Abstract
Background and Purpose
Between half to 1 million people die annually from malaria. Anopheles gambiae mosquitoes are major malaria vectors. Unfortunately, resistance has emerged to the agents currently used to control A. gambiae, creating a demand for novel control measures. The pentameric glutamate‐gated chloride channel (GluCl) expressed in the muscle and nerve cells of these organisms are a potentially important biological target for malaria control. The pharmacological properties of Anophiline GluCl receptors are, however, largely unknown. Accordingly, we compared the efficacy of four insecticides (lindane, fipronil, picrotoxin, and ivermectin) on two A. gambiae GluCl receptor splice variants with the aim of providing a molecular basis for designing novel anti‐malaria treatments.
Experimental Approach
The A. gambiae GluCl receptor b1 and c splice variants were expressed homomerically in Xenopus laevis oocytes and studied with electrophysiological techniques, using two‐electrode voltage‐clamp.
Key Results
The b1 and c GluCl receptors were activated with similar potencies by glutamate and ivermectin. Fipronil was more potent than picrotoxin and lindane at inhibiting glutamate‐ and ivermectin‐gated currents. Importantly, b1 GluCl receptors exhibited reduced sensitivity to picrotoxin and lindane. They also recovered from these effects to a greater extent than c GluCl receptors
Conclusions and Implications
The two splice variant subunits exhibited differential sensitivities to multiple, structurally divergent insecticides, without accompanying changes in the sensitivity to the endogenous neurotransmitter, glutamate, implying that drug resistance may be caused by alterations in relative subunit expression levels, without affecting physiological function. Our results strongly suggest that it should be feasible to develop novel subunit‐specific pharmacological agents.
Abbreviations
- FIP
fipronil
- GluCl
glutamate‐gated chloride channel
- IVM
ivermectin
- LND
lindane
- pLGIC
pentameric ligand‐gated ion channel
- PTX
picrotoxin
1.
What is already known
Malaria is transmitted by the mosquito Anopheles gambiae.
A major target of insecticides is the glutamate‐gated chloride channel, found at inhibitory synapses.
What this study adds
Structurally different insecticides were tested at splice isoforms of glutamate‐gated chloride channels of A. gambiae.
The four tested insecticides exhibited isoform‐specific potencies.
What is the clinical significance
The different drug sensitivities are attributable to elements of the channels that mediate channel activation.
Expression of splice isoforms of glutamate‐gated chloride channels may confer insecticide resistance to A. gambiae.
2. INTRODUCTION
Anopheles is a genus of mosquitoes comprising over 450 species. More than 40 of these are efficient vectors of malaria parasites in humans, the most efficient being Anopheles gambiae (Phillips et al., 2017). A. gambiae is endemic to tropical and sub‐tropical regions of sub‐Saharan Africa, where it is responsible for over 90% of the world's malaria deaths, most of which are children (Ashley, Pyae Phyo, & Woodrow, 2018; Phillips et al., 2017). The mosquito is long‐lived, thrives in human environments, and prefers human blood (Killeen et al., 2017; White et al., 2014). Insecticide‐treated mosquito bed nets and indoor residual spraying of pyrethroid‐based insecticides have been highly successful in malaria control programmes (Bhatt et al., 2015; Phillips et al., 2017). However, significant resistance to pyrethroids and other insecticides threatens to undermine the recent positive trends in malaria control (Hemingway et al., 2016; Phillips et al., 2017).
Ion channels are the molecular targets of many neuroactive insecticides. Pyrethroids, for instance, target https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=82 (Raymond‐Delpech, Matsuda, Sattelle, Rauh, & Sattelle, 2005) that underlie action potential generation and propagation along axons. Pentameric ligand‐gated ion channels (pLGICs), which mediate fast neurotransmission at synapses, are also major targets for insecticides (Raymond‐Delpech et al., 2005). Invertebrate pLGICs include the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=76, which is the target for neonicotinoids and spinosyns (Ihara, Buckingham, Matsuda, & Sattelle, 2017; Raymond‐Delpech et al., 2005) and the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=72 receptor that is targeted by insecticides such as cyclodienes (e.g., dieldrin), avermectins, lindane (LND), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4051 (PTX), and fipronil (FIP; Buckingham, Ihara, Sattelle, & Matsuda, 2017; Raymond‐Delpech et al., 2005). Invertebrates, including A. gambiae, also express glutamate‐gated chloride channel (GluCl) receptors (Meyers et al., 2015). These receptors are anion‐selective pLGICs found at neuronal and neuromuscular inhibitory synapses (Wolstenholme, 2012) and, unlike GABAA receptors and nAChRs, are unique to invertebrates (Wolstenholme, 2012). They are high affinity targets for insecticides, including negative modulators such as FIP (Narahashi, Zhao, Ikeda, Salgado, & Yeh, 2010) and positive modulators, such as https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2373 (IVM; Atif, Estrada‐Mondragon, Nguyen, Lynch, & Keramidas, 2017; Cully et al., 1994). GluCl receptors are also sensitive to PTX (Atif et al., 2019; Cully et al., 1994; Hibbs & Gouaux, 2011) and LND (Hirata et al., 2008; Ihara, Ishida, Okuda, Ozoe, & Matsuda, 2005). However, the pharmacological properties of GluCl receptors expressed by mosquitoes are largely unknown, even though recent reports have highlighted the effectiveness of insecticides such as IVM in reducing mosquito survival and malarial burden (Alout et al., 2014).
The documented mosquitocidal efficacy of IVM (Alout et al., 2014) and FIP (Poche et al., 2017) against Anopheline mosquitoes provides an incentive for exploring their molecular targets. Both drugs exhibit sensitivities to invertebrate anion‐selective pLGICs (GluCl and GABAA receptors) that are 1–2 orders of magnitude greater than those found in vertebrate receptors (GABAA and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=73; Islam & Lynch, 2012; Li & Akk, 2008; Narahashi et al., 2010). LND and PTX are believed to act by blocking the ion pore of anion‐selective pLGICs (Hibbs & Gouaux, 2011; Islam & Lynch, 2012; Masiulis et al., 2019). Notably, mutations to pore‐lining residues that confer LND and PTX insensitivity also exhibit cross‐resistance to FIP at invertebrate GABAA receptors (resistance to dieldrin; Hosie, Baylis, Buckingham, & Sattelle, 1995) and GluCl receptors (Hirata et al., 2008), suggesting an overlapping mechanism of action. In addition, insecticides such as FIP, LND, and PTX often exhibit profound pharmacological differences between otherwise functionally similar receptors.
We selected two modulators, IVM and FIP, and two pore blockers, PTX and LND, to explore differences in the pharmacological properties of two homomeric A. gambiae GluCl receptors comprising differentially spliced variant subunits (splice variants, b1 and c, Meyers et al., 2015, Figure 1a). Our data shed light on key functional differences between these subunits and provide a molecular basis for drug design that will complement current strategies aimed at controlling this important disease vector (Benelli & Beier, 2017; Shaw & Catteruccia, 2019).
Figure 1.
