Graphical abstract
Keywords: Caco-2 cells, Octanol–water distribution coefficient, Multivariate prediction equation, Fraction absorbed, No-observed-effect level
Highlights
-
•
Permeability values of 90 industry chemicals were measured by a Caco-2 system.
-
•
A multivariate prediction equation for permeability of chemicals was proposed.
-
•
Chemical permeability coefficients were inversely associated with hepatic NOELs.
Abstract
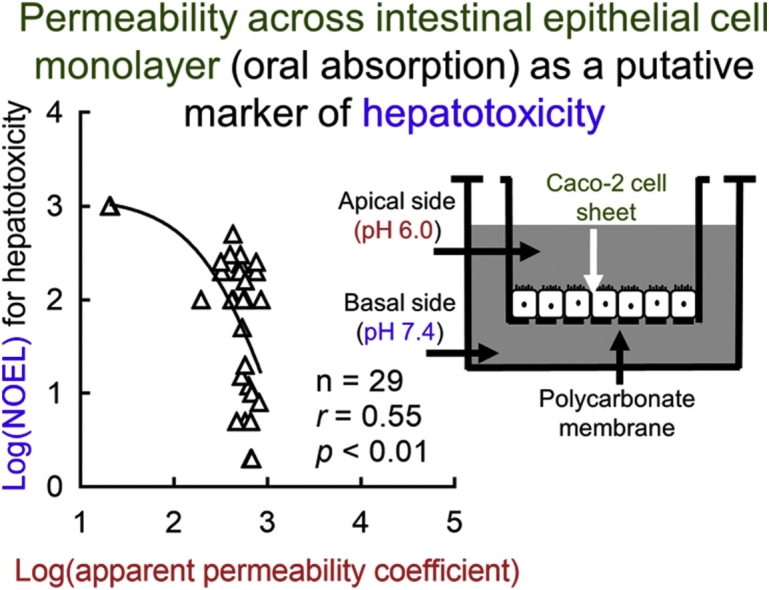
Apparent permeability coefficients (Papp) across a human intestinal epithelial Caco-2 cell monolayer were measured for a range of industrial/drug chemicals. A predictive equation for determining in vitro Papp values of fifty-six substances was set up using multivariate regression analysis based on in silico-estimated physicochemical properties (molecular weights and water distribution coefficients for apical and basal pH environments) (r = 0.77, p < 0.01). Predicted logPapp values of a secondary set of 34 compounds were correlated with the measured values. Under the medicinal logPapp values associated with their reported fraction absorbed, a significant inverse non-linear correlation was found between the logarithmic transformed values of observed Papp values and reported hepatic no-observed-effect levels of industrial chemicals (r = –0.55, p < 0.01, n = 29). In vitro determination and/or in silico prediction of permeability across intestinal cells could be effective for estimating oral absorption as a putative indicator for hepatotoxicity.
1. Introduction
Current experimental testing methods for estimation of the human risks of industrial chemicals generally require toxicological studies in experimental animals. Such studies include repeated oral doses to rodents for 28 days and employ procedures that adhere to guidance such as Organization for Economic Co-operation and Development test guidelines. Although big toxicity databases have been widely set up, limited numbers of chemicals only possess adequate toxicokinetic data in vivo regarding parameters (such as oral absorption rates) for assessing human potential hazards [1]. The in vitro permeability assay for oral absorption in pharmaceutical research is a kind of established methods and is principally based on using human colon cancer cell line Caco-2 systems [[2], [3], [4], [5], [6]]. Studies that attempted to predict the permeability of drugs and druglike chemicals across Caco-2 cell monolayers have been performed as part of preclinical drug development [[7], [8], [9]]. However, little information has been provided on the oral absorption of industrial chemicals through gastrointestinal absorption and/or the mucosa, which is a necessary phase before such chemicals could exert their potential toxicity. It would be of great benefit for industrial chemicals if it were possible to derive the oral absorption parameters in vivo of general chemicals from established in vitro permeability values.
In the present study, we evaluated the permeability of a broad range of general chemical substances (for which the oral absorption is not commonly investigated) using a pH-dependent Caco-2 monolayer system. A multivariate prediction equation derived from the permeability coefficients of 56 disparate compounds was proposed. The input parameters for this equation were the in silico physicochemical properties of the compounds. This prediction equation was then used to estimate the permeability of a secondary set of 34 compounds. We report herein that the Caco-2 cell permeability coefficients of 28 industrial chemicals and acetaminophen were inversely associated with their hepatic no-observed-effect levels (NOELs).
