Abstract
Background and Purpose
Adolescents are regularly exposed to ∆9‐tetrahydrocannabinol (THC) via smoking and, more recently, vaping cannabis extracts. Growing legalization of cannabis for medical and recreational purposes, combined with decreasing perceptions of harm, makes it increasingly important to determine the consequences of frequent adolescent exposure for motivated behaviour and lasting tolerance in response to THC.
Experimental Approaches
Male and female rats inhaled THC vapour, or that from the propylene glycol (PG) vehicle, twice daily for 30 min from postnatal day (PND) 35–39 and PND 42–46 using an e‐cigarette system. Thermoregulatory responses to vapour inhalation were assessed by radio‐telemetry during adolescence and from PND 86–94. Chow intake was assessed in adulthood. Blood samples were obtained from additional adolescent groups following initial THC inhalation and after 4 days of twice daily exposure. Additional groups exposed repeatedly to THC or PG during adolescence were evaluated for intravenous self‐administration of oxycodone as adults.
Key Results
Female, not male, adolescents developed tolerance to the hypothermic effects of THC inhalation in the first week of repeated exposure despite similar plasma THC levels. Each sex exhibited tolerance to THC hypothermia in adulthood after repeated adolescent THC. However, enhanced potency was found in females. Repeated THC male rats consumed more food than their PG‐treated control group, without significant bodyweight differences. Adolescent THC did not alter oxycodone self‐administration in either sex but increased fentanyl self‐administration in females.
Conclusions and Implications
Repeated THC vapour inhalation in adolescent rats has lasting consequences observable in adulthood.
What is already known
Repeated injection of ∆9‐tetrahydrocannabinol (THC) produces tolerance in rats.
Effects of injected THC last six hours in rats, unlike effects of human cannabis inhalation.
What this study adds
Repeated vapour inhalation of THC in adolescent rats induces tolerance only in females.
Male rats consume more food, and female rats self‐administer more fentanyl, after repeated adolescent THC.
What is the clinical significance
Repeated cannabis use during adolescence alters physiology and behaviour in adulthood.
What is the clinical significance
Repeated cannabis use during adolescence alters physiology and behaviour in adulthood.
Abbreviations
- PG
propylene glycol
- THC
∆9‐tetrahydrocannabinol
1. INTRODUCTION
Significant numbers of adolescents are exposed to https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2424 (THC) on a regular basis via the smoking and, more recently, vaping of cannabis and/or cannabis extracts. Epidemiological data confirm that 5–6% of 12th grade students in the United States use cannabis nearly daily, and 13.9% have used in the past month (Miech et al., 2018). About 10% of 12th grade students have vaped cannabis at least once in the past year and 5% in the past month (Miech et al., 2018). Furthermore, growing legalization of cannabis use for medical and recreational purposes, and a decreasing perception of harm, suggests that these populations will only grow in coming years. Human epidemiological evidence poses clear limitations for interpretation and it is therefore critical to determine the consequences of frequent adolescent exposure to THC in well‐controlled, and translationally valid, animal models.
Repeated injection of THC in adolescent rats produces lasting effects in adulthood, including decreased bodyweight (Rubino et al., 2008), impaired spatial working memory (Rubino et al., 2009), increased https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9082 self‐administration (Ellgren, Spano, & Hurd, 2007), increased reinstatement of heroin seeking (Stopponi et al., 2014), and greater sensitivity to learning impairments produced by THC (Winsauer et al., 2011). Most prior investigations have employed the injected route of administration (Cha, Jones, Kuhn, Wilson, & Swartzwelder, 2007; Ellgren et al., 2007; Rubino et al., 2008; Winsauer et al., 2011) which may not match the human condition very well. For example, the duration of effect of THC on hypothermia in the rat lasts hours longer following i.p. administration compared to a vapour inhalation regimen that produces a similar temperature nadir and peak plasma THC levels (Nguyen, Aarde, et al., 2016; Taffe, Creehan, & Vandewater, 2015). The majority of human use of cannabis is via inhalation which entails a comparatively rapid onset and offset with a shorter overall duration of activity. Thus, the study of inhaled delivery of cannabis constituents in rodent models may improve translational inferences.
The first goal of this study was to determine if vapour inhalation of THC reduces body temperature in the adolescent rat, as this is a key measure of THC activity in rodents. Our e‐cigarette‐based model has been validated previously in adult rats, producing THC‐typical effects on nociception and body temperature (Javadi‐Paydar et al., 2018; Nguyen, Aarde, et al., 2016) and plasma THC levels comparable to those reached by human marijuana users (Hartman et al., 2015; Huestis, Henningfield, & Cone, 1992). We have not shown efficacy in adolescent rats which is a critical gap in the validation of this model and therefore an important goal.
The second, and major, goal was to determine if twice daily THC exposure over consecutive days during adolescence produces tolerance, indicative of a degree of THC exposure sufficient to induce lasting changes in the CNS. Our recent study in adult rats showed tolerance to the hypothermic and antinociceptive responses to THC after twice (female) or three times (male or female) daily inhalation (Nguyen et al., 2018).
The third goal was to test the hypothesis that the development of tolerance differs across rat sex, since prior work has shown that female rats develop tolerance more rapidly and at a lower mg·kg−1 adjusted dose following twice daily parenteral injection of THC (Wakley, Wiley, & Craft, 2014). Our prior work shows that male and female adult rats achieve similar plasma THC levels after identical inhalation conditions (Javadi‐Paydar et al., 2018) but that adult female rats become tolerant with less intensive THC exposure compared with males (Nguyen, Grant, et al., 2018). Thus, it was hypothesized that female adolescent rats would be more sensitive than the males, developing tolerance after fewer sessions and to a greater extent.
The fourth goal was to determine if there were lasting consequences of adolescent THC inhalation in the adult rat in terms of (a) tolerance to acute THC exposure; (b) alterations in weight gain and feeding behaviour (Sofia & Barry, 1974); and (c) in the propensity to self‐administer https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7093 (Nguyen, et al., 2019) or https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1626, as has previously been reported for heroin (Ellgren et al., 2007; Stopponi et al., 2014).
2. METHODS
2.1. Animals
All animal care and experimental procedures were conducted under protocols approved by the Institutional Care and Use Committee of The Scripps Research Institute in a manner consistent with the principles of the NIH Guide for the Care and Use of Laboratory Animals. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. Female (N = 40) and male (N = 48) Wistar (RRID:RGD_737929) rats (Charles River, Livermore, CA) were shipped on postnatal day (PND) 19 and entered the laboratory on PND 22. Rats were housed in humidity and temperature‐controlled (23 ± 2°C) vivaria on 12:12‐hr light:dark cycles, and all studies were conducted in the rats' scotophase. Animals had ad libitum access to food and water in their home cages. Gelatin nutritional support (DietGel® Recovery, ClearH2O, Westbrook, ME, USA) was provided during weekdays from PND 32–46 given the age of the animals, the radio‐telemetry surgery, and the unfamiliar experimental apparatus.
Studies were designed to contrast primary comparison groups (i.e., the vapour inhalation conditions) with equal group sizes. Groups of 16 male and 16 female rats were used in the radio‐telemetry experiments, groups of 24 male and 16 female rats were used in the self‐administration experiments, and groups of eight male and eight female rats were used in the plasma THC concentration assessments. The principles of reduction, replacement, and refinement were addressed in several ways in this study. Thermoregulatory assessment in radio‐telemeterized animals was used to reduce invasiveness. Repeated measures designs were selected for many studies to minimize the number of groups required and to enhance statistical power for comparisons. In addition, the same group was used in multiple sub‐studies, thereby reducing the total number of animals required for the purpose. Finally, the overall goal of developing vapour inhalation as a method of drug delivery offers future widespread refinement of approaches compared with parenteral injection of drugs. Subjects were randomly assigned to the major treatment groups (see Section 2.6, below), and dose substitutions in the self‐administration experiments were in a counterbalanced order. Computer‐automated data collection (radio‐telemetry and self‐administration) and the automated vapour exposures were conducted in parallel across groups; thus, no blinding of investigator to treatment group was included in the approach. The experimental apparatus locations were balanced across the groups. Blood collection and food weighing were conducted in a balanced treatment group order but not blinded, since these objective measures were unlikely to be influenced by investigator knowledge of treatment group.
2.2. Radio‐telemetry
Rats (N = 16 per sex) were implanted with sterile radio‐telemetry transmitters (Data Sciences International, St Paul, MN; TA11TA‐F20) in the abdominal cavity as previously described (Taffe et al., 2015; Wright et al., 2012) on PND 25. Group sizes were selected based on prior work with this model and anticipated size of the drug effect on temperature, for example, in Javadi‐Paydar, Creehan, Kerr, and Taffe (2019), Javadi‐Paydar, Kerr, Harvey, Cole, and Taffe (2019), and Nguyen, Aarde, et al. (2016). Animals were recorded in a dark testing room separate from the vivarium in either the vapour inhalation chambers (males) or in separate clean home cages (females) in the same room. We have previously shown that this moderate difference in procedure has negligible effect on the body temperature response to cannabinoids (Javadi‐Paydar et al., 2018). This difference in procedure resulted in a 30‐min recording time point for male rats but not for female rats. Radio‐telemetry transmissions were collected via telemetry receiver plates (Data Sciences International; RPC‐1 or RMC‐1) placed under the cages as described in prior investigations (Aarde et al., 2013; Miller et al., 2013; Wright et al., 2012). The sexes were treated identically up until PND 86, and thereafter, there were some slight differences; the order and details of studies are outlined in Table 1.
Table 1.
Order of inhalation and feeding studies for male and female radio‐telemetry cohorts
| Female and male | PND 29 | Air inhalation for 30 min |
| PND 30 | PG vapour inhalation for 30 min | |
| PND 31 | THC (100 mg·ml−1) vapour inhalation for 30 min | |
| PND 35–39, 42–46 | PG or THC (100 mg·ml−1) vapour inhalation for 30 min, b.i.d., qa. 5 hr | |
| PND 85 | PG vapour inhalation for 30 min | |
| PND 86 | THC (100 mg·ml−1) vapour inhalation for 30 min | |
| Female only | PND 91–94 | THC (25, 50 mg·ml−1) vapour inhalation |
| PND 105–109 | Feeding study | |
| PND 168–179 | Feeding study | |
| PND 200, 207 | Nociception | |
| Male only | PND 93–94 | PG and THC (200 mg·ml−1) vapour inhalation in counterbalanced order |
| PND 100–101 | PG and THC (200 mg·ml−1) vapour inhalation for 40 min in counterbalanced order | |
| PND154–165 | Feeding study |
2.3. Intravenous catheterization
Rats (N = 16 female; N = 24 male) were anaesthetized with an isoflurane/oxygen vapour mixture (isoflurane 5% induction and 1–3% maintenance) and prepared with chronic indwelling intravenous catheters as described previously (Aarde, Huang, & Taffe, 2017; Miller et al., 2015; Nguyen, Grant, Creehan, Vandewater, & Taffe, 2017) on PND 84–87, that is, after adolescent vapour exposure (see below). Group sizes were selected based on prior work explicating group differences in the self‐administration of oxycodone and other drugs, including reduced variability in female rats (Creehan, Vandewater, & Taffe, 2015; Miller et al., 2015; Nguyen, Bremer, et al., 2016; Nguyen, Hwang, Grant, Janda, & Taffe, 2018; Vandewater, Creehan, & Taffe, 2015). Briefly, the intravenous catheters consisted of a 14.5‐cm length of polyurethane‐based tubing (Micro‐Renathane®, Braintree Scientific, Inc, Braintree, MA) fitted to a guide cannula (Plastics One, Roanoke, VA) curved at an angle and encased in dental cement anchored to an ~3‐cm circle of durable mesh. Catheter tubing was passed subcutaneously from the animal's back to the right jugular vein. Catheter tubing was inserted into the vein and tied gently with suture thread. A liquid tissue adhesive was used to close the incisions (3M™ Vetbond™ Tissue Adhesive: 1469SB, 3M, St. Paul, MN). A minimum of 4 days was allowed for surgical recovery prior to starting an experiment. For the first 3 days of the recovery period, an antibiotic (cefazolin) and an analgesic (flunixin) were administered daily. During testing and training, intravenous catheters were flushed with ~0.2‐ to 0.3‐ml heparinized (166.7 USP·ml−1) saline before sessions and ~0.2‐ to 0.3‐ml heparinized saline containing cefazolin (100 mg·ml−1) after sessions. Catheter patency was assessed once a week after the last session of the week, via administration of the ultra‐short‐acting barbiturate anaesthetic Brevital sodium (1% methohexital sodium; ~0.2 ml [10 mg·ml−1]; Eli Lilly, Indianapolis, IN) through the catheter. Animals with patent catheters exhibit prominent signs of anaesthesia (pronounced loss of muscle tone) within 3 s after infusion. Animals that failed to display these signs were considered to have faulty catheters, and if catheter patency failure was detected, data that were collected after the previous passing of this test were excluded from analysis.
2.4. Administration of drugs
THC was administered by vapour inhalation. Doses are varied, and therefore described, by altering the concentration in the propylene glycol (PG) vehicle, the puff schedule, and the duration of inhalation sessions in this approach. The ethanolic THC stock was aliquoted in the appropriate volume, the ethanol evaporated off, and the THC was then dissolved in the PG to achieve target concentrations. (−)‐Oxycodone HCl and fentanyl citrate were dissolved in saline (0.9% NaCl), for injection. The THC was provided by the U.S. National Institute on Drug Abuse and the PG, oxycodone, and fentanyl were obtained from Sigma‐Aldrich Corporation (St. Louis, MO, USA).
2.5. Inhalation procedure
The inhalation procedure followed methods that have been recently described (Javadi‐Paydar et al., 2018; Nguyen, Aarde, et al., 2016). Sealed exposure chambers were modified from the 259 mm × 234 mm × 209 mm Allentown, Inc. (Allentown, NJ) rat cage to regulate airflow and the delivery of vaporized drug to rats. An e‐vape controller (Model SSV‐1; La Jolla Alcohol Research, Inc, La Jolla, CA, USA) was triggered to deliver the scheduled series of puffs from Protank 3 Atomizer (Kanger Tech; Shenzhen Kanger Technology Co., LTD; Fuyong Town, Shenzhen, China) e‐cigarette cartridges by a computerized controller designed by the equipment manufacturer (Control Cube 1; La Jolla Alcohol Research, Inc., La Jolla, CA, USA). Type 2 sealed exposure chambers (La Jolla Alcohol Research, Inc.) and second generation e‐vape controllers (Model SSV‐2; La Jolla Alcohol Research, Inc.) with Herakles Sub‐Ohm Tank e‐cigarette cartridges (Sense; Shenzhen Sense Technology Co., LTD; Baoan Dist, Shenzhen, China) or Smok Baby Beast Brother TFV8 Sub‐Ohm Tank (with the V8 X‐Baby M2 0.25‐Ω coil; SMOKTech, Nanshan, Shenzhen, China), triggered by MedPC IV software (Med Associates, St. Albans, VT, USA), were used for adolescent PG/THC exposure in the groups destined for the pharmacokinetic and self‐administration experiments. The chamber air was vacuum controlled by a chamber exhaust valve (i.e., a “pull” system) to flow room ambient air through an intake valve at ~1 L per min. This also functioned to ensure that vapour entered the chamber on each device triggering event. The vapour stream was integrated with the ambient air stream once triggered. For all studies, the system delivered four 10‐s vapour puffs, with 2‐s intervals, every 5 min for 30 min (i.e., last puff at 25 min with 5‐min inhalation time).
2.6. Adolescent vapour exposure
Each experimental cohort (i.e., destined for radio‐telemetry, intravenous self‐administration, or plasma collection experiments) was randomly divided into experimental (repeated adolescent THC inhalation) and control (repeated adolescent PG inhalation) subgroups, which received 30‐min episodes of vapour exposure, twice per day at a 5‐hr interval (~2 and 7 hr from the start of dark) to either THC (100 mg·ml−1) or the PG vehicle on sequential days PND 35–39 and again on PND 42–46 (plasma assessment groups received vapour exposure only for the first 4 days, see below). Plasma THC declines rapidly in the first hour after ceasing inhalation exposure and reaches low levels by 4 hr after vapour initiation (Javadi‐Paydar et al., 2018; Nguyen, Aarde, et al., 2016). The experimental timeline for the radio‐telemetry cohorts is outlined in Table 1. The self‐administration groups received intravenous catheter implant surgery on PND 84–87 and initiated the intravenous self‐administration (IVSA) of oxycodone (0.15 mg·kg−1 per infusion; 8‐hr sessions; Fixed Ratio 1 response contingency) on PND 112. Following an acquisition interval of 17 sessions, the rats completed six sessions of Fixed Ratio (8 hr) and progressive ratio (3 hr) dose substitution (0.006, 0.06, and 0.15 mg·kg−1 per infusion) in a counterbalanced order. Treatment of the plasma concentration assessment cohorts is described below.
2.7. Feeding procedure
To begin a test day, all chow was removed at the start of the dark cycle in the vivarium. Rats were moved to a procedure room, weighed, and placed individually in separate rat cages equipped with J‐style hanging chow dispensers (Guinea Pig Feeder; Ancare, Bellmore, NY, USA) starting 2 hr into the dark period. Chow was removed and weighed every 2 hr for 6 hr; rats were weighed again at the end of the test interval. The experimenter was not blinded to treatment condition since the measurements are objective. The feeding study was conducted PND 105–109 and 168–179 in the female radio‐telemetry groups and PND 154–165 in the male radio‐telemetry groups.
2.8. Plasma THC levels
Separate groups of eight male and eight female rats were received on PND 22. These rats received a single inhalation session (THC 100 mg·ml−1; 30 min) on PND 31, after which a blood sample (~500 μl) was obtained by acute venipuncture under inhalation anaesthesia. The rats then received twice daily inhalation sessions from PND 36–39 with a second blood sample obtained after the first session on PND 39. This timing was designed to capture the first session of complete tolerance observed in the female rats in the telemetry study, that is, Day 4. Blood samples were also obtained from these groups on PND 86 following a THC 100 mg·ml−1 (30 min) inhalation session and on PND 100 and 107 following THC 50 or 200 mg·ml−1 inhalation for 30 min in a counterbalanced order. The experimenter was not blinded to treatment condition for blood collection, since the measurements of drug concentration by MS are objective.
Blood samples were collected (~500 μl) via jugular needle insertion under anaesthesia with an isoflurane/oxygen vapour mixture (isoflurane 5% induction and 1–3% maintenance) 35 min post‐initiation of vapour inhalation. Plasma THC content was quantified using fast LC/MS adapted from Irimia, Polis, Stouffer, and Parsons (2015), Lacroix and Saussereau (2012), and Nguyen, Grant, et al. (2018); 5 μl of plasma were mixed with 50 μl of deuterated internal standard (100 ng·ml−1 cannabidiol [CBD]‐d3 and THC‐d3; Cerilliant), and cannabinoids were extracted into 300‐μl acetonitrile and 600 μl of chloroform and then dried. Samples were reconstituted in 100 μl of an acetonitrile/methanol/water (2:1:1) mixture. Separation was performed on an Agilent LC1100 using an Eclipse XDB‐C18 column (3.5 μm, 2.1 mm × 100 mm) using gradient elution with water and methanol, both with 0.2% formic acid (300 μl·min−1; 73–90%). Cannabinoids were quantified using an Agilent MSD6140 single quadrupole using electrospray ionization and selected ion monitoring (CBD [m/z = 315.2], CBD‐d3 [m/z = 318.3], THC [m/z = 315.2] and THC‐d3 [m/z = 318.3]). Although rats were not exposed to any CBD in this experiment, the assay included it for consistency and comparison of the plasma concentrations with other studies ongoing in the laboratory which include simultaneous analysis of CBD and THC. Calibration curves were conducted for each assay at a concentration range of 0–200 ng·ml−1 and observed correlation coefficients were .999.
2.9. Oxycodone self‐administration
Intravenous self‐administration was conducted in operant boxes (Med Associates) located inside sound‐attenuating chambers, in an experimental room (ambient temperature 22 ± 1°C; illuminated by red light) outside of the housing vivarium, starting on PND 112. The experimenter was not blinded to treatment condition since the data collection and recording is by computer. To begin a session, the catheter fittings on the animals' backs were connected to polyethylene tubing contained inside a protective spring suspended into the operant chamber from a liquid swivel attached to a balance arm. Each operant session started with the extension of two retractable levers into the chamber. Following each completion of the response requirement (response ratio), a white stimulus light (located above the reinforced lever) signalled delivery of the reinforcer and remained on during a 20‐s post‐infusion timeout, during which responses were recorded but had no scheduled consequences. Drug infusions were delivered via syringe pump. The training dose (0.15 mg·kg−1 per infusion; ~0.1 ml per infusion) was selected from prior oxycodone self‐administration studies (Nguyen, Hwang, et al., 2018; Wade, Vendruscolo, Schlosburg, Hernandez, & Koob, 2015). The rats were trained in 8‐hr sessions using a Fixed Ratio 1 (FR1) response contingency during weekdays (5 days per week) for 17 (male) or 16 (female) sessions. Thereafter, the animals were assessed in a dose substitution procedure (with dose order counterbalanced within the groups) in 8‐hr sessions under an FR1 contingency. Following this, the dose substitution (with dose order counterbalanced within the groups) was completed under a progressive ratio (PR) contingency procedure. In the PR paradigm, the required response ratio was increased after each reinforcer delivery within a session (Hodos, 1961; Segal & Mandell, 1974) as determined by the following equation (rounded to the nearest integer): Response Ratio = 5e^(injection number*j)–5 (Richardson & Roberts, 1996). The j value was set to 0.2, and sessions were terminated when 60 min elapsed without a response, or after 3 hr. Female rats also completed two successive dose substitution tests under FR1 in 4‐hr sessions involving first fentanyl (0.0, 1.25, 2.5, and 5.0 μg·kg−1 per inf) and then fentanyl (0.0, 0.625, 2.5, and 10.0 μg·kg−1 per inf), each in a counterbalanced order, starting PND 200 (see Supplemental Methods and Figure S6 for additional experiments completed prior to this experiment for these animals).
2.10. Data and statistical analysis
Body temperature and activity rate (counts per min) were collected on a 5‐min schedule but are expressed as 30‐min averages for analysis in the study. The time identifier is at the end of the interval, that is, the 60‐min time point is the average from 35 to 60 min after the start of inhalation. The time courses for data collection are expressed relative to the start of the inhalation. Any missing temperature values were interpolated from the values before and after the lost time point, this typically involves fewer than 10% of observations. Missing activity rate data points were not replaced because values can change dramatically from one 5‐min interval to another, thus there is no rational basis for interpolating. The 60‐min time point was selected for follow‐up comparison across sexes and/or treatment conditions because our prior work (Javadi‐Paydar et al., 2018; Nguyen, Aarde, et al., 2016; Nguyen, Grant, et al., 2018) found that this time is consistent when the maximum hypothermia is observed after THC inhalation.
Statistical analysis of temperature, activity, plasma THC concentrations, bodyweight, infusions earned, and lever discrimination was conducted with ANOVA. Between‐groups factors included sex or adolescent treatment condition, as appropriate. Within‐subjects factors of session, dose, postnatal day (PND), and vapour inhalation condition were included as appropriate. Significant main effects or effects of the interaction of factors were followed with post hoc analysis using Tukey (multi‐level factors) or Sidak (two‐level factors) correction for multiple comparisons. A P value <.05 was used as the criterion for a significant result. Statistical analysis was undertaken only for studies where group size was at least n = 5. Group sizes indicate independent animals, and there were no exclusions for outlying data points employed. The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). All analyses used GraphPad Prism, RRID:SCR_002798 for Windows (v. 6.02, 7.03, 8.1.1; GraphPad Software, Inc., San Diego CA). Where the data are presented as bar charts, an examination of the individual data did not reveal any unusual or interesting aspects of the data that are not made obvious from the bar chart (George et al., 2017).
2.11. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
3. RESULTS
3.1. THC‐induced hypothermia in adolescent rats
The initial experiment confirmed that vapour inhalation of THC induces hypothermia in adolescent rats of each sex (Figure 1a,b). Furthermore, the male and female groups destined for repeated THC versus repeated PG were not different in response to PG on PND 30 and to THC on PND 31. The three‐factor analysis confirmed significant effects of vapour inhalation condition, of time after vapour initiation and the interaction of time with vapour inhalation condition, in female rats. There were no significant main effects or interactions with the group of eventual assignment. The three‐way analysis for the male groups confirmed significant effects of vapour inhalation condition, of time after vapour initiation, and the interaction of time with vapour inhalation condition, and the interaction of the group of eventual assignment with vapour inhalation condition. Post hoc analysis of this latter interaction failed to confirm any differences between groups destined for repeated PG and repeated THC in either PG or THC inhalation conditions.
Figure 1.
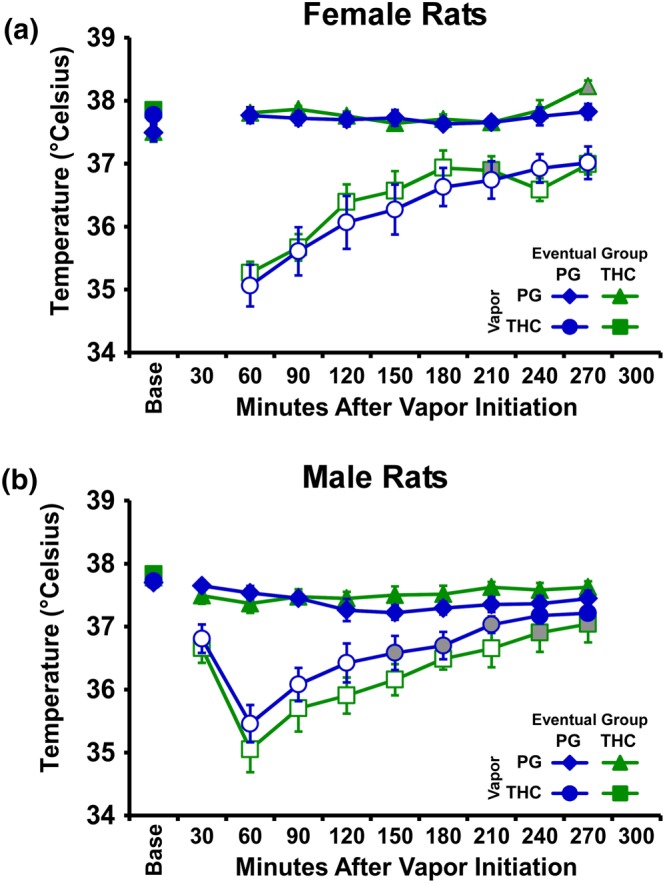
Mean (N = 8 per group; ±SEM) body temperature responses after inhalation of PG (on PND30) or THC (100 mg·ml−1; on PND31) vapour for 30 min in the subgroups eventually assigned to the repeated PG or repeated THC (a) female and (b) male groups. Open symbols indicate a significant difference from both vehicle at a given time point and the within‐treatment baseline (Base), while shaded symbols indicate a significant difference from the baseline only
Follow‐up analysis of body temperature 60 min after the start of inhalation including the repeated PG and repeated THC cohorts of each sex confirmed a main effect of vapour inhalation condition, but not of group or of the interaction. The post hoc test confirmed a significantly lower temperature after THC vapour compared with PG within each group and did not confirm any differences across all the groups within PG or THC vapour inhalation conditions.
3.2. Effect of repeated THC or PG inhalation on hypothermia in adolescent rats
There were no changes in the body temperature response of the repeated PG groups across the recording interval for any of the recording days (Figure S1), but both sexes in the repeated THC groups became hypothermic following inhalation during each of the chronic exposure weeks (Figure 2). Tolerance to the hypothermic effects differed between the sexes. Statistical analysis of the female animals' temperature confirmed significant effects of time after vapour initiation and day in Week 1 and interaction. For Week 2 there was a significant effects of time after vapour initiation, but not for day, and a significant interaction. Statistical analysis of the male animals' temperature confirmed significant effects in Week 1 and Week 2 for time, day and interaction. The post hoc test confirmed that a progressive tolerance to hypothermia developed in the female rats across the first 4 days of exposure with no hypothermia observed on Days 4 and 5. The post hoc test also confirmed that significant tolerance developed in Days 3–5 relative to Day 1 in the males, although this was limited to the 120‐ to 180‐min time points. In Week 2, the post hoc test confirmed a significant reduction in body temperature relative to the PG day 60 min after the start of inhalation on Days 7–9 for the female rats and Days 6–10 for the male rats. Follow‐up analysis was conducted on the temperature recorded 60 min after the start of inhalation for repeated THC cohorts of each sex to directly compare the magnitude of hypothermia across days and sex (Figure 3). The ANOVA confirmed a main effect of day, of sex and of the interaction. The post hoc test further confirmed that significant reductions in temperature following THC inhalation (relative to the PG condition) were observed for each THC session in male rats.
Figure 2.
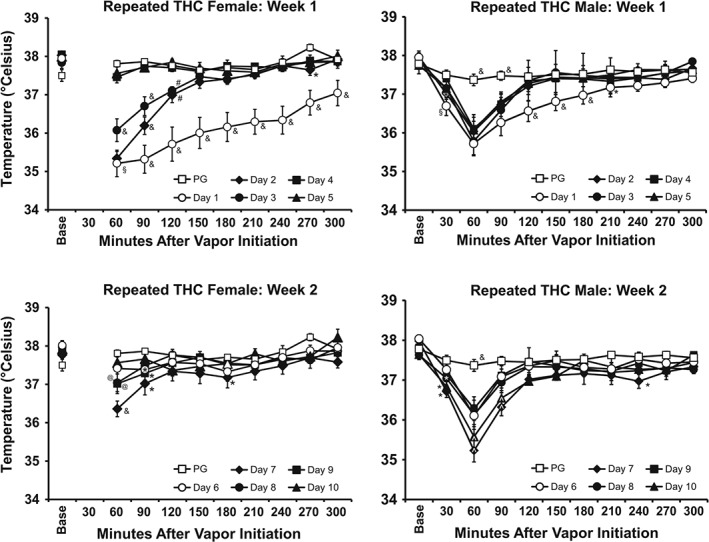
Mean (N = 8 per sex; ±SEM) body temperature recorded for the first vapour inhalation session of each day of the repeated treatment weeks is shown for the repeated THC groups; Week 1 = PND 35–39; Week 2 = PND 42–46. The PND 30 PG inhalation data are shown in upper and lower panels for comparison. The pre‐inhalation baseline temperature is indicated by “Base.” & P <.05, significantly different from all other days at a given time after the start of inhalation; §a P <.05, significantly different from all days except Day 2; # P <.05, significantly different from PG, Days 4 and 5; @ P <.05, significantly different from PG, Days 7 and 10; ^ P <.05, significantly different from PG, Days 6, 8, and 9; * P <.05, significantly different from PG.
Figure 3.
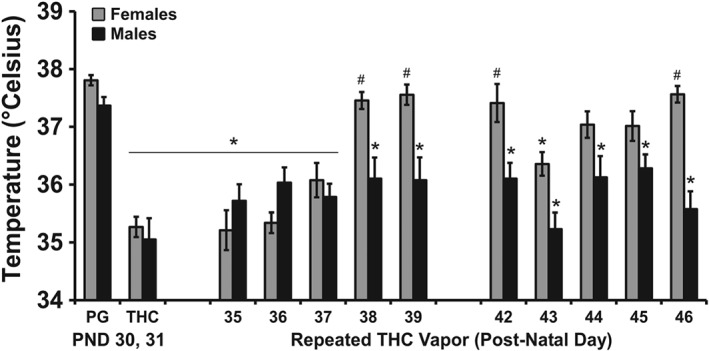
Mean (N = 8 per sex; ±SEM) body temperature 60 min after the start of inhalation for all inhalation days during adolescence. * P <.05, significant difference from the PG value, within group; # P <.05, significant sex difference on a given day.
Significant reductions in body temperature were also confirmed for female rats on PND Days 31, 35–37, and 43; a significant sex difference was confirmed for PND 38–42 and 46. A reduction in body temperature was observed during the first daily session of all 10 days of repeated THC inhalation in the males, but no change in body temperature was observed during PG inhalation. Bodyweight was most significantly reduced by repeated THC during the adolescent weeks in male rats (see Figure S3).
3.3. Adult THC inhalation
Tolerance to the hypothermic effects of THC inhalation were observed in adulthood in the repeated THC groups as compared with their respective repeated PG control groups (Figure 4). The statistical analysis of the temperature of the repeated PG females after inhalation during adulthood confirmed a significant effect of time after vapour initiation, of vapour concentration condition, and of the interaction of time with vapour concentration condition. Analysis of the temperature of the repeated THC females confirmed a significant effect of time after vapour initiation, of vapour concentration condition, and of the interaction of factors. The analysis of the temperature of the repeated PG males confirmed a significant effect of time after vapour initiation, and of the interaction of time with vapour concentration condition. Analysis of the temperature of the repeated THC males confirmed a significant effect of time after vapour initiation, of vapour concentration condition, and of the interaction of factors.
Figure 4.
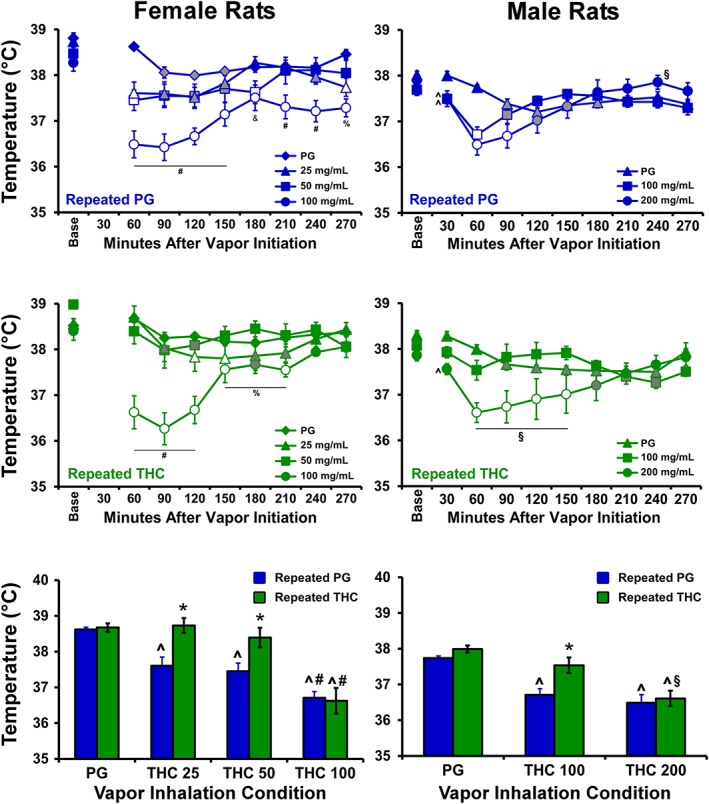
Mean (±SEM) body temperature of female and male rat cohorts (N = 8 per group) exposed to repeated PG or THC during adolescence and challenged in acute sessions with vapour from PG or varying concentrations of THC (25–200 mg·ml−1) from PND 85–94. Open symbols indicate a significant difference from the pre‐inhalation baseline (Base) and the corresponding time after PG inhalation, and grey symbols represent a difference from the baseline only. ^ P <.05, significantly different from the PG condition; # P <.05, significantly different from the 25 and 50 mg·ml−1 condition; § P <.05, significantly different from the 100 mg·ml−1 condition; % P <.05, significantly different from the 50 mg·ml−1 condition, & P <.05, significantly different from the 25 mg·ml−1 condition; * P <.05, significantly different between treatment groups at given dose, within sex.
Analysis of the temperature for the 60‐min interval in the female groups confirmed a significant effect of group, of vapour inhalation condition and of the interaction of factors, on body temperature. The post hoc analysis confirmed a group difference following inhalation of THC 25–50 mg·ml−1. The analysis of temperature for the 60‐min interval for the male groups likewise confirmed a significant effect of group, and of vapour inhalation condition, but not of the interaction of factors. The post hoc test confirmed a significant difference between the groups in the THC 100 mg·ml−1 condition.
3.4. Plasma THC levels
The plasma THC concentrations did not differ between samples obtained from adolescent male and female rats in either the acute (PND 31) or chronic (PND 39) THC inhalation experiments (Figure 5). The analysis confirmed a significant effect of acute compared with chronic experiment phase, without significant effect of sex or of the interaction of factors. During adulthood, plasma THC levels varied significantly with vapour inhalation condition, and sex. The post hoc test confirmed a significant sex difference at the 50 mg·ml−1 THC concentration and a difference from the 50 mg·ml−1 concentration after 100 or 200 mg·ml−1 inhalation for each sex.
Figure 5.
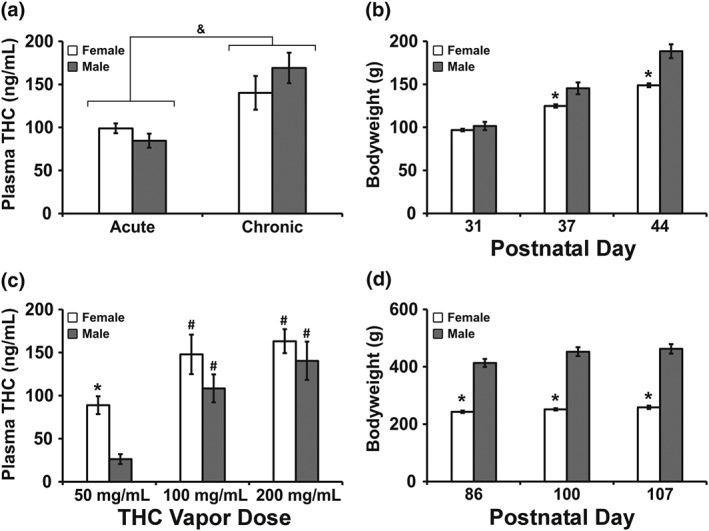
(a,c) Mean (±SEM) plasma THC levels from female and male rats (N = 8 per group) exposed to (a) THC (100 mg·ml−1) vapour on PND 31 (acute), 36–39 (chronic) or (c) THC (50–200 mg·ml−1) on PND 86, 100, and 107. Corresponding bodyweights are presented for the adolescent (b) and adult (d) age intervals. * P <.05, significant sex difference for a given dose or day;, & P <.05, significant difference from the acute condition across groups; # P <.05, significant difference from the 50 mg·ml−1 condition.
Analysis of the bodyweights confirmed significant differences in the adolescent age range for PND, sex, and interaction and in the adult age range for PND, sex and interaction. The post hoc tests confirmed that there were significant sex differences in weight on PND 37 and PND 44 during adolescences and across all 3 days of the adult age range.
3.5. Adult food consumption
Feeding was assessed in 6‐hr sessions during PND 154–158 and PND 161–165 in the male rats and during PND 105–109, PND 168–172, and PND 175–179 in the female rats (Figure 6). One of the female rats from the original THC group was in apparently unrelated ill health and was not included in the PND 168–179 feeding study. No significant difference in food intake was confirmed for the female rats in any of the feeding studies although, interestingly, the female repeated THC group remained slightly less sensitive to antinociceptive effects of THC when assessed from PND 200–207 (Figure S4). The male THC group consumed more chow than the male PG group with the analysis confirming significant effects of group, of day, and of the interaction of group with day. The post hoc test further confirmed that the repeated THC group consumed more food on PND 154, 155, 161, 162, and 165. Bodyweight prior to the session was not significantly different between the groups for either sex.
Figure 6.
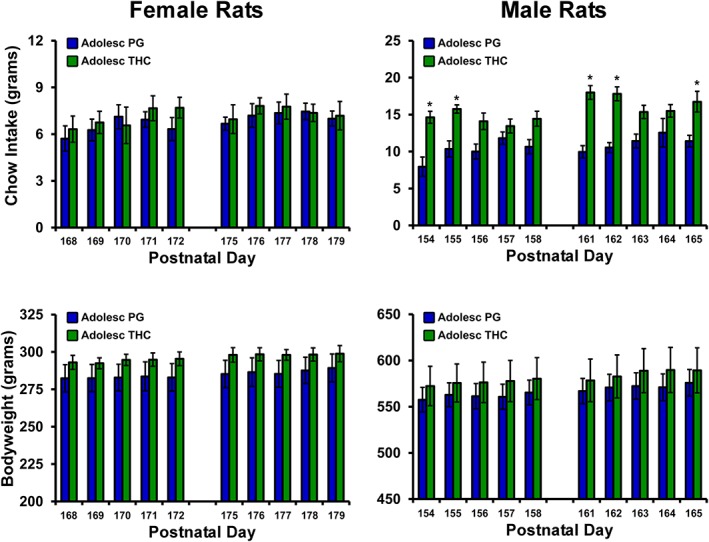
Mean (±SEM) chow intake and bodyweight of female and male rat cohorts exposed to repeated PG or THC during adolescence (N = 8 per group except N = 7 female/THC) and assessed during adulthood. * P <.05, significant difference between treatment groups, within sex.
3.6. Oxycodone self‐administration in adulthood
The male rats that were exposed to repeated vapour inhalation of either THC or PG vehicle during adolescence significantly escalated their oxycodone self‐administration during 17 sessions of acquisition training as adults. There was no significant effect of group (Figure 7a). Both groups exhibited a significant increase in appropriate drug‐lever responding (lever discrimination) across sessions (Figure 7b) as the ANOVA confirmed significant effects of session and of the interaction Group × Session. Analysis of oxycodone infusions during dose substitution experiments under an FR1 schedule confirmed a significant effect of dose, but no effect of group (Figure 7c). Similarly, analysis of the breakpoints under PR confirmed a significant effect of dose, but no effect of group (Figure 7d).
Figure 7.
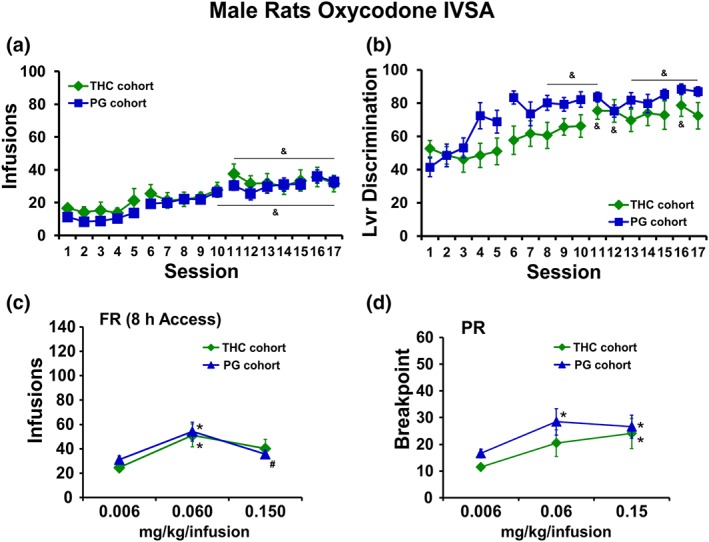
(a) Mean infusions and (b) percent drug‐appropriate lever responding (Lvr discrimination) for male rats trained to self‐administer oxycodone (0.15 mg·kg−1 per infusion) within 8‐hr‐extended access sessions, starting on PND112. (c) Mean (THC cohort, N = 11; PG cohort, N = 12; ±SEM) infusions during self‐administration under an FR1 schedule and (d) breakpoint values during self‐administration under a PR schedule following acute injection of THC (0.006–0.15 mg·kg−1, i.p.). & P <.05, significantly different from the first session; * P <.05, significantly different from 0.006 mg·kg−1 per infusion; # P <.05, significantly different from the 0.06 mg·kg−1 per infusion.
The female rats that were exposed to repeated vapour inhalation of either THC or PG vehicle during adolescence also significantly escalated their oxycodone self‐administration during the initial 16 sessions of acquisition training as adults, but there was no significant effect of group (Figure 8a). Both groups exhibited a significant increase in appropriate drug‐lever responding (lever discrimination) across sessions (Figure 8b) as the ANOVA confirmed significant effects of session. Analysis of oxycodone infusions during dose substitution experiments under an FR1 schedule confirmed a significant effect of dose, but no effect of group (Figure 8c). Similarly, analysis of the breakpoints under PR confirmed a significant effect of dose, but no effect of group (Figure 8d).
Figure 8.
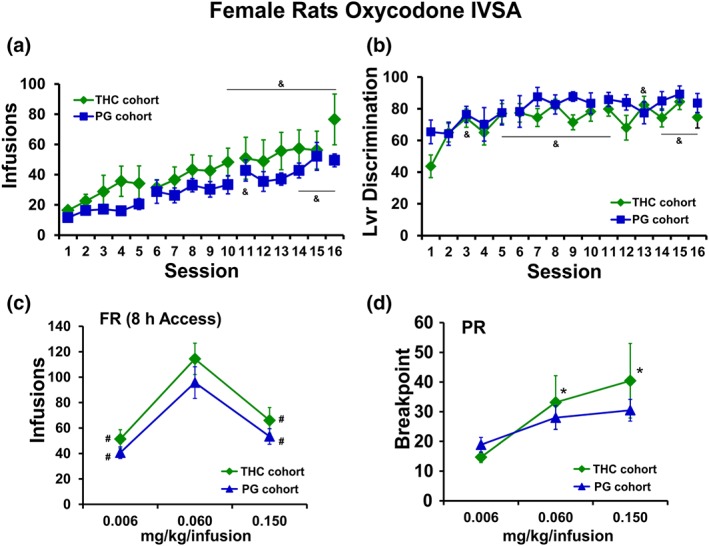
(a) Mean (N = 8; ±SEM) infusions and (b) percent drug‐appropriate lever responding (Lvr discrimination) for female rats trained to self‐administer oxycodone (0.15 mg·kg−1 per infusion) within 8‐hr‐extended access sessions, starting on PND112. (c) Mean (THC cohort, N = 7; PG cohort, N = 8; ±SEM) infusions during self‐administration under an FR1 schedule and (d) breakpoint values during self‐administration under a PR schedule following acute injection of THC (0.006–0.15 mg·kg−1, i.p.). & P <.05, significantly different from the first session; * P <.05, significantly different from 0.006 mg·kg−1 per infusion; # P <.05, significantly different from the 0.06 mg·kg−1 per infusion.
3.7. Fentanyl self‐administration in female rats
Preliminary analysis of the two fentanyl dose substitution experiments in the female rats (the catheters of N = 6 repeated THC remained patent) found no difference in the two overlapping doses in the first and second series. Therefore, the average of the vehicle and 2.5 μg·kg−1 per infusion doses were used for formal analysis of the entire dose range. The analysis confirmed that the THC exposed female rats self‐administered more fentanyl, showing a significant effect of adolescent treatment and of dose, and the post hoc test confirmed a group difference at the 0.625 μg·kg−1 per inf dose (Figure 9). Recalculation of the oxycodone FR dose substitution excluding the THC‐exposed animal who was not patent for the fentanyl study resulted in no change of the statistical significances.
Figure 9.
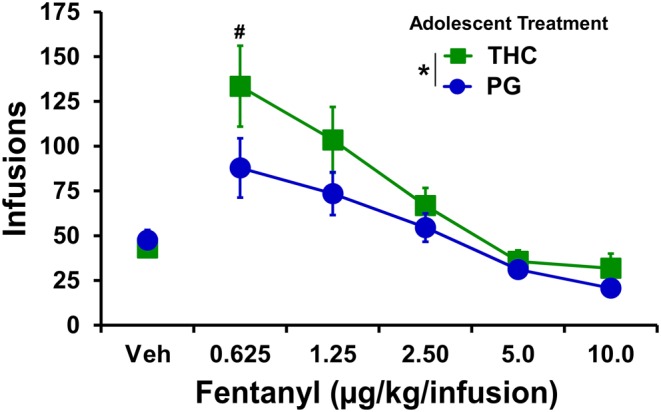
Mean (adolescent repeated THC cohort, N = 6; adolescent repeated PG cohort, N = 8; ±SEM) infusions of fentanyl obtained by female rats during self‐administration under an FR1 schedule. * P <.05, significant main effect of group (across doses); # P <.05, significant group difference at a specific dose.
3.8. Sex differences in oxycodone self‐administration
Follow‐up analysis of the apparent sex difference in oxycodone self‐administration, collapsed across adolescent treatment, confirmed that the female rats obtained more infusions during acquisition than did the males, with significant effects for sex, session and interaction. The post hoc test confirmed a significant difference between the sexes on Sessions 14–16 (Figure 10a). Lever discrimination was affected by sex in interaction with sessions, although the post hoc test confirmed a sex difference only in Session 3 (Figure 10b). This secondary analysis also confirmed that female rats self‐administered more oxycodone in the FR procedure (Figure 10c) compared with male rats, with significant effects for sex, dose and the interaction. The post hoc test confirmed a sex difference at the 0.06 and 0.15 mg·kg−1 per infusion doses. The post hoc test of the marginal mean for dose confirmed significant differences in the infusions obtained in all three dose conditions. In the PR dose substitution (Figure 10d), there was a significant effect of dose, but there were no significant effects of sex confirmed. The post hoc test of the marginal mean for dose confirmed significant differences in the infusions obtained in the 0.06 and 0.15 mg·kg−1 per infusion condition compared with the 0.006 mg·kg−1 per infusion dose condition. See Figure S5 for the self‐administration data for all adolescent treatment groups, that is, collapsed across sex.
Figure 10.
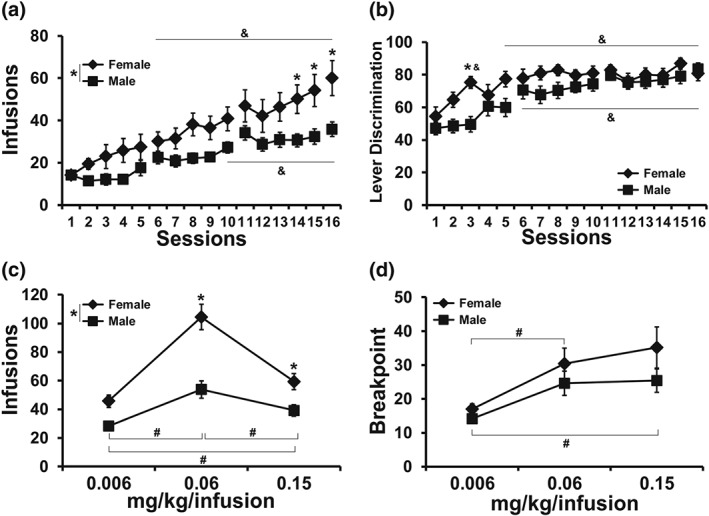
(a) Mean (±SEM) infusions and (b) lever discrimination during acquisition for the female (N = 15) and male (N = 24) animals collapsed across adolescent treatment groups. (c) Mean (±SEM) infusions obtained during the FR dose substitution and (d) breakpoints reached in the PR dose substitution procedures are also depicted. * P <.05, significant difference between sexes; & P <.05, a significant change from the first session within group; # P <.05, significant difference between doses, collapsed across sex.
4. DISCUSSION
This study showed that an e‐cigarette‐based method of THC inhalation produces hypothermia in adolescent rats, and tolerance with repeated exposure. Adolescent rats of each sex became hypothermic after vapour inhalation of THC. However, repeated inhalation produced rapid tolerance only in the females and a persisting tolerance in both sexes when assessed as adults. This was observed after 2 weeks (M–F) of twice daily exposure to an inhalation regimen which produced similar plasma levels of THC across the sexes (Figure 5a), thus confirming the enhanced sensitivity of female rats to the acute development of tolerance given a similar exposure to THC. In addition, tolerance to the thermoregulatory effects of inhaled THC lasted long past the chronic regimen, as adults of each sex from the repeated THC groups were less sensitive to THC‐induced hypothermia compared with their respective repeated PG control groups when evaluated on PND 86 (Figure 4). The tolerance was dose‐specific in each sex since an increase in the THC concentration resulted in a similar hypothermia in each adolescent treatment group. Relatedly, there was a sex difference in the tolerance threshold during adulthood, that is, the concentration of THC which produced minimal hypothermia in the repeated THC group but a significant response in the repeated PG group. This dose‐effect difference may have been due to a sex difference in plasma THC concentrations produced during adulthood, particularly at lower effective dosing conditions (Figure 5c).
The THC‐induced temperature change in the repeated PG groups was smaller on PND 86 compared with PND 31, potentially due to the maturation of the rats and improved thermoregulation. This interpretation is consistent with the fact that body temperature observed 60 min after the start of inhalation on PND 86 was nearly identical to that observed in groups of naïve adult Wistar rats of the respective sexes following 30‐min inhalation of THC (100 mg·ml−1) with no exposure during adolescence (Javadi‐Paydar et al., 2018). As with our prior studies (Javadi‐Paydar et al., 2018; Nguyen, Aarde, et al., 2016), locomotor behaviour was not consistently affected by repeated THC. THC vapour inhalation increased activity rates slightly for about 30 min after inhalation ceased, and some tolerance to this appeared to be present in the female rats during the second week (Figure S2).
These results are qualitatively congruent with prior findings using parenteral injections or inhalation of THC. For example, adult female rats developed tolerance to THC to a greater extent than males, even with a lower per‐injection dose (Wakley, Wiley, & Craft, 2015). Tolerance to the acute locomotor stimulant effects of injected THC developed more rapidly in adolescent female rats compared with adult male rats (Wiley, Evans, Grainger, & Nicholson, 2011), and locomotor tolerance to THC injections was approximately equivalent in adolescent male and female rats after 9 days of repeated THC injection (Wiley & Burston, 2014). Our prior work found that female adult rats became tolerant after a repeated THC vapour inhalation regimen that did not produce tolerance in male adults (Nguyen, Grant, et al., 2018). Adult male rats (females were not assessed) exposed to repeated marijuana smoke did not exhibit cross‐tolerance to the locomotor suppressing effects of the endogenous cannabinoid agonist https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2364 (Bruijnzeel et al., 2016). The finding of significantly lower bodyweight in the males during the second treatment week (Figure S3) is similar to evidence that repeated THC injections during adolescence reduced weight gain in male and female Wistar and Long–Evans rats in a 14‐day treatment interval (Keeley, Trow, & McDonald, 2015) and in male Wistar rats during an 8‐day‐repeated injection study (Sofia & Barry, 1974). As a minor caveat, this study held age of drug exposure constant across sex, given a desire for face validity and the fact that human onset of marijuana use across sexes is better matched to chronological age rather than pubertal onset (Crane, Schuster, Mermelstein, & Gonzalez, 2015; Miech et al., 2018). Nevertheless, it would be of interest to compare a wider set of age ranges in future studies to further determine the role of pubertal events in any sex‐mediated differences.
The present findings are likely to be mechanistically attributable, in part, to plasticity in the expression and/or function of the endogenous https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56 receptors. One prior study of repeated adolescent THC exposure found decreased CB1 receptor expression in the hippocampus of female, but not male rats (Weed, Filipeanu, Ketchum, & Winsauer, 2016). Another study found greater CB1 receptor desensitization in adolescents, compared with adults, and in female adolescents compared with male adolescents, following repeated THC injection. Changes were found in several brain regions including, importantly for the present thermoregulatory data, hypothalamus (Burston, Wiley, Craig, Selley, & Sim‐Selley, 2010). One reason that adolescent female rats may be more sensitive to developing tolerance at a similar dose, brain, or plasma THC level is that an active THC metabolite, 11‐OH‐THC, reaches higher concentrations in brain, compared with male rats (Wiley & Burston, 2014).
There were no apparent effects of adolescent THC exposure on the intravenous self‐administration (IVSA) of oxycodone in either male or female rats. Analysed across sex (Figure S5), there were no treatment‐group differences in FR or PR dose substitution or infusions obtained during acquisition; however, the THC‐exposed animals increased their lever discrimination more slowly. There was a significant sex difference in acquisition and the FR dose effect (Figure 10) which contrasts with one prior report (Mavrikaki, Pravetoni, Page, Potter, & Chartoff, 2017), although this did not interact with adolescent treatment. The Mavrikaki et al. (2017) study used 1‐hr access sessions and the extended access model used here might be the reason for the sex difference in the current study. Alternatively, it has previously been reported that female rats self‐administer more https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1627, heroin, or fentanyl than do male rats (Cicero, Aylward, & Meyer, 2003; Klein, Popke, & Grunberg, 1997). Thus, it is not clear if sex differences in oxycodone self‐administration exist, apart from the procedural differences, across a very limited number of comparisons. The acquisition in the males was consistent with that observed using similar procedures in a group of experimentally naïve adult males (Nguyen et al., 2019). Unfortunately, the majority of rat oxycodone IVSA studies published so far have been in male rats (Austin Zamarripa et al., 2018; Blackwood et al., 2019; Bossert et al., 2018; Jordan et al., 2019; Leri & Burns, 2005; Mavrikaki et al., 2019; Nawarawong et al., 2019; Neelakantan et al., 2017; Nguyen et al., 2019; Nguyen, Hwang, et al., 2018; Pravetoni et al., 2014; Townsend et al., 2017; Wade et al., 2015; You et al., 2017; You et al., 2019), with one exception of a study in pregnant rats (Vassoler, Oranges, Toorie, & Byrnes, 2018). Interestingly, when female animals were evaluated on a fentanyl dose substitution under an FR procedure, the THC‐exposed rats self‐administered more drug at the lowest dose (Figure 9). Additional investigation of specific opioids, particularly those that differ in potency, might be warranted in future studies.
Lasting effects of adolescent THC exposure have been sometimes, but not always, found in other studies. Prior studies identified decreased bodyweight (Rubino et al., 2008), impaired spatial working memory (Rubino et al., 2009), and greater sensitivity to THC on a learning task (Winsauer et al., 2011) after repeated adolescent THC exposure. In contrast, repeated THC injection (3.2 mg·kg−1; 8 days) during adolescence did not affect THC place or taste conditioning in adulthood (Wakeford, Flax, Pomfrey, & Riley, 2016). Some prior studies have reported lasting motivational consequences; however, the two available studies regarding the effect of repeated adolescent THC exposure on heroin IVSA reached different conclusions. One study in male Wistar rats used a regimen of twice daily injection in an escalating sequence of 2.5 mg·kg−1 from PND 35–37, 5 mg·kg−1 from PND 38–41, and 10 mg·kg−1 from PND 42–45 (Stopponi et al., 2014). Another study injected male Long–Evans rats with THC (1.5 mg·kg−1, i.p.) every third day from PND 28–49 (Ellgren et al., 2007). Ellgren and colleagues reported increased heroin IVSA during acquisition as well as in a post‐acquisition dose substitution procedure under an FR1 contingency in the repeated THC group when evaluated as adults. In contrast, Stopponi and colleagues found no differences in the acquisition of heroin IVSA. The present study is consistent with the latter study and further work could determine if intermittency of exposure, overall dose, specific opioid drug, or some other factors are responsible for the differences. Due to limited prior study, and varying THC exposure regimens used, it is not possible to draw strong conclusions about the breadth or selectivity of lasting motivational consequences of repeated adolescent exposure at this time. Related to this, feeding behaviour was altered by repeated THC as the males consumed more food in 6‐hr focal feeding sessions compared with the PG control group. It will be interesting to determine in future studies if this reflects a motivational change, akin to that reported for heroin IVSA by Ellgren and colleagues.
In conclusion, the e‐cigarette inhalation method is effective for the repeated daily exposure of adolescent rats, generating physiologically significant THC exposure and plasma levels consistent with those reported for humans after marijuana smoking or vaping (Hartman et al., 2015; Huestis et al., 1992). The exposure produced tolerance in the female adolescents but not in the males, consistent with a sex difference found previously with repeated THC injections. There are also lasting consequences of adolescent exposure on the adult animal including thermoregulatory tolerance in each sex, nociceptive tolerance in female rats (Figure S4), a feeding phenotype in male rats, and increased fentanyl IVSA in female rats. This new approach has many advantages including that it avoids the stress of repeated injection, entails improved face validity, and may provide improved pharmacokinetic fidelity with human exposure patterns.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
M.A.T. and J.D.N. designed the studies, with refinements contributed by K.M.C. and T.M.K. J.D.N., K.M.C., and T.M.K. performed the research and conducted initial data analysis. J.D.N. and M.A.T. conducted statistical analysis of data, created figures, and wrote the paper. All authors approved of the submitted version of the manuscript.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/abs/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/abs/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1: Mean (N = 8 per sex; ±SEM) body temperature recorded for the first vapor inhalation session of each day is depicted for the repeated PG groups. The PND 30 PG data are depicted in upper and lower panels.
Figure S2: Mean (N = 8 per sex; ±SEM) activity rate (counts/minute) recorded for the first vapor inhalation session of each day is depicted for the repeated THC groups. The PND30 PG data are depicted in upper and lower panels. A significant difference from all other days at a given time after the start of inhalation is depicted by &, a significant difference from PG and the first day of the week by #, a significant difference from Days 6 and 7 by ^, and a significant difference from PG by *.
Figure S3: Mean (N = 8 per sex; ±SEM) bodyweight of the repeated‐PG and repeated‐THC groups of male and female rats are depicted. A significant difference between groups is indicated by *. A significant increase in weight within‐group relative to PND36 is indicated with # and an increase relative to PND42 with &.
Figure S4: Mean (N = 8 PG, N = 7 THC; +SEM) tail withdrawal latency following vapor inhalation of PG or THC (100 mg/mL). Open symbols indicate a significant difference from the pre‐inhalation baseline and the respective timepoint after PG inhalation, within‐group. A significant difference between groups is indicated with *.
Figure S5: Mean (±SEM) infusions and lever discrimination during acquisition for the PG (N = 20) vs THC (N = 19) treatment groups, collapsed across female (N = 15) and male (N = 24) sex. A significant difference from the first session across group is indicated with %, a difference from the first session within group by & and a significant difference between doses, collapsed across group is indicated with #.
Figure S6: Mean (±SEM) breakpoints reached during Oxycodone self‐administration grouped by A) adolescent‐treatment or; B) per‐infusion dose. C) Mean (±SEM) breakpoints reached in the Fentanyl dose substitution grouped by adolescent‐treatment.
ACKNOWLEDGEMENTS
This work was supported by USPHS Grants R01 DA035482 (Taffe, PI), R01 DA035281 (Taffe, PI) and R44 DA041967 (Cole, PI). The National Institutes of Health/NIDA had no direct influence on the design, conduct, analysis, or decision to publication of the findings. LJARI likewise did not influence the study designs, the data analysis, or the decision to publish findings. The authors are grateful to Shawn M. Aarde, PhD, for significant contributions to the invention and initial validation of the vapour inhalation method, to Mr Howard Britton for prototyping inhalation equipment, and to Eric Zorrilla, PhD, Allison Kreisler, PhD, and Sophia A. Vandewater for assistance with the feeding assay. This is manuscript #29759 from The Scripps Research Institute.
Substantial portions of this work were made available in pre‐print format (Nguyen, Creehan, Kerr, & Taffe, 2018), consistent with the policies of the National Institutes of Health which funded the work.
Nguyen JD, Creehan KM, Kerr TM, Taffe MA. Lasting effects of repeated ∆9‐tetrahydrocannabinol vapour inhalation during adolescence in male and female rats. Br J Pharmacol. 2020;177:188–203. 10.1111/bph.14856
REFERENCES
- Aarde, S. M. , Angrish, D. , Barlow, D. J. , Wright, M. J. Jr. , Vandewater, S. A. , Creehan, K. M. , … Taffe, M. A. (2013). Mephedrone (4‐methylmethcathinone) supports intravenous self‐administration in Sprague–Dawley and Wistar rats. Addiction Biology, 18, 786–799. 10.1111/adb.12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde, S. M. , Huang, P. K. , & Taffe, M. A. (2017). High ambient temperature facilitates the acquisition of 3,4‐methylenedioxymethamphetamine (MDMA) self‐administration. Pharmacology, Biochemistry, and Behavior, 163, 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin Zamarripa, C. , Edwards, S. R. , Qureshi, H. N. , Yi, J. N. , Blough, B. E. , & Freeman, K. B. (2018). The G‐protein biased mu‐opioid agonist, TRV130, produces reinforcing and antinociceptive effects that are comparable to oxycodone in rats. Drug and Alcohol Dependence, 192, 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood, C. A. , Hoerle, R. , Leary, M. , Schroeder, J. , Job, M. O. , McCoy, M. T. , … Cadet, J. L. (2019). Molecular adaptations in the rat dorsal striatum and hippocampus following abstinence‐induced incubation of drug seeking after escalated oxycodone self‐administration. Molecular Neurobiology, 56, 3603–3615. 10.1007/s12035-018-1318-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert, J. M. , Hoots, J. K. , Fredriksson, I. , Adhikary, S. , Zhang, M. , Venniro, M. , & Shaham, Y. (2018). Role of mu, but not ∆ or κ, opioid receptors in context‐induced reinstatement of oxycodone seeking. The European Journal of Neuroscience. 10.1111/ejn.13955 [DOI] [PubMed] [Google Scholar]
- Bruijnzeel, A. W. , Qi, X. , Guzhva, L. V. , Wall, S. , Deng, J. V. , Gold, M. S. , … Setlow, B. (2016). Behavioral characterization of the effects of cannabis smoke and anandamide in rats. PLoS ONE, 11, e0153327 10.1371/journal.pone.0153327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burston, J. J. , Wiley, J. L. , Craig, A. A. , Selley, D. E. , & Sim‐Selley, L. J. (2010). Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated ∆‐tetrahydrocannabinol exposure. British Journal of Pharmacology, 161, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha, Y. M. , Jones, K. H. , Kuhn, C. M. , Wilson, W. A. , & Swartzwelder, H. S. (2007). Sex differences in the effects of ∆9‐tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behavioural Pharmacology, 18, 563–569. [DOI] [PubMed] [Google Scholar]
- Cicero, T. J. , Aylward, S. C. , & Meyer, E. R. (2003). Gender differences in the intravenous self‐administration of mu opiate agonists. Pharmacology, Biochemistry, and Behavior, 74, 541–549. [DOI] [PubMed] [Google Scholar]
- Crane, N. A. , Schuster, R. M. , Mermelstein, R. J. , & Gonzalez, R. (2015). Neuropsychological sex differences associated with age of initiated use among young adult cannabis users. Journal of Clinical and Experimental Neuropsychology, 37, 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creehan, K. M. , Vandewater, S. A. , & Taffe, M. A. (2015). Intravenous self‐administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology, 92, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgren, M. , Spano, S. M. , & Hurd, Y. L. (2007). Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology, 32, 607–615. [DOI] [PubMed] [Google Scholar]
- George, C. H. , Stanford, S. C. , Alexander, S. , Cirino, G. , Docherty, J. R. , Giembycz, M. A. , … Ahluwalia, A. (2017). Updating the guidelines for data transparency in the British Journal of Pharmacology—Data sharing and the use of scatter plots instead of bar charts. British Journal of Pharmacology, 174, 2801–2804. 10.1111/bph.13925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman, R. L. , Brown, T. L. , Milavetz, G. , Spurgin, A. , Gorelick, D. A. , Gaffney, G. , & Huestis, M. A. (2015). Controlled cannabis vaporizer administration: Blood and plasma cannabinoids with and without alcohol. Clinical Chemistry, 61, 850–869. 10.1373/clinchem.2015.238287 [DOI] [PubMed] [Google Scholar]
- Hodos, W. (1961). Progressive ratio as a measure of reward strength. Science, 134, 943–944. [DOI] [PubMed] [Google Scholar]
- Huestis, M. A. , Henningfield, J. E. , & Cone, E. J. (1992). Blood cannabinoids. I. Absorption of THC and formation of 11‐OH‐THC and THCCOOH during and after smoking marijuana. Journal of Analytical Toxicology, 16, 276–282. [DOI] [PubMed] [Google Scholar]
- Irimia, C. , Polis, I. Y. , Stouffer, D. , & Parsons, L. H. (2015). Persistent effects of chronic ∆9‐THC exposure on motor impulsivity in rats. Psychopharmacology, 232, 3033–3043. [DOI] [PubMed] [Google Scholar]
- Javadi‐Paydar, M. , Creehan, K. M. , Kerr, T. M. , & Taffe, M. A. (2019). Vapor inhalation of cannabidiol (CBD) in rats. Pharmacology, Biochemistry, and Behavior, 184, 172741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi‐Paydar, M. , Kerr, T. M. , Harvey, E. L. , Cole, M. , & Taffe, M. A. (2019). Effects of nicotine and THC vapor inhalation administered by an electronic nicotine delivery system (ENDS) in male rats. Drug and Alcohol Dependence, 198, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi‐Paydar, M. , Nguyen, J. D. , Kerr, T. M. , Grant, Y. , Vandewater, S. A. , Cole, M. , & Taffe, M. A. (2018). Effects of ∆9‐THC and cannabidiol vapor inhalation in male and female rats. Psychopharmacology, 235, 2541–2557. 10.1007/s00213-018-4946-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, C. J. , Humburg, B. , Rice, M. , Bi, G. H. , You, Z. B. , Shaik, A. B. , … Xi, Z. X. (2019). The highly selective dopamine D3R antagonist, RVK4‐40, attenuates oxycodone reward and augments analgesia in rodents. Neuropharmacology, 107597 10.1016/j.neuropharm.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley, R. J. , Trow, J. , & McDonald, R. J. (2015). Strain and sex differences in puberty onset and the effects of THC administration on weight gain and brain volumes. Neuroscience, 305, 328–342. [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, L. C. , Popke, E. J. , & Grunberg, N. E. (1997). Sex differences in effects of predictable and unpredictable footshock on fentanyl self‐administration in rats. Experimental and Clinical Psychopharmacology, 5, 99–106. [DOI] [PubMed] [Google Scholar]
- Lacroix, C. , & Saussereau, E. (2012). Fast liquid chromatography/tandem mass spectrometry determination of cannabinoids in micro volume blood samples after dabsyl derivatization. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 905, 85–95. [DOI] [PubMed] [Google Scholar]
- Leri, F. , & Burns, L. H. (2005). Ultra‐low‐dose naltrexone reduces the rewarding potency of oxycodone and relapse vulnerability in rats. Pharmacology, Biochemistry, and Behavior, 82, 252–262. [DOI] [PubMed] [Google Scholar]
- Mavrikaki, M. , Anastasiadou, E. , Ozdemir, R. A. , Potter, D. , Helmholz, C. , Slack, F. J. , & Chartoff, E. H. (2019). Overexpression of miR‐9 in the nucleus accumbens increases oxycodone self‐administration. The International Journal of Neuropsychopharmacology, 22, 383–393. 10.1093/ijnp/pyz015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrikaki, M. , Pravetoni, M. , Page, S. , Potter, D. , & Chartoff, E. (2017). Oxycodone self‐administration in male and female rats. Psychopharmacology, 234, 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech, R. A. , Johnston, L. D. , O'Malley, P. M. , Bachman, J. G. , Schulenberg, J. E. , & Patrick, M. E. (2018). Monitoring the future national survey results on drug use, 1975‐2017 (Vol. I) (p. 590). Ann Arbor, MI: Secondary school students Institute for Social Research, The University of Michigan. [Google Scholar]
- Miller, M. L. , Aarde, S. M. , Moreno, A. Y. , Creehan, K. M. , Janda, K. D. , & Taffe, M. A. (2015). Effects of active anti‐methamphetamine vaccination on intravenous self‐administration in rats. Drug and Alcohol Dependence, 153, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M. L. , Creehan, K. M. , Angrish, D. , Barlow, D. J. , Houseknecht, K. L. , Dickerson, T. J. , & Taffe, M. A. (2013). Changes in ambient temperature differentially alter the thermoregulatory, cardiac and locomotor stimulant effects of 4‐methylmethcathinone (mephedrone). Drug and Alcohol Dependence, 127, 248–253. 10.1016/j.drugalcdep.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawarawong, N. N. , Slaker, M. , Muelbl, M. , Shah, A. S. , Chiariello, R. , Nelson, L. D. , … Olsen, C. M. (2019). Repeated blast model of mild traumatic brain injury alters oxycodone self‐administration and drug seeking. The European Journal of Neuroscience. 50(3) 10.1111/ejn.14281. Epub 2018 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan, H. , Holliday, E. D. , Fox, R. G. , Stutz, S. J. , Comer, S. D. , Haney, M. , … Cunningham, K. A. (2017). Lorcaserin suppresses oxycodone self‐administration and relapse vulnerability in rats. ACS Chemical Neuroscience, 8, 1065–1073. 10.1021/acschemneuro.6b00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, J. D. , Aarde, S. M. , Vandewater, S. A. , Grant, Y. , Stouffer, D. G. , Parsons, L. H. , … Taffe, M. A. (2016). Inhaled delivery of ∆(9)‐tetrahydrocannabinol (THC) to rats by e‐cigarette vapor technology. Neuropharmacology, 109, 112–120. 10.1016/j.neuropharm.2016.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, J. D. , Bremer, P. T. , Ducime, A. , Creehan, K. M. , Kisby, B. R. , Taffe, M. A. , & Janda, K. D. (2016). Active vaccination attenuates the psychostimulant effects of α‐PVP and MDPV in rats. Neuropharmacology, 116, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, J. D. , Creehan, K. M. , Kerr, T. M. , & Taffe, M. A. (2018). Repeated Δ9‐tetrahydrocannabinol (THC) vapor inhalation during adolescence: Sex differences in acute thermoregulatory tolerance and in feeding during adulthood. bioRxiv.: 10.1101/426064 Version: https://www.biorxiv.org/content/10.1101/426064v1 [DOI]
- Nguyen, J. D. , Grant, Y. , Creehan, K. M. , Hwang, C. S. , Vandewater, S. A. , Janda, K. D. , … Taffe, M. A. (2019). ∆(9)‐tetrahydrocannabinol attenuates oxycodone self‐administration under extended access conditions. Neuropharmacology, 151, 127–135. 10.1016/j.neuropharm.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, J. D. , Grant, Y. , Creehan, K. M. , Vandewater, S. A. , & Taffe, M. A. (2017). Escalation of intravenous self‐administration of methylone and mephedrone under extended access conditions. Addiction Biology, 22, 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, J. D. , Grant, Y. , Kerr, T. M. , Gutierrez, A. , Cole, M. , & Taffe, M. A. (2018). Tolerance to hypothermic and antinoceptive effects of 9‐tetrahydrocannabinol (THC) vapor inhalation in rats. Pharmacology, Biochemistry, and Behavior, 172, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, J. D. , Hwang, C. S. , Grant, Y. , Janda, K. D. , & Taffe, M. A. (2018). Prophylactic vaccination protects against the development of oxycodone self‐administration. Neuropharmacology, 138, 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni, M. , Pentel, P. R. , Potter, D. N. , Chartoff, E. H. , Tally, L. , & LeSage, M. G. (2014). Effects of an oxycodone conjugate vaccine on oxycodone self‐administration and oxycodone‐induced brain gene expression in rats. PLoS ONE, 9, e101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson, N. R. & Roberts, D.C. (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of Neuroscience Methods, 66, 1-11 [DOI] [PubMed] [Google Scholar]
- Rubino, T. , Realini, N. , Braida, D. , Guidi, S. , Capurro, V. , Vigano, D. , … Parolaro, D. (2009). Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus, 19, 763–772. 10.1002/hipo.20554 [DOI] [PubMed] [Google Scholar]
- Rubino, T. , Vigano, D. , Realini, N. , Guidali, C. , Braida, D. , Capurro, V. , … Parolaro, D. (2008). Chronic ∆ 9‐tetrahydrocannabinol during adolescence provokes sex‐dependent changes in the emotional profile in adult rats: Behavioral and biochemical correlates. Neuropsychopharmacology, 33, 2760–2771. 10.1038/sj.npp.1301664 [DOI] [PubMed] [Google Scholar]
- Segal, D. S. , & Mandell, AJ (1974). Long‐term administration of d‐amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacology Biochemistry and Behavior, 2, 249–255. [DOI] [PubMed] [Google Scholar]
- Sofia, R. D. , & Barry, H. 3rd (1974). Acute and chronic effects of ∆9‐tetrahydrocannabinol on food intake by rats. Psychopharmacologia, 39, 213–222. [DOI] [PubMed] [Google Scholar]
- Stopponi, S. , Soverchia, L. , Ubaldi, M. , Cippitelli, A. , Serpelloni, G. , & Ciccocioppo, R. (2014). Chronic THC during adolescence increases the vulnerability to stress‐induced relapse to heroin seeking in adult rats. European Neuropsychopharmacology, 24, 1037–1045. [DOI] [PubMed] [Google Scholar]
- Taffe, M. A. , Creehan, K. M. , & Vandewater, S. A. (2015). Cannabidiol fails to reverse hypothermia or locomotor suppression induced by ∆(9)‐tetrahydrocannabinol in Sprague–Dawley rats. British Journal of Pharmacology, 172, 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend, E. A. , Naylor, J. E. , Negus, S. S. , Edwards, S. R. , Qureshi, H. N. , McLendon, H. W. , … Freeman, K. B. (2017). Effects of nalfurafine on the reinforcing, thermal antinociceptive, and respiratory‐depressant effects of oxycodone: Modeling an abuse‐deterrent opioid analgesic in rats. Psychopharmacology, 234, 2597–2605. 10.1007/s00213-017-4652-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewater, S. A. , Creehan, K. M. , & Taffe, M. A. (2015). Intravenous self‐administration of entactogen‐class stimulants in male rats. Neuropharmacology, 99, 538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler, F. M. , Oranges, M. L. , Toorie, A. M. , & Byrnes, E. M. (2018). Oxycodone self‐administration during pregnancy disrupts the maternal–infant dyad and decreases midbrain OPRM1 expression during early postnatal development in rats. Pharmacology, Biochemistry, and Behavior, 173, 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade, C. L. , Vendruscolo, L. F. , Schlosburg, J. E. , Hernandez, D. O. , & Koob, G. F. (2015). Compulsive‐like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology, 40, 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeford, A. G. , Flax, S. M. , Pomfrey, R. L. , & Riley, A. L. (2016). Adolescent ∆‐9‐tetrahydrocannabinol (THC) exposure fails to affect THC‐induced place and taste conditioning in adult male rats. Pharmacology, Biochemistry, and Behavior, 140, 75–81. [DOI] [PubMed] [Google Scholar]
- Wakley, A. A. , Wiley, J. L. , & Craft, R. M. (2014). Sex differences in antinociceptive tolerance to ∆‐9‐tetrahydrocannabinol in the rat. Drug and Alcohol Dependence, 143, 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakley, A. A. , Wiley, J. L. , & Craft, R. M. (2015). Gonadal hormones do not alter the development of antinociceptive tolerance to ∆‐9‐tetrahydrocannabinol in adult rats. Pharmacology, Biochemistry, and Behavior, 133, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed, P. F. , Filipeanu, C. M. , Ketchum, M. J. , & Winsauer, P. J. (2016). Chronic ∆9‐tetrahydrocannabinol during adolescence differentially modulates striatal CB1 receptor expression and the acute and chronic effects on learning in adult rats. The Journal of Pharmacology and Experimental Therapeutics, 356, 20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley, J. L. , & Burston, J. J. (2014). Sex differences in ∆(9)‐tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neuroscience Letters, 576, 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley, J. L. , Evans, R. L. , Grainger, D. B. , & Nicholson, K. L. (2011). Locomotor activity changes in female adolescent and adult rats during repeated treatment with a cannabinoid or club drug. Pharmacological Reports, 63, 1085–1092. [DOI] [PubMed] [Google Scholar]
- Winsauer, P. J. , Daniel, J. M. , Filipeanu, C. M. , Leonard, S. T. , Hulst, J. L. , Rodgers, S. P. , … Sutton, J. L. (2011). Long‐term behavioral and pharmacodynamic effects of ∆‐9‐tetrahydrocannabinol in female rats depend on ovarian hormone status. Addiction Biology, 16, 64–81. 10.1111/j.1369-1600.2010.00227.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, M. J. Jr. , Angrish, D. , Aarde, S. M. , Barlow, D. J. , Buczynski, M. W. , Creehan, K. M. , … Taffe, M. A. (2012). Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4‐methylmethcathinone in Wistar and Sprague‐Dawley rats. PLoS ONE, 7, e44652 10.1371/journal.pone.0044652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, Z. B. , Bi, G. H. , Galaj, E. , Kumar, V. , Cao, J. , Gadiano, A. , … Newman, A. H. (2019). Dopamine D3R antagonist VK4‐116 attenuates oxycodone self‐administration and reinstatement without compromising its antinociceptive effects. Neuropsychopharmacology, 44(8), 1415–1424. 10.1038/s41386-018-0284-5. Epub 2018 Nov 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, Z. B. , Gao, J. T. , Bi, G. H. , He, Y. , Boateng, C. , Cao, J. , … Xi, Z. X. (2017). The novel dopamine D3 receptor antagonists/partial agonists CAB2‐015 and BAK4‐54 inhibit oxycodone‐taking and oxycodone‐seeking behavior in rats. Neuropharmacology, 126, 190–199. 10.1016/j.neuropharm.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Mean (N = 8 per sex; ±SEM) body temperature recorded for the first vapor inhalation session of each day is depicted for the repeated PG groups. The PND 30 PG data are depicted in upper and lower panels.
Figure S2: Mean (N = 8 per sex; ±SEM) activity rate (counts/minute) recorded for the first vapor inhalation session of each day is depicted for the repeated THC groups. The PND30 PG data are depicted in upper and lower panels. A significant difference from all other days at a given time after the start of inhalation is depicted by &, a significant difference from PG and the first day of the week by #, a significant difference from Days 6 and 7 by ^, and a significant difference from PG by *.
Figure S3: Mean (N = 8 per sex; ±SEM) bodyweight of the repeated‐PG and repeated‐THC groups of male and female rats are depicted. A significant difference between groups is indicated by *. A significant increase in weight within‐group relative to PND36 is indicated with # and an increase relative to PND42 with &.
Figure S4: Mean (N = 8 PG, N = 7 THC; +SEM) tail withdrawal latency following vapor inhalation of PG or THC (100 mg/mL). Open symbols indicate a significant difference from the pre‐inhalation baseline and the respective timepoint after PG inhalation, within‐group. A significant difference between groups is indicated with *.
Figure S5: Mean (±SEM) infusions and lever discrimination during acquisition for the PG (N = 20) vs THC (N = 19) treatment groups, collapsed across female (N = 15) and male (N = 24) sex. A significant difference from the first session across group is indicated with %, a difference from the first session within group by & and a significant difference between doses, collapsed across group is indicated with #.
Figure S6: Mean (±SEM) breakpoints reached during Oxycodone self‐administration grouped by A) adolescent‐treatment or; B) per‐infusion dose. C) Mean (±SEM) breakpoints reached in the Fentanyl dose substitution grouped by adolescent‐treatment.


