptor agonists induced concentration‐dependent relaxations in precontracted human bronchial preparations. The maximum relaxations induced by iloprost and/or treprostinil were significantly greater than those to other IP receptor agonists (beraprost and MRE‐269) in either control or PH patients (Figure 2a–d, Table 2). The pEC50 of iloprost and relaxation induced by iloprost at 1 μM were significantly lower in human bronchial preparations from PH patients compared with control patients, while there was no difference for the other IP receptor agonists (Figure 2a–d, Table 2). These results comparing relaxation of control bronchial preparations by various PG agonists show that EP4 receptor agonists were 10‐ to 50‐fold more active than IP receptor agonists (Tables 1 and 2).
Figure 2.
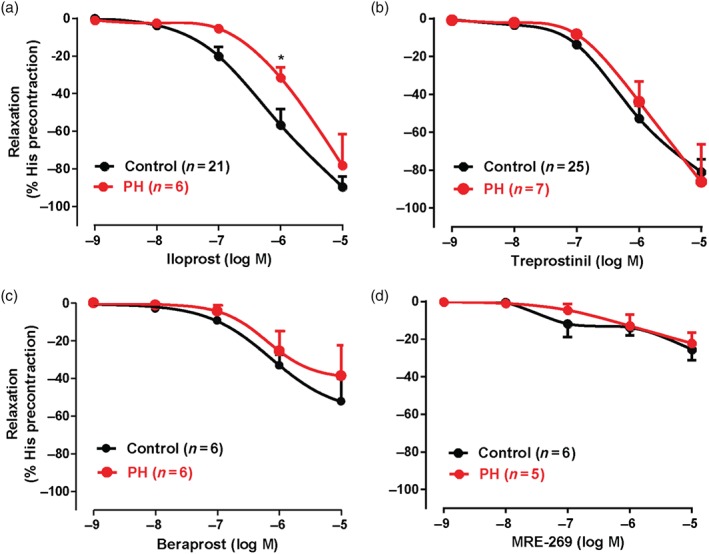
Relaxation induced by IP receptor agonists in human bronchial preparations derived from control and pulmonary hypertensive (PH) Group III patients. Cumulative concentration–response curves induced by IP receptor agonists (iloprost, treprostinil, beraprost, and MRE‐269). All rings were treated (30 min) with indomethacin (COX inhibitor, 1.7 μM). Responses are expressed as a percentage of precontraction induced by histamine (His, 50 μM). Values are means ± SEM; n indicates the number of patients. * P < .05, significantly different from control patients; two‐way ANOVA. See Table 2 for pEC50, Emax values, relevant and statistics
Table 2.
Relaxation induced by IP receptor agonists in human bronchial preparations derived from control or pulmonary hypertensive (PH) Group III patients
| Control patients | PH patients | |||||
|---|---|---|---|---|---|---|
| Agonists | Emax (%) | pEC50 | n | Emax (%) | pEC50 | n |
| Iloprost | −94 ± 06* , # | 6.24 ± 0.13 | 21 | −94 ± 19# | 5.68 ± 0.15† | 6 |
| Treprostinil | −87 ± 08# | 6.15 ± 0.10 | 25 | −93 ± 21# | 6.02 ± 0.05 | 7 |
| Beraprost | −61 ± 08# | 6.31 ± 0.18 | 6 | −40 ± 17 | 5.91 ± 0.21 | 6 |
| MRE‐269 | −26 ± 07* | NC | 6 | −24 ± 06 | NC | 5 |
Note. Human bronchial preparations were precontracted with histamine (50 μM; control: 1.41 ± 0.12 g; PH: 1.44 ± 0.17 g). The rings were incubated for 30 min with indomethacin (1.7 μM). The maximal relaxations (Emax) and the pEC50 values are presented. Values represent means ± SEM and are derived from cumulative concentration–response curves induced by IP receptor agonists and from (n) different patients. For each group of patients;
P < .05, Emax values significantly different from that of beraprost;
P < .05, Emax values significantly different from that of MRE‐269;
P <.05, significantly different from control pEC50; one‐way ANOVA or Student's t test. NC, not calculable.
3.3. Protein and mRNA levels of prostanoid receptors in human bronchial preparations derived from control and PH patients
A significant decrease in both protein and mRNA levels was observed for EP4 and IP receptors in the human bronchial preparations derived from PH patients, compared to control patients. However, mRNA (preliminary results) and/or protein levels of EP2 or DP1 receptors were not different (Figure 3a–c).
Figure 3.
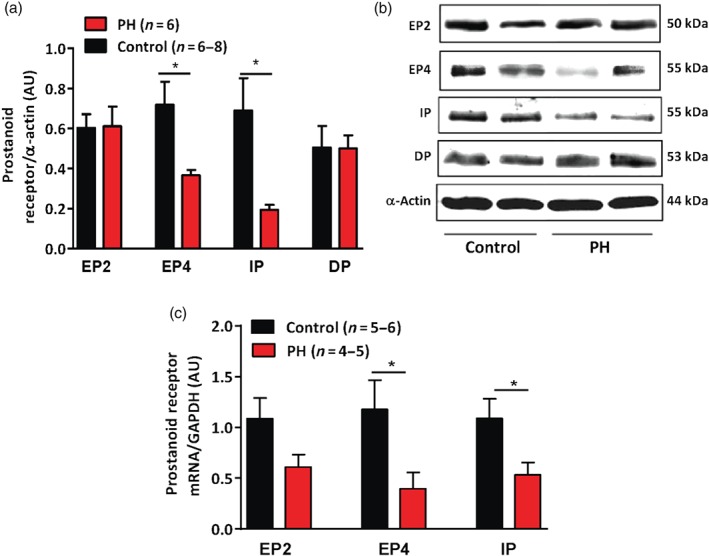
Expression of the prostanoid receptors in human bronchial preparations derived from control and pulmonary hypertensive (PH) Group III patients. (a) Western blot analysis for prostanoid receptors (EP2, EP4, IP, and DP) normalized by α‐actin in human bronchial preparations. (b) A representative photograph of western blot of EP2, EP4, IP, and DP receptors and actin. (c) Relative expression of EP2, EP4, and IP mRNA normalized by GAPDH (housekeeping gene) in human bronchial preparations. Values are means ± SEM; n indicates the number of patients. * P < .05, significantly different from control patients; Student's t test.