2. Materials and methods
2.1. Materials and chemical properties
The chemicals tested for permeability in the Caco-2 cell system (shown in Table 1, Table 2) were of analytical grade and were obtained from Fujifilm Wako Pure Chemical (Osaka, Japan), Tokyo Chemical Industry (Tokyo, Japan) or from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum, nonessential amino acids, penicillin–streptomycin–amphotericin B suspension, and Hank’s balanced salt solution (HBSS) were obtained from Fujifilm Wako Pure Chemical. 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 2-morpholinoethanesulfonic acid monohydrate (MES) were purchased from Sigma-Aldrich. Cell culture dishes (100 mm) and Transwell plates (12-well, pore size: 0.4 μm, growth area: 1.12 cm2) were obtained from Corning (Corning, NY, USA).
Table 1.
Measured Permeability Coefficients of 56 Compounds with Their Physicochemical Properties and Reported Fraction Absorbed (Fa) and/or Hepatic No-observed-effect Levels (NOEL).
| compound | Cas No. | Pappa, nm/s | molecular weight | logP | logDapical | logDbasal | reported human Fa, % | hepatic NOEL, mg/kg/day |
|---|---|---|---|---|---|---|---|---|
| 2-aminobiphenyl | 90-41-5 | 576 ± 11 a | 169 | 3.05 | 3.05 | 3.05 | 100 | |
| 3-aminobenzenesulfonic acid | 121-47-1 | 21 ± 3 a | 173 | –4.26 | –5.65 | –5.71 | 1000 | |
| 5-amino-2-chlorotoluene-4-sulfonic acid | 88-53-9 | 20 ± 2 a | 222 | –3.17 | –3.31 | –3.33 | 1000 | |
| 3-aminophenol | 591-27-5 | 513 ± 23 a | 109 | –0.05 | –0.06 | –0.05 | 240 | |
| aniline | 62-53-3 | 544 ± 26 | 93 | 1.03 | 1.01 | 1.03 | ||
| atenolol | 29122-68-7 | 5 ± 1 | 266 | 0.01 | –2.97 | –1.56 | 50 [7] | |
| atomoxetine | 83015-26-3 | 27 ± 1 | 255 | 4.21 | 1.01 | 2.33 | 100 [8] | |
| benzimidazole | 51-17-2 | 730 ± 6 | 118 | 0.24 | 0.13 | 0.24 | ||
| benzoic acid | 65-85-0 | 1490 ± 160 | 122 | 1.55 | –0.42 | –1.92 | ||
| benzydamine | 642-72-8 | 16 ± 1 | 309 | 4.39 | 1.81 | 2.63 | 100 [23] | |
| bisphenol A | 80-05-7 | 321 ± 13 a | 228 | 4.48 | 4.48 | 4.47 | 200 | |
| caffeine | 58-08-2 | 544 ± 12 | 194 | 0.95 | 0.95 | 0.95 | 100 [24] | |
| 2-chloroaniline | 95-51-2 | 893 ± 26 | 128 | 1.74 | 1.74 | 1.74 | ||
| cotinine | 486-56-6 | 412 ± 29 | 176 | 1.02 | 1.01 | 1.02 | 90 [8] | |
| 3-cyanopyridine | 100-54-9 | 569 ± 70 a | 104 | 0.58 | 0.58 | 0.58 | 5 | |
| dexamethasone | 50-02-2 | 95 ± 14 | 392 | 2.63 | 2.63 | 2.63 | 90 [8] | |
| diclofenac | 15307-86-5 | 756 ± 6 | 296 | 4.14 | 2.25 | 0.77 | 82 [25] | |
| dihydrocodeine | 125-28-0 | 24 ± 1 | 301 | 2.99 | 0.47 | 0.95 | ||
| diphenylamine | 122-39-4 | 151 ± 11 | 169 | 3.53 | 3.53 | 3.53 | ||
| 1,3-dinitrobenzene | 99-65-0 | 536 ± 35 | 168 | 1.51 | 1.51 | 1.51 | ||
| 2,3-dimethylaniline | 87-59-2 | 624 ± 30 a | 121 | 1.90 | 1.87 | 1.90 | 12 | |
| 2,4-dimethylaniline | 95-68-1 | 661 ± 20 a | 121 | 1.92 | 1.88 | 1.92 | 2 | |
| 3,4-dimethylaniline | 95-64-7 | 541 ± 58 a | 121 | 2.01 | 1.93 | 2.01 | 50 | |
| 3,5-dimethylaniline | 108-69-0 | 674 ± 113 a | 121 | 2.08 | 2.03 | 2.08 | 10 | |
| hippuric acid | 495-69-2 | 6 ± 1 | 179 | –0.46 | –2.49 | –3.79 | ||
| 2-hydroxybenzimidazole | 615-16-7 | 507 ± 16 | 134 | –0.97 | –1.52 | –1.53 | ||
| 4-hydroxybiphenyl | 92-69-3 | 441 ± 28 | 170 | 3.89 | 3.89 | 3.88 | ||
| isophthalonitrile | 626-17-5 | 805 ± 8 a | 128 | 1.48 | 1.48 | 1.48 | 8 | |
| lenalidomide | 191732-72-6 | 7 ± 1 | 259 | –1.03 | –1.03 | –1.04 | 90 [26] | |
| lucifer yellow | 67769-47-5 | 7 ± 1 | 445 | –4.80 | –13.1 | –13.3 | 0 [27] | |
| m-cresol | 108-39-4 | 851 ± 35 a | 108 | 2.21 | 2.21 | 2.21 | 100 | |
| 2-mercaptobenzimidazole | 583-39-1 | 673 ± 18 a | 150 | 0.64 | –3.74 | –3.76 | 2 | |
| metoprolol | 51384-51-1 | 34 ± 1 | 267 | 2.20 | –0.80 | 0.61 | 98 [7] | |
| midazolam | 59467-70-8 | 318 ± 19 | 326 | 4.54 | 4.20 | 4.49 | 60 [28] | |
| mono(2-ethylhexyl) phthalate | 4376-20-9 | 467 ± 18 | 278 | 4.93 | 3.16 | 1.67 | ||
| monobutyl phthalate | 131-70-4 | 318 ± 8 | 222 | 3.35 | 1.58 | 0.09 | ||
| N,N-dimethylaniline | 121-69-7 | 999 ± 129 | 121 | 2.17 | 2.07 | 2.16 | ||
| N-ethylaniline | 103-69-5 | 660 ± 10 a | 121 | 2.10 | 2.04 | 2.10 | 5 | |
| nicotine | 54-11-5 | 57 ± 6 | 162 | 2.07 | 0.23 | 1.56 | 100 [8] | |
| nifedipine | 21829-25-4 | 424 ± 44 | 346 | 2.21 | 2.21 | 2.21 | 100 [8] | |
| 3-nitroaniline | 99-09-2 | 520 ± 50 a | 138 | 1.92 | 1.92 | 1.92 | 15 | |
| 2-nitrotoluene | 88-72-2 | 576 ± 46 | 137 | 2.28 | 2.28 | 2.28 | ||
| N-methylaniline | 100-61-8 | 463 ± 50 a | 107 | 1.59 | 1.55 | 1.59 | 5 | |
| o-cresol | 95-48-7 | 905 ± 64 | 108 | 1.82 | 1.82 | 1.82 | ||
| p-cresol | 106-44-5 | 507 ± 45 | 108 | 2.21 | 2.21 | 2.21 | ||
| phthalimide | 85-41-6 | 933 ± 69 | 147 | 0.30 | 0.30 | 0.30 | ||
| p-hydroxybenzoic acid | 99-96-7 | 609 ± 30 | 138 | 1.73 | –0.10 | –1.61 | ||
| pomalidomide | 19171-19-8 | 466 ± 57 | 273 | –0.03 | –0.03 | –0.04 | 73 [29] | |
| progesterone | 57-83-0 | 113 ± 20 | 315 | 4.18 | 4.18 | 4.18 | ||
| propranolol | 525-66-6 | 29 ± 3 | 259 | 3.07 | 0.15 | 1.57 | 90 [24] | |
| quetiapine | 111974-69-7 | 38 ± 4 | 384 | 2.61 | 2.29 | 2.60 | 73 [30] | |
| terephthalonitrile | 623-26-7 | 573 ± 13 a | 128 | 1.48 | 1.48 | 1.48 | 20 | |
| thalidomide | 50-35-1 | 235 ± 19 | 258 | 0.36 | 0.36 | 0.34 | ||
| tolbutamide | 64-77-7 | 1220 ± 110 | 270 | 2.58 | 2.06 | 0.71 | 88 [25] | |
| trimethylamine | 75-50-3 | 33 ± 1 | 59 | 0.76 | –1.77 | –1.43 | ||
| warfarin | 81-81-2 | 1210 ± 50 | 308 | 2.33 | 1.83 | 0.49 | 98 [8] |
Observed Papp value represents the mean of triplicate determinations with standard deviation in this study. Physicochemical properties were calculated using the SPARC physicochemical calculator as mentioned in Materials and Methods. NOEL values for hepatotoxicity of chemical substances were obtained from the Hazard Evaluation Support System Integrated Platform [12].