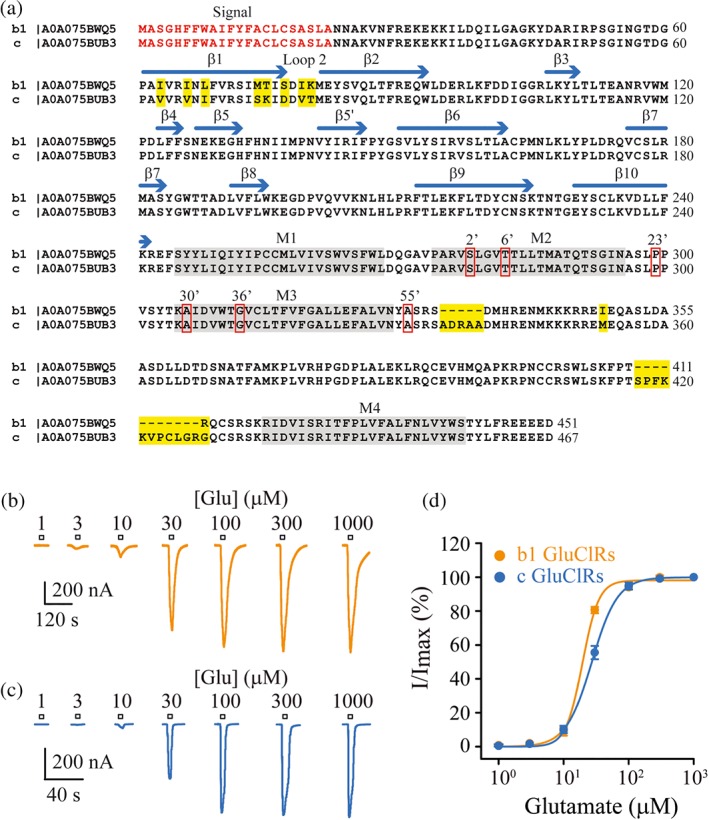
Glutamate concentration–response relationships for homomeric b1 and c GluCl receptors. (a) Sequence alignment of b1 and c subunits of Anopheles gambiae. The signal sequence is shown in red. Secondary structures are indicated by horizontal blue arrows (β‐strands) or grey shade (α‐helical transmembrane domains, M1–M4). The amino acid differences between the b1 and c subunits are shaded yellow. The differences are concentrated at the β1 strand and the loop connecting it to the β2 strand (Loop 2) and regions of the intracellular domain. Residues boxed in red have been implicated in insecticide resistance. (b, c) A series of currents from single oocytes elicited by the indicated glutamate concentrations for b1 GluCl receptors (b) and c GluCl receptors (c). The clamped membrane potential was −40 mV. (d) Concentration–response plots of group date for b1 and c receptors fitted to Hill equations. No significant difference in glutamate sensitivity was observed between the two receptors. Data points are means ± SEM
3. METHODS
3.1. Molecular biology
We will use the subunit nomenclature as found in the protein database, UniprotKB (RRID:SCR_004424). GluCl receptor subunit cDNAs encoding the b1 (UniprotKB ID, A0A075BWQ5) or c (UniprotKB ID, A0A075BUB3) subunits (both in the pUNIV vector) of A. gambiae were expressed in Xenopus laevis oocytes (NASCO, WI, USA).
3.2. Oocyte preparation
The handling and surgical procedures on X. laevis were conducted under conditions approved by the University of Queensland Animal Ethics Committee (approval number: QBI/AIBN/087/16/NHMRC/ARC). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. Mature stage V or VI X. laevis oocytes were selected, defolliculated with 1.5 mg·ml−1 collagenase for 2 hr, and rinsed with calcium‐free OR‐2 solution containing (in mM) 82.5 NaCl, 2 KCl, 1 MgCl2, 5 HEPES, pH 7.4; 42 nl at a concentration of 50 ng·μl−1 of RNA encoding the b1 or c subunits were injected into the oocytes using a Nanolitre 2000 microinjector (WPI Inc). Injected oocytes were incubated in ND96 storage solution (in mM) 96 NaCl, 2 KCl, 1 MgCl2.6H2O, 1.8 CaCl2, 5 HEPES, 50 μg ml−1 gentamicin, 2.5 sodium pyruvate, 0.5 theophylline, pH 7.4 at 16°C for 2–3 days before experimentation. Optimal expression was achieved at 4–5 days post‐injection.
3.3. Two‐electrode voltage‐clamp
Two‐electrode voltage‐clamp was performed on oocytes that were secured in a cell bath and continuously perfused with ND96 recording solution (ND96 storage solution without pyruvate, theophylline, and gentamicin). https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1369 and all test drugs were diluted in ND96 recording solution and were applied to the recorded oocyte via bath perfusion. The two microelectrodes contained 3‐M KCl and had resistances of 0.2–2 MΩ. Recordings were done using Clampex 10.2 software (Molecular Devices) at a clamped voltage of −40 mV. Currents were low‐pass filtered at 200 Hz, sampled at 2 kHz using a Gene Clamp 500B amplifier, and digitised by a Digidata 1440A interface. All experiments were carried out at room temperature (22 ± 1°C).
3.4. Test drug application protocols
The IVM (IVM B1a) concentration–response experiments were standardised by using an application protocol that consisted of successively applying increasing concentrations of IVM for, respectively, 3, 3, 3, 2, 1, 1, and 0.5 min. The IVM‐induced currents were normalised to a saturating glutamate concentration of 5 mM. The effects of LND, FIP, and PTX on IVM‐elicited currents were examined using a protocol that consisted of a 10‐min application of IVM followed by successive applications of increasing concentrations of test drug. These drug application protocols were used because IVM was not reversible over the course of the experiment (Cully et al., 1994; Lynagh & Lynch, 2010; Shan, Haddrill, & Lynch, 2001). The inhibitory effects and recovery times of FIP, LND, and PTX were tested by alternating applications of the test drug and EC50 concentrations of glutamate.
3.5. Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). Currents were normalised to glutamate‐gated controls for each oocyte to minimise variation. The oocytes were obtained from multiple surgeries from different animals throughout the project. Each n (oocyte) in a given data set represents an independent experiment obtained from a different frog. Only one drug was tested on each recorded oocyte. Because the experimenter designed the experiment for a selected drug, none of the experiments were blinded or randomised during the recording or analysis. To minimise animal use, oocytes harvested for a given frog contributed to experiments for multiple, different data sets. These data sets were designed to generate groups of equal size (n = 6), except for the glutamate concentration–response group for the c GluCl receptors (n = 7). This ensured that for an alpha of .05, an expected difference in means of 20%, and an SD of 10%, n = 6 would achieve a power of 0.8. Homogeneity of variance was confirmed using Levine's test for equality of variances in IMB SPSS Statistics 26 (RRID:SCR_002865). Statistical analysis was carried out only on data sets comprising at least n = 5. Group data are expressed as mean ± SEM and were analysed for significance using unpaired t tests or repeated measures ANOVA for the time course of recovery experiments in SigmaPlot 13.0 (RRID:SCR_003210) or IMB SPSS Statistics 26, where P < .05 was taken as the significance threshold. Tests for normally distributed data are built into the analysis software. Only experiments that included measurements over the entire ligand concentration range were included in the analysis. Oocyte concentration–response data were fit to Hill equations to obtain an IC50s or EC50s and Hill coefficient values for each oocyte recording. These parameters were then averaged across multiple oocyte experiments of the same type. Unpaired t‐test comparisons were made between the two receptor isoforms in response to the same ligand. Parameter comparisons include EC50s, IC50s, Hill coefficients (nH), % inhibition, % recovery, and time constants and are indicated in Tables 1, 2, 3, 4, 5.
Table 1.
Glutamate concentration–response parameters
| GluCl receptor | Glutamate | |||
|---|---|---|---|---|
| EC50 (μM) | nH | Imax (nA) | n | |
| b1 | 19 ± 1 | 3.4 ± 0.3 | 1.0 ± 0.2 | 6 |
| c | 27 ± 1 | 2.1 ± 0.1 | 0.8 ± 0.1 | 7 |
Note. Values are means ± SEM.
Table 2.
Effects of IVM and FIP
| GluCl receptor | IVM | FIP | ||||||
|---|---|---|---|---|---|---|---|---|
| EC50 (nM) | nH | %ImaxGlu | n | IC50 (μM) | nH | %Inhib. | n | |
| b1 | 145 ± 13 | 1.3 ± 0.1 | 18 ± 1 | 6 | 0.5 ± 0.1 | 1.2 ± 0.1 | 99 ± 1 | 6 |
| c | 202 ± 50 | 1.2 ± 0.2 | 28 ± 1* | 6 | 0.8 ± 0.1 | 1.3 ± 0.2 | 99 ± 1 | 6 |
Note. Values are means ± SEM.
P < .05, significantly different from b1 GluCl receptors for the equivalent experiment.
Table 3.
Effects of LND and PTX
| GluCl receptor | LND | PTX | ||||||
|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | nH | %Inhib. | n | IC50 (μM) | nH | %Inhib. | n | |
| b1 | 8.0 ± 1.0 | 1.2 ± 0.1 | 65 ± 5 | 6 | 23 ± 3 | 1.1 ± 0.1 | 80 ± 9 | 6 |
| c | 5.8 ± 1.3 | 1.6 ± 0.4 | 95 ± 1* | 6 | 7.1 ± 0.2* | 1.5 ± 0.1 | 97 ± 1* | 6 |
Note. Values are means ± SEM.
P < .05, significantly different from b1 GluCl receptors for the equivalent experiment.
Table 4.
Recovery from inhibition by LND and FIP
| GluCl receptor | LND | Time constant (s) | FIP | Time constant (s) | ||
|---|---|---|---|---|---|---|
| %Recovery (μM) | n | %Recovery | n | |||
| b1 | 72 ± 2 | 6 | 64 ± 4 | 37 ± 6 | 6 | 56 ± 14 |
| c | 42 ± 3* | 6 | 60 ± 9 | 18 ± 2* | 6 | 22 ± 2 |
Note. Values are means ± SEM.
P < .05, significantly different from b1 GluCl receptors for the equivalent experiment.
Table 5.
Inhibition of IVM‐gated currents
| GluCl receptor | LND | FIP | PTX | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | nH | %Inhib. | n | IC50 (μM) | nH | %Inhib. | n | IC50 (μM) | nH | %Inhib. | n | |
| b1 | 3.0 ± 0.9 | 0.7 ± 0.3 | 17 ± 3 | 6 | 0.2 ± 0.1 | 0.8 ± 0.1 | 50 ± 6 | 6 | 6 ± 1 | 1.5 ± 0.3 | 20 ± 2 | 6 |
| c | 11.0 ± 0.5* | 1.0 ± 0.3 | 19 ± 6 | 6 | 0.6 ± 0.1* | 1.0 ± 0.1 | 68 ± 10* | 6 | 16 ± 5 | 1.5 ± 0.4 | 45 ± 7* | 6 |
Note. Values are means ± SEM.
P < .05, significantly different from b1 GluCl receptors for the equivalent experiment.
3.6. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
4. RESULTS
4.1. Activation of homomeric c and b1 GluCl receptors by glutamate
Oocytes injected with the RNAs encoding the b1 or c subunits expressed clearly defined currents in response to glutamate concentrations ranging from 1 μM to 1 mM. Optimal currents were expressed 3–5 days post‐injection for both receptor isoforms (Figure 1b,c). The maximal currents elicited by saturating glutamate concentrations were similar between the two receptors, being 1.0 and 0.8 nA for b1 and c GluCl receptors, respectively (Table 1), suggesting similar levels of receptor expression in oocytes. Glutamate concentration–response plots yielded comparable EC50 values for b1 and for c GluCl receptors (Figure 1d, Table 1). A similar EC50 value has been reported for c GluCl receptors expressed in oocytes (Meyers et al., 2015).
4.2. Actions of IVM and FIP at homomeric c and b1 GluCl receptors
Direct application of IVM elicited a slowly developing, quasi‐irreversible current in both receptor isoforms (Figure 2a,b). Successive application of increasing concentrations of IVM from 3 nM to 3 μM produced concentration‐dependent increases in current that produced similar EC50 values, for c and b1 GluCl receptors (Table 2). IVM was more efficacious at c GluCl receptorsproducing a significantly greater maximal current relative to glutamatecompared to b1 GluCl receptors (Figure 2c, Table 2). The smaller relative peak current generated by b1 GluCl receptors for surface expression levels that were comparable to c GluCl receptors, suggests that b1 GluCl receptors are less sensitive to the potentiating effects of IVM.
Figure 2.
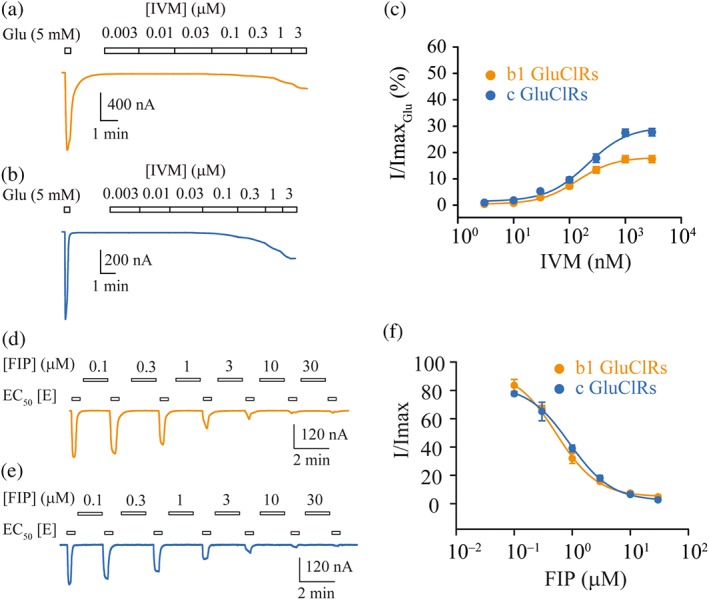
Actions of IVM and FIP at b1 and c GluCl receptors. (a, b) Example currents obtained from oocytes expressing b1 GluCl receptors (a) and c GluCl receptors (b) in response to increasing concentrations of IVM. The IVM‐elicited currents were normalised to a saturating glutamate‐gated current (5 mM). (c) Group concentration–response plots of normalised IVM‐gated currents for b1 and c GluCl receptors fitted to Hill equations. The EC50s were not significantly different, whereas the maximum current for the b1 GluCl receptors was significantly smaller. (d, e) Example currents gated by an EC50 concentration of glutamate in response to increasing concentrations of FIP for b1 GluCl receptors (d) and c GluCl receptors (e). (f) Group concentration–response plots for inhibition by FIP, fitted to Hill equations. No significant difference was observed in EC50s or maximum inhibition. The clamped membrane potential was −40 mV. Data points are means ± SEM
Alternating applications between a solution containing an EC50 concentration of glutamate and one containing a progressively increasing FIP concentration (from 0.1 to 30 μM) produced strong concentration‐dependent inhibition of currents (Figure 2d,e), which was near complete for both receptor isoforms at 30‐μM FIP. Hill equation fits to the group data yielded the IC50 concentrations shown in Table 2 for b1 and c GluCl receptors , respectively (Figure 2f). We infer that FIP effectively binds to non‐conducting states of the receptor to inhibit receptor activation by glutamate with a similar potency at both receptor isoforms. This mode of inhibition by FIP has been observed for other anion‐selective pLGICs, including invertebrate GABAA receptors (Hosie et al., 1995; Zhao, Salgado, Yeh, & Narahashi, 2003) and human GlyRs (Islam & Lynch, 2012).
4.3. Inhibitory potencies of LND and PTX
In our next series of experiments, we tested current inhibition by LND and PTX. LND was tested using a similar drug application protocol to that of FIP, whereas PTX was tested on current‐conducting receptors, consistent with it being an open channel blocker (Hibbs & Gouaux, 2011; Masiulis et al., 2019).
Applications of LND at concentrations of 1 to 300 μM strongly inhibited currents elicited by EC50 concentrations of glutamate (Figure 3a,b), demonstrating that LND has a similar mode of action to FIP. However, LND was an order of magnitude less potent than FIP and not equipotent at the two receptor isoforms. b1 GluCl receptors had a sensitivity to LND, similar to that of c GluCl receptors (Figure 3c, Table 3). However, LND was significantly less efficacious at inhibiting the maximum current mediated by b1 GluCl receptors, compared to c GluCl receptors . Our data show that LND is an effective inhibitor of GluCl receptors of A. gambiae with an efficacy that is splice variant‐dependent. In addition, our data demonstrate that channel block by LND, like FIP, can occur via non‐conducting receptors, as has been shown for the human α1 homomeric glycine receptors (Islam & Lynch, 2012).
Figure 3.