3.4. Effects of the prostanoid receptor antagonists on the relaxation induced by IP receptor agonists
In the presence of the IP receptor antagonist (RO3244019, 1 μM), the relaxations induced by treprostinil and iloprost were completely blocked until 0.1 μM and partly blocked at 10 μM in human bronchial preparations from control patients (Figure 4a,b; Table 3). On the other hand, EP4 receptor antagonist (GW627368, 1 and 10 μM), DP receptor antagonist (L‐877499, 10 μM), EP1 receptor antagonist (ONO‐8713 or SC‐51322, 10 μM), EP3 receptor antagonist (L‐826266, 3 μM, or DG‐041, 1 μM), or TP receptor antagonist (BAY‐u3405, 1 μM) did not modify the maximal relaxations induced by iloprost or treprostinil (Figure 4a–d; Table 3). In contrast, only the pEC50 values calculated for treprostinil were significantly reduced in the presence of the EP4 (GW627368, 10 μM) or DP (L‐877499, 10 μM) receptor antagonists (Table 3). While the EP1 receptor antagonist did not modify iloprost‐induced broncodilations, iloprost induced very small dose‐dependent contractions in the presence of the IP receptor antagonist (CAY10441, 1 μM: Emax = 0.15 ± 0.04 g, n = 5) probably via activation of EP1 receptors in our control bronchial preparations at basal tone.
Figure 4.
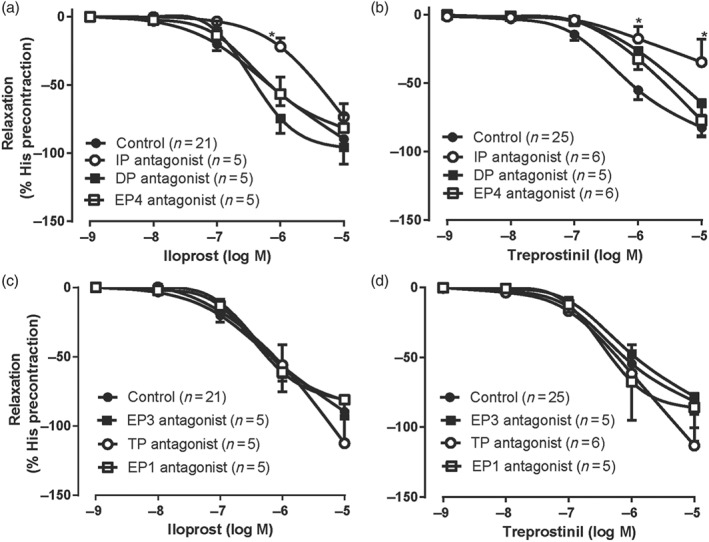
Effect of the prostanoid receptor antagonists on the relaxations induced by IP receptor agonists in human bronchial preparations derived from control patients. Cumulative concentration–response curves induced by IP receptor agonists (iloprost and treprostinil) were performed after an incubation period (30 min) with or without one of the antagonists. The treatments used are DP receptor antagonist (L‐877499, 10 μM), EP4 receptor antagonist (GW627368, 10 μM), IP receptor antagonist (RO3244019 [AGN230933], 1 μM), EP1 receptor antagonist (ONO‐8713 or SC‐51322, 10 μM), EP3 receptor antagonist (L‐826266, 3 μM, or DG‐041, 1 μM), and TP receptor antagonist (BAY‐u3405, 1 μM). Responses are expressed as a percentage of precontraction induced by histamine (His, 50 μM). Values are means ± SEM; n indicates the number of patients. * P < .05, significantly different from control patients; two‐way ANOVA. See Table 3 for pEC50, Emax values, and statistics
Table 3.
Effect of prostanoid receptor antagonists on the relaxation induced by iloprost and treprostinil in human bronchial preparations derived from control patients
| IP receptor agonist | Antagonist | Emax (%) | pEC50 | n |
|---|---|---|---|---|
| Iloprost | Control | −94 ± 06 | 6.24 ± 0.13 | 21 |
| RO3244019 (IP, 1 μM) | −87 ± 10 | 5.65 ± 0.14* | 5 | |
| GW627368 (EP4) | ||||
| 1 μM | −103 ± 08 | 5.97 ± 0.28 | 5 | |
| 10 μM | −83 ± 10 | 6.33 ± 0.22 | 5 | |
| L‐877499 (DP, 10 μM) | −97 ± 13 | 6.38 ± 0.12 | 5 | |
| ONO‐8713 (EP1, 10 μM) | −84 ± 10 | 6.25 ± 0.20 | 5 | |
| L‐826266 (EP3, 3 μM) | −93 ± 13 | 6.37 ± 0.12 | 5 | |
| BAY‐u3405 (TP, 1 μM) | −91 ± 10 | 6.11 ± 0.24 | 5 | |
| Treprostinil | Control | −87 ± 08 | 6.15 ± 0.10 | 25 |
| RO3244019 (IP, 1 μM) | −44 ± 20* | NC | 6 | |
| GW627368 (EP4, 10 μM) | −97 ± 13 | 5.65 ± 0.14* | 6 | |
| L‐877499 (DP, 10 μM) | −112 ± 18 | 5.49 ± 0.23* | 5 | |
| SC‐51322 (EP1, 10 μM) | −92 ± 23 | 6.18 ± 0.21 | 5 | |
| DG‐041 (EP3, 1 μM) | −89 ± 12 | 5.82 ± 0.23 | 5 | |
| BAY‐u3405 (TP, 1 μM) | −122 ± 13 | 6.07 ± 0.21 | 6 |
Note. Human bronchial preparations were precontracted with histamine (50 μM). The rings were incubated for 30 min with indomethacin (1.7 μM). The maximal relaxations (Emax) and the pEC50 values are presented. The selectivity of receptor agonists or antagonists is indicated in parentheses. Values represent means ± SEM and are derived from cumulative concentration–response curves induced by IP receptor agonists (iloprost and treprostinil) and from (n) different patients.
P < .05, significantly different from respective control values; one‐way ANOVA or Student's t test. NC, not calculable.
3.5. Basal production of cAMP, PGI2, PGE2, and correlations
Endogenous levels of PGE2, PGI2 (measured as its stable metabolite 6‐keto‐PGF1α), and cAMP in bronchial preparations derived from control and PH patients were not significantly different (Table S4). There was a significant positive correlation between PGI2 and cAMP or PGE2 levels, while no correlation was found between PGE2 and cAMP levels (Figure 5).
Figure 5.
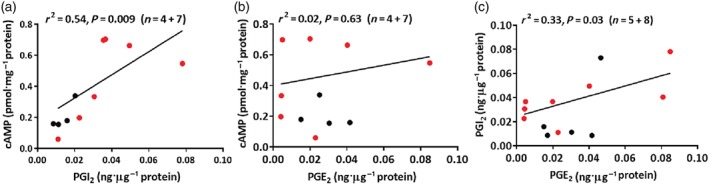
Correlations between the endogenous levels of PGE2 and PGI2 (measured as its stable metabolite 6‐keto‐PGF1α) or cAMP. Coefficients of determination have been calculated (r2, Pearson analysis). Significant correlations (P<.05) were found in Figure 5A and 5C. In Figure 5B, the correlation was not significant. Data derived from homogenates of human bronchial preparations: control (black circle, n=4‐5) and pulmonary hypertension (PH) Group III patients (red circle, n=7‐8).
3.6. Results of pulmonary function tests of the patients from the TRIUMPH trial
Lung function testing with 216 μg·day−1 (nine inhalations four times daily) inhaled treprostinil (Tyvaso®) was performed at baseline and after 12 weeks of treatment in a population of PH Group I patients (Table 4). There was no evidence of adverse effects of inhaled treprostinil on lung function, as assessed by FVC (median change from baseline 0.0% in both groups) and FEV in 1 s (median change from baseline 0.0% in the active treatment group and −0.5% in the placebo group).
Table 4.
Data from the TRIUMPH study; respiratory baseline characteristics (placebo and active) for pulmonary hypertensive Group I patients receiving treprostinil by inhalation
| Statistic | Placebo | Active | P valuea |
|---|---|---|---|
| Total number of patients | |||
| n | 120 | 115 | |
| FVC at baseline force | |||
| n | 120 | 115 | .461 NP |
| Median (min, max) | 82 (0.0, 123) | 83 (20.0, 139) | |
| FVC at Week 12 | |||
| n | 109 | 103 | .741 NP |
| Median (min, max) | 82 (0.0, 127) | 82 (0.0, 149) | |
| FVC change from baseline to Week 12 | |||
| n | 109 | 103 | .925 NP |
| Median (min, max) | 0.0 (−87, 98) | 0.0 (−99, 23) | |
| FEV at baseline | |||
| n | 120 | 115 | .201 NP |
| Median (min, max) | 74.5 (0.0, 122) | 76.0 (19.0, 130) | |
| FEV at Week 12 | |||
| n | 109 | 103 | .334 NP |
| Median (min, max) | 73 (0.0, 113) | 74 (0.0, 129) | |
| FEV change from baseline to Week 12 | |||
| n | 109 | 103 | .917 NP |
| Median (min, max) | −1.0 (−78, 77) | 0.0 (−86, 23) | |
Abbreviations: FEV, forced expiratory volume; FVC, forced vital capacity; NP, non‐parametric test.
For differences between placebo and active.
4. DISCUSSION
In this current work, we have demonstrated that the maximal relaxations induced by PGE2 and the two potent, selective EP4 receptor agonists (L‐902688 and ONO‐AE1‐329) were strongly and significantly decreased, by 35–75%, in human bronchial preparations derived from PH Group III patients, compared to controls (Figure 1a–c, Table 1). This decreased reactivity could be explained by the reduced expression of EP4 receptors (≥50%, mRNA and protein) in PH bronchial preparations (Figure 3a–c). In contrast, the maximal relaxations produced by the PGI2–mimetics were not modified, even though IP receptor expression was also reduced in PH bronchial preparations.
Crosstalk between human airways and pulmonary vessels in terms of vascular tone and remodelling has been described for many years (Farah, Li, McIntyre, Pan, & Belik, 2009). PH is associated not only with increased pulmonary vascular tone but also with increased respiratory system resistance (Fernandez‐Bonetti et al., 1983; Meyer et al., 2002; Schindler, Bohn, Bryan, Cutz, & Rabinovitch, 1995). In this context, the efficacy of inhaled PH treatments may be partly related to their direct effect on bronchial tone. Human clinical studies have demonstrated that inhaled PGE2 exhibited consistent bronchodilation which could be reduced in some pathological conditions (such as asthma; Kawakami, Uchiyama, Irie, & Murao, 1973; Melillo, Woolley, Manning, Watson, & O'Byrne, 1994; Pavord, Wong, Williams, & Tattersfield, 1993; Seth, Clarke, Lewis, & Tattersfield, 1981; Walters, Bevan, & Davies, 1982), yet supportive ex vivo and in vitro studies to further explain these observations are limited.
Although several beneficial effects of PGE2 or EP4 receptor agonists, such as anti‐inflammatory, antiproliferative, and bronchodilatory effects (Aso et al., 2013; Birrell et al., 2015; Mori et al., 2011), have been observed in human airway cells and tissues, their effects in the presence of underlying PH have not been investigated. In the present report, we show that EP4 receptor‐mediated bronchodilation is strongly reduced in PH patients. This is consistent with observations from a pulmonary model of inflammation, where EP4 receptor expression is down‐regulated (Clayton, Holland, Pang, & Knox, 2005). Inflammation has an important role in the development of PH (Pugliese et al., 2015), and increased inflammatory mediators may be responsible for the decreased EP4 receptor expression that was observed in PH bronchi (Figure 3). Taken together, our results and those already published point to the important and complex roles of the EP4 receptor in different lung diseases.