Results (without SD values) of 17 compounds are reported in our study [10].
Table 2.
Predicted and Observed logPapp Values of a Secondary Set of 34 Compounds and Their Reported Fraction Absorbed (Fa) and/or Hepatic NOEL Values.
| compound | CAS No. | molecular weight | logDapical | logDbasal | predicteda logPapp | observed Papp nm/s | observed logPapp | reported human Fa, % | hepatic NOEL mg/kg/day |
|---|---|---|---|---|---|---|---|---|---|
| acetaminophen | 103-90-2 | 151 | 0.09 | 0.09 | 2.42 | 319 ± 14 | 2.50 | 100 [23] | 250 [11] |
| azamethiphos | 35575-96-3 | 325 | 2.58 | 2.58 | 2.14 | 402 ± 18 | 2.60 | ||
| bisphenol F | 620-92-8 | 200 | 3.61 | 3.60 | 2.66 | 415 ± 21 | 2.62 | 100 | |
| bisphenol S | 80-09-1 | 250 | 1.26 | 1.11 | 2.29 | 503 ± 35 | 2.70 | 200 | |
| carbamazepine | 298-46-4 | 236 | 3.64 | 3.64 | 2.54 | 380 ± 14 | 2.58 | ||
| 4-chloro-o-cresol | 1570-64-5 | 143 | 2.51 | 2.51 | 2.71 | 754 ± 39 | 2.88 | 250 | |
| 2-chlorophenol | 95-57-8 | 129 | 2.13 | 2.07 | 2.73 | 752 ± 83 | 2.88 | 200 | |
| 4-chlorophenol | 106-48-9 | 129 | 2.26 | 2.26 | 2.73 | 431 ± 37 | 2.63 | 500 | |
| cimetidine | 51481-61-9 | 252 | –0.79 | –0.58 | 1.92 | 17 ± 2 | 1.22 | 68 [7] | |
| coumarin | 91-64-5 | 146 | 0.85 | 0.85 | 2.52 | 806 ± 54 | 2.91 | 100 [13] | |
| 4-α-cumylphenol | 599-64-4 | 212 | 4.99 | 4.99 | 2.77 | 195 ± 34 | 2.29 | 100 | |
| dabigatran | 211915-06-9 | 472 | 0.26 | −1.19 | 1.97 | 38 ± 17 | 1.58 | ||
| disopyramide | 3737-09-5 | 340 | –0.70 | 0.79 | 1.17 | 14 ± 3 | 1.16 | 83 [7] | |
| 7-ethoxycoumarin | 31005-02-4 | 190 | 1.94 | 1.94 | 2.50 | 750 ± 48 | 2.88 | ||
| 3-ethylphenol | 620-17-7 | 122 | 2.75 | 2.75 | 2.80 | 515 ± 50 | 2.71 | 300 | |
| 4-ethylphenol | 123-07-9 | 122 | 2.76 | 2.75 | 2.81 | 437 ± 23 | 2.64 | 100 | |
| fluvoxamine | 54739-18-3 | 318 | 1.85 | 2.88 | 1.69 | 23 ± 3 | 1.37 | ||
| 2-hydroxybiphenyl | 90-43-7 | 170 | 3.72 | 3.72 | 2.76 | 334 ± 29 | 2.52 | ||
| 3-hydroxybiphenyl | 580-51-8 | 170 | 3.88 | 3.88 | 2.78 | 284 ± 22 | 2.45 | ||
| 7-hydroxycoumarin | 93-35-6 | 162 | 0.24 | 0.01 | 2.49 | 1030 ± 170 | 3.01 | ||
| itopride | 122898-67-3 | 358 | 0.15 | 1.40 | 1.29 | 12 ± 3 | 1.09 | ||
| lovastatin | 75330-75-5 | 405 | 4.04 | 4.04 | 2.05 | 21 ± 1 | 1.32 | 31 [7] | |
| mefenamic acid | 61-68-7 | 241 | 3.78 | 2.29 | 3.11 | 1804 ± 83 | 3.26 | ||
| 2-mercaptoimidazole | 872-35-5 | 100 | –4.91 | –4.91 | 2.02 | 91 ± 3 | 1.96 | ||
| methotrexate | 59-05-2 | 454 | –4.60 | –7.46 | 2.03 | 11 ± 2 | 1.04 | 20 [24] | |
| 2-methoxy-4-nitroaniline | 97-52-9 | 168 | 1.71 | 1.71 | 2.54 | 552 ± 70 | 2.74 | 100 | |
| mirtazapine | 85650-52-8 | 265 | 2.10 | 3.03 | 1.93 | 46 ± 3 | 1.66 | 80 [7] | |
| olanzapine | 132539-06-1 | 312 | 3.00 | 3.29 | 2.12 | 35 ± 3 | 1.54 | ||
| omeprazole | 73590-58-6 | 345 | 1.60 | 1.63 | 1.96 | 674 ± 69 | 2.83 | 95 [7] | |
| p-aminobenzoic acid | 150-13-0 | 137 | 0.40 | −1.08 | 3.06 | 587 ± 40 | 2.77 | ||
| p-phenetidine | 156-43-4 | 137 | 1.42 | 1.46 | 2.59 | 582 ± 31 | 2.76 | 160 | |
| pravastatin | 81093-37-0 | 425 | –0.11 | –1.57 | 2.08 | 9 ± 1 | 0.95 | 13 [31] | |
| 4-sec-butylphenol | 99-71-8 | 150 | 3.70 | 3.70 | 2.82 | 402 ± 19 | 2.60 | 300 | |
| verapamil | 52-53-9 | 455 | 0.58 | 2.01 | 0.97 | 23 ± 1 | 1.36 | 100 [7] |
Predicted using the following equation: LogPapp = 2.9 − 0.0032 × (molecular weight) + 0.49 × (logDapical) − 0.38 × (logDbasal). Observed Papp value represents the mean of triplicate determinations with standard deviation in this study.
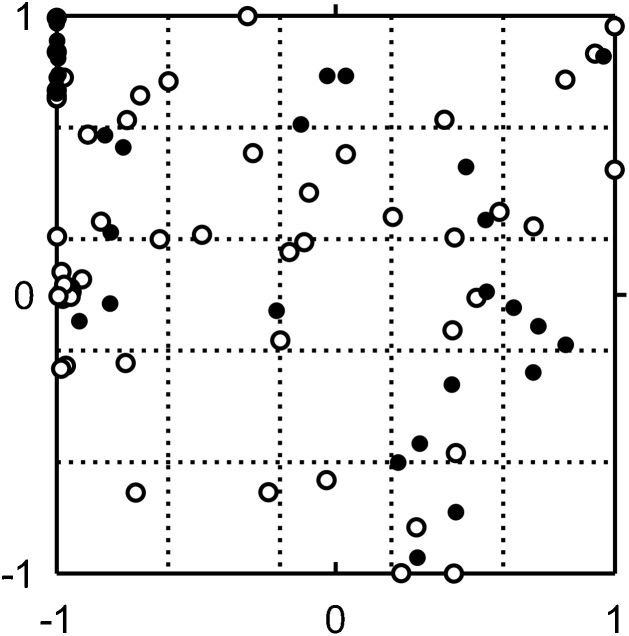
The broad diversity of the tested chemical substances is illustrated in a two-dimensional plane depicting the wide chemical space (Fig. 1), as described previously [10]. Briefly, the structures described by 196 chemical descriptors were calculated using the chemoinformatics tool RDKit and projected using generative topographic mapping methods onto a two-dimensional plane [10]. In Fig. 1, closer plots in the illustrated chemical space could indicate some similarity in their material properties. The molecular weights (MW), the octanol–water partition coefficients (logP), and the octanol–water distribution coefficients for Caco-2 cell apical and basal pH environments (logDapical and logDbasal, respectively) of the tested chemical substances and drugs were calculated based on their chemical structures using the Sparc physicochemical calculator (ARChem, Atlanta, GA, USA).
