The authors of Murillo‐Carretero et al. (2017) have supplied the following correction to their article.
There is an error that affects the structure of one of the non‐active products described in the article, the so‐called EOF2, which we described as 7,8,12‐tri‐O‐acetyl‐3‐O‐(4‐methoxyphenyl)acetylingol (CAS number: 944799‐47‐7). Chemical transformations on the compound used for the work led us to conclude that the compound described as EOF2 is actually 3,8,12‐tri‐O‐acetyl‐7‐O‐(4‐methoxyphenyl)acetylingol (CAS number: 2230806‐06‐9). This error does not affect the results and conclusions presented in the article.
The corrected structure from Figure 1 is shown below.
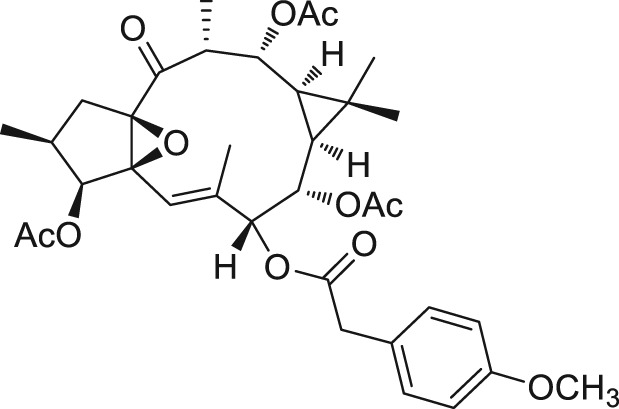
The authors apologize for the errors and any inconvenience it may have caused.
CORRECTION. Br J Pharmacol. 2020;177:235–235. 10.1111/bph.14944
REFERENCE
- Murillo‐Carretero, M. , Geribaldi‐Doldán, N. , Flores‐Giubi, E. , García‐Bernal, F. , Navarro‐Quiroz, E. A. , Carrasco, M. , … Castro, C. (2017). ELAC (3,12‐di‐O‐acetyl‐8‐O‐tigloilingol), a plant‐derived lathyrane diterpene, induces subventricular zone neural progenitor cell proliferation through PKCβ activation. British Journal of Pharmacology, 174(14), 2373–2392. 10.1111/bph.13846 [DOI] [PMC free article] [PubMed] [Google Scholar]