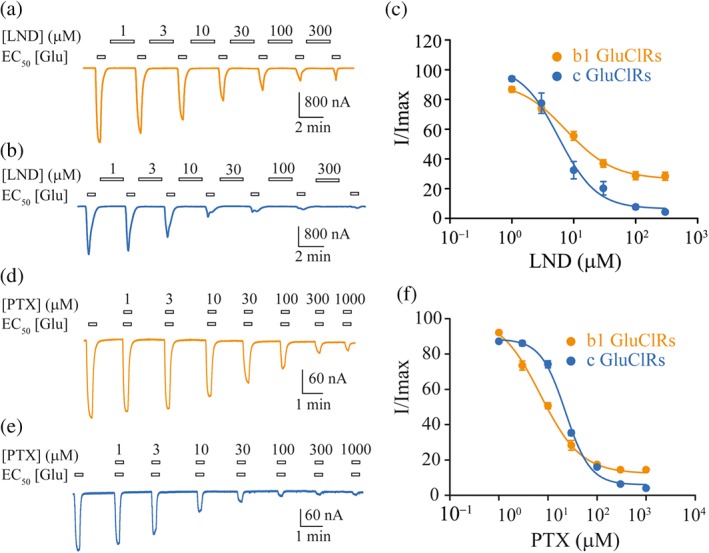
Glutamate‐gated current inhibition by LND and PTX. (a, b) Example currents gated by an EC50 concentration of glutamate in response to increasing concentrations of LND for b1 GluCl receptors (a) and c GluCl receptors (b). (c) Group concentration–response plots for inhibition by LND, fitted to Hill equations. The IC50s were not significantly different, whereas the inhibition at maximum concentration of LND was significantly different. (d, e) Typical currents gated by an EC50 concentration of glutamate in response to increasing concentrations of PTX for b1 GluCl receptors (d) and c GluCl receptors (e). (f) Group concentration–response plots for inhibition by PTX, fitted to Hill equations. The IC50s were significantly different as was the inhibition at maximum concentration of PTX. The clamped membrane potential was −40 mV. Data points are means ± SEM
PTX inhibited currents activated by EC50 concentrations of glutamate in a dose‐dependent manner at both receptor isoforms (Figure 3d,e). This insecticide exhibited a significantly different sensitivity between the two receptor isoforms, with the c GluCl receptors having a significantly lower IC50 value than the b1 GluCl receptors (Figure 3f, Table 3). The extent of inhibition also differed significantly, being less for b1 than for c GluCl receptors. This result demonstrates that PTX is less effective at inhibiting currents mediated by b1 GluCl receptors. Given that the receptors have identical pore‐lining residues (Figure 1a), LND and PTX must have more complex mechanisms of action than being simple pore blockers of GluCl receptors, as has been demonstrated for PTX at vertebrate glycine receptors (Lynch, Rajendra, Barry, & Schofield, 1995).
Overall, our data demonstrate that b1 GluCl receptors are significantly less sensitive to the insecticides, IVM, LND, and PTX than c GluCl receptors.
4.4. Recovery profile from inhibition by LND and FIP
We then explored glutamate current restoration after inhibition induced by LND and FIP. Recovery of peak currents elicited by an EC50 concentration of glutamate was monitored after first measuring a baseline control current. A 2‐min application of either LND or FIP at 30 μM was then followed by successive applications of an EC50 concentration of glutamate, which were separated by 2‐min wash periods with drug‐free extracellular solution. Sample time‐dependent recovery recordings are shown in Figure 4. Recovery from inhibition from both drugs reached a steady state after about 6 min. A repeated measures ANOVA showed that recovery from LND was statistically greater at b1 GluCl receptors from 6 min onwards compared to c GluCl receptors. Standard exponential fits to the data revealed that recovery from LND inhibition occurred at a similar rate (Figure 4c) to recovery from FIP (Figure 4f) for both receptors. However, b1 GluCl receptors recovered to a greater extent than c GluCl receptors (Table 4). A similar pattern was observed for FIP. b1 GluCl receptors demonstrated greater recovery throughout the experiment, compared with the c GluCl receptors (Figure 4c,f, Table 4). Current recovery was less pronounced after inhibition by FIP than LND, consistent with FIP being a more potent inhibitor. These recovery experiments also provide further evidence that the b1 receptors were less sensitive to LND and FIP than the c GluCl receptors.
Figure 4.
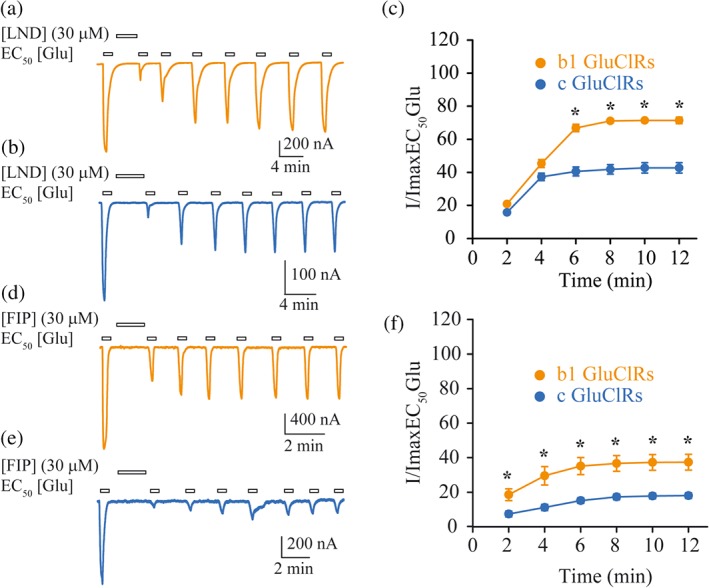
Recovery profile of glutamate‐gated currents from inhibition from LND and FIP. (a, b) Typical currents gated by an EC50 concentration of glutamate in response to a single application of a maximal concentration of LND for b1 GluCl receptors (a) and c GluCl receptors (b). (c) Group plots of time‐dependent current recovery showing that b1 GluCl receptors recover to a significantly greater extent than c GluCl receptors. (d, e) Typical currents gated by an EC50 concentration of glutamate in response to a single application of a maximal concentration of FIP for b1 GluCl receptors (d) and c GluCl receptors (e). (f) Group plots of time‐dependent current recovery showing that b1 GluCl receptors recover to a significantly greater extent, but at a similar rate as c GluCl receptors. The clamped membrane potential was −40 mV. Data points are means ± SEM. *P < .05, significantly different from c GluCl receptors.
4.5. Inhibitory potency of LND, FIP, and PTX at IVM‐elicited currents
In our final series of experiments, we aimed at examining the potency with which LND, FIP, and PTX inhibited IVM‐activated currents at b1 and c GluCl receptors. These experiments involved eliciting control currents using a saturating concentration of glutamate (5 mM), followed by a 10‐min exposure to a saturating concentration of IVM (150 nM), which produced a steady‐state, maximum IVM current. The inhibitory effect of the test drug was then determined by applying increasing concentrations of drug in the absence of IVM (Figure 5).
Figure 5.
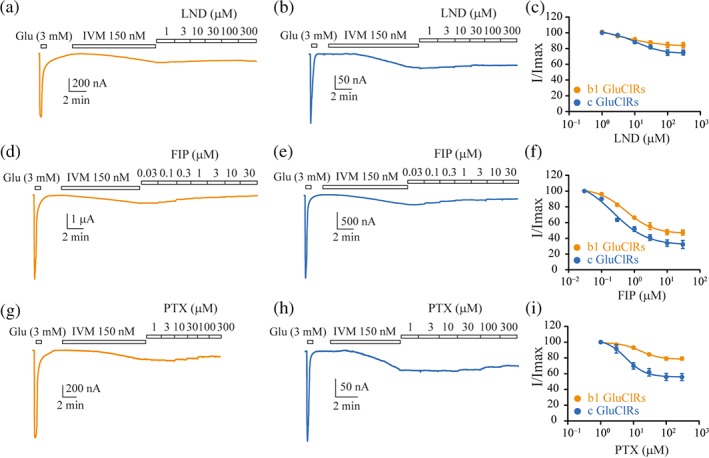
Inhibition of IVM‐gated currents by LND, FIP, and PTX. (a, b) Currents activated by a saturating concentration of IVM followed by successive applications of increasing concentrations of LND for b1 GluCl receptors (a) and c GluCl receptors (b). (c) Group plots of IVM‐gated current inhibition by LND fitted to Hill equations. Maximum inhibition for the two receptors was similar. (d, e) Currents activated by a saturating concentration of IVM followed by successive applications of increasing concentrations of FIP for b1 GluCl receptors (d) and c GluCl receptors (e). (f) Group plots of IVM‐gated current inhibition by FIP fitted to Hill equations. Maximum inhibition was significantly greater for c GluCl receptors. (g, h) Currents activated by a saturating concentration of IVM followed by successive applications of increasing concentrations of PTX for b1 GluCl receptors (g) and c GluCl receptors (h). (i) Group plots of IVM‐gated current inhibition by FIP fitted to Hill equations. Maximum inhibition was significantly greater for c GluCl receptors. All currents were normalised to the saturating glutamate‐gated current. The clamped membrane potential was −40 mV. Data points are means ± SEM
LND inhibited maximum IVM currents to a similar extent in both receptor isoforms (Figure 5a,b). Group data fitted to a Hill equation produced significantly lower IC50 values for b1 than for c GluCl receptors (Figure 5c, Table 5). FIP produced greater inhibition than LND at both receptors (Figure 5d,e), consistent with its higher potency at reducing glutamate‐gated currents. There was a significantly greater inhibition of currents mediated by c GluCl receptors, compared with that of b1 GluCl receptor‐mediated currents. The IC50 values for FIP at b1 and c GluCl receptors were also significantly different (Figure 5f, Table 5).
The greatest difference in inhibitory potency between the two receptors was exhibited by PTX. This compound inhibited IVM‐mediated currents at b1 GluCl receptors by almost half with a low IC50 value, whereas the inhibitory effect of PTX on current amplitude was significantly less at c GluCl receptors with a higher IC50 (Figure 5g–i, Table 5). This result re‐enforces the inference that PTX is not a simple pore blocker of GluCl receptors.
Our data clearly show that FIP is a potent inhibitor of glutamate‐ and IVM‐gated currents, with PTX and LND being less potent. Furthermore, GluCl receptors comprising individual splice variant subunits exhibited differential sensitivity to insecticides with b1 GluCl receptors being more resistant to their direct inhibitory effects. They also recovered from these effects to a greater extent than c GluCl receptors.
5. DISCUSSION
To reduce the transmission of malaria, the use of insecticides is an essential complement to bed nets and the targeted infiltration of wild populations by genetically modified mosquitoes using methods such as CRISPR/Cas‐9 (Kyrou et al., 2018). However, emerging resistance to insecticides and other methods of insect control is threatening to undermine recent advances at curbing malaria transmission (Anopheles gambiae 1000 Genomes Consortium et al., 2017; Hemingway et al., 2016). This is because genetic diversity amongst mosquito populations confers a great capacity for adaptation to both genetic and insecticidal methods of control (Anopheles gambiae 1000 Genomes Consortium et al., 2017). Insecticide resistance in Anopheline mosquitoes is now being reported across sub‐Saharan Africa (Du et al., 2005; Taylor‐Wells, Brooke, Bermudez, & Jones, 2015; Wondji et al., 2011). Compounding the threat presented by resistance is the prediction that the global distribution of malaria will increase in response to climate change, particularly in highland regions of Africa, parts of South America, and Southeast Asia (Caminade et al., 2014). These factors make it imperative to identify new insecticide targets and map the molecular determinants of drug sensitivity in an effort to design new drugs.
5.1. Key pLGIC mutations conferring insecticide resistance
Invertebrate GABAA receptor‐mediated resistance to cyclodienes, LND, FIP, and PTX is mainly due to a widespread mutation found in the resistance to dieldrin gene that encodes the receptor subunits (Ffrench‐Constant, Rocheleau, Steichen, & Chalmers, 1993). The resulting amino acid substitutions are located at the conserved A2′ position in the ion pore‐forming M2 domains (A2′S or A2′G) of various insect orders (Ffrench‐Constant et al., 1993; Lees et al., 2014; Thompson, Steichen, & ffrench‐Constant, 1993), including Anopheline (Du et al., 2005; Taylor‐Wells et al., 2015; Wondji et al., 2011) and other mosquitoes (Thompson, Shotkoski, & ffrench‐Constant, 1993; Figure 1a).
Mutations in the GluCl receptors of agricultural pests that confer insecticide resistance have been identified in segments that are essential to receptor activation and modulation (Keramidas & Lynch, 2013; Soh, Estrada‐Mondragon, Durisic, Keramidas, & Lynch, 2017), such as the M3 domain and segments that flank it. In particular, missense mutations to the M2–M3 linker and N‐terminal portion of the M3 domain confer IVM resistance in Tetranychus urticae (G36′D, G36′E; Kwon, Yoon, Clark, & Lee, 2010; Mermans, Dermauw, Geibel, & Van Leeuwen, 2017) and Plutella xylostella (A30′V; Wang et al., 2017), as well as in Drosophila melanogaster (P23′; Kane et al., 2000). A mutation just beyond the M3 (A55′V) has also been identified in the human head lice, Pediculus humanus (Amanzougaghene et al., 2018). However, all of these residues are conserved in the b1 and c subunits of A. gambiae (Figure 1a) and thus are unlikely to underlie the pharmacological difference between the two splice variants observed in this study. The splice variants have identical transmembrane domain sequences including the residues comprising M2, which at the critical 2′ position, is occupied by a serine (S2′, Figure 1a). When mutated to alanine (S2′A) in GluCl receptors of Musca domestica, the sensitivity of this receptor to LND and FIP increases (Hirata et al., 2008), whereas the reverse mutation to GABAA receptors of D. melanogaster (A2′S) reduces the sensitivity of these receptors to PTX, IVM, and FIP (Lees et al., 2014). Another highly conserved pore residue implicated in the sensitivity to LND, FIP, and PTX is the T6′, which is present in the sequences of b1 and c subunits of A. gambiae (Figure 1a) and other vertebrate and invertebrate pLGIC subunits (Horoszok, Raymond, Sattelle, & Wolstenholme, 2001; Nakao, Banba, Nomura, & Hirase, 2013; Shan et al., 2001). Insensitivity to inhibition by LND, FIP, and PTX at human α1β glycine receptors (Islam & Lynch, 2012; Shan et al., 2001) and GABAA receptors of Spodoptera litura (Nakao et al., 2013) has been attributed to non‐threonine residues at 6′ in these pLGICs. We infer that the sensitivity to LND, FIP, and PTX at b1 and c GluCl receptors measured here is, at least in part, due to the countervailing effects of the sensitivity reducing S2′ and the sensitivity enhancing T6′ in both b1 and c GluCl receptors.
5.2. pLGICs sensitivities to LND, FIP, PTX, and IVM
Vertebrate anion‐selective glycine receptors and GABAA receptors are generally less sensitive to the four insecticides investigated in this study than their invertebrate counterparts. The EC50 concentrations for IVM for α1β2γ2 GABAA and glycine receptors range between 1 and 20 μM (Estrada‐Mondragon & Lynch, 2015; Lynagh & Lynch, 2010). The IC50 values of LND at glycine and GABAA receptors range between 0.4 and 2 μM (Islam & Lynch, 2012; Maskell, Wafford, & Bermudez, 2001), whereas α homomeric glycine receptors have reported IC50 values for PTX of 18 μM (Shan et al., 2001). GABAA receptors are more sensitive to PTX with an IC50 value of about 1 μM (Gurley, Amin, Ross, Weiss, & White, 1995). FIP IC50 values at glycine and GABAA receptors of vertebrates range between 0.4 and 2 μM (Islam & Lynch, 2012; Li & Akk, 2008; Narahashi et al., 2010).
The sensitivity to LND, FIP, PTX, and IVM amongst invertebrate GABAA receptors and GluCl receptors varies greatly and depends on species, splice variant subunits, and RNA editing. Direct activation by IVM is achieved at a half‐maximal concentration of 40 and 140–190 nM at the GluCl receptors of Haemonchus contortus (Lynagh & Lynch, 2010) and Caenorhabditis elegans (Cully et al., 1994), respectively. IVM potentiation of glutamate‐gated currents at GluCl receptors of these species occurs at 5–10 nM (Atif et al., 2017; Cully et al., 1994). IVM has a bi‐phasic effect at invertebrate GABAA receptors, such as those of A. gambiae and D. melanogaster. GABA‐gated currents elicited by >EC50 concentration of GABA are inhibited by IVM with an IC50 of around 0.5 to 1 μM (Lees et al., 2014; Taylor‐Wells, Senan, Bermudez, & Jones, 2018), whereas IVM potentiates GABA‐gated currents elicited by EC20 concentrations of GABA (Taylor‐Wells et al., 2018). Our study reports an EC50 for direct activation by IVM of 150–200 nM for GluCl receptors of A. gambiae, which is close to the value reported for C. elegans. The GluCl receptors of M. domestica and Periplaneta americana respond to LND with IC50 concentrations of about 0.2 to 0.8 μM (Hirata et al., 2008; Ihara et al., 2005). GABAA receptors of P. americana exhibit an exquisite sensitivity to LND with a reported IC50 value of 0.002 μM (Ihara et al., 2005). By contrast, the GluCl receptors of A. gambiae studied here have IC50 values between 6 and 8 μM, which is closer to those reported for vertebrate receptors. PTX has an IC50 of 22 μM at GluCl receptors of M. domestica (Hirata et al., 2008) and 0.4 μM at GABAA receptors of D. melanogaster (Lees et al., 2014). The sensitivities to PTX at the GluCl receptors of A. gambiae are closer to those of M. domestica, being between 7 and 23 μM. The GluCl receptors of M. domestica and P. americana have IC50 values for FIP of 0.01 to 0.8 μM (Hirata et al., 2008; Narahashi et al., 2010). Notably, the IC50 values of GluCl receptors of A. gambiae (0.5 to 0.8 μM) are closer to those for GABAA receptors of P. americana, D. melanogaster, S. litura, and A. gambiae, which are inhibited by FIP with IC50 values of 0.03 to 2 μM (Lees et al., 2014; Nakao et al., 2013; Narahashi et al., 2010; Taylor‐Wells et al., 2018).
Overall, our study demonstrates that the b1 subunit confers greater resistance to the insecticides studied here, as reflected by higher IC50s, more extensive recovery to the inhibitory effects of LND, PTX, and FIP, and smaller relative maximal currents in response to IVM. PTX showed the greatest differential potency between the two receptors, with a significantly higher IC50 and greater resistance to inhibition of peak currents elicited by glutamate and IVM at b1 GluCl receptors. Although b1 GluCl receptors were more resistant to the effects of LND, FIP, and IVM than c GluCl receptors, the differential potencies of these drugs were less pronounced, suggesting differences in the mechanisms of action between the test drugs. This is consistent with work carried out on glycine receptors, which propose an additional binding site for FIP that is likely to be within the transmembrane domain, but outside the pore (Islam & Lynch, 2012) and GLC‐3 GluCl receptors of C. elegans, which are refractory to inhibition by PTX, but retain sensitivity to FIP (Horoszok et al., 2001). This study also revealed that the inhibitory potency on LND, PTX, and FIP is reduced when the receptors are activated by IVM, rather than by glutamate. This is likely to be due to LND, PTX, FIP, and IVM all having binding sites within the transmembrane domains, resulting in reciprocal effects of local structural mechanisms when IVM is bound.
5.3. Structural determinants of insecticidal potency
The putative transmembrane domain binding sites for LND, PTX, FIP, and IVM at the b1 and c GluCl receptors comprise identical amino acid sequences (Figure 1a). This strongly suggests that other structural elements are responsible to the different potencies of the test insecticides at the two receptors. A sequence alignment of the b1 and c subunits reveals two regions of heterogeneity: (1) the β1 strand and Loop 2, which connects it to the β2 strand of the extracellular domain, and (2) three sites in the intracellular domain that connects M3 to M4 (Figure 1a; Meyers et al., 2015). The β1 strand and Loop 2 are of particular interest as the β1 strand forms part of the orthosteric binding pocket (loop G) for glutamate (Hibbs & Gouaux, 2011) and Loop 2 is a critical element in receptor activation in pLGICs (Chakrapani, Bailey, & Auerbach, 2004; Kash, Jenkins, Kelley, Trudell, & Harrison, 2003; Soh et al., 2017). Both of these structures undergo conformational rearrangements in response to ligands that bind to the orthosteric binding pocket in the extracellular domain (Chakrapani et al., 2004; Hibbs & Gouaux, 2011; Soh et al., 2017) and, importantly, in response to ligands that bind within the transmembrane domains and pore regions of pLGICs (Hibbs & Gouaux, 2011; Masiulis et al., 2019). These coupled, bi‐directional conformational changes suggest that the potency of a drug is not simply determined by its binding affinity (1/Kd) but depends also on structural rearrangements that are distant from its binding site that confer drug efficacy. A case in point is PTX, which binds with relatively high affinity to open pores of anion‐selective pLGICs. Cryo‐EM methods have demonstrated that once bound, PTX induces a shut pore structure. The PTX‐induced structural change in the pore back‐propagates to the extracellular domains to modulate orthosteric agonist affinity, giving rise to apparent competitive antagonism (Masiulis et al., 2019). A key residue in GluCl receptors that mediates the effects of IVM is the G36′ position in the M3 domain (Figure 1a) that forms part of the IVM binding pocket (Hibbs & Gouaux, 2011). Mutations to glutamate (G36′E), aspartate (G36′D), or alanine (G36′A) produce marked reductions to IVM sensitivity (Atif et al., 2017; Kwon et al., 2010; Lynagh & Lynch, 2010; Mermans et al., 2017). The G36′ is likely to act as a hinge that facilitates activation and positive modulation by IVM (Atif et al., 2017). In addition, residues within or segments of the intracellular M3–M4 loop have been shown to affect drug sensitivity (Moraga‐Cid, Yevenes, Schmalzing, Peoples, & Aguayo, 2011) and the activation properties (Langlhofer & Villmann, 2016) of pLGICs. Further studies will be required to determine which areas of heterogeneity between the two splice variant subunits mediate the differences in drug potency.
In conclusion, an emerging theme in receptor‐mediated insecticide resistance is the regulation of receptor subunits that confer resistance. For example, recent reports suggest that A. gambiae GluCl receptors comprising either b1 or c subunits exhibit differential sensitivities to IVM (Meyers et al., 2015). Similarly, reports of splice variant‐dependent sensitivity to FIP have been reported in GluCl receptors of the herbivorous insect pest, P. xylostella (Wang et al., 2018). The expression of GluCl receptors isoforms by the agricultural endoparasite H. contortus that contain IVM insensitive subunits has also been suggested as a possible mechanism for IVM resistance in this species (Atif et al., 2019). RNA A‐to‐I editing has also been postulated as a mechanism of insecticide resistance (Lees et al., 2014; Taylor‐Wells et al., 2018). Our study has demonstrated that splice variant subunits can confer reduced sensitivity to multiple, structurally divergent insecticides. Notably, this can be achieved without inducing changes to the sensitivity to the neurotransmitter, glutamate, and hence, inhibitory input to target neurons or muscle. Our study also emphasises the relevance of structural elements of receptor activation and modulation as determinants of drug potency.
AUTHOR CONTRIBUTIONS
A.K. and J.W.L. conceptualised the study and designed the experiments. M.A. performed the experiments and analysis. All authors wrote and edited the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers, and other organisations engaged with supporting research.
Atif M, Lynch JW, Keramidas A. The effects of insecticides on two splice variants of the glutamate‐gated chloride channel receptor of the major malaria vector, Anopheles gambiae . Br J Pharmacol. 2020;177:175–187. 10.1111/bph.14855
Contributor Information
Joseph W. Lynch, Email: j.lynch@uq.edu.au.
Angelo Keramidas, Email: a.keramidas@uq.edu.au.