Fig. 1.
Coordinate values in a two-dimensional plane illustrating variety in the chemical space for the primary set of 56 compounds (open circles) and the secondary set of 34 (solid circles) compounds evaluated using Caco-2 permeability assays.
2.2. Permeation studies and permeability coefficients
The general procedures employed to prepare in vitro human intestinal Caco-2 monolayers were described previously [10]. Briefly, Caco-2 cells (American Type Culture Collection, Manassas, VA, USA; passages: 20–65) were cultured in DMEM supplemented with nonessential amino acids, penicillin–streptomycin–amphotericin B, and fetal bovine serum at 37 °C under a 5 % CO2 atmosphere. For experimental use, the cells were seeded on permeable polycarbonate Transwell membranes at a density of 1.0 × 105 cells/cm2 and were cultured for 21–28 days. Before and after the experiments, the integrity of the Caco-2 cell monolayers was evaluated by measuring the transepithelial electrical resistance (TEER) using a Voltohmmeter (Millicell ERS-2, Merck, Darmstadt, Germany); only Caco-2 cell monolayers with a TEER value of > 200 Ω·cm2 at pre-and post-incubations were used for the current experiments.
The apparent experimental permeability coefficients (Papp, nm/s) were calculated for time-dependent absorption in vitro from the apical side of the Caco-2 monolayer in HBSS with 10 mM MES (pH 6.0) to the basal side of the Caco-2 monolayer in HBSS with 10 mM HEPES (pH 7.4), as described previously [10] with slight modification. Briefly, 1–100 μM (dependent on the solubility of each substrate) of test substance in a final concentration of < 0.1 % dimethyl sulfoxide (originally dissolved in dimethyl sulfoxide and diluted with Hank’s balanced salt solution) was applied to the apical side of Caco-2 cells cultured on Transwell plates. Caffeine and lucifer yellow were used as positive and negative permeability controls, respectively. The amounts of the test substances in permeation samples from the basal sides were measured by high-performance liquid chromatography or liquid chromatography–mass spectrometry [10]. The experiment for each chemical substance was performed in triplicate determinations.
2.3. Statistical analysis
Univariate and multivariate linear regression analyses were performed using Prism software (GraphPad Software, San Diego, CA, USA). The relationships among logPapp values of chemicals experimentally determined in vitro, their physicochemical properties estimated in silico, and reported in vivo toxicological properties [the no-observed-effect level (NOEL)] for hepatotoxicity taken from the Hazard Evaluation Support System Integrated Platform in Japan and literature were investigated [11,12].
