Abstract
Background and Purpose
CR4056 is a first‐in‐class imidazoline‐2 (I2) receptor ligand characterized by potent analgesic activity in different experimental animal models of pain. In a recent phase II clinical trial, CR4056 effectively reduced pain in patients with knee osteoarthritis. In the present study, we investigated the effects of CR4056 on PKCε translocation in vitro and on PKCε activation in vivo in dorsal root ganglia (DRG) neurons.
Experimental Approach
Effects of CR4056 on bradykinin‐induced PKCε translocation were studied in rat sensory neurons by immunocytochemistry. PKCε activation was investigated by immunohistochemistry analysis of DRG from complete Freund's adjuvant‐treated animals developing local hyperalgesia. The analgesic activity of CR4056 was tested on the same animals.
Key Results
CR4056 inhibited PKCε translocation with very rapid and long‐lasting activity. CR4056 decreased hyperalgesia and phospho‐PKCε immunoreactivity in the DRG neurons innervating the inflamed paw. The effect of CR4056 on PKCε translocation was blocked by pertussis toxin, implying that the intracellular pathways involved Gi proteins. The inhibition of PKCε translocation by CR4056 was independent of the α2‐adrenoeceptor and, surprisingly, was also independent of idazoxan‐sensitive I2 binding sites. The I2 agonist 2BFI had no effect alone but potentiated the activity of low concentrations of CR4056.
Conclusions and Implications
Our results demonstrate that CR4056 shares the ability to inhibit PKCε translocation with other analgesics. Whether the inhibition of PKCε involves binding to specific subtype(s) of I2 receptors should be further investigated. If so, this would be a new mode of action of a highly specific I2 receptor ligand.
Abbreviations
- 2BFI
2‐(2‐benzofuranyl)‐2‐imidazoline
- ARA‐C
cytosine 1‐d‐arabinofuranoside
- BK
bradykinin
- CFA
complete Freund's adjuvant
- CP
carrier peptide
- DRG
dorsal root ganglia
- IB4−/+
isolectin B4‐negative/positive
- PAR
protease‐activated receptors
- PBS‐T
PBS with 0.3% Triton X‐100
- PK2
prokineticin 2
- PMA
phorbol 12‐myristate 13‐acetate
- pPKCε/phospho‐PKCε
phosphorylated PKCε
- PTX
pertussis toxin
- RACK
receptor for activated C kinase
1. What is already known
CR4056 is an imidazoline‐2 (I2) receptor ligand with demonstrated analgesic properties in animals and humans.
What this study adds
CR4056 significantly inhibits the translocation of PKCε induced by inflammatory mediators in sensory neurons.
In vivo CR4056 decreases the phospho‐PKCε levels in the DRG neurons innervating the inflamed tissues.
What is the clinical significance
These results provide novel mechanisms of action for CR4056
These results also are consistent with its analgesic properties.
1. INTRODUCTION
Imidazoline binding sites/receptors are largely expressed in the central and peripheral nervous system, liver, kidneys, pancreas, and heart. Currently, three subtypes of imidazoline binding sites/receptors, termed I1, I2, and I3, have been proposed based on their pharmacological affinity and functional characterization. To date, only the I1 receptor subtype has been molecularly identified as the non‐G protein‐coupled imidazoline receptor antisera‐selected protein, and cloned (Piletz et al., 2000). I1 is defined as a high‐affinity receptor for https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=516, is present both in the medulla oblongata and in sympathetic nerve endings, and is important for the clonidine‐like hypotensive effect (Bousquet, 2000). Conversely, I2 receptors have only been identified functionally as allosteric modulators of specific subpopulations of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2490 with a high affinity for idazoxan (Carpene et al., 1995; McDonald, Olivieri, Ramsay, & Holt, 2010; Ozaita et al., 1997; Tesson et al., 1995) and as modulators of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2767 (Holt et al., 2008) and brain creatine kinase (Kimura et al., 2009). The modulation of these enzymes supports the importance of I2 receptors in several pathological conditions of the nervous system (Garcia‐Sevilla, Escriba, & Guimon, 1999; Halaris & Piletz, 2003), including inflammatory and neuropathic pain (Aricioglu, Korcegez, Bozkurt, & Ozyalcin, 2003; Boronat, Olmos, & Garcia‐Sevilla, 1998; Fairbanks et al., 2000). Pharmacologically, I2 receptors can be further subdivided into I2A and I2B, depending on their affinity for https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2421 (Bektas, Nemutlu, & Arslan, 2015; Diamant, Eldar‐Geva, & Atlas, 1992). It is now clear that I2 binding sites are not classic receptors but represent a heterogeneous class of proteins that are able to bind and respond with different functional effects to well‐defined ligands. These ligands are characterized by the selective interaction with non‐α2 idazoxan binding sites, by a lack of interaction with classic monoamine receptors, and by a common pharmacological signature in vivo (i.e., analgesic efficacy in selected pain models; synergy with opiates in controlling pain; ability to counteract opiate tolerance and, sometimes, opiate physical dependence; and neuroprotective activity). The neuropharmacology of imidazoline I2 receptor ligands has been recently reviewed by Li (2017).
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10453 (2‐phenyl‐6‐(1H‐imidazol‐1yl)quinazoline) is a synthetic drug developed by Rottapharm Biotech with high competitive selectivity for I2 imidazoline receptors/binding sites (Ferrari et al., 2011; Li & Zhang, 2011). This drug displays strong in vivo analgesic properties in several animal models of inflammatory, chronic, and neuropathic pain (Comi et al., 2017; Ferrari et al., 2011; Lanza, Ferrari, Menghetti, Tremolada, & Caselli, 2014; Meregalli et al., 2012; Siemian, Wang, Zhang, & Li, 2018). A recent phase II clinical trial of CR4056 in patients with knee osteoarthritis chronic pain demonstrated for the first time the efficacy of an I2 ligand in humans (Rovati et al., 2019). The understanding of its pharmacological and molecular mechanisms of action leading to analgesia is therefore highly relevant to evaluate its potential use in human therapies and to clarify the functional pathways activated by I2 ligands.
https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1486ε is the only isoform of PKC (Alexander et al., 2017) expressed in the neurons of the dorsal root ganglia (DRG) that translocates to the plasma membrane after activation of membrane receptors coupled to Gq (Cesare, Dekker, Sardini, Parker, & McNaughton, 1999; Vellani & McNaughton, 2004; Vellani et al., 2006; Vellani et al., 2010; Vellani, Prandini, et al., 2011). The inhibition of translocation, as well as the inhibition of catalytic activity, prevents the phosphorylation of membrane proteins, particularly ion channels (Numazaki, Tominaga, Toyooka, & Tominaga, 2002), a mechanism involved in the development of hyperalgesia and allodynia. Inhibitors of translocation have recently raised interest as novel treatments for pain and hyperalgesia. For example, the inhibition of PKCε translocation by synthetic peptides that compete for the binding site on its specific receptor for activated C kinase (RACK) protein, RACK2, was able to largely reduce the carrageenan‐induced chronic inflammatory pain (Aley, Messing, Mochly‐Rosen, & Levine, 2000). The importance of PKCε in inflammatory hypernociception and sensitization was also evident in the PKCε mutant mouse model (Khasar et al., 1999) and is supported by a large number of studies on specific diseases and by animal models of hyperalgesia and neuropathy (Aley et al., 2000; Dina et al., 2000; Dina, Chen, Reichling, & Levine, 2001; Dina, Levine, & Green, 2008; Shumilla, Liron, Mochly‐Rosen, Kendig, & Sweitzer, 2005; Summer et al., 2006).
We have previously shown that anti‐inflammatory and analgesic drugs such as https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7401, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5239, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5483 (Vellani et al., 2013; Vellani & Giacomoni, 2017; Vellani, Franchi, et al., 2011) inhibit PKCε translocation in cultured DRG neurons stimulated with inflammatory mediators. In the present study, we showed that CR4056 tested in vitro is a rapidly‐acting and powerful inhibitor of PKCε translocation, and we have investigated the mechanisms leading to this inhibition. In addition, we showed that CR4056 is also effective in reducing PKCε activation in vivo in an inflammatory pain model.
2. METHODS
2.1. PKCε catalytic activity assay
PKCε kinase activity was measured in a cell‐free assay (Cerep, currently Eurofins, France) as described in Chen et al. (1993). Briefly, human recombinant PKCε was incubated at 22°C for 60 min in the presence of the substrate biotinyl‐bAbAbAKIQASFRGHMARKK (400 nM) and ATP, with or without CR4056 (1 μM). The production of the phosphorylated substrate was detected by a homogeneous time‐resolved fluorescence phospho‐assay.
2.2. Culturing isolated neurons from DRG
All the animal care and experimental procedures described here were in compliance with international laws and policies (Directive 2010/63/EU revising Directive 86/609/EEC on the protection of animals used for scientific purposes; Guide for the Care and Use of Laboratory Animals, U.S. National Research Council, 1996) and were approved by the Institutional Animal Care Ethical Committee of the University of Modena and Reggio Emilia and the Italian Ministry of Health. The in vivo experimental procedures, performed at Rottapharm Biotech, were also approved by Rottapharm Biotech review board. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010), with the editorial on reporting animal studies (McGrath & Lilley, 2015) and with the recommendations made by the British Journal of Pharmacology.
For neuronal cultures, a total of 89 Sprague–Dawley rats (2–3 weeks old) were used. Animals were anaesthetized prior to cervical dislocation and decapitation with protocols in agreement with the guidelines of the Committee for Research and Ethical Issues of IASP, Italian and European legislation (Kilkenny et al., 2010; McGrath et al., 2010; Zimmermann, 1983).
DRG were obtained from freshly isolated spines after carefully removing nerve trunks and connective tissue. Larger ganglia, chopped into 2–4 smaller pieces, were then incubated for 1 hr at 37°C in 0.125% collagenase (Worthington, Freehold, NJ) dissolved in DMEM containing 10% FBS plus 1% penicillin/streptomycin and 1% l‐glutamine (Euroclone, Milan, Italy). After enzymatic digestion, ganglia were mechanically dissociated, and neurons were plated at a density such that neurons would cover approximately 30% of the coverslip surface in a single layer in Petri dishes containing wells with a glass‐bottom coverslip (pre‐coated with 10 μg·ml−1 of poly‐l‐lysine and 20 μg·ml−1 of laminin, Sigma‐Aldrich, Milan, Italy). Cells were incubated for 2–3 days in DMEM, as described above, plus 1.5 μg·ml−1 cytosine 1‐d‐arabinofuranoside (ARA‐C, Sigma‐Aldrich) to slow the proliferation of non‐neuronal cells and 100 ng·ml−1 of nerve growth factor (Sigma‐Aldrich) to increase cell health and the expression of receptors that are linked to PKCε translocation upon stimulation (Vellani et al., 2006; Vellani, Zachrisson, & McNaughton, 2004).
2.3. Immunocytochemistry experimental design
The immuno‐related procedures used are reported in agreement with the editorial on immunoblotting and immunohistochemistry (Alexander et al., 2018) and comply with the recommendations made by the British Journal of Pharmacology. Activation of membrane receptors coupled to the PLC pathway leads to PKCε translocation from the cytoplasm to the plasma membrane. To study PKCε behaviour, we employed a well‐established technique (Vellani et al., 2004; Vellani et al., 2006; Vellani et al., 2010; Vellani et al., 2013; Vellani, Franchi, et al., 2011; Vellani & Giacomoni, 2017; Vellani, Prandini, et al., 2011). This technique involves activation of PKCε translocation in cultured DRG neurons rapidly induced (30 s) by inflammatory mediators, such as https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=649 (BK), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3717 (PK2), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4453, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=989, followed by fixation with 4% paraformaldehyde and 4% sucrose in PBS (50% dilution), staining for PKCε, and quantification of the number of neurons in which translocation is observed. To increase the time precision of treatments and fixation, an automated system was used in all experiments (FSC‐1, CV Scientific, Modena, Italy). CR4056 and other drugs under examination were pre‐applied in the culture medium for 10 min or longer (up to overnight) or, in some cases, co‐applied with the inflammatory mediators used to elicit PKCε translocation. All treatments (including the vehicle), were pre‐dissolved in the culture medium and were directly applied to the cells at 37°C.
After fixation, cells were permeabilized with 0.2% Triton X‐100 (Sigma‐Aldrich, Milan, Italy) and exposed overnight to a rabbit polyclonal antibody highly specific for PKCε that was previously validated with transgenic animals (Cesare, Dekker, et al., 1999). After extensive rinsing, PKCε was visualized with a secondary antibody (1:200 dilution Alexa Fluor 488 goat anti‐rabbit IgG, Thermo Fisher Scientific, Monza, Italy, Cat# A‐11008, RRID:AB_143165) applied for 2–4 hr at room temperature in the dark. https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1482α was stained with an mouse monoclonal antibody coupled to Alexa 594 (Santa Cruz Biotechnology, Santa Cruz, CA, USA, Cat# sc‐8393‐AF594, RRID:AB_628142). Teleostean fish skin gelatine (0.1%, from Sigma) was present throughout the immunocytochemistry procedures after fixation to avoid nonspecific labelling. Isolectin B4 staining was obtained with isolectin B4 from Griffonia simplicifolia coupled to Alexa 594 (Thermo Fisher Scientific, Cat# I21413, RRID:AB_2313921) and applied at a 1:1,000 concentration for 10 min in a calcium‐containing solution (PBS). All cultures were also stained with DAPI (Thermo Fisher Scientific).
Cells showing PKCε translocation were detected with a confocal microscope (Leica SP2, Leica, Switzerland) by measuring the fluorescence intensity along a line drawn through the cytoplasm and the membrane, thus avoiding the nucleus. Neurons in which the fluorescence intensity of the plasma membrane throughout the cell was 1.5‐fold or higher than the mean cytoplasmatic intensity were considered positive (Cesare, Dekker, et al., 1999). All neurons were either well above or below the cut‐off threshold, so, according to this criterion, we considered using the percentage of positive neurons to be appropriate to quantify translocation. At the time of treatment, coverslips were assigned a random number and then randomly assigned to an experimental condition. The analyst was blinded to the treatment of each coverslip.
2.4. Western blotting
Cell cultures, prepared as described above, were pre‐incubated with CR4056 for 10 min and then stimulated for 30 s with 1 μM BK. Control samples were pre‐incubated with CR4056 or medium only. Immediately after, the supernatant was removed, and the cells were scraped with subcellular fractionation ice‐cold buffer (250 mM sucrose, 20 mM HEPES (pH 7.4), 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, plus a protease and phosphatase inhibitor cocktail). The lysate was passed through a 25‐gauge needle and centrifuged at 11,000xg for 1 hr at 4°C, and the pellet (containing the membrane fraction) was resuspended in Mammalian Protein Extraction Reagent (Thermo Scientific) with the protease and phosphatase inhibitor cocktail and further passed through a 25‐gauge needle.
Protein concentrations were determined using a Bradford assay (Sigma‐Aldrich), and 2 μg of protein was loaded onto a NuPAGE 4–12% Bis‐Tris Gel (Invitrogen) and blotted onto a PVDF membrane (GE Healthcare). Membranes were blocked with StartingBlock Blocking Buffer (Thermo Scientific) for 1 hr and then incubated overnight at 4°C with the same anti‐PKCε antibody used for immunocytochemistry (diluted 1:1,000 in Tris‐buffered saline with 0.1% Tween 20 + 5% BSA), followed by an HRP‐conjugated anti‐rabbit antibody (Abcam, Cat# ab6721, RRID:AB_955447) diluted 1:20,000 in Tris‐buffered saline with 0.1% Tween 20 + 5% BSA. A β‐actin‐specific antibody (Thermo Fisher Scientific, Cat# MA5‐11869, RRID:AB_11004139) followed by an HRP‐conjugated anti‐mouse antibody (Bethyl, Cat# A90‐146P, RRID:AB_10682243) was used as a loading control. Target proteins were visualized with an enhanced chemiluminescence substrate (SuperSignal West Dura, Thermo Scientific), and the images were obtained using the Luminescent Image Analyzer Fujifilm LAS300.
2.5. Complete Freund's adjuvant model of chronic inflammatory pain
Male Wistar Han rats (Charles River, Calco, LC, Italy) weighing 175–300 g at the time of arrival were housed three or two (if weighing >250 g) per cage in polycarbonate cages (42.5 × 26.6 × 18.5 cm) with ad libitum access to food and water; the rats were acclimatized for at least 1 week before performing the tests in a temperature‐ and humidity‐controlled room (20°C ± 2°C, relative humidity within the range of 55 ± 10%) with a 12‐hr light/dark cycle (7:00 a.m.–7:00 p.m.). Animals were assigned to three groups (sham, complete Freund's adjuvant [CFA], and CFA+CR4056) with simple randomization. Baseline measurements of mechanical hyperalgesia were comparable across CFA‐treated groups (block randomization). The total number of rats used was 18, considering six animals per group is the minimum number reported in the literature for similar experiments.
Inflammatory pain was induced by a monolateral injection of 100 μl CFA (Sigma‐Aldrich, Milan, Italy) at a 1 mg·ml−1 concentration diluted 1:1 with saline into the plantar surface of the right hind paw. A sham vehicle group was present for comparison (Ferrari et al., 2011). CR4056 (6 mg·kg−1, p.o.) or its vehicle (0.5% hydroxypropylmethyl cellulose) was administered 72 hr after CFA injection. The Randall–Selitto test was employed to assess the analgesic effect of CR4056 on the response thresholds to mechanical pressure stimulation. The nociceptive withdrawal threshold was assessed by a Randall–Selitto algesimeter (Ugo Basile, Comerio, Varese, Italy). The evaluation of pain was performed just before CFA injection (basal control value) and 3 days after CFA injury. On Day 3, two measurements were carried out, first prior to treatment (pain control value) and then 90 min after CR4056 or vehicle administration.
Immediately, after behavioural assessment (Randall–Selitto test), rats were deeply anaesthetized with an overdose of urethane (1.5 g·kg−1, i.p.) and then transcardially perfused with 250 ml 0.9% saline containing 1% heparin (5,000 IU·ml−1), followed by 500 ml 10% formalin (4% paraformaldehyde, Bio‐Optica Spa, Milan, Italy). L4 and L5 DRG (both ipsilateral and contralateral) were dissected and post‐fixed in the same fixative (10% formalin) for 24 hr at 4°C. After that, samples were switched to 0.01% NaN3 (sodium azide, Sigma‐Aldrich) in PBS (PBS tablets, Sigma‐Aldrich). Each sample was then dehydrated through a graded series of ethanol solutions, cleared in xylene (BioClear, Bio‐Optica Spa, Milan, Italy), and embedded in paraffin blocks for sectioning. Each DRG (L4 and L5) was sliced (one section, 5‐μm thickness) with a fully automated rotary microtome (RM2255, Leica Microsystem Srl, Milan, Italy) and mounted on poly‐l‐lysine‐coated slides (Thermo Fisher Scientific, Waltham, MA, USA) coupled to corresponding contralateral DRG. Before starting the immunofluorescence assay, sections were cleared with xylene and rehydrated through a graded series of ethanol solutions. Antigen unmasking was performed using 0.05% Tween (Tween 20, Sigma‐Aldrich) in 10‐mM citrate buffer pH 6.0 (prepared with citric acid, Sigma‐Aldrich) for 20 min at 95°C. Sections were then washed in PBS and PBS with 0.3% Triton X‐100 (PBS‐T, Sigma‐Aldrich), blocked in 10% normal donkey serum (Sigma‐Aldrich) in PBS‐T, and incubated overnight at 4°C with the primary antibody in 2% normal donkey serum/PBS‐T.
DRG sections were stained with a rabbit polyclonal antibody anti‐phospho‐S729 PKCε (Abcam, Cat# ab63387, RRID:AB_1142277), diluted 1:25. After primary antibody incubation, sections were washed again in PBS and PBS‐T and incubated with a secondary antibody (Thermo Fisher Scientific, Cat# A21207, RRID:AB_141637) diluted 1:400 in 2% normal donkey serum in PBS‐T. Sections were dehydrated again and covered with a coverslip with Fluoroshield mounting medium containing DAPI (Sigma‐Aldrich); the slides were then visualized with Invitrogen EVOS FL Auto Cell Imaging System (Thermo Fisher Scientific, Waltham, MA, USA).
The entire area of the DRG section was analysed at 20× magnification. The number of neurons positive to staining for phospho‐PKCε (pPKCε) was normalized to the total number of DAPI‐positive neurons (i.e., with a clearly visible nucleus). The L4–L5 mean value was considered for each sample.
2.6. Data analysis
The experimental design, the data and the statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). For estimation of the percentage of cells showing PKCε translocation, all experiments were performed for each condition in at least five different cultures (replicates: see Vaux, Fidler, & Cumming, 2012), for dose–response experiments (Figures 2a,b and 6a), and in six cultures for all other experiments. According to power analysis based on our previous studies on PKCε translocation, five replicates would be sufficient to achieve 80% power in detecting a 5% difference with α set at 0.05. Each condition was tested in at least three different coverslips from the same culture (repeats), with largely consistent results within each group. At least 700 neurons per coverslip were analysed. The choice of coverslips for different treatments was randomized, and coverslip treatment blinded to the analyst (see above). The values from repeats were averaged to obtain the final data for one condition from one experiment, which in turn was averaged with other replicates. Translocation data were therefore expressed as the actual average percentage of PKCε translocated cells (mean of replicates), with the exception of Figure 1b,c, where the value of each replicate was normalized to its matched control. The latter method was used to facilitate the comparison among different treatments.
Figure 2.
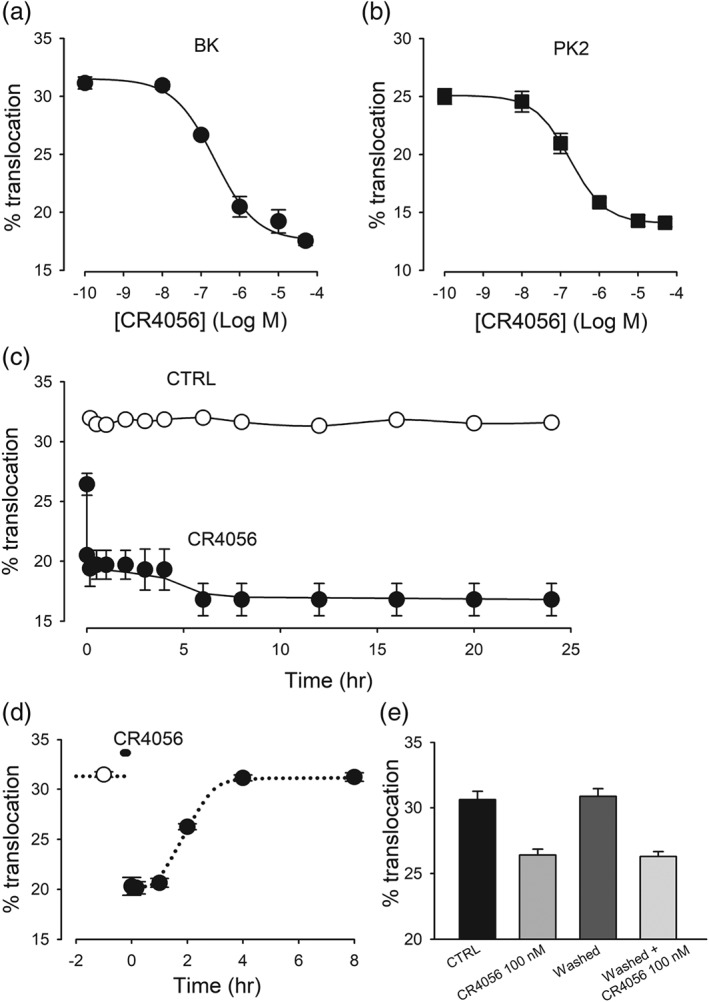
Dose–response, time course, and washout of CR4056 effect on PKCε translocation. (a, b) dose–response of CR4056 inhibition of PKCε translocation induced by bradykinin (BK, 1 μM) or prokineticin 2 (PK2, 10 nM). CR4056 was pre‐applied for 10 min at different concentrations, and cells were exposed to BK/PK2 for 30 s and then quickly fixed and stained for PKCε. Each datapoint shows the average data from five to six different cultures. Data were fitted by the Hill equation, with the following parameters: (a) IC50: 2.0 ± 0.6 × 10−7 M; min = 18.1 ± 0.7; max = 31.4 ± 0.7; Hill slope = 0.93 ± 0.23; (b) IC50: 1.7 ± 0.1 × 10−7 M; min = 18.1 ± 0.1; max = 25.1 ± 0.1; Hill slope = 0.96 ± 0.05. (c) Time course of CR4056 effect. Open symbols represent the percentage of neurons positive for PKCε translocation following BK treatment, stimulated at different times during 24 hr. Filled symbols represent neurons treated with CR4056 (10 μM) applied from time zero onwards. The first filled symbol indicates the effect of CR4056 co‐applied for 30 s with BK, and the second filled symbol indicates 10 s of pre‐exposure. The effect of CR4056 was highly significant at all times (t test, P < .05, n = 6). (d) Reversibility of CR4056 effect. BK‐induced PKCε translocation was evaluated before (open symbol) and after CR4056 (10 μM, 10 min application, filled symbols), at different time intervals. The first datapoint was obtained at the end of the 10 min treatment, when CR4056 was still present. The subsequent datapoints were obtained after extensive rinsing to ensure complete removal of the drug from extracellular solution (six cultures for each point). (e) Recovery of CR4056 effect. The graph is showing that after a 5 hr recovery from a first treatment with CR4056 (0.1 μM), a second treatment had a comparable effect (n = 6)
Figure 6.
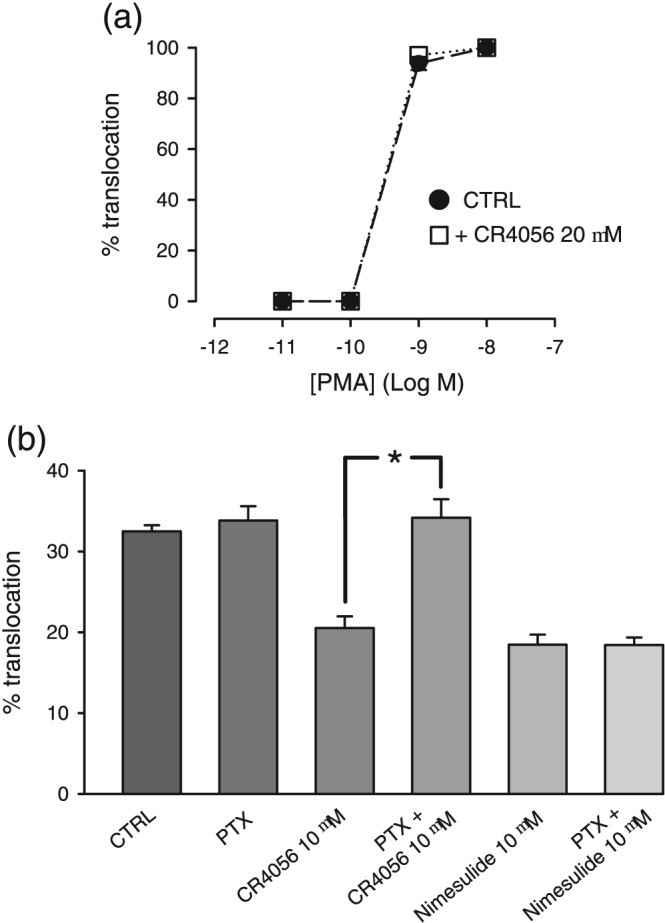
Mechanisms underlying the block by CR4056, of PKCε translocation. (a) Cultured dorsal root ganglia neurons were stimulated with a range of concentrations of phorbol 12‐myristate 13‐acetate (PMA) for exactly 30 s and then rapidly fixed. PKCε translocation was measurable above 10−10 M PMA concentration and was clearly visible in the vast majority of neurons. Pre‐incubation for 30 min with 20 μM CR4056 before PKC activation did not change the effect of PMA, in comparison with PMA alone (n = 5). (b) Experiments performed in neurons pretreated with pertussis toxin (PTX, 200 nM overnight). Incubation with PTX alone did not modulate bradykinin‐induced translocation of PKCε but blocked the translocation induced by CR4056. By contrast, PTX did not block the effect of nimesulide, another inhibitor of PKCε translocation, *P < .05, significantly different as indicated (n = 6)
Figure 1.
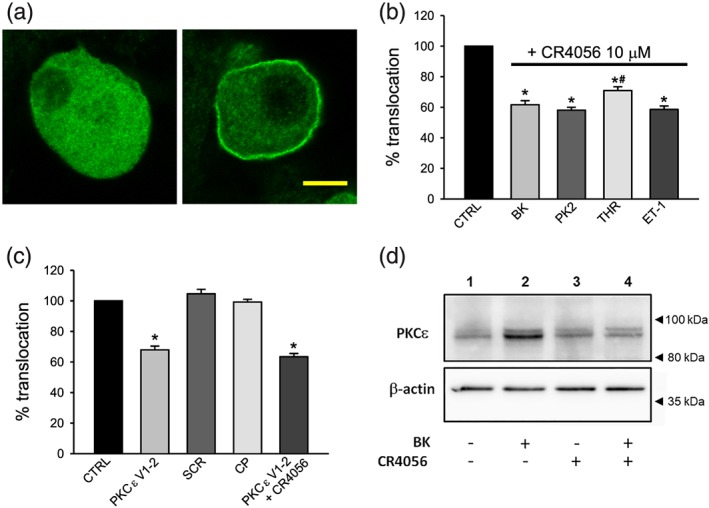
Translocation of PKCε induced by inflammatory mediators is blocked by CR4056 and by the specific PKCε translocation blocking peptide in cultured sensory neurons. (a) Unresponsive neuron (left) compared with a neuron which responded to bradykinin (BK) with translocation of PKCε (right), visualized by immunocytochemistry and confocal imaging. Confocal section of cultured dorsal root ganglia neurons treated with a saturating concentration of BK (1 μM) for 30 s and then fixed and stained with a rabbit polyclonal antibody against PKCε. In unresponsive neurons (not expressing functional BK receptors, left) PKCε signal is diffused in the cytoplasm, while in responsive neurons (right), most fluorescence is localized in the plasma membrane. The images shown are taken from two areas of the same confocal image and have identical scale and acquisition settings. No contrast enhancement was employed. Scale bar: 10 μm. Image stacks and three‐dimensional reconstructions of responsive and unresponsive cells are available in Video S1. (b) Effect of CR4056 on PKCε translocation induced by different inflammatory mediators. CR4056 (10 μM) was pre‐applied for 10 min, and cells were then exposed for 30 s to saturating concentrations of one of the following inflammatory mediators: BK (1 μM), prokineticin 2 (PK2, 100 nM), thrombin (THR, 100 nM), or endothelin‐1 (ET‐1, 500 nM), and then quickly fixed and stained for PKCε (n = 6). CR4056 significantly reduced the percentage of neurons responding to any one of the inflammatory mediators, compared with the matched control value (CTRL; set to 100%). *P < .05, significantly different from CTRL; t test, performed on non‐normalized data. Each replicate value was then normalized to its matched control to compare the effect of CR4056 on translocation induced by the different inflammatory mediators. CR4056 was slightly, but significantly, less effective in inhibiting the translocation of PKCε elicited by THR than that elicited by BK, PK2 or ET‐1. # P < .05, significantly different from BK, PK2 or ET‐1; ANOVA followed by Bonferroni's post hoc test. (c) Effect of the PKCε blocking peptide on PKCε translocation. The specific PKCε translocation blocker εV1–2 coupled to a membrane‐permeant carrier peptide (PKCε V1–2, 10 μM) was pre‐applied for 60 min to allow cell loading, with or without CR4056 (10 μM). Cells were then challenged with BK. The scrambled peptide coupled to the carrier peptide (SCR) and the carrier peptide alone (CP) were used as negative controls (same concentrations and loading time as PKCε V1–2). Treatment with PKCε V1–2 and with the negative controls were significantly different (P < .05). Co‐application of CR4056 and PKCεV1–2 did not cause further inhibition of PKCε translocation in comparison with PKCε V1–2 alone (n = 6). Data displayed were normalized to matched control. (d) Effect of CR4056 on PKCε translocation induced by BK visualized by Western blot assay in the membrane fraction. BK (1 μM) was added for 30 s to cultured sensory neurons (lane 2) inducing enrichment of PKCε in the membrane fraction, compared with vehicle control (lane 1). CR4056 (10 μM) added 10 min before BK application prevents the PKCε translocation (lane 4). CR4056 (10 μM) alone (lane 3) was not different from vehicle control
GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA) and QI Macros (KnowWare International, Denver, CO, USA) were used for statistical analyses, except for power analysis, performed using free software available online (https://clincalc.com/stats/samplesize.aspx). Data were analysed with t test or with ANOVA where appropriate, after being tested for normality (Shapiro‐Wilk test) and homogeneity (Brown‐Forsythe and Bartlett's test, automatically run by Prism software when performing ANOVA, and Levene's test when using QI Macros). ANOVA was followed by Bonferroni's or Tukey's multiple comparisons post hoc tests, with P < .05 considered statistically significant. Data are presented as the mean ± SEM.
Data are presented as bar graphs when a scatter plot did not reveal any unusual or interesting aspect of the data, in agreement with current recommendations (George et al., 2017).
2.7. Materials
All drugs were obtained from Sigma‐Aldrich (Milan, Italy) except for PK2 (PeproTech, London, UK) and CR4056 (Rottapharm Biotech, Monza, Italy) and the PKCε membrane‐permeant blocking peptides, which were from American Peptide Company (Sunnyvale, CA, USA). The active blocking peptide PKCεV1–2 (NH2–EAVSLKPT–COOH) was coupled via a cysteine S–S bond to the https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=773‐(47‐57) arginine‐rich (NH2–YGRKKRRQRRR–COOH) carrier peptide (CP). As a negative control, the scrambled peptide (NH2–LSETKPAV–COOH) and the CP alone were used as controls. Peptides were loaded into cultured neurons at a 10‐μM concentration for 60 min and were also present in the extracellular solution when cells were challenged with BK. This combination of loading time and peptide concentration was determined in preliminary experiments (with BK, thrombin, and PK2 as stimuli, not shown) to be the combination producing the maximum blocking effect: neither longer application times nor higher peptide concentrations displayed a higher efficacy.
2.8. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, S. P. H., Christopoulos et al., 2017; Alexander, S. P. H., Fabbro, et al., 2017).
3. RESULTS
3.1. PKCε catalytic activity is not modulated by CR4056
Purified human recombinant PKCε was incubated with a suitable substrate and ATP, with or without CR4056 (1 μM). The substrate phosphorylation, detected using a homogeneous time‐resolved fluorescence phospho‐assay, was identical with or without CR4056. The concurrent positive control (Ro 31‐8425) potently reduced phosphorylation of the substrate (IC50 = 6.8 nM), as expected.
3.2. PKCε translocation induced by inflammatory mediators is dose‐dependently inhibited by CR4056
A typical example of PKCε translocation routinely obtained after 30 s of exposure to 1‐μM BK is shown in the immunocytochemistry experiment in Figure 1a. The confocal image of a BK unresponsive neuron (left) was compared with that of a responsive neuron (right). In unstimulated cultures, all neurons displayed the same pattern of PKCε immunostaining as neurons unresponsive to BK, and there was no sign of spontaneous PKCε translocation. The PKCε translocation induced by other Gq‐coupled inflammatory mediators produced a qualitatively indistinguishable response when the same experimental protocol was used, as previously reported (Vellani et al., 2004; Vellani et al., 2010; Vellani, Prandini, et al., 2011). Figure 1b shows the fractional blocking effect of CR4056 (10 μM) on PKCε translocation caused by BK, PK2, the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=59 (PAR)‐1, ‐3, and ‐4 agonist thrombin (Vellani et al., 2010), and endothelin‐1 (Vellani, Prandini, et al., 2011).
The translocation of PKCε induced by BK was completely independent of the cellular localization of PKCα, which is also expressed in most sensory neurons with a small–medium diameter. In cells showing the translocation of the ε isoform, the α isoform could be expressed in the cytoplasm, in the membrane, or both. In several cells that did not respond with PKCε translocation, as well as in approximately 50% of unstimulated cells, PKCα was detected in the plasma membrane (Figure S1). We conclude that PKCα translocation to the plasma membrane is independent of BK stimulation.
The translocation of PKCε was suppressed by CR4056 in a similar fraction of neurons independent of the different mediators, except for the neurons activated by thrombin, that are slightly, but significantly, less sensitive to CR4056 than those activated by BK. We previously observed that PKCε translocation caused by thrombin, PK2, and endothelin‐1 occurs mostly in DRG neurons negative for staining with isolectin B4, a marker of non‐peptidergic nociceptors (Vellani et al., 2006; Vellani et al., 2010; Vellani, Prandini et al., 2011), while translocation induced by BK is visible in both isolectin B4‐negative (IB4−) and isolectin B4‐positive (IB4+) neurons (Vellani et al., 2004). In the neuronal subpopulation activated by BK, the percentage of IB4+ neurons was 50.6 ± 1.6%. In cultures treated with CR4056 (10 μM, 10 min), the remaining BK‐responsive IB4+ neurons were 52.6 ± 1.0%, indicating that CR4056 equally decreased the IB4+ and the IB4− BK‐sensitive subpopulations (n = 5).
To test a different PKCε blocking agent for comparison with CR4056, the specific PKCε blocking peptide ε V1–2 (Figure 1c) was loaded into DRG neurons using a membrane‐permeable form, coupled to the TAT CP. This peptide was able to mimic the blocking activity of CR4056. The scrambled εV1–2 peptide coupled to the same CP (negative control) and the CP alone did not produce any effects (n = 6). The effect of PKCεV1–2 in combination with CR4056 was similar to that of either treatment alone.
The translocation of PKCε induced by BK and the inhibition by CR4056 was also confirmed by an alternative approach (Western blotting) using the same anti‐PKCε antibody employed in the immunocytochemistry assay. Cultured DRG neurons were set up in four experimental groups: vehicle/vehicle, vehicle/BK, CR4056/vehicle, and CR4056/BK. After the incubation period, cells were subjected to lysis, and the proteins present in the membrane fraction were detected as shown in Figure 1d. Western blot analysis confirmed both the BK‐induced translocation of PKCε to the plasma membrane and the inhibitory effect of CR4056.
Figure 2 shows the CR4056 dose–response curves for PKCε translocation obtained with either BK (a) or PK2 (b). Data fitting returned IC50 values of 0.20 and 0.17 μM, respectively (further parameters in the figure legend). When CR4056 was applied for 10 min, the dose–response curves approached saturation at approximately 10 μM (a, b). This concentration was tested at different time intervals to investigate the kinetics of the CR4056 effect. When CR4056 was co‐applied with BK as a PKCε translocation inducer for 30 s (the shortest feasible application time in our protocol, see Section 2), approximately 40% of the maximum effect was present, as shown in Figure 2c. When the drug was pre‐applied (10 s) before BK stimulation (30 s), the inhibitory effect on translocation increased significantly, reaching its maximum. Indeed, pre‐exposures longer than 10 s before translocation activation did not produce any further significant inhibition. The effect of CR4056 was therefore stable for 10 s–24 hr pre‐exposure, with no sign of adaptation/tachyphylaxis.
Next, we analysed the time necessary to eliminate the effect of this drug. In Figure 2d, PKCε translocation is reported at different timepoints after CR4056 (10 μM) exposure: first, after the 10 min application and then after repeated, long‐lasting washes with large volumes of culture medium (DMEM + 10% FBS, 37°C) expected to remove any trace of CR4056 from the extracellular environment (washout).
The effect of CR4056 remained unchanged for up to 1 hr after washout, and then it slowly decreased and was completely reversed after 3–4 hr. We conclude that the CR4056 effect has a fast onset, but it is very slowly removed from the cells. This experiment also shows that there is no apparent aftereffect of the drug on cells responding to BK in this time frame, as the percentage of responsive cells returns to the exact initial level. Figure 2e shows that repeating the stimulation procedure (challenge with BK in the presence or absence of 0.1 μM CR4056) after a 5 hr washout (i.e., when the cells fully recovered their sensitivity to BK) gave the same inhibitory effect by CR4056, thus confirming the full reversibility of the phenomenon and the absence of tolerance. A further experiment demonstrated that the pre‐application of ineffective concentrations of CR4056 (0.1 and 10 nM) for 1 hr did not produce any modulation of the effect of 0.1 μM of the drug (data not shown). This result suggests that no priming effect was triggered by CR4056 in this experimental condition.
3.3. Idazoxan‐sensitive I2 binding sites do not appear to be involved in the inhibition of PKCε translocation by CR4056
The in vivo analgesic activity of CR4056 was reported to be mediated by its interaction with I2 receptors and, at least in part, inhibited by idazoxan, the putative I2 receptor antagonist. To test whether the mechanism leading to the inhibition of PKCε translocation was mediated by, or in some way related to, I2 receptor interaction, we evaluated the effects of the so‐called imidazoline receptor agonists and antagonists, both individually and in combination with CR4056.
Idazoxan was originally developed as a α2‐adrenoceptor antagonist, but it has also been proposed as the only known functional I2 receptor antagonist, even if it shows a distinctive agonist‐like behaviour in discrimination tests (for a review, see Li, 2017). Therefore, we tested the sensitivity of the PKCε translocation assay to idazoxan itself and pre‐applied idazoxan for 10 min at high concentrations (10 and 100 μM). We also tested idazoxan on the effect of 1 μM CR4056, a concentration inducing a submaximal block of PKCε translocation. In both experimental conditions, idazoxan was completely ineffective. The data are shown in Figure 3a.
Figure 3.
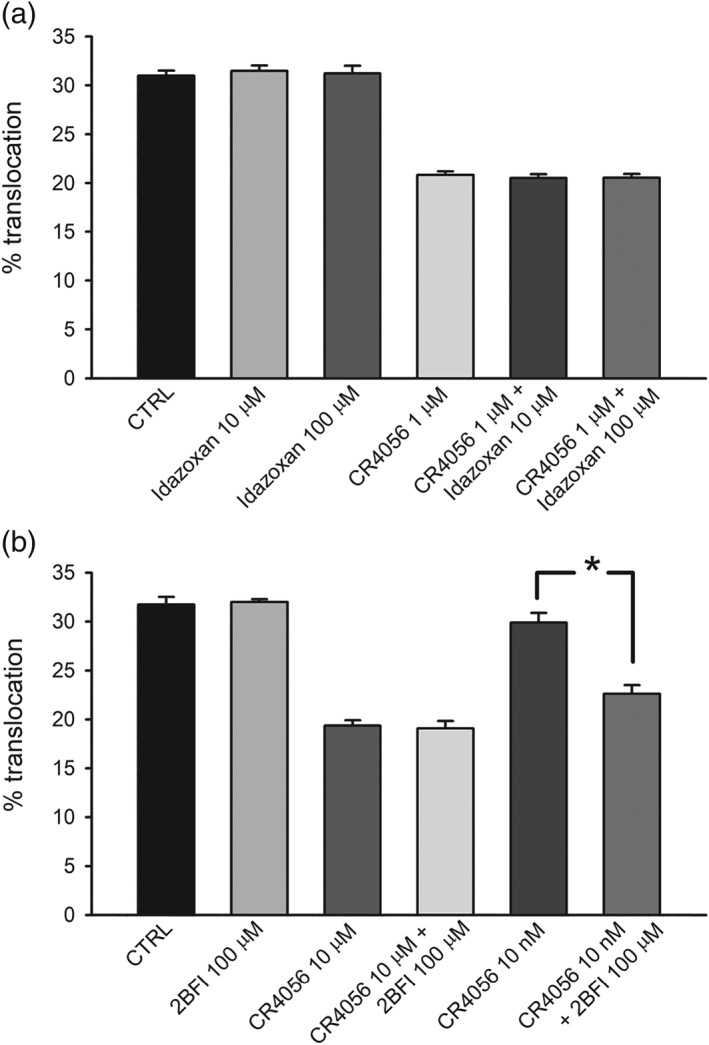
Effects of imidazoline‐2 receptor antagonist idazoxan and of the agonist 2BFI. (a) Idazoxan, a purported I2 receptor antagonist, was pre‐applied (10 and 100 μM) onto cultured dorsal root ganglia neurons for 10 min before stimulation with bradykinin. The application of idazoxan alone had no effect on translocation. As expected (see Fig 2a), CR4056 (1 μM for 10 min) applied alone induced a significant reduction of translocation. Application of CR4056 in combination with idazoxan did not affect the response to CR4056 (n = 6). (b) 2BFI applied for 10 min at the high dose of 100 μM did not affect PKCε translocation. In the same set of experiments, 2BFI (100 μM) even applied overnight was completely ineffective (n = 6, data not shown). Furthermore, 2BFI at 100 μM (10 min) did not increase the effect of a saturating concentration of CR4056 (10 μM, 10 min) but increased the effect of 10 nM CR4056 which per se was ineffective. *P < .05, significantly different as indicated (n = 6)
We then tested the effect of the purported I2‐receptor specific agonist 2‐(2‐benzofuranyl)‐2‐imidazoline (2BFI) by itself, both pre‐applied for 10 min and co‐applied with BK used to stimulate PKCε translocation. Under these conditions, 2BFI at high concentrations (100 μM, Figure 3b) or lower concentrations (10 and 1 μM, not shown) did not change the percentage of neurons showing PKCε translocation in response to BK. Moreover, 2BFI was not able to interfere with the effect of 10 μM CR4056 (Figure 3b). Intriguingly, however, the co‐application of 2BFI (100 μM) with CR4056 at a sub‐effective concentration (10 nM) elicited the effect of CR4056, showing a possible synergy between these two I2 ligands. The lack of potentiation by 2BFI on the 10 μM CR4056 effect is probably due to the maximum possible inhibition observed for CR4056 at this concentration. Further investigations at lower concentrations would confirm this positive modulation.
3.4. α2‐Adrenoceptor blocking by yohimbine does not modulate the effect of CR4056 on PKCε translocation
The in vivo analgesic effect of CR4056 is reported to be partially (~30%) sensitive to block of α2 receptors (Lanza et al., 2014). We therefore checked with the use of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=102, a specific α2 receptor antagonist, if such receptors could be involved in the CR4056 effect on PKCε translocation. As shown in Figure 4, yohimbine had no effect per se on translocation and did not modify the responses to CR4056 when tested at two different concentrations.
Figure 4.
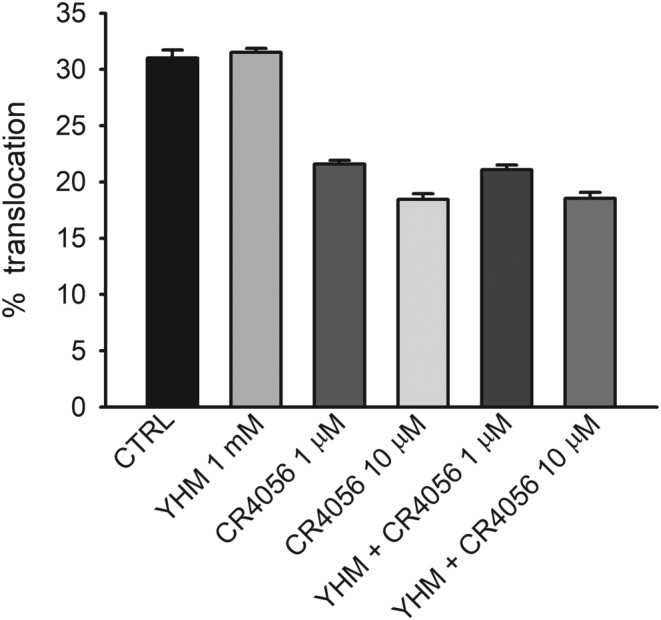
Yohimbine (YHM) does not interfere with CR4056 inhibition of PKCε translocation. The selective α2 adrenoceptor antagonist yohimbine (1 mM) was applied 10, 30, or 120 min before CR4056 (1 or 10 μM, for 10 min) followed by the translocation protocol. Bars show the pooled results of these experiments (n = 6). Yohimbine given alone, did not affect the translocation of PKCε and did not modulate the inhibition of translocation by CR4056, at either concentration
3.5. Gabapentin but not valproic acid modulates the effects of CR4056
Gabapentin and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7009 are anticonvulsant drugs that also exhibit antinociceptive properties with multifactorial mechanism that is not fully understood (Mathiesen, Moiniche, & Dahl, 2007; Moore, Wiffen, Derry, & Mcquay, 2011; Wiffen et al., 2013; Ximenes et al., 2013). As gabapentin previously showed several largely different effects, including the modulation of PKC activity (Zhang et al., 2015) and translocation in cultured sensory neurons (Vellani & Giacomoni, 2017), we checked whether these two drugs could modulate the effect of CR4056. In these experiments, BK was used to stimulate PKCε translocation (Vellani & Giacomoni, 2017). Figure 5a shows that gabapentin inhibited PKCε translocation and that the presence of a sub‐effective concentration of CR4056 (10 nM) had a potentiating effect on gabapentin. A similar effect was obtained when gabapentin was co‐applied with paracetamol (Vellani & Giacomoni, 2017). However, gabapentin (200 μM) did not increase the maximum effect obtained with a saturating concentration of CR4056 when co‐applied (experiment performed with PK2 as well as BK, data not shown).
Figure 5.
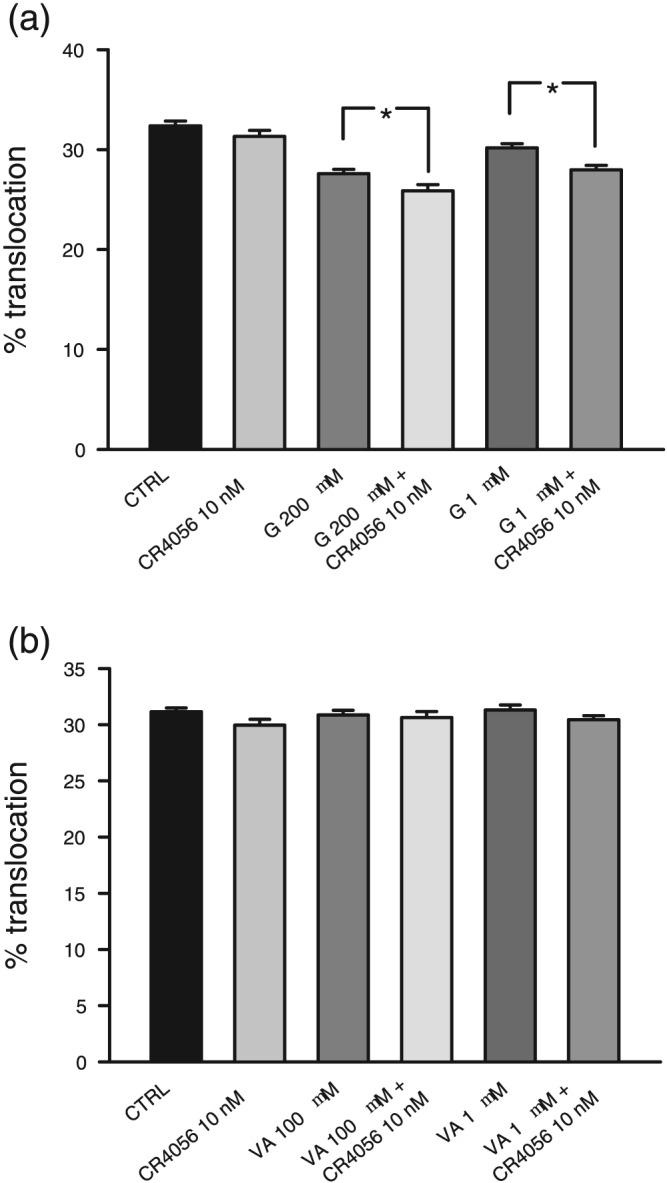
Gabapentin (G), valproic acid (VA), and CR4056. (a) Gabapentin in all experiments was applied for 90 min. The effect of gabapentin was modestly potentiated by the per se ineffective concentration (10 nM) of CR4056 on bradykinin‐induced translocation (n = 6). *P < .05, significantly different as indicated. (b) VA, applied for 90 min, did not modulate bradykinin‐induced PKCε translocation or potentiate the ineffective concentration (10 nM) of CR4056 (n = 6)
Valproic acid (Figure 5b), another anticonvulsant drug with established antinociceptive properties, had no effect per se nor did it show any potentiation of the CR4056 response.
We conclude that the small potentiating effect of gabapentin on CR4056 activity is not reasonably related to the sodium channel block induced by gabapentin, as this action is shared with valproic acid, which does not modulate the CR4056 effect.
3.6. Phorbol ester‐induced PKCε translocation is not modulated by CR4056
To further investigate the mechanism of PKCε inhibition, we checked whether CR4056 could modulate not only the “physiological” PKCε translocation via activation of the Gq pathway but also the “direct” translocation that can be obtained with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2341 (PMA). PMA is the classic activator of the DAG‐sensitive isoforms of PKC, and it can quickly and completely translocate PKCε to the plasma membrane in DRG neurons (Cesare, Dekker, et al., 1999; Vellani et al., 2004) by binding to the C1 domain on PKC (Hurley, Newton, Parker, Blumberg, & Nishizuka, 1997). In Figure 6a, we report the effect of PMA after a very short application (30 s). The activation of PKCε translocation ranged from 0% to 100% with a very steep dose–response curve and a threshold above 10−10 M. Protein translocation was observed in 100% of neurons positive for PKCε signal, as expected by the direct mechanism of activation of PMA on PKC. CR4056 produced no changes to the PMA effect, even at the very high concentration of 20 μM and pre‐applied for a long time (30 min). Based on these results, we may conclude that CR4056 is not acting by blocking the C1 domain of PKCε.
3.7. CR4056 block of PKCε translocation is sensitive to pertussis toxin
Pertussis toxin (PTX) is a specific blocker of Gi/o proteins. We compared the effect of CR4056 on naïve cultures pretreated overnight with PTX (200 nM). The effect of CR4056 was completely blocked by PTX, thus supporting that Gi/o activation is necessary for CR4056 inhibition of PKCε translocation. As expected, PTX alone did not cause any change in the translocation behaviour induced by treatment with BK, consistent with similar observations reported for the PK2 response (Vellani et al., 2006). In the same set of experiments, PTX treatment caused no changes in the effect of nimesulide, an anti‐inflammatory and analgesic drug that is also a blocker of PKCε translocation (Figure 6b; Vellani, Franchi, et al., 2011; Vellani et al., 2013).
3.8. Effects of CR4056 acute administration on pain behaviour in CFA‐treated rats
Chronic inflammatory pain, induced in adult rats by injection of CFA in the hind paw, was evaluated 72 hr after CFA injection. The mean withdrawal threshold (Randall–Selitto test) in CFA rats was significantly lower than the one measured in sham rats. CR4056, following acute administration (6 mg·kg−1, p.o.), caused a significant increase of the withdrawal threshold. Thus, CR4056 reversed the CFA‐induced mechanical hyperalgesia (Figure 7a).
Figure 7.
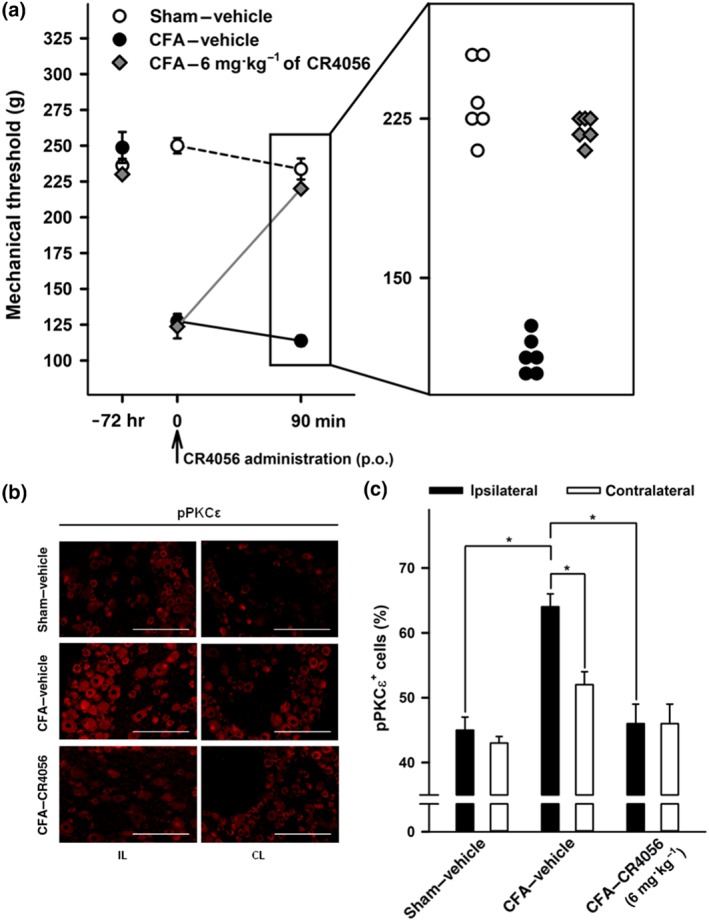
CR4056 blocks complete Freund's adjuvant (CFA)‐induced inflammatory pain in rats and the phosphorylation of PKCε in dorsal root ganglia (DRG) in vivo. (a) Anti‐hyperalgesic effect of CR4056 in CFA‐injected rats (Randall–Selitto test). CR4056 was administered (6 mg·kg−1, p.o.) 72 hr after CFA injection. Data represent the pain threshold values expressed in grams, reported as mean ± SEM (n = 6 per group). Magnification inset shows individual threshold values 90 min after vehicle/CR4056 administration. (b) Immunofluorescence staining for pPKCε in L4–L5 ipsilateral (IL) and contralateral (CL) DRG sections from sham and CFA‐injected rats. Pictures are representative of pPKCε immunoreactivity in sham animals (top), vehicle‐treated CFA‐injected animals (middle), and CR4056‐treated CFA‐injected animals (bottom), 90 min after single administration. (c) Quantitative pPKCε staining in L4–L5 DRG sections (IL and CL) from sham and CFA‐injected rats, acutely treated with vehicle or CR4056. Data represent mean percentage of pPKCε‐positive cells normalized to total number of cells (n = 6 per group). *P < .05, significantly different as indicated; one‐way ANOVA followed by Tukey's multiple comparisons test
3.9. Effects of CR4056 on PKCε phosphorylation in DRG neurons from CFA rats
DRG from the same animals tested for hyperalgesia were quickly collected for assessment of the levels of PKCε phosphorylation. Chronic hind limb inflammation induced by CFA administration was associated with a significant increase in PKCε phosphorylation, a sign of its activation (Zhou, Li, & Zhao, 2003), in ipsilateral L4–L5 DRG neurons compared with those from sham rats. CR4056, after a single acute treatment (6 mg·kg−1, p.o.), was able to significantly reduce PKCε phosphorylation in ipsilateral L4–L5 DRG neurons (Figure 7b,c). The CR4056‐treated group was significantly different versus the CFA‐treated animals but was not significantly different from sham animals.
No significant difference in PKCε phosphorylation was detected between the ipsilateral and contralateral L4–L5 DRG of sham animals.
4. DISCUSSION
The imidazoline I2 receptors are expressed in several brain areas, many of which are involved in responses to noxious stimuli and in pain perception (Ferrari et al., 2011; Gentili et al., 2006). As already discussed, the term I2 receptors refers to a heterogeneous class of proteins whose activity, upon binding to their selective imidazol(in)e ligands, is somehow modified. Among them, MAO (A and B), brain creatine kinase, and semicarbazide‐sensitive amine oxidase have been identified, whereas others are still unidentified. While I2 ligands share a characteristic pharmacological spectrum (analgesic activity and interaction with opiates), their ultimate behaviour in specific tests could sensibly vary. The compound 2BFI is generally regarded as the classic I2 “agonist.” An attempt to further sub‐classify I2 binding sites was made with the supposed subtypes I2A (sensitive to the “antagonist” amiloride) and I2B (insensitive to amiloride) (Bektas et al., 2015; Diamant et al., 1992). Idazoxan is generally considered the classic I2 receptor “antagonist,” even if the activities of some I2 ligands are idazoxan‐insensitive and even if idazoxan itself behaves as an “agonist” in some paradigms (i.e., in blocking opiate tolerance). Other I2 ligands, such as BU‐224, show a very high affinity, but their efficiency as “agonists” is very low in most paradigms. This complex picture is well described by the elegant studies on in vivo cross‐discrimination between I2 ligands published by Li and co‐workers (Qiu, He, Zhang, & Li, 2014; Qiu, Thorn, Zhang, He, & Li, 2014; Qiu, Zhang, & Li, 2015). In these papers, it is possible to observe a full range of substitutions between I2 ligands, from complete to partial, up to no substitution at all.
Contrary to the in vivo situation, in vitro characterization of I2 ligands, which could clarify the different pathways involved in their activity, is still largely lacking. CR4056 is an ideal candidate for such analysis: It specifically binds I2 receptors with no or negligible interactions with over 60 pharmacologically relevant targets (in either binding or functional assays, Rottapharm Biotech, data on file). Moreover, this is the first I2 ligand able to control pain in humans (Rovati et al., 2019).
In the present study, we demonstrated that CR4056 blocked the translocation of PKCε in activated sensory neurons. The potency of CR4056 was in the same range of concentrations as nimesulide, paracetamol, and other non‐steroidal anti‐inflammatory drugs (Vellani, Franchi, et al., 2011; Vellani et al., 2013) but was approximately 10 times higher than that of gabapentin (Vellani & Giacomoni, 2017).
The effect is very rapid in its onset, visible as early as 30 s after application (when CR4056 was co‐applied with the translocation stimulus). This very rapid effect is comparable with the time the drug takes to enter the cell. Therefore, we believe that the effect is direct—that is, CR4056 is unlikely to inhibit PKCε translocation via a metabolite or a slowly activating intracellular pathway. Another peculiarity of the effect of CR4056 is the long duration of this activity, which is consistent with the long‐lasting analgesic effect observed in pain models in rats (Comi et al., 2017). No tachyphylaxis and no priming effects are present.
Prokineticin receptors activate PKCε translocation exclusively in IB4−, peptidergic DRG neurons (Vellani et al., 2006). Additionally, endothelin receptors (Vellani, Prandini, et al., 2011) and thrombin receptors PAR‐1, ‐3, and ‐4 (Vellani et al., 2010) are expressed in IB4− sensory neurons, but they all can be up‐regulated in IB4+ neurons by glia‐derived neurotropic factors and other RET receptor agonists. However, other receptors are also expressed in IB4+ neurons under basal conditions, such as BK https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=42 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=41 (Cesare, Moriondo, Vellani, & McNaughton, 1999; Vellani et al., 2004). Translocation therefore occurs both in peptidergic and in non‐peptidergic sensory neurons (mostly nociceptors), where PKCε is expressed at high levels. The dose–response of CR4056 and its fractional inhibitory effect on PKCε translocation are largely comparable when induced by agonists of BK, PK2, and PAR receptors. Similarly, CR4056 is equally active in the different subpopulations expressing these receptors, that is, in both peptidergic and non‐peptidergic DRG neurons, as shown in this paper with isolectin B4 staining. These observations suggest that the effect of CR4056 on PKCε translocation is not subpopulation‐specific. It remains to be explained why both CR4056 and the specific translocation blocking peptide PKCε V1–2 (Figure 1c) block translocation of only a fraction of BK‐activated neurons but do not affect the remaining neurons. We know that the translocation block induced by the peptide is achieved by competitive inhibition of the ε‐specific receptor for activated C kinase (RACK2) binding site. The similarity of the CR4056 effect to the peptide effect and the lack of additivity when both treatments were applied together (Figure 1c) suggest that their mechanisms of action may be similar. However, the effect of CR4056 on translocation is completely blocked by PTX (see below), which suggests that a more complex, metabotropic mechanism is involved (Figure 6). On the other hand, RACKs are membrane‐associated proteins with several other functions. In addition to recruiting PKCs in an active conformation and transporting them to a specific membrane compartment, they can be allosterically modulated and interact with several proteins (Steinberg, 2008). In particular, RACKs share a seven‐WD40‐motif repeat structure, similar to the protein–protein binding motifs found in heterotrimeric G‐protein β‐subunits. It is possible that CR4056 acts indirectly via Gi protein to modulate RACK2 affinity for PKCε rather than by blocking its PKCε binding site, obtaining a similar effect to the blocking peptide effect.
The binding of the PKC blocking peptide to an isoform‐specific RACK is a well‐demonstrated mechanism of PKC translocation that allows isoform‐specific targeting to specific subcellular locations, which can be different for the same isoform in different cell types, depending on the cellular localization of its specific RACK. However, there is ample evidence that this is not the only mechanism. In fact, PKCs also localize in cells by means of RACK‐independent interactions with the cytoskeleton and with scaffolding proteins, such as A‐kinase anchoring proteins and caveolin (Steinberg, 2008). It is possible that different mechanisms of PKCε translocation take place in different subpopulations of DRG neurons and that CR4056 can interfere with only one of them. The observation that CR4056 is slightly but significantly less effective on the thrombin‐responsive subpopulation than on the BK‐responsive subpopulation (Figure 1b) agrees with this hypothesis.
Our data show that the effect of CR4056 on PKCε is highly specific for its translocation, but according to our in vitro results with human recombinant PKCε, there is no interference on PKCε catalytic activity. Our data, however, do not rule out possible effects on the translocation of other isoforms of PKC expressed in DRG neurons. Among these, PKCβ1 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1483 are translocated to the plasma membrane under unstimulated conditions, and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1491 is not translocated by BK or PMA (Cesare, Dekker, et al., 1999, Vellani & McNaughton, 2004). The remaining PKC isoforms include https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1482 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1485, both of which can translocate to the plasma membrane. PKCα is activated by intracellular calcium and, in principle, can be activated by calcium signalling produced by inflammatory mediators. In this study, we found that PKCα is spontaneously translocated in several unstimulated neurons (similarly to β1/2 isoforms) and that it may or may not be translocated to the plasma membrane in BK‐responsive neurons (Figure S1). This suggests that the pathway(s) leading to PKCα translocation are largely distinguished from those controlling PKCε, which is normally in the cytoplasm and only translocates to the membrane following stimulation with inflammatory mediators. PKCδ is another member of the “novel” PKC class, which, as PKCε, is calcium independent and is translocated to the plasma membrane by phorbol esters (Cesare, Dekker, et al., 1999, Vellani, Mapplebeck, Moriondo, Davis, & McNaughton, 2001). In PKCε−/− mice, the phorbol ester PMA produces a strong sensitization of membrane ion currents induced by heat and by capsaicin, which can be blocked by the PKC inhibitor https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5259 (Vellani & McNaughton, 2004). These findings show that not only PKCε but also other PKC isoforms in some conditions can translocate to the plasma membrane, where they are able to phosphorylate membrane ion channels that are highly relevant to pain and hyperalgesia. Whether the effect of CR4056 is PKC isoform‐specific remains to be determined.
The in vitro action of CR4056 on the signalling pathway activated by BK (as well by other inflammatory stimuli) in cultured sensory neurons, that is, PKCε translocation, was consistent with its analgesic effect in the CFA‐induced inflammatory pain model; this action was paralleled by the reduction in PKCε activation. The involvement of PKCε in the painful response to inflammation was shown in the DRG innervating CFA‐inflamed tissue, demonstrating that this isoform participates not only in the early phases (response to inflammatory mediators, such as BK and thrombin, at the site of injury) but also in the chronic phases of inflammation (Reichling & Levine, 2009), which we observed as an increase in pPKCε in neuronal cell bodies. CR4056 quickly (90 min) and significantly reduced the amount of pPKCε expressed in DRG neurons, demonstrating similar effects on two different indicators of PKCε activation: translocation to the plasma membrane and phosphorylation (Parekh, Ziegler, & Parker, 2000; Zhou et al., 2003).
In summary, the pharmacology of CR4056 activity on PKCε translocation is multifaceted, sharing the general complexity of the “I2 receptor system.”
First, the α2 adrenoceptors, the other system heavily affected by imidazole/imidazoline‐containing drugs, do not have a role in this CR4056 effect. The in vivo α2 receptor blockade by yohimbine displayed only a minor reduction in CR4056 analgesia, as observed in a rat model of post‐operative pain (Lanza et al., 2014), or displayed no effect at all, as observed in a rat model of neurogenic inflammation obtained with a capsaicin sub‐plantar injection (Ferrari et al., 2011).
Second, it is remarkable that the effect of CR4056 on PKCε translocation is completely blocked by PTX, indicating the involvement of intracellular pathways involving Gi proteins. Interestingly, some analgesic properties of I2 ligands, including the morphine potentiation effect (a feature exhibited by CR4056; Ferrari et al., 2011; Lanza et al., 2014), are blocked by PTX pretreatment (Sanchez‐Blazquez, Boronat, Olmos, Garcia‐Sevilla, & Garzon, 2000). Nevertheless, the putative Gi‐coupled GPCR involved should be identified.
Even more complex are the profiles of 2BFI, considered a sort of prototypical I2 “agonist,” and idazoxan, a putative I2 “antagonist.” The former is ineffective in inhibiting PKCε translocation but is effective in potentiating CR4056 when applied at a suboptimal concentration, showing that these I2 ligands could somehow synergize in this respect. Surprisingly, idazoxan does not block the effect of CR4056, suggesting the involvement of an idazoxan‐resistant I2 subtype site.
Therefore, a further dissection of this pathway could contribute to a better understanding of the role of I2 receptors in pain control and to explain the differences and similarities of I2 ligands endowed with different analgesic properties.
AUTHOR CONTRIBUTIONS
V.V., C.S., C.M., and C.G. carried out the experiments. V.V., M.L., C.S., O.L., G.C., C.G., and L.C.R. contributed in the conception and design of the study, in the analysis and interpretation of the data, in drafting the article, and in the final approval of the version to be submitted. V.V. and L.C.R. contributed also in the obtaining of funding.
CONFLICT OF INTEREST
V.V. obtained a grant from Rottapharm Biotech. C.S., G.C., M.L., O.L., and L.C.R. are employees of Rottapharm Biotech. C.G. has no competing interests.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206 and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Supporting information
I cannot see the correct figure hereFigure S1. PKCα and PKCε localization in DRG neurons treated with bradykinin
In neurons where BK caused translocation of PKCε (green), PKCα (red) could either be translocated to the membrane and in intracellular sites (A); be diffused in the cytoplasm and absent from the plasma membrane (B); or located in intracellular spots (panel C). Plasma membrane localization of PKCα was observed in unstimulated neurons (not shown) and in neurons non responsive to BK (panel D). Scale bar: 10 μm.
Video S1. High magnification image stack and 3D reconstruction of cultured DRG neurons responsive and non‐responsive to BK, stained for PKCε. Translocation of PKCε in BK‐responsive neurons occurs uniformly to the whole surface of the plasma membrane, including neurites.
Attached file: PKCepsilon membrane translocation in sensory neurons.mp4
ACKNOWLEDGEMENTS
This work was supported by Rottapharm Biotech (Monza, Italy), by Ministero dell'Università e della Ricerca Scientifica e Tecnologica (Rome, Italy), and by Consorzio Futuro in Ricerca (Ferrara, Italy). Imaging facilities in the VV lab were provided by Fondazione Cassa di Risparmio di Modena and Fondazione Cassa di Risparmio di Carpi (Italy).
Vellani V, Sabatini C, Milia C, et al. CR4056, a powerful analgesic imidazoline‐2 receptor ligand, inhibits the inflammation‐induced PKCε phosphorylation and membrane translocation in sensory neurons. Br J Pharmacol. 2020;177:48–64. 10.1111/bph.14845
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … Collaborators, C. G. T. P. (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford S. C., … Collaborators . (2018). Goals and practicalities of immunoblottingand immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley, K. O. , Messing, R. O. , Mochly‐Rosen, D. , & Levine, J. D. (2000). Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. The Journal of Neuroscience, 20, 4680–4685. 10.1523/JNEUROSCI.20-12-04680.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aricioglu, F. , Korcegez, E. , Bozkurt, A.with the recommendations made , & Ozyalcin, S. (2003). Effect of agmatine on acute and mononeuropathic pain. Annals of the new York Academy of Sciences, 1009, 106–115. 10.1196/annals.1304.010 [DOI] [PubMed] [Google Scholar]
- Bektas, N. , Nemutlu, D. , & Arslan, R. (2015). The imidazoline receptors and ligands in pain modulation. Indian Journal of Pharmacology, 47, 472–478. 10.4103/0253-7613.165196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronat, M. A. , Olmos, G. , & Garcia‐Sevilla, J. A. (1998). Attenuation of tolerance to opioid‐induced antinociception and protection against morphine‐induced decrease of neurofilament proteins by idazoxan and other I2‐imidazoline ligands. British Journal of Pharmacology, 125, 175–185. 10.1038/sj.bjp.0702031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet, P. (2000). Identification and characterization of I1 imidazoline receptors: Their role in blood pressure regulation. American Journal of Hypertension, 13, 84S–88S. 10.1016/S0895-7061(00)00223-5 [DOI] [PubMed] [Google Scholar]
- Carpene, C. , Collon, P. , Remaury, A. , Cordi, A. , Hudson, A. , Nutt, D. , … Lafontan, M. (1995). Inhibition of amine oxidase activity by derivatives that recognize imidazoline I2 sites. The Journal of Pharmacology and Experimental Therapeutics, 272, 681–688. [PubMed] [Google Scholar]
- Cesare, P. , Dekker, L. V. , Sardini, A. , Parker, P. J. , & McNaughton, P. A. (1999). Specific involvement of PKC‐ε in sensitization of the neuronal response to painful heat. Neuron, 23, 617–624. 10.1016/S0896-6273(00)80813-2 [DOI] [PubMed] [Google Scholar]
- Cesare, P. , Moriondo, A. , Vellani, V. , & McNaughton, P. A. (1999). Ion channels gated by heat. Proceedings of the National Academy of Sciences of the United States of America, 96, 7658–7663. 10.1073/pnas.96.14.7658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. J. , Klann, E. , Gower, M. C. , Powell, C. M. , Sessoms, J. S. , & Sweatt, J. D. (1993). Studies with synthetic peptide substrates derived from the neuronal protein neurogranin reveal structural determinants of potency and selectivity for protein kinase C. Biochemistry, 32, 1032–1039. 10.1021/bi00055a006 [DOI] [PubMed] [Google Scholar]
- Comi, E. , Lanza, M. , Ferrari, F. , Mauri, V. , Caselli, G. , & Rovati, L. C. (2017). Efficacy of CR4056, a first‐in‐class imidazoline‐2 analgesic drug, in comparison with naproxen in two rat models of osteoarthritis. Journal of Pain Research, 10, 1033–1043. 10.2147/JPR.S132026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A ., … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant, S. , Eldar‐Geva, T. , & Atlas, D. (1992). Imidazoline binding sites in human placenta: Evidence for heterogeneity and a search for physiological function. British Journal of Pharmacology, 106, 101–108. 10.1111/j.1476-5381.1992.tb14300.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina, O. A. , Barletta, J. , Chen, X. J. , Mutero, A. , Martin, A. , Messing, R. O. , … Levine, J. D. (2000). Key role for the epsilon isoform of protein kinase C in painful alcoholic neuropathy in the rat. Journal of Neuroscience, 20, 8614–8619. 10.1523/JNEUROSCI.20-22-08614.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina, O. A. , Chen, X. , Reichling, D. , & Levine, J. D. (2001). Role of protein kinase Cε and protein kinase A in a model of paclitaxel‐induced painful peripheral neuropathy in the rat. Neuroscience, 108, 507–515. 10.1016/S0306-4522(01)00425-0 [DOI] [PubMed] [Google Scholar]
- Dina, O. A. , Levine, J. D. , & Green, P. G. (2008). Muscle inflammation induces a protein kinase Cε‐dependent chronic‐latent muscle pain. Journal of Pain, 9, 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks, C. A. , Schreiber, K. L. , Brewer, K. L. , Yu, C. G. , Stone, L. S. , Kitto, K. F. , … Wilcox, G. L. (2000). Agmatine reverses pain induced by inflammation, neuropathy, and spinal cord injury. Proceedings of the National Academy of Sciences of the United States of America, 97, 10584–10589. 10.1073/pnas.97.19.10584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari, F. , Fiorentino, S. , Mennuni, L. , Garofalo, P. , Letari, O. , Mandelli, S. , … Caselli, G. (2011). Analgesic efficacy of CR4056, a novel imidazoline‐2 receptor ligand, in rat models of inflammatory and neuropathic pain. Journal of Pain Research, 4, 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Sevilla, J. A. , Escriba, P. V. , & Guimon, J. (1999). Imidazoline receptors and human brain disorders. Annals of the new York Academy of Sciences, 881, 392–409. 10.1111/j.1749-6632.1999.tb09388.x [DOI] [PubMed] [Google Scholar]
- Gentili, F. , Cardinaletti, C. , Carrieri, A. , Ghelfi, F. , Mattioli, L. , Perfumi, M. , … Pigini, M. (2006). Involvement of I2‐imidazoline binding sites in positive and negative morphine analgesia modulatory effects. European Journal of Pharmacology, 553, 73–81. 10.1016/j.ejphar.2006.09.031 [DOI] [PubMed] [Google Scholar]
- George, C. H. , Stanford, S. C. , Alexander, S. , Cirino, G. , Docherty, J. R. , Giembycz, M. A. , … Ahluwalia, A. (2017). Updating the guidelines for data transparency in the British Journal of Pharmacology—Data sharing and the use of scatter plots instead of bar charts. British Journal of Pharmacology, 174, 2801–2804. 10.1111/bph.13925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaris, A. , & Piletz, J. E. (2003). Relevance of imidazoline receptors and agmatine to psychiatry: A decade of progress. Annals of the new York Academy of Sciences, 1009, 1–20. 10.1196/annals.1304.001 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, A. , Smith, D. J. , Cendron, L. , Zanotti, G. , Rigo, A. , & Di Paolo, M. L. (2008). Multiple binding sites for substrates and modulators of semicarbazide‐sensitive amine oxidases: Kinetic consequences. Molecular Pharmacology, 73, 525–538. 10.1124/mol.107.040964 [DOI] [PubMed] [Google Scholar]
- Hurley, J. H. , Newton, A. C. , Parker, P. J. , Blumberg, P. M. , & Nishizuka, Y. (1997). Taxonomy and function of C1 protein kinase C homology domains. Protein Science, 6, 477–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar, S. G. , Lin, Y. H. , Martin, A. , Dadgar, J. , McMahon, T. , Wang, D. , … Messing, R. O. (1999). A novel nociceptor signaling pathway revealed in protein kinase C ε mutant mice. Neuron, 24, 253–260. 10.1016/S0896-6273(00)80837-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, A. , Tyacke, R. J. , Robinson, J. J. , Husbands, S. M. , Minchin, M. C. , Nutt, D. J. , … Hudson, A. L. (2009). Identification of an imidazoline binding protein: Creatine kinase and an imidazoline‐2 binding site. Brain Research, 1279, 21–28. 10.1016/j.brainres.2009.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza, M. , Ferrari, F. , Menghetti, I. , Tremolada, D. , & Caselli, G. (2014). Modulation of imidazoline I2 binding sites by CR4056 relieves postoperative hyperalgesia in male and female rats. British Journal of Pharmacology, 171, 3693–3701. 10.1111/bph.12728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. X. (2017). Imidazoline I2 receptors: An update. Pharmacology & Therapeutics, 178, 48–56. 10.1016/j.pharmthera.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. X. , & Zhang, Y. (2011). Imidazoline I2 receptors: Target for new analgesics? European Journal of Pharmacology, 658, 49–56. 10.1016/j.ejphar.2011.02.038 [DOI] [PubMed] [Google Scholar]
- Mathiesen, O. , Moiniche, S. , & Dahl, J. B. (2007). Gabapentin and postoperative pain: A qualitative and quantitative systematic review, with focus on procedure. BMC Anesthesiology, 7, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, G. R. , Olivieri, A. , Ramsay, R. R. , & Holt, A. (2010). On the formation and nature of the imidazoline I2 binding site on human monoamine oxidase‐B. Pharmacological Research, 62, 475–488. 10.1016/j.phrs.2010.09.001 [DOI] [PubMed] [Google Scholar]
- McGrath, J. C. , Drummond, G. B. , McLachlan, E. M. , Kilkenny, C. , & Wainwright, C. L. (2010). Guidelines for reporting experiments involving animals: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1573–1576. 10.1111/j.1476-5381.2010.00873.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meregalli, C. , Ceresa, C. , Canta, A. , Carozzi, V. A. , Chiorazzi, A. , Sala, B. , … Cavaletti, G. (2012). CR4056, a new analgesic I2 ligand, is highly effective against bortezomib‐induced painful neuropathy in rats. Journal of Pain Research, 5, 151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, R. A. , Wiffen, P. J. , Derry, S. , & Mcquay, H. J. (2011). Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews, 16, CD007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazaki, M. , Tominaga, T. , Toyooka, H. , & Tominaga, M. (2002). Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cε and identification of two target serine residues. The Journal of Biological Chemistry, 277, 13375–13378. 10.1074/jbc.C200104200 [DOI] [PubMed] [Google Scholar]
- Ozaita, A. , Olmos, G. , Boronat, M. A. , Lizcano, J. M. , Unzeta, M. , & Garcia‐Sevilla, J. A. (1997). Inhibition of monoamine oxidase A and B activities by imidazol(ine)/guanidine drugs, nature of the interaction and distinction from I2‐imidazoline receptors in rat liver. British Journal of Pharmacology, 121, 901–912. 10.1038/sj.bjp.0701214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh, D. B. , Ziegler, W. , & Parker, P. J. (2000). Multiple pathways control protein kinase C phosphorylation. The EMBO Journal, 19, 496–503. 10.1093/emboj/19.4.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piletz, J. E. , Ivanov, T. R. , Sharp, J. D. , Ernsberger, P. , Chang, C. H. , Pickard, R. T. , … Reis, D. J. (2000). Imidazoline receptor antisera‐selected (IRAS) cDNA: Cloning and characterization. DNA and Cell Biology, 19, 319–329. 10.1089/10445490050043290 [DOI] [PubMed] [Google Scholar]
- Qiu, Y. , He, X. H. , Zhang, Y. , & Li, J. X. (2014). Discriminative stimulus effects of the novel imidazoline I2 receptor ligand CR4056 in rats. Scientific Reports, 4, 6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, Y. , Thorn, D. A. , Zhang, Y. , He, X. , & Li, J. X. (2014). Behavioral effects of the imidazoline I2 receptor ligand BU99006 in rats. Behavioural Pharmacology, 25, 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, Y. , Zhang, Y. , & Li, J. X. (2015). Discriminative stimulus effects of the imidazoline I2 receptor ligands BU224 and phenyzoline in rats. European Journal of Pharmacology, 749, 133–141. 10.1016/j.ejphar.2015.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Blazquez, P. , Boronat, M. A. , Olmos, G. , Garcia‐Sevilla, J. A. , & Garzon, J. (2000). Activation of I2‐imidazoline receptors enhances supraspinal morphine analgesia in mice: A model to detect agonist and antagonist activities at these receptors. British Journal of Pharmacology, 130, 146–152. 10.1038/sj.bjp.0703294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumilla, J. A. , Liron, T. , Mochly‐Rosen, D. , Kendig, J. J. , & Sweitzer, S. M. (2005). Ethanol withdrawal‐associated allodynia and hyperalgesia: Age‐dependent regulation by protein kinase Cε and γ isoenzymes. The Journal of Pain, 6, 535–549. [DOI] [PubMed] [Google Scholar]
- Siemian, J. N. , Wang, K. , Zhang, Y. , & Li, J. X. (2018). Mechanisms of imidazoline I2 receptor agonist‐induced antinociception in rats: Involvement of monoaminergic neurotransmission. British Journal of Pharmacology, 175, 1519–1534. 10.1111/bph.14161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg, S. F. (2008). Structural basis of protein kinase C isoform function. Physiological Reviews, 88, 1341–1378. 10.1152/physrev.00034.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summer, G. J. , Puntillo, K. A. , Miaskowski, C. , Dina, O. A. , Green, P. G. , & Levine, J. D. (2006). TrkA and PKC‐epsilon in thermal burn‐induced mechanical hyperalgesia in the rat. The Journal of Pain, 7, 884–891. [DOI] [PubMed] [Google Scholar]
- Tesson, F. , Limon‐Boulez, I. , Urban, P. , Puype, M. , Vandekerckhove, J. , Coupry, I. , … Parini, A. (1995). Localization of I2‐imidazoline binding sites on monoamine oxidases. The Journal of Biological Chemistry, 270, 9856–9861. 10.1074/jbc.270.17.9856 [DOI] [PubMed] [Google Scholar]
- Vaux, D. L. , Fidler, F. , & Cumming, G. (2012). Replicates and repeats‐what is the difference and is it significant? A brief discussion of statistics and experimental design. EMBO Reports, 13, 291–296. 10.1038/embor.2012.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani, V. , Colucci, M. , Lattanzi, R. , Giannini, E. , Negri, L. , Melchiorri, P. , … McNaughton, P. A. (2006). Sensitization of transient receptor potential vanilloid 1 by the prokineticin receptor agonist Bv8. Journal of Neuroscience, 26, 5109–5116. 10.1523/JNEUROSCI.3870-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani, V. , Franchi, S. , Prandini, M. , Moretti, S. , Castelli, M. , Giacomoni, C. , … Sacerdote, P. (2013). Effects of NSAIDs and paracetamol (acetaminophen) on protein kinase C epsilon translocation and on substance P synthesis and release in cultured sensory neurons. Journal of Pain Research, 6, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani, V. , Franchi, S. , Prandini, M. , Moretti, S. , Pavesi, G. , Giacomoni, C. , … Sacerdote, P. (2011). Nimesulide inhibits protein kinase C epsilon and substance P in sensory neurons—Comparison with paracetamol. Journal of Pain Research, 4, 177–187. 10.2147/JPR.S21931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani, V. , & Giacomoni, C. (2017). Gabapentin inhibits protein kinase C epsilon translocation in cultured sensory neurons with additive effects when coapplied with paracetamol (acetaminophen). ScientificWorldJournal, 2017, 3595903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani, V. , Kinsey, A. M. , Prandini, M. , Hechtfischer, S. C. , Reeh, P. , Magherini, P. C. , … McNaughton, P. A. (2010). Protease activated receptors 1 and 4 sensitize TRPV1 in nociceptive neurones. Molecular Pain, 6, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani, V. , Mapplebeck, S. , Moriondo, A. , Davis, J. B. , & McNaughton, P. A. (2001). Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. The Journal of Physiology, 534, 813–825. 10.1111/j.1469-7793.2001.00813.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani, V. , & McNaughton, P. A. (2004). Involvement of PKC in the sensation of pain In Dekker L. V. (Ed.), Protein kinase C (Second ed.) (pp. 134–146). New York: Kluwer Academic/Plenum Publisher, Landes Bioscience. [Google Scholar]
- Vellani, V. , Prandini, M. , Giacomoni, C. , Pavesi, G. , Ravegnani, L. , & Magherini, P. C. (2011). Functional endothelin receptors are selectively expressed in isolectin B4‐negative sensory neurons and are upregulated in isolectin B4‐positive neurons by neurturin and glia‐derived neurotropic factor. Brain Research, 1381, 31–37. 10.1016/j.brainres.2011.01.019 [DOI] [PubMed] [Google Scholar]
- Vellani, V. , Zachrisson, O. , & McNaughton, P. A. (2004). Functional bradykinin B1 receptors are expressed in nociceptive neurones and are upregulated by the neurotrophin GDNF. The Journal of Physiology, 560, 391–401. 10.1113/jphysiol.2004.067462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiffen, P. J. , Derry, S. , Moore, R. A. , Aldington, D. , Cole, P. , Rice, A. S. , … Kalso, E. A. (2013). Antiepileptic drugs for neuropathic pain and fibromyalgia—An overview of Cochrane reviews. The Cochrane Database of Systematic Reviews, 11, CD010567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ximenes, J. C. , de Oliveira, G. D. , Siqueira, R. M. , Neves, K. R. , Santos, C. G. , Correia, A. O. , … Viana, G. S. (2013). Valproic acid: An anticonvulsant drug with potent antinociceptive and anti‐inflammatory properties. Naunyn‐Schmiedeberg's Archives of Pharmacology, 386, 575–587. 10.1007/s00210-013-0853-4 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. B. , Guo, Z. D. , Li, M. Y. , Fong, P. , Zhang, J. G. , Zhang, C. W. , … Lv, G. W. (2015). Gabapentin effects on PKC‐ERK1/2 signaling in the spinal cord of rats with formalin‐induced visceral inflammatory pain. PLoS ONE, 10(10), e0141142 10.1371/journal.pone.0141142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Li, G. D. , & Zhao, Z. Q. (2003). State‐dependent phosphorylation of ε‐isozyme of protein kinase C in adult rat dorsal root ganglia after inflammation and nerve injury. Journal of Neurochemistry, 85, 571–580. 10.1046/j.1471-4159.2003.01675.x [DOI] [PubMed] [Google Scholar]
- Zimmermann, M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain, 16, 109–110. 10.1016/0304-3959(83)90201-4 [DOI] [PubMed] [Google Scholar]
- McGrath, J. C. , & Lilley, E. (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. British Journal of Pharmacology, 172(13), 3189–3193. 10.1111/bph.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling, D. B. , & Levine, J. D. (2009). Critical role of nociceptor plasticity in chronic pain. Trends in Neurosciences, 32(12), 611–618. 10.1016/j.tins.2009.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovati, L. C. , Brambilla, N. , Blicharski, T. , Connell, J. , Vitalini, C. , Bonazzi, A. , … D'Amato, M. (2019). Efficacy and safety of the first‐in‐classimidazoline‐2 receptor ligand CR4056 in pain from knee osteoarthritis and diseasephenotypes: a randomized, double‐blind, placebo‐controlled phase 2 trial. Osteoarthritis and Cartilage, in press. 10.1016/j.tins.2009.07.007doi:10.1016/j.joca.2019.09.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
I cannot see the correct figure hereFigure S1. PKCα and PKCε localization in DRG neurons treated with bradykinin
In neurons where BK caused translocation of PKCε (green), PKCα (red) could either be translocated to the membrane and in intracellular sites (A); be diffused in the cytoplasm and absent from the plasma membrane (B); or located in intracellular spots (panel C). Plasma membrane localization of PKCα was observed in unstimulated neurons (not shown) and in neurons non responsive to BK (panel D). Scale bar: 10 μm.
Video S1. High magnification image stack and 3D reconstruction of cultured DRG neurons responsive and non‐responsive to BK, stained for PKCε. Translocation of PKCε in BK‐responsive neurons occurs uniformly to the whole surface of the plasma membrane, including neurites.
Attached file: PKCepsilon membrane translocation in sensory neurons.mp4