REFERENCES
- Alexander, S. P.H. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174(Suppl 1), S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alout, H. , Krajacich, B. J. , Meyers, J. I. , Grubaugh, N. D. , Brackney, D. E. , Kobylinski, K. C. , … Foy, B. D. (2014). Evaluation of ivermectin mass drug administration for malaria transmission control across different West African environments. Malaria Journal, 13, 417 10.1186/1475-2875-13-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanzougaghene, N. , Fenollar, F. , Diatta, G. , Sokhna, C. , Raoult, D. , & Mediannikov, O. (2018). Mutations in GluCl associated with field ivermectin‐resistant head lice from Senegal. International Journal of Antimicrobial Agents, 52, 593–598. 10.1016/j.ijantimicag.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Anopheles gambiae 1000 Genomes Consortium , Data analysis group , Partner working group , Sample collections—Angola:; Burkina Faso:; Cameroon:; Gabon:; Guinea:; Guinea‐Bissau:; Kenya:; Uganda:; Crosses , Sequencing and data production , Web application development , & Project coordination (2017). Genetic diversity of the African malaria vector Anopheles gambiae . Nature, 552, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley, E. A. , Pyae Phyo, A. , & Woodrow, C. J. (2018). Malaria. Lancet, 391, 1608–1621. 10.1016/S0140-6736(18)30324-6 [DOI] [PubMed] [Google Scholar]
- Atif, M. , Estrada‐Mondragon, A. , Nguyen, B. , Lynch, J. W. , & Keramidas, A. (2017). Effects of glutamate and ivermectin on single glutamate‐gated chloride channels of the parasitic nematode H. contortus . PLoS Pathogens, 13, e1006663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atif, M. , Smith, J. J. , Estrada‐Mondragon, A. , Xiao, X. , Salim, A. A. , Capon, R. J. , … Keramidas, A. (2019). GluClR‐mediated inhibitory postsynaptic currents reveal targets for ivermectin and potential mechanisms of ivermectin resistance. PLoS Pathogens, 15, e1007570 10.1371/journal.ppat.1007570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli, G. , & Beier, J. C. (2017). Current vector control challenges in the fight against malaria. Acta Tropica, 174, 91–96. 10.1016/j.actatropica.2017.06.028 [DOI] [PubMed] [Google Scholar]
- Bhatt, S. , Weiss, D. J. , Cameron, E. , Bisanzio, D. , Mappin, B. , Dalrymple, U. , … Gething, P. W. (2015). The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature, 526, 207–211. 10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham, S. D. , Ihara, M. , Sattelle, D. B. , & Matsuda, K. (2017). Mechanisms of action, resistance and toxicity of insecticides targeting GABA receptors. Current Medicinal Chemistry, 24, 2935–2945. [DOI] [PubMed] [Google Scholar]
- Caminade, C. , Kovats, S. , Rocklov, J. , Tompkins, A. M. , Morse, A. P. , Colon‐Gonzalez, F. J. , … Lloyd, S. J. (2014). Impact of climate change on global malaria distribution. Proceedings of the National Academy of Sciences of the United States of America, 111, 3286–3291. 10.1073/pnas.1302089111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrapani, S. , Bailey, T. D. , & Auerbach, A. (2004). Gating dynamics of the acetylcholine receptor extracellular domain. The Journal of General Physiology, 123, 341–356. 10.1085/jgp.200309004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully, D. F. , Vassilatis, D. K. , Liu, K. K. , Paress, P. S. , Van der Ploeg, L. H. , Schaeffer, J. M. , & Arena, J. P. (1994). Cloning of an avermectin‐sensitive glutamate‐gated chloride channel from Caenorhabditis elegans . Nature, 371, 707–711. 10.1038/371707a0 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, W. , Awolola, T. S. , Howell, P. , Koekemoer, L. L. , Brooke, B. D. , Benedict, M. Q. , … Zheng, L. (2005). Independent mutations in the Rdl locus confer dieldrin resistance to Anopheles gambiae and An. arabiensis . Insect Molecular Biology, 14, 179–183. 10.1111/j.1365-2583.2005.00544.x [DOI] [PubMed] [Google Scholar]
- Estrada‐Mondragon, A. , & Lynch, J. W. (2015). Functional characterization of ivermectin binding sites in α1β2γ2L GABA(A) receptors. Frontiers in Molecular Neuroscience, 8, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench‐Constant, R. H. , Rocheleau, T. A. , Steichen, J. C. , & Chalmers, A. E. (1993). A point mutation in a Drosophila GABA receptor confers insecticide resistance. Nature, 363, 449–451. 10.1038/363449a0 [DOI] [PubMed] [Google Scholar]
- Gurley, D. , Amin, J. , Ross, P. C. , Weiss, D. S. , & White, G. (1995). Point mutations in the M2 region of the alpha, beta, or gamma subunit of the GABAA channel that abolish block by picrotoxin. Receptors & Channels, 3, 13–20. [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway, J. , Ranson, H. , Magill, A. , Kolaczinski, J. , Fornadel, C. , Gimnig, J. , … Hamon, N. (2016). Averting a malaria disaster: Will insecticide resistance derail malaria control? Lancet, 387, 1785–1788. 10.1016/S0140-6736(15)00417-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs, R. E. , & Gouaux, E. (2011). Principles of activation and permeation in an anion‐selective Cys‐loop receptor. Nature, 474, 54–60. 10.1038/nature10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata, K. , Ishida, C. , Eguchi, Y. , Sakai, K. , Ozoe, F. , Ozoe, Y. , & Matsuda, K. (2008). Role of a serine residue (S278) in the pore‐facing region of the housefly l‐glutamate‐gated chloride channel in determining sensitivity to noncompetitive antagonists. Insect Molecular Biology, 17, 341–350. 10.1111/j.1365-2583.2008.00806.x [DOI] [PubMed] [Google Scholar]
- Horoszok, L. , Raymond, V. , Sattelle, D. B. , & Wolstenholme, A. J. (2001). GLC‐3: A novel fipronil and BIDN‐sensitive, but picrotoxinin‐insensitive, l‐glutamate‐gated chloride channel subunit from Caenorhabditis elegans . British Journal of Pharmacology, 132, 1247–1254. 10.1038/sj.bjp.0703937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie, A. M. , Baylis, H. A. , Buckingham, S. D. , & Sattelle, D. B. (1995). Actions of the insecticide fipronil, on dieldrin‐sensitive and‐ resistant GABA receptors of Drosophila melanogaster . British Journal of Pharmacology, 115, 909–912. 10.1111/j.1476-5381.1995.tb15896.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara, M. , Buckingham, S. D. , Matsuda, K. , & Sattelle, D. B. (2017). Modes of action, resistance and toxicity of insecticides targeting nicotinic acetylcholine receptors. Current Medicinal Chemistry, 24, 2925–2934. 10.2174/0929867324666170206142019 [DOI] [PubMed] [Google Scholar]
- Ihara, M. , Ishida, C. , Okuda, H. , Ozoe, Y. , & Matsuda, K. (2005). Differential blocking actions of 4′‐ethynyl‐4‐n‐propylbicycloorthobenzoate (EBOB) and γ‐hexachlorocyclohexane (γ‐HCH) on γ‐aminobutyric acid‐ and glutamate‐induced responses of American cockroach neurons. Invertebrate Neuroscience, 5, 157–164. 10.1007/s10158-005-0008-5 [DOI] [PubMed] [Google Scholar]
- Islam, R. , & Lynch, J. W. (2012). Mechanism of action of the insecticides, lindane and fipronil, on glycine receptor chloride channels. British Journal of Pharmacology, 165, 2707–2720. 10.1111/j.1476-5381.2011.01722.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane, N. S. , Hirschberg, B. , Qian, S. , Hunt, D. , Thomas, B. , Brochu, R. , … Cully, D. F. (2000). Drug‐resistant Drosophila indicate glutamate‐gated chloride channels are targets for the antiparasitics nodulisporic acid and ivermectin. Proceedings of the National Academy of Sciences of the United States of America, 97, 13949–13954. 10.1073/pnas.240464697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash, T. L. , Jenkins, A. , Kelley, J. C. , Trudell, J. R. , & Harrison, N. L. (2003). Coupling of agonist binding to channel gating in the GABA(A) receptor. Nature, 421, 272–275. 10.1038/nature01280 [DOI] [PubMed] [Google Scholar]
- Keramidas, A. , & Lynch, J. W. (2013). An outline of desensitization in pentameric ligand‐gated ion channel receptors. Cellular and Molecular Life Sciences, 70, 1241–1253. 10.1007/s00018-012-1133-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen, G. F. , Kiware, S. S. , Okumu, F. O. , Sinka, M. E. , Moyes, C. L. , Massey, N. C. , … Tusting, L. S. (2017). Going beyond personal protection against mosquito bites to eliminate malaria transmission: Population suppression of malaria vectors that exploit both human and animal blood. BMJ Global Health, 2, e000198 10.1136/bmjgh-2016-000198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, D. H. , Yoon, K. S. , Clark, J. M. , & Lee, S. H. (2010). A point mutation in a glutamate‐gated chloride channel confers abamectin resistance in the two‐spotted spider mite, Tetranychus urticae Koch. Insect Molecular Biology, 19, 583–591. [DOI] [PubMed] [Google Scholar]
- Kyrou, K. , Hammond, A. M. , Galizi, R. , Kranjc, N. , Burt, A. , Beaghton, A. K. , … Crisanti, A. (2018). A CRISPR‐Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nature Biotechnology, 36, 1062–1066. 10.1038/nbt.4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlhofer, G. , & Villmann, C. (2016). The intracellular loop of the glycine receptor: It's not all about the size. Frontiers in Molecular Neuroscience, 9, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees, K. , Musgaard, M. , Suwanmanee, S. , Buckingham, S. D. , Biggin, P. , & Sattelle, D. (2014). Actions of agonists, fipronil and ivermectin on the predominant in vivo splice and edit variant (RDLbd, I/V) of the Drosophila GABA receptor expressed in Xenopus laevis oocytes. PLoS ONE, 9, e97468 10.1371/journal.pone.0097468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, P. , & Akk, G. (2008). The insecticide fipronil and its metabolite fipronil sulphone inhibit the rat α1β2γ2L GABAA receptor. British Journal of Pharmacology, 155, 783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynagh, T. , & Lynch, J. W. (2010). A glycine residue essential for high ivermectin sensitivity in Cys‐loop ion channel receptors. International Journal for Parasitology, 40, 1477–1481. 10.1016/j.ijpara.2010.07.010 [DOI] [PubMed] [Google Scholar]
- Lynch, J. W. , Rajendra, S. , Barry, P. H. , & Schofield, P. R. (1995). Mutations affecting the glycine receptor agonist transduction mechanism convert the competitive antagonist, picrotoxin, into an allosteric potentiator. The Journal of Biological Chemistry, 270, 13799–13806. 10.1074/jbc.270.23.13799 [DOI] [PubMed] [Google Scholar]
- Masiulis, S. , Desai, R. , Uchanski, T. , Serna Martin, I. , Laverty, D. , Karia, D. , … Aricescu, A. R. (2019). GABAA receptor signalling mechanisms revealed by structural pharmacology. Nature, 565, 454–459. 10.1038/s41586-018-0832-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskell, P. D. , Wafford, K. A. , & Bermudez, I. (2001). Effects of γ‐HCH and δ‐HCH on human recombinant GABAA receptors: Dependence on GABAA receptor subunit combination. British Journal of Pharmacology, 132, 205–212. 10.1038/sj.bjp.0703824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermans, C. , Dermauw, W. , Geibel, S. , & Van Leeuwen, T. (2017). A G326E substitution in the glutamate‐gated chloride channel 3 (GluCl3) of the two‐spotted spider mite Tetranychus urticae abolishes the agonistic activity of macrocyclic lactones. Pest Management Science, 73, 2413–2418. 10.1002/ps.4677 [DOI] [PubMed] [Google Scholar]
- Meyers, J. I. , Gray, M. , Kuklinski, W. , Johnson, L. B. , Snow, C. D. , Black, W. C. , … Foy, B. D. (2015). Characterization of the target of ivermectin, the glutamate‐gated chloride channel, from Anopheles gambiae . The Journal of Experimental Biology, 218, 1478–1486. 10.1242/jeb.118570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraga‐Cid, G. , Yevenes, G. E. , Schmalzing, G. , Peoples, R. W. , & Aguayo, L. G. (2011). A single phenylalanine residue in the main intracellular loop of α1 γ‐aminobutyric acid type A and glycine receptors influences their sensitivity to propofol. Anesthesiology, 115, 464–473. 10.1097/ALN.0b013e31822550f7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao, T. , Banba, S. , Nomura, M. , & Hirase, K. (2013). Meta‐diamide insecticides acting on distinct sites of RDL GABA receptor from those for conventional noncompetitive antagonists. Insect Biochemistry and Molecular Biology, 43, 366–375. 10.1016/j.ibmb.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Narahashi, T. , Zhao, X. , Ikeda, T. , Salgado, V. L. , & Yeh, J. Z. (2010). Glutamate‐activated chloride channels: Unique fipronil targets present in insects but not in mammals. Pesticide Biochemistry and Physiology, 97, 149–152. 10.1016/j.pestbp.2009.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, M. A. , Burrows, J. N. , Manyando, C. , van Huijsduijnen, R. H. , Van Voorhis, W. C. , & Wells, T. N. C. (2017). Malaria. Nature Reviews. Disease Primers, 3, 17050 10.1038/nrdp.2017.50 [DOI] [PubMed] [Google Scholar]
- Poche, R. M. , Githaka, N. , van Gool, F. , Kading, R. C. , Hartman, D. , Polyakova, L. , … Lozano‐Fuentes, S. (2017). Preliminary efficacy investigations of oral fipronil against Anopheles arabiensis when administered to Zebu cattle (Bos indicus) under field conditions. Acta Tropica, 176, 126–133. 10.1016/j.actatropica.2017.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond‐Delpech, V. , Matsuda, K. , Sattelle, B. M. , Rauh, J. J. , & Sattelle, D. B. (2005). Ion channels: Molecular targets of neuroactive insecticides. Invertebrate Neuroscience, 5, 119–133. 10.1007/s10158-005-0004-9 [DOI] [PubMed] [Google Scholar]
- Shan, Q. , Haddrill, J. L. , & Lynch, J. W. (2001). A single β subunit M2 domain residue controls the picrotoxin sensitivity of αβ heteromeric glycine receptor chloride channels. Journal of Neurochemistry, 76, 1109–1120. 10.1046/j.1471-4159.2001.00124.x [DOI] [PubMed] [Google Scholar]
- Shaw, W. R. , & Catteruccia, F. (2019). Vector biology meets disease control: Using basic research to fight vector‐borne diseases. Nature Microbiology, 4, 20–34. 10.1038/s41564-018-0214-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh, M. S. , Estrada‐Mondragon, A. , Durisic, N. , Keramidas, A. , & Lynch, J. W. (2017). Probing the structural mechanism of partial agonism in glycine receptors using the fluorescent artificial amino acid, ANAP. ACS Chemical Biology, 12, 805–813. 10.1021/acschembio.6b00926 [DOI] [PubMed] [Google Scholar]
- Taylor‐Wells, J. , Brooke, B. D. , Bermudez, I. , & Jones, A. K. (2015). The neonicotinoid imidacloprid, and the pyrethroid deltamethrin, are antagonists of the insect Rdl GABA receptor. Journal of Neurochemistry, 135, 705–713. 10.1111/jnc.13290 [DOI] [PubMed] [Google Scholar]
- Taylor‐Wells, J. , Senan, A. , Bermudez, I. , & Jones, A. K. (2018). Species specific RNA A‐to‐I editing of mosquito RDL modulates GABA potency and influences agonistic, potentiating and antagonistic actions of ivermectin. Insect Biochemistry and Molecular Biology, 93, 1–11. 10.1016/j.ibmb.2017.12.001 [DOI] [PubMed] [Google Scholar]
- Thompson, M. , Shotkoski, F. , & ffrench‐Constant, R. (1993). Cloning and sequencing of the cyclodiene insecticide resistance gene from the yellow fever mosquito Aedes aegypti. Conservation of the gene and resistance associated mutation with Drosophila . FEBS Letters, 325, 187–190. 10.1016/0014-5793(93)81070-G [DOI] [PubMed] [Google Scholar]
- Thompson, M. , Steichen, J. C. , & ffrench‐Constant, R. H. (1993). Conservation of cyclodiene insecticide resistance‐associated mutations in insects. Insect Molecular Biology, 2, 149–154. [DOI] [PubMed] [Google Scholar]
- Wang, X. , O Reilly, A. O. , Williamson, M. S. , Puinean, A. M. , Yang, Y. , Wu, S. , & Wu, Y. (2018). Function and pharmacology of glutamate‐gated chloride channel exon 9 splice variants from the diamondback moth Plutella xylostella . Insect Biochemistry and Molecular Biology, 104, 58–64. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Puinean, A. M. , O Reilly, A. O. , Williamson, M. S. , Smelt, C. L. C. , Millar, N. S. , & Wu, Y. (2017). Mutations on M3 helix of Plutella xylostella glutamate‐gated chloride channel confer unequal resistance to abamectin by two different mechanisms. Insect Biochemistry and Molecular Biology, 86, 50–57. 10.1016/j.ibmb.2017.05.006 [DOI] [PubMed] [Google Scholar]
- White, N. J. , Pukrittayakamee, S. , Hien, T. T. , Faiz, M. A. , Mokuolu, O. A. , & Dondorp, A. M. (2014). Malaria. Lancet, 383, 723–735. 10.1016/S0140-6736(13)60024-0 [DOI] [PubMed] [Google Scholar]
- Wolstenholme, A. J. (2012). Glutamate‐gated chloride channels. The Journal of Biological Chemistry, 287, 40232–40238. 10.1074/jbc.R112.406280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondji, C. S. , Dabire, R. K. , Tukur, Z. , Irving, H. , Djouaka, R. , & Morgan, J. C. (2011). Identification and distribution of a GABA receptor mutation conferring dieldrin resistance in the malaria vector Anopheles funestus in Africa. Insect Biochemistry and Molecular Biology, 41, 484–491. 10.1016/j.ibmb.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Salgado, V. L. , Yeh, J. Z. , & Narahashi, T. (2003). Differential actions of fipronil and dieldrin insecticides on GABA‐gated chloride channels in cockroach neurons. The Journal of Pharmacology and Experimental Therapeutics, 306, 914–924. 10.1124/jpet.103.051839 [DOI] [PubMed] [Google Scholar]