3. Results
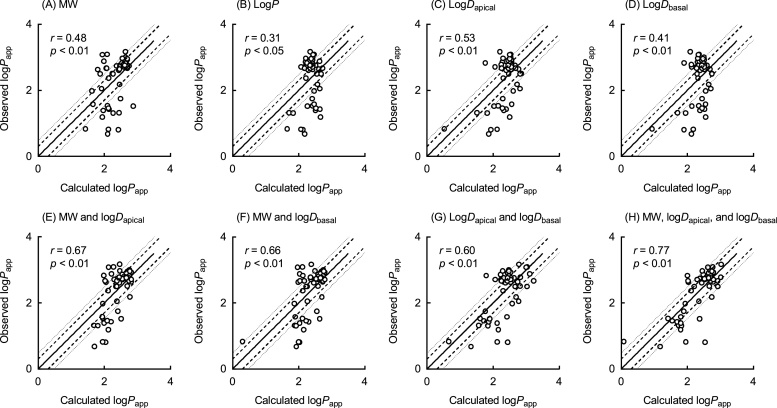
The Papp values of more than 50 disparate types of chemicals (Fig. 1) were measured and are shown in Table 1. The observed Papp values of 56 compounds varied in the range 5–1490 nm/s. The physicochemical properties (MW, logP, logDapical, and logDbasal) of the 56 chemicals were estimated using in silico methods and are shown in Table 1. To investigate the feasibility of establishing a predictive equation, we carried out various analyses to identify the relationships between logPapp values and the compounds’ physicochemical parameters. Univariate linear regression analyses revealed that, under the present conditions, the observed logPapp values (Table 1) were correlated with the corresponding MW (r = 0.48, p < 0.01, n = 56, Fig. 2A), logP values (r = 0.31, p < 0.05, n = 56, Fig. 2B), logDapical values (r = 0.53, p < 0.01, n = 56, Fig. 2C), and logDbasal values (r = 0.41, p < 0.01, n = 56, Fig. 2D). Because logP values univariately showed a low correlation coefficient, further analyses were performed with the rest of three chemical parameters, MW, logDapical and logDbasal values. Bivariate analyses established that logPapp values were correlated with the MW and logDapical values (r = 0.67, p < 0.01, n = 56, Fig. 2E), MW and logDbasal values (r = 0.66, p < 0.01, n = 56, Fig. 2F), and logDapical and logDbasal values (r = 0.60, p < 0.01, n = 56, Fig. 2G) in combination. Moreover, logPapp values were multivariately correlated with the MW, logDapical, and logDbasal values in combination (r = 0.77, p < 0.01, n = 56; Fig. 2H), which led to the following equation: Predicted logPapp value = 2.9 − 0.0032 × (MW) + 0.49 × (logDapical) − 0.38 × (logDbasal). These results suggest that multiple physicochemical properties are the determinants of the permeability coefficient of a variety of chemicals in the pH-dependent Caco-2 monolayer assays.
Fig. 2.
Relationships between logPapp values experimentally observed in the Caco-2 cell system and those calculated using univariate (A–D), bivariate (E–G) and multivariate (H) linear regression analyses of the primary set of 56 compounds, as a function of physicochemical properties (MW, logP, logDapical, and logDbasal). Each observed logPapp value represents the mean of triplicate determinations with standard deviation as shown in Table 1. Solid and dashed/dotted lines indicate linear regression and twofold/threefold ranges, respectively.
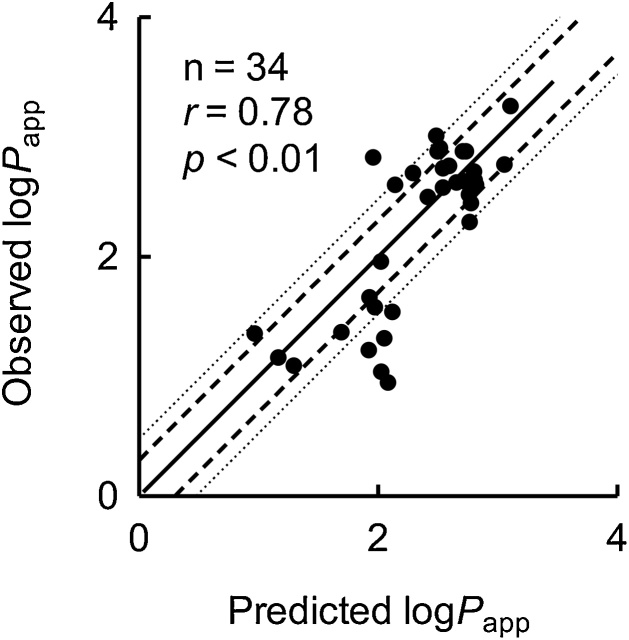
To verify the multivariate prediction equation, logPapp values for a secondary set of 34 compounds (Table 2) were predicted using the above equation in silico before Papp values were measured in in vitro experiments. The Caco-2 cell permeability coefficients of these additional 34 compounds were determined and are shown in Table 2. Estimated logPapp values were well correlated with the experimentally observed logPapp values (r = 0.78, p < 0.01, n = 34; Fig. 3). Under the present conditions, the predicted Papp values of 23 and 27 of the 34 additional compounds were within twofold and threefold errors, respectively, of the experimentally observed values. Under these conditions, predicted logPapp values of some medicines, namely olanzapine, lovastatin, methotrexate, pravastatin, and cimetidine were overestimated in comparison with the observed values, presumably because of partly contributