Graphical abstract
Keywords: Cryptococcus, Acute myeloid leukemia, Skeletal infection
Highlights
-
•
Cryptococcus-related infection developed after tooth extraction.
-
•
Cryptococcal infection of mandible is rare.
-
•
Surgical treatment was performed under poor general conditions.
Abstract
Cryptococcus is a mycosis founded in immunocompromised patients. Cryptococcus in the oral cavity is rare and skeletal infection is uncommon.
We report the case of a 31-year-old man in whom cellulitis developed due to infection after tooth extraction complicated by acute myeloid leukemia (AML). Cellulitis of the left mandible did not improve after conservative therapy, including antimicrobial therapy, because of AML and chemotherapy, and gas was generated in the left cervical and supraclavicular regions. We considered the infection symptoms to be life-threatening, and surgery was performed for the infection of the head and neck under poor general conditions. As histopathological examination of the removed tissue revealed cryptococcus, antifungal agents were administered for cryptococcal infection. The surgical site healed after the operation.
Surgical treatment, including debridement and drainage, should be avoided for patients with a poor general condition caused by AML and chemotherapy. However, the detection of Cryptococcus in the surgical site in such a condition is important.
Introduction
Cryptococcosis is common in patients with malignant disease and HIV infection [1,2]. However, detection of Cryptococcus infection in the oral cavity is rare and skeletal infection is uncommon [1,3]. We performed debridement and drainage for skeletal infection after third molar extraction in an acute myeloid leukemia (AML) patient, after which Cryptococcus was detected in the removed tissue.
Case
A 31-year-old male presented to the emergency department of our hospital with pain, swelling and redness on left side of his face and respiratory difficulty. He had undergone extraction of the left mandibular third molar at another hospital 1 week prior to presentation. He had no history of illness.
His maximum temperature was 37.8 °C. Laboratory tests revealed a hemoglobin concentration of 8.0 g/dl, white blood cell count of 11.984 × 104/μl, absolute neutrophil count of 0/μl, myeloblast advantage of 98.5%, blood platelet count of 1.2 × 104/μl, and C-reactive protein concentration of 38.0 mg/dl. Contrast-enhanced computed tomography (CT) imaging of the head and neck demonstrated accentuated permeability around his left mandible.
We diagnosed cellulitis due to infection after tooth extraction complicated by AML and he was immediately hospitalized. Antimicrobial therapy using doripenem was initiated because of severe cellulitis and decrease in the neutrophil count on day 1 of hospitalization. Thereafter, remission induction therapy using idarubicin and cytosine arabinoside was performed at the hematology department because leukemic infiltration of the lungs due to AML was advanced on day 3 of hospitalization. Transfusions of red blood cells, platelets and fresh frozen plasma were frequently performed for anemia, decrease in the blood platelet count and fibrinolytic system hyperactivity due to AML and disseminated intravascular coagulation. Further administration of teicoplanin as an anti-MRSA (methicillin-resistant Staphylococcus aureus) drug and micafungin as an antifungal agent was started. Although myeloblasts disappeared from the peripheral blood two weeks after starting the remission induction therapy, the cellulitis did not improve by conservative therapy. Moreover, contrast-enhanced CT revealed gas in the left cervical and supraclavicular regions on day 17 of hospitalization (Fig. 1A). Following consultation with the otolaryngology and hematology departments, we selected surgical treatment after repeated red blood cell and platelet transfusions because the infection symptoms of the head and neck were considered life-threatening on day 20 of hospitalization.
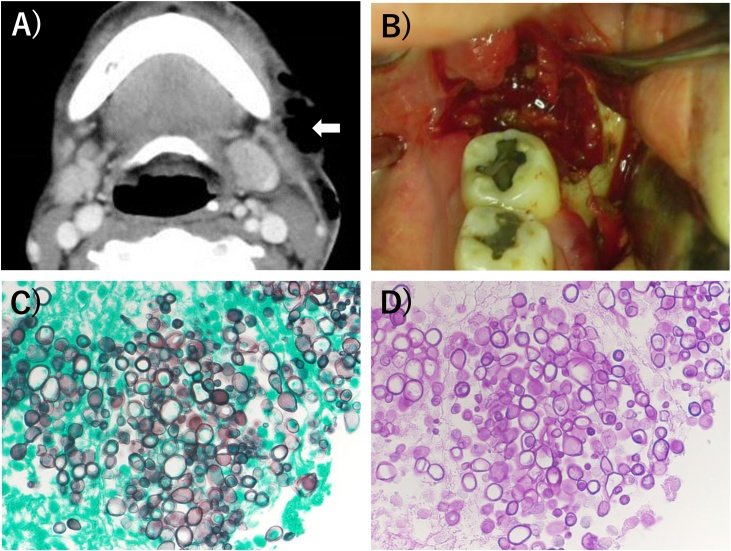
Fig. 1.
(A) Contrast-enhanced computed tomography (CT) imaging showed gas in the left cervical and supraclavicular regions (arrow). (B) The infected granulation tissue, necrotic tissue and irregular bone in surgical treatment. (C) Periodic Acid Schiff (PAS) staining for examination of the removed bone (×400). (D) Grocott staining for examination of the removed bone (×400).
Our department was in charge of the oral cavity, and the otolaryngology department was in charge of the left cervical and supraclavicular regions during anti-inflammatory surgery under general anesthesia. We removed as much of the infected granulation tissue, necrotic tissue and irregular bone-like sequestrum as possible in the region of the left mandibular third molar (Fig. 1B). On histopathological examination of the removed bone by periodic acid-Schiff staining and Grocott staining, Cryptococcus was observed as oval capsules around the fragments of non-vital bone (Fig. 1C and D).
Micafungin was changed to liposomal amphotericin B (3−4 mg/kg per day) as the antifungal agent because of yeast-like fungi growing on the day of surgery. As induction therapy for cryptococcal infection, liposomal amphotericin B plus flucytosine (6000 mg per day orally) was administered for 1 week, followed by consolidation therapy with fluconazole (400 mg per day orally) for 6 weeks. After induction and consolidation therapies, maintenance therapy with fluconazole (200 mg per day orally) was continued for 6 months.
The surgical site in the oral cavity healed, although there was scarring.
Discussion
Cryptococcus is a representative mycosis characterized as an encapsulated yeast fungus [1]. The infection spreads through the blood stream following inhalation of the spores [1]. Although cell-mediated immunity is markedly low, it is possible for Cryptococcus to enter the circulation and cause disseminated disease [1]. In reports between 1970 and 2014, hematological malignancies accounted for 82% of the cases of cryptococcal infection in the setting of malignancy [2]. According to US national cancer statistics (2008–2012), leukemia accounted for 32% of hematological malignancies [2]. Detection of Cryptococcus in the oral cavity is rare in non-HIV patients, but there are some reports of oral cryptococcal infections in the palate, maxilla and tongue [[3], [4], [5], [6], [7], [8]]. To our knowledge, this is the first report of Cryptococcus-related infection after tooth extraction.
Cryptococcus is diagnosed by organizational identification from samples collected from bronchoalveolar lavage fluid, sputum or cerebrospinal fluid, or biopsy of cutaneous or subcutaneous diseases such as cutaneous lesions [[9], [10], [11]]. Isolation of skeletal cryptococcal infections is rare. In this case, we performed anti-inflammatory surgery, and excluded the possibility that the irregular tissue and bone in the region of the left mandibular third molar was malignant. Therefore, the removed tissues were submitted to the pathology department during surgery and Cryptococcus was found. Although no increase in β-d-glucan was observed in the serum, cryptococcal antigen was not detected in the serum after the detection of Cryptococcus by histopathological examination, and fungal identification of Cryptococcus was not possible in the lavage fluid from the wound after the operation.
Cryptococcal infection of bone is rare [1]. Cryptococcal infections may develop in any region of bone, and disease of the vertebrae, pelvis and ribs is common [[12], [13], [14]]. As there are few reports of cryptococcal osteomyelitis, it is unclear whether debridement or only medical management should be performed [5].
Hematological malignancies, such as leukemia and lymphoma, were reported to be risk factors [1]. Although cryptococcal infection of the mandible may develop via the blood system from an initial pulmonary source, it is possible that the infection spread through the skin and neighboring tissues. In addition, the oral cavity is the first target of infection in immunocompromised patients, resulting in osteomyelitis and cellulitis after tooth extraction, as in this case [9].
Radiography findings are non-specific, and bone resorption with or without periosteal reactions is characteristic [1,15]. Periosteal reactions were not visible on radiography in our patient. The radiological features were difficult to diagnose because of the extraction socket. On contrast-enhanced CT imaging of the head and neck, it was difficult to confirm infection related to Cryptococcus because of other infections and malignant disease. The diagnosis of Cryptococcus infection was made by tissue identification in the region of the left mandibular third molar.
Surgical treatment for patients with a poor general condition should be avoided. However, in this case, it enabled the detection of Cryptococcus in a sample from the surgical site.
Declarations of interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Approval was not required.
Consent
The patient was dead and consent of the patient's family to publish the case report has not been obtained. I called the patient's family and attempted to obtain consent to publish the case report, however it was not possible. Therefore, I plan to withdraw my submission if consent of the patient's family is required in this case.
Author contribution
Hiroshi Inoue designed the report, and wrote the initial draft of the manuscript. Tomokazu Motohashi assisted in the preparation of the manuscript. All other authors have contributed to data collection and interpretation, and critically reviewed the manuscript. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
We are grateful to Dr. Takaya Mitsuyoshi, Dr. Takao Yoshida and Dr. Kazunori Imada of Osaka Red Cross Hospital for helpful discussions and comments, and Dr. Toshihide Shimada of Osaka Red Cross Hospital for reviewing and reporting the histopathology.
Contributor Information
Hiroshi Inoue, Email: hiro7536@hotmail.co.jp.
Tomokazu Motohashi, Email: motohashi@cc.osaka-dent.ac.jp.
Yusuke Ioku, Email: ioku2003jp@yahoo.co.jp.
Masahiro Watanabe, Email: watanabe-m@osaka-med.jrc.or.jp.
Masahiro Nakajima, Email: masa-na@cc.osaka-dent.ac.jp.
Mitsuchika Sugitatsu, Email: sugitatsu-m@osaka-med.jrc.or.jp.
References
- 1.Ramkillawan Y., Dawood H., Ferreira N. Isolated cryptococcal osteomyelitis in an immune-competent host: a case report. Int J Infect Dis. 2013;17 doi: 10.1016/j.ijid.2013.04.013. e1229-31. [DOI] [PubMed] [Google Scholar]
- 2.Schmalzle S.A., Buchwald U.K., Gilliam B.L., Riedel D.J. Cryptococcus neoformans infection in malignancy. Mycoses. 2016;59:542–552. doi: 10.1111/myc.12496. [DOI] [PubMed] [Google Scholar]
- 3.Patel S., Navas M., Batt C., Jump R.L. Oral Cryptococcosis in a patient with chronic lymphocytic leukemia. Int J Infect Dis. 2016;50:18–20. doi: 10.1016/j.ijid.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiNardo A.R., Schmidt D., Mitchell A., Kaufman Y., Tweardy D.J. First description of oral Cryptococcus neoformans causing osteomyelitis of the mandible, manubrium and third rib with associated soft tissue abscesses in an immunocompetent host. Clin Microbiol Case Rep. 2015;1(3):017. [PMC free article] [PubMed] [Google Scholar]
- 5.Glick M., Cohen S.G., Cheney R.T., Crooks G.W., Greenberg M.S. Oral manifestations of disseminated Cryptococcus neoformans in a patient with acquired immunodeficiency syndrome. Oral Surg Oral Med Oral Pathol. 1987;64(4):454–459. doi: 10.1016/0030-4220(87)90152-6. [DOI] [PubMed] [Google Scholar]
- 6.Tzerbos F.1, Kabani S., Booth D. Cryptococcosis as an exclusive oral presentation. J Oral Maxillofac Surg. 1992;50(7):759–760. doi: 10.1016/0278-2391(92)90115-g. [DOI] [PubMed] [Google Scholar]
- 7.Dodson T.B., Perrott D.H., Leonard M.S. Nonhealing ulceration of oral mucosa. J Oral Maxillofac Surg. 1989;47(8):849–852. doi: 10.1016/s0278-2391(89)80045-x. [DOI] [PubMed] [Google Scholar]
- 8.Lynch D.P., Naftolin L.Z. Oral Cryptococcus neoformans infection in AIDS. Oral Surg Oral Med Oral Pathol. 1987;64(4):449–453. doi: 10.1016/0030-4220(87)90151-4. [DOI] [PubMed] [Google Scholar]
- 9.Iatta R., Napoli C., Borghi E., Montagna M.T. Rare mycoses of the oral cavity: a literature epidemiologic review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):647–655. doi: 10.1016/j.tripleo.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Armonda R.A., Fleckenstein J.M., Brandvold B., Ondra S.L. Cryptococcal skull infection: a case report with review of the literature. Neurosurgery. 1993;32(6):1034–1036. doi: 10.1227/00006123-199306000-00028. [DOI] [PubMed] [Google Scholar]
- 11.Meredith H.C., John J.F., Jr, Rogers C.I., Gooneratne N., Kreutner A., Jr Case report 89. Skeletal Radiol. 1979;10(1):53–55. doi: 10.1007/BF00350599. [DOI] [PubMed] [Google Scholar]
- 12.Chleboun J., Nade S. Skeletal cryptococcosis. J Bone Jt Surg Am. 1977;59(4):509–514. [PubMed] [Google Scholar]
- 13.Behrman R.E., Masci J.R., Nicholas P. Cryptococcal skeletal infections: case report and review. Rev Infect Dis. 1990;12(2):181–190. doi: 10.1093/clinids/12.2.181. [DOI] [PubMed] [Google Scholar]
- 14.Wood L., Miedzinski L. Skeletal cryptococcosis: case report and review of the literature. Can J Infect Dis. 1996;7(2):125–132. doi: 10.1155/1996/102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qadir I., Ali F., Malik U.Z., Umer M. Isolated cryptococcal osteomyelitis in an immunocompetent patient. J Infect Dev Ctries. 2011;5(9):669–673. doi: 10.3855/jidc.1352. [DOI] [PubMed] [Google Scholar]