In PH preparations with end‐stage pathology, among the agents tested in our study, iloprost and treprostinil induced the greater bronchodilations (Figure 2). The reduction in IP and EP4 receptor expression slightly affects iloprost (IP/weak EP4 receptor agonist) induced bronchorelaxation and does not affect treprostinil (DP/EP2/IP and weak EP4 receptor agonist) responses (Abramovitz et al., 2000; Whittle et al., 2012). The relaxation induced by 1‐μM iloprost was significantly decreased (by 33%), as was the pEC50 value, in PH patients (Figure 2a, Table 2). On the other hand, relaxations produced by treprostinil in preparations from PH patients were not different from control and may be explained by a compensatory DP component (Norel et al., 1999; Whittle et al., 2012).
In control preparations, the bronchodilations induced by iloprost and treprostinil were significantly inhibited by RO3244019, a very selective IP receptor antagonist (Figure 4a,b; Table 2). These antagonistic effects were surmountable with agonist doses >1 μM, possibly in a competitive manner or due to some activity of iloprost and treprostinil at EP4 and/or DP receptors (Abramovitz et al., 2000; Whittle et al., 2012). In particular, a DP receptor antagonist significantly decreased the sensitivity of treprostinil‐induced bronchial (Table 3) and pulmonary vein relaxations (Benyahia et al., 2013). On the other hand, the greater potency of iloprost at the IP receptor when compared with treprostinil could account for the EP4 receptor antagonist (GW627368) lacking effects on the iloprost‐induced bronchorelaxations in control patients (Figure 4a).
In addition, a significant decrease in IP receptor expression was observed in bronchi derived from PH Group III patients (Figure 3a–c). Other studies demonstrated similar results either in pulmonary artery smooth muscle cells (PASMC) derived from PH Group I patients or in an experimental rat model of PH (Falcetti et al., 2010; Lai et al., 2008). Despite a reduction in IP receptor expression in PH Group III patients, the ability of PGI2–mimetics to induce maximal relaxation of bronchi, ex vivo, was not impaired (Figure 2). A similar discrepancy has been demonstrated in PASMC derived from PH Group I patients, where even in the presence of a strong decrease in IP receptor density, treprostinil was still able to increase cAMP levels (Falcetti et al., 2010). In the current study, the sensitivity (pEC50) and relaxation induced by iloprost at high concentrations were attenuated in bronchi derived from PH Group III. These results may be explained by the fact that iloprost is likely to have affinity for both IP and EP4 receptors at these concentrations, and both receptors are down‐regulated in PH patient preparations. Globally, other prostanoid receptors (DP and EP2) and/or https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=86, depending on the PGI2 analogue tested, could account for the maintained bronchorelaxation in PH preparations (Ali et al., 2006; Falcetti et al., 2010; Patel et al., 2018; Turcato & Clapp, 1999) (Figure 6).
Figure 6.
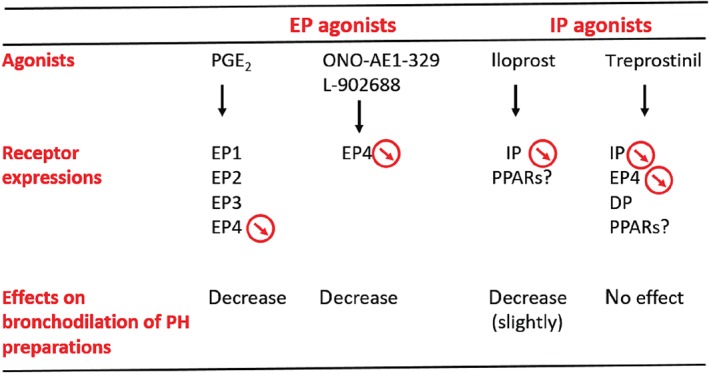
Proposed mechanisms of bronchorelaxation induced by IP and EP receptor agonists in pulmonary hypertension (PH) Group III patients. Downward arrows indicate decreased expression of prostanoid receptors.
The different effects of IP and EP4 receptor down‐regulation on vascular tone may be explained by differences in their signalling pathways and mechanisms of action. For example, cAMP accumulation via Gs activation in vascular smooth muscle cells is thought to be the main mechanism of IP receptor‐induced vasorelaxation, whereas EP4 receptor signalling is associated not only with Gs but also with Gi (Leduc et al., 2009), https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=673 (Regan, 2003), β‐arrestin (Buchanan et al., 2006; Kim, Lakshmikanthan, Frilot, & Daaka, 2010), and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5371 (Banu, Lee, Speights, Starzinski‐Powitz, & Arosh, 2009; Jang et al., 2012). As shown in Figure 5, cAMP levels were positively correlated with PGI2 levels but not with PGE2 levels in homogenates of human bronchial preparations. This suggests that the mechanism of action for IP receptor agonists involving cAMP activation was still functional in PH lungs as shown in hPASMC (Falcetti et al., 2010; Patel et al., 2018), whereas cAMP signalling through EP4 receptors was impaired. The down‐regulations described here related to PH and/or to the underlying respiratory pathologies could have global implications.
Our in vitro results are complemented by in vivo data (courtesy of United Therapeutics Corporation) from the TRIUMPH study (Benza et al., 2011), which demonstrated that inhalation of treprostinil does not change respiratory function parameters in patients with PH Group I. However, in one small prospective study in PH Group III patients with chronic obstructive pulmonary disease (Bajwa et al., 2017), the effects of nebulized treprostinil on pulmonary function tests showed a small but statistically significant decrease in FEV in 1 s (median change −0.18 L; P = .004). As inhaled treprostinil may cause airway irritation, it is unknown if this change is clinically relevant given the numerical increases in functional improvement. This is in contrast with another study in PH patients (n = 7) with pulmonary fibrosis, where no significant changes in pulmonary function tests (FEV, FVC, and FEV/FVC ratio) were detected following 12 weeks of parenteral treprostinil monotherapy (Saggar et al., 2014). On the other hand, in these two studies (Benza et al., 2011; Saggar et al., 2014), treprostinil administration improved 6MWD, haemodynamic function, or oxygenation in these PH patients.
The respiratory function data presented from the TRIUMPH study are comparable to data from other studies of nebulized iloprost in PH Group III patients (Dernaika, Beavin, & Kinasewitz, 2010; Hegewald & Elliott, 2009; Lasota, Skoczynski, Mizia‐Stec, & Pierzchala, 2013; Olschewski et al., 1999; Reichenberger et al., 2007; Richter et al., 2015). Iloprost treatment demonstrated an absence of effect (or a trend to improvement) on respiratory function and/or oxygenation (Dernaika et al., 2010; Hegewald & Elliott, 2009; Lasota et al., 2013; Reichenberger et al., 2007) and was associated with functional improvement (mPAP and 6MWD) in most of the PH Group III patients with severe PH (mPAP >35 mmHg). However, high doses of PGI2 analogues could induce airway irritation in some patients (Reichenberger et al., 2007). In terms of dose, one study (Voswinckel et al., 2006) suggests that similar doses of inhaled iloprost (7.5 μg) or treprostinil (7.5–15 μg) result in a similar efficiency to reduce pulmonary vascular resistance and mPAP in patients with severe precapillary PH. These data are surprising as iloprost has been always regarded as more potent than treprostinil (Abramovitz et al., 2000; Benyahia et al., 2013; Hiremath et al., 2010; Hoeper et al., 2009; Kumar, Thudium, Laliberte, Zaccardelli, & Nelsen, 2016; Olschewski et al., 2004; Whittle et al., 2012). Yet our human bronchial preparation data (see Table 2) are supported by the Voswinckel et al. results, where in vivo, the same potency was calculated for these PGI2 analogues, and airways appear to behave differently from vasculature, with potency differences abolished between iloprost and treprostinil.
These clinical studies and our in vitro results support that agonists such as iloprost and treprostinil are the most suitable PGI2 analogues for patients with (severe) PH Group III. Inhalation of these PGI2 analogues may be a preferred route of administration, where patients could concurrently benefit from airway dilatation, blood oxygenation, and pulmonary vasodilation to reduce hypoxic pulmonary vasoconstriction observed in PH Group III patients. Our study also reveals the down‐regulation of IP and EP4 receptor expression levels in the human airways and loss of EP4 receptor agonist‐induced relaxation in PH Group III bronchial preparation, which could be contributing factors for PH Group III. For this reason, the most potent therapies to activate IP receptor and those to target the prevention/reversal of EP4 receptor down‐regulation may be the most effective for treating respiratory dysfunction in these patients.
AUTHOR CONTRIBUTIONS
X.N., G.O., and C.B. contributed to the conception and design. Y.C., H.M., C.D., D.L., and X.N. obtained pulmonary samples and recruited patients. G.O., C.B., X.N., K.B., R.B., A.M.S., A.C.N., and S.M. collected the data. X.N., G.O., and C.B. performed the analysis and/or interpretation. G.O., X.N., D.L., and C.B. wrote the manuscript for intellectual content. D.L., A.M.S., A.C.N., and L.H.C. reviewed the manuscript.
CONFLICT OF INTEREST
This work was funded by an educational research grant from United Therapeutics to X.N. L.H.C. has received educational research grants from United Therapeutics and honoraria UTC. A.M.S. and A.C.N. are employees of United Therapeutics.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Supporting information
Table S1: Characteristics of PH Group‐III patients (in vitro study)
Table S2: Characteristics of control patients (in vitro study)
Table S3: Primers used for Real‐time PCR
Table S4: Basal production of cAMP, PGI2 and PGE2
ACKNOWLEDGEMENTS
We would like to thank Elisabeth Brunet and Amina El Hilali from the Anapathology laboratory, CHU X. Bichat for their help. The authors would like to thank Erik Borg (United Therapeutics Corporation) for his helpful corrections on the manuscript. We thank Dr. Takayuki Maruyama for providing the ONO compounds. We would like also to thank Merck, deCODE Genetics, and Allergan companies for gifts of some compounds. Gulsev Ozen is a recipient of a postgraduate fellowship (BIDEB‐2214) from the Scientific and Technological Research Council of Turkey (TUBITAK).
Ozen G, Benyahia C, Mani S, et al. Bronchodilation induced by PGE2 is impaired in Group III pulmonary hypertension. Br J Pharmacol. 2020;177:161–174. 10.1111/bph.14854
Gulsev Ozen and Chabha Benyahia contributed equally to this work.
REFERENCES
- Abramovitz, M. , Adam, M. , Boie, Y. , Carriere, M. , Denis, D. , Godbout, C. , … Metters, K. M. (2000). The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochimica et Biophysica Acta, 1483, 285–293. 10.1016/S1388-1981(99)00164-X [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174(Suppl 1), S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, F. Y. , Egan, K. , FitzGerald, G. A. , Desvergne, B. , Wahli, W. , Bishop‐Bailey, D. , … Mitchell, J. A. (2006). Role of prostacyclin versus peroxisome proliferator‐activated receptor beta receptors in prostacyclin sensing by lung fibroblasts. American Journal of Respiratory Cell and Molecular Biology, 34, 242–246. 10.1165/rcmb.2005-0289OC [DOI] [PubMed] [Google Scholar]
- Aso, H. , Ito, S. , Mori, A. , Suganuma, N. , Morioka, M. , Takahara, N. , … Hasegawa, Y. (2013). Differential regulation of airway smooth muscle cell migration by E‐prostanoid receptor subtypes. American Journal of Respiratory Cell and Molecular Biology, 48, 322–329. 10.1165/rcmb.2012-0158OC [DOI] [PubMed] [Google Scholar]
- Bajwa, A. A. , Shujaat, A. , Patel, M. , Thomas, C. , Rahaghi, F. , & Burger, C. D. (2017). The safety and tolerability of inhaled treprostinil in patients with pulmonary hypertension and chronic obstructive pulmonary disease. Pulm Circ, 7, 82–88. 10.1086/689291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu, S. K. , Lee, J. , Speights, V. O. Jr. , Starzinski‐Powitz, A. , & Arosh, J. A. (2009). Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NFκB, and β‐catenin pathways and activation of intrinsic apoptotic mechanisms. Molecular Endocrinology, 23, 1291–1305. 10.1210/me.2009-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyahia, C. , Boukais, K. , Gomez, I. , Silverstein, A. , Clapp, L. , Fabre, A. , … Norel, X. (2013). A comparative study of PGI2 mimetics used clinically on the vasorelaxation of human pulmonary arteries and veins, role of the DP‐receptor. Prostaglandins & Other Lipid Mediators, 107, 48–55. 10.1016/j.prostaglandins.2013.07.001 [DOI] [PubMed] [Google Scholar]
- Benyahia, C. , Gomez, I. , Kanyinda, L. , Boukais, K. , Danel, C. , Leseche, G. , … Norel, X. (2012). PGE2 receptor (EP4) agonists: Potent dilators of human bronchi and future asthma therapy? Pulmonary Pharmacology & Therapeutics, 25, 115–118. 10.1016/j.pupt.2011.12.012 [DOI] [PubMed] [Google Scholar]
- Benza, R. L. , Seeger, W. , McLaughlin, V. V. , Channick, R. N. , Voswinckel, R. , Tapson, V. F. , … Rubin, L. J. (2011). Long‐term effects of inhaled treprostinil in patients with pulmonary arterial hypertension: The Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension (TRIUMPH) study open‐label extension. The Journal of Heart and Lung Transplantation, 30, 1327–1333. 10.1016/j.healun.2011.08.019 [DOI] [PubMed] [Google Scholar]
- Birrell, M. A. , Maher, S. A. , Dekkak, B. , Jones, V. , Wong, S. , Brook, P. , & Belvisi, M. G. (2015). Anti‐inflammatory effects of PGE2 in the lung: Role of the EP4 receptor subtype. Thorax, 70, 740–747. 10.1136/thoraxjnl-2014-206592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourge, R. C. , Tapson, V. F. , Safdar, Z. , Benza, R. L. , Channick, R. N. , Rosenzweig, E. B. , … Rubin, L. J. (2013). Rapid transition from inhaled iloprost to inhaled treprostinil in patients with pulmonary arterial hypertension. Cardiovascular Therapeutics, 31, 38–44. 10.1111/1755-5922.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan, F. G. , Gorden, D. L. , Matta, P. , Shi, Q. , Matrisian, L. M. , & DuBois, R. N. (2006). Role of β‐arrestin 1 in the metastatic progression of colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America, 103, 1492–1497. 10.1073/pnas.0510562103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley, J. , Birrell, M. A. , Maher, S. A. , Nials, A. T. , Clarke, D. L. , & Belvisi, M. G. (2011). EP4 receptor as a new target for bronchodilator therapy. Thorax, 66, 1029–1035. 10.1136/thx.2010.158568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp, L. H. , & Gurung, R. (2015). The mechanistic basis of prostacyclin and its stable analogues in pulmonary arterial hypertension: Role of membrane versus nuclear receptors. Prostaglandins & Other Lipid Mediators, 120, 56–71. 10.1016/j.prostaglandins.2015.04.007 [DOI] [PubMed] [Google Scholar]
- Clayton, A. , Holland, E. , Pang, L. , & Knox, A. (2005). Interleukin‐1β differentially regulates β2 adrenoreceptor and prostaglandin E2‐mediated cAMP accumulation and chloride efflux from Calu‐3 bronchial epithelial cells. Role of receptor changes, adenylyl cyclase, cyclo‐oxygenase 2, and protein kinase A. The Journal of Biological Chemistry, 280, 23451–23463. 10.1074/jbc.M502242200 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernaika, T. A. , Beavin, M. , & Kinasewitz, G. T. (2010). Iloprost improves gas exchange and exercise tolerance in patients with pulmonary hypertension and chronic obstructive pulmonary disease. Respiration, 79, 377–382. 10.1159/000242498 [DOI] [PubMed] [Google Scholar]
- Falcetti, E. , Hall, S. M. , Phillips, P. G. , Patel, J. , Morrell, N. W. , Haworth, S. G. , & Clapp, L. H. (2010). Smooth muscle proliferation and role of the prostacyclin (IP) receptor in idiopathic pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine, 182, 1161–1170. 10.1164/rccm.201001-0011OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah, O. R. , Li, D. , McIntyre, B. A. , Pan, J. , & Belik, J. (2009). Airway epithelial‐derived factor relaxes pulmonary vascular smooth muscle. American Journal of Physiology. Lung Cellular and Molecular Physiology, 296, L115–L120. 10.1152/ajplung.90391.2008 [DOI] [PubMed] [Google Scholar]
- Fein, D. G. , Zaidi, A. N. , & Sulica, R. (2016). Pulmonary hypertension due to common respiratory conditions: Classification, evaluation and management strategies. Journal of Clinical Medicine, 5(9), E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Bonetti, P. , Lupi‐Herrera, E. , Martinez‐Guerra, M. L. , Barrios, R. , Seoane, M. , & Sandoval, J. (1983). Peripheral airways obstruction in idiopathic pulmonary artery hypertension (primary). Chest, 83, 732–738. 10.1378/chest.83.5.732 [DOI] [PubMed] [Google Scholar]
- Galie, N. , Humbert, M. , Vachiery, J. L. , Gibbs, S. , Lang, I. , Torbicki, A. , … ESC Scientific Document Group (2016). 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). European Heart Journal, 37, 67–119. [DOI] [PubMed] [Google Scholar]
- Harari, S. , Elia, D. , & Humbert, M. (2018). Pulmonary hypertension in parenchymal lung diseases: Any future for new therapies? Chest, 153, 217–223. [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haye‐Legrand, I. , Bourdillat, B. , Labat, C. , Cerrina, J. , Norel, X. , Benveniste, J. , & Brink, C. (1987). Relaxation of isolated human pulmonary muscle preparations with prostacyclin (PGI2) and its analogs. Prostaglandins, 33, 845–854. 10.1016/0090-6980(87)90113-4 [DOI] [PubMed] [Google Scholar]
- Hegewald, M. J. , & Elliott, C. G. (2009). Sustained improvement with iloprost in a COPD patient with severe pulmonary hypertension. Chest, 135, 536–537. 10.1378/chest.08-1515 [DOI] [PubMed] [Google Scholar]
- Hill, N. S. , Badesch, D. , Benza, R. L. , D'Eletto, T. A. , Farber, H. W. , Gomberg‐Maitland, M. , … Preston, I. (2015). Perspectives on oral pulmonary hypertension therapies recently approved by the U.S. Food and Drug Administration. Annals of the American Thoracic Society, 12, 269–273. 10.1513/AnnalsATS.201501-020AS [DOI] [PubMed] [Google Scholar]
- Hiremath, J. , Thanikachalam, S. , Parikh, K. , Shanmugasundaram, S. , Bangera, S. , Shapiro, L. , … White, R. J. (2010). Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: A placebo‐controlled trial. The Journal of Heart and Lung Transplantation, 29, 137–149. 10.1016/j.healun.2009.09.005 [DOI] [PubMed] [Google Scholar]
- Hoeper, M. M. , Gall, H. , Seyfarth, H. J. , Halank, M. , Ghofrani, H. A. , Winkler, J. , … Ewert, R. (2009). Long‐term outcome with intravenous iloprost in pulmonary arterial hypertension. The European Respiratory Journal, 34, 132–137. 10.1183/09031936.00130408 [DOI] [PubMed] [Google Scholar]
- Hoeper, M. M. , McLaughlin, V. V. , Dalaan, A. M. , Satoh, T. , & Galie, N. (2016). Treatment of pulmonary hypertension. The Lancet Respiratory Medicine, 4, 323–336. 10.1016/S2213-2600(15)00542-1 [DOI] [PubMed] [Google Scholar]
- Jang, M. W. , Yun, S. P. , Park, J. H. , Ryu, J. M. , Lee, J. H. , & Han, H. J. (2012). Cooperation of Epac1/Rap1/Akt and PKA in prostaglandin E2‐induced proliferation of human umbilical cord blood derived mesenchymal stem cells: Involvement of c‐Myc and VEGF expression. Journal of Cellular Physiology, 227, 3756–3767. 10.1002/jcp.24084 [DOI] [PubMed] [Google Scholar]
- Kawakami, Y. , Uchiyama, K. , Irie, T. , & Murao, M. (1973). Evaluation of aerosols of prostaglandins E1 and E2 as bronchodilators. European Journal of Clinical Pharmacology, 6, 127–132. 10.1007/BF00562439 [DOI] [PubMed] [Google Scholar]
- Kim, J. I. , Lakshmikanthan, V. , Frilot, N. , & Daaka, Y. (2010). Prostaglandin E2 promotes lung cancer cell migration via EP4‐βArrestin1‐c‐Src signalsome. Molecular Cancer Research, 8, 569–577. 10.1158/1541-7786.MCR-09-0511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, P. , Thudium, E. , Laliberte, K. , Zaccardelli, D. , & Nelsen, A. (2016). A comprehensive review of treprostinil pharmacokinetics via four routes of administration. Clinical Pharmacokinetics, 55, 1495–1505. 10.1007/s40262-016-0409-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, Y. J. , Pullamsetti, S. S. , Dony, E. , Weissmann, N. , Butrous, G. , Banat, G. A. , … Schermuly, R. T. (2008). Role of the prostanoid EP4 receptor in iloprost‐mediated vasodilatation in pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine, 178, 188–196. 10.1164/rccm.200710-1519OC [DOI] [PubMed] [Google Scholar]
- Lasota, B. , Skoczynski, S. , Mizia‐Stec, K. , & Pierzchala, W. (2013). The use of iloprost in the treatment of ‘out of proportion' pulmonary hypertension in chronic obstructive pulmonary disease. International Journal of Clinical Pharmacy, 35, 313–315. 10.1007/s11096-013-9762-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc, M. , Breton, B. , Galés, C. , Le Gouill, C. , Bouvier, M. , Chemtob, S. , & Heveker, N. (2009). Functional selectivity of natural and synthetic prostaglandin EP4 receptor ligands. The Journal of Pharmacology and Experimental Therapeutics, 331, 297–307. 10.1124/jpet.109.156398 [DOI] [PubMed] [Google Scholar]
- McLaughlin, V. V. , Benza, R. L. , Rubin, L. J. , Channick, R. N. , Voswinckel, R. , Tapson, V. F. , … Seeger, W. (2010). Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: A randomized controlled clinical trial. Journal of the American College of Cardiology, 55, 1915–1922. 10.1016/j.jacc.2010.01.027 [DOI] [PubMed] [Google Scholar]
- Melillo, E. , Woolley, K. L. , Manning, P. J. , Watson, R. M. , & O'Byrne, P. M. (1994). Effect of inhaled PGE2 on exercise‐induced bronchoconstriction in asthmatic subjects. American Journal of Respiratory and Critical Care Medicine, 149, 1138–1141. 10.1164/ajrccm.149.5.8173753 [DOI] [PubMed] [Google Scholar]
- Meyer, F. J. , Ewert, R. , Hoeper, M. M. , Olschewski, H. , Behr, J. , Winkler, J. , … for the German PPH Study Group (2002). Peripheral airway obstruction in primary pulmonary hypertension. Thorax, 57, 473–476. 10.1136/thorax.57.6.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, A. , Ito, S. , Morioka, M. , Aso, H. , Kondo, M. , Sokabe, M. , & Hasegawa, Y. (2011). Effects of specific prostanoid EP receptor agonists on cell proliferation and intracellular Ca2+ concentrations in human airway smooth muscle cells. European Journal of Pharmacology, 659, 72–78. 10.1016/j.ejphar.2011.03.001 [DOI] [PubMed] [Google Scholar]
- Norel, X. , Walch, L. , Labat, C. , Gascard, J. P. , Dulmet, E. , & Brink, C. (1999). Prostanoid receptors involved in the relaxation of human bronchial preparations. British Journal of Pharmacology, 126, 867–872. 10.1038/sj.bjp.0702392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olschewski, H. , Ghofrani, H. A. , Walmrath, D. , Schermuly, R. , Temmesfeld‐Wollbruck, B. , Grimminger, F. , & Seeger, W. (1999). Inhaled prostacyclin and iloprost in severe pulmonary hypertension secondary to lung fibrosis. American Journal of Respiratory and Critical Care Medicine, 160, 600–607. 10.1164/ajrccm.160.2.9810008 [DOI] [PubMed] [Google Scholar]
- Olschewski, H. , Rose, F. , Schermuly, R. , Ghofrani, H. A. , Enke, B. , Olschewski, A. , & Seeger, W. (2004). Prostacyclin and its analogues in the treatment of pulmonary hypertension. Pharmacology & Therapeutics, 102, 139–153. 10.1016/j.pharmthera.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Patel, J. A. , Shen, L. , Hall, S. M. , Benyahia, C. , Norel, X. , McAnulty, R. J. , … Clapp, L. H. (2018). Prostanoid EP2 receptors are up‐regulated in human pulmonary arterial hypertension: A key anti‐proliferative target for treprostinil in smooth muscle cells. International Journal of Molecular Sciences, 19(8), E2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavord, I. D. , Wong, C. S. , Williams, J. , & Tattersfield, A. E. (1993). Effect of inhaled prostaglandin E2 on allergen‐induced asthma. The American Review of Respiratory Disease, 148, 87–90. 10.1164/ajrccm/148.1.87 [DOI] [PubMed] [Google Scholar]
- Pluchart, H. , Khouri, C. , Blaise, S. , Roustit, M. , & Cracowski, J. L. (2017). Targeting the prostacyclin pathway: Beyond pulmonary arterial hypertension. Trends in Pharmacological Sciences, 38, 512–523. 10.1016/j.tips.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Pugliese, S. C. , Poth, J. M. , Fini, M. A. , Olschewski, A. , El Kasmi, K. C. , & Stenmark, K. R. (2015). The role of inflammation in hypoxic pulmonary hypertension: From cellular mechanisms to clinical phenotypes. American Journal of Physiology. Lung Cellular and Molecular Physiology, 308, L229–L252. 10.1152/ajplung.00238.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan, J. W. (2003). EP2 and EP4 prostanoid receptor signaling. Life Sciences, 74, 143–153. 10.1016/j.lfs.2003.09.031 [DOI] [PubMed] [Google Scholar]
- Reichenberger, F. , Mainwood, A. , Doughty, N. , Fineberg, A. , Morrell, N. W. , & Pepke‐Zaba, J. (2007). Effects of nebulised iloprost on pulmonary function and gas exchange in severe pulmonary hypertension. Respiratory Medicine, 101, 217–222. 10.1016/j.rmed.2006.05.019 [DOI] [PubMed] [Google Scholar]
- Richter, M. J. , Ghofrani, H. A. , Voswinckel, R. , Seeger, W. , Schulz, R. , Reichenberger, F. , & Gall, H. (2015). Acute hemodynamic effects of nebulized iloprost via the I‐neb Adaptive Aerosol Delivery system in pulmonary hypertension. Pulm Circ, 5, 162–170. 10.1086/679722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safholm, J. , Manson, M. L. , Bood, J. , Delin, I. , Orre, A. C. , Bergman, P. , … Adner, M. (2015). Prostaglandin E2 inhibits mast cell‐dependent bronchoconstriction in human small airways through the E prostanoid subtype 2 receptor. The Journal of Allergy and Clinical Immunology, 136, 1232–1239 e1231. [DOI] [PubMed] [Google Scholar]
- Saggar, R. , Khanna, D. , Vaidya, A. , Derhovanessian, A. , Maranian, P. , Duffy, E. , … Saggar, R. (2014). Changes in right heart haemodynamics and echocardiographic function in an advanced phenotype of pulmonary hypertension and right heart dysfunction associated with pulmonary fibrosis. Thorax, 69, 123–129. 10.1136/thoraxjnl-2013-204150 [DOI] [PubMed] [Google Scholar]
- Schindler, M. B. , Bohn, D. J. , Bryan, A. C. , Cutz, E. , & Rabinovitch, M. (1995). Increased respiratory system resistance and bronchial smooth muscle hypertrophy in children with acute postoperative pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine, 152, 1347–1352. 10.1164/ajrccm.152.4.7551393 [DOI] [PubMed] [Google Scholar]
- Seth, R. V. , Clarke, V. S. , Lewis, R. A. , & Tattersfield, A. E. (1981). Effect of propranolol on the airway response to prostaglandin E2 in normal man. British Journal of Clinical Pharmacology, 12, 731–735. 10.1111/j.1365-2125.1981.tb01297.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, M. , Imanishi, J. , Takano, T. , & Miwa, Y. (2011). Disproportionate pulmonary hypertension in a patient with early‐onset pulmonary emphysema treated with specific drugs for pulmonary arterial hypertension. Internal Medicine, 50, 2341–2346. 10.2169/internalmedicine.50.5995 [DOI] [PubMed] [Google Scholar]
- Simonneau, G. , Montani, D. , Celermajer, D. S. , Denton, C. P. , Gatzoulis, M. A. , Krowka, M. , … Souza, R. (2019). Haemodynamic definitions and updated clinical classification of pulmonary hypertension. The European Respiratory Journal, 53, 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcato, S. , & Clapp, L. H. (1999). Effects of the adenylyl cyclase inhibitor SQ22536 on iloprost‐induced vasorelaxation and cyclic AMP elevation in isolated guinea‐pig aorta. British Journal of Pharmacology, 126, 845–847. 10.1038/sj.bjp.0702383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voswinckel, R. , Enke, B. , Reichenberger, F. , Kohstall, M. , Kreckel, A. , Krick, S. , … Olschewski, H. (2006). Favorable effects of inhaled treprostinil in severe pulmonary hypertension: Results from randomized controlled pilot studies. Journal of the American College of Cardiology, 48, 1672–1681. 10.1016/j.jacc.2006.06.062 [DOI] [PubMed] [Google Scholar]
- Walters, E. H. , Bevan, M. , & Davies, B. H. (1982). Interactions between response to inhaled prostaglandin E2 and chronic beta‐adrenergic agonist treatment. Thorax, 37, 430–437. 10.1136/thx.37.6.430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle, B. J. , Silverstein, A. M. , Mottola, D. M. , & Clapp, L. H. (2012). Binding and activity of the prostacyclin receptor (IP) agonists, treprostinil and iloprost, at human prostanoid receptors: Treprostinil is a potent DP1 and EP2 agonist. Biochemical Pharmacology, 84, 68–75. 10.1016/j.bcp.2012.03.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Characteristics of PH Group‐III patients (in vitro study)
Table S2: Characteristics of control patients (in vitro study)
Table S3: Primers used for Real‐time PCR
Table S4: Basal production of cAMP, PGI2 and PGE2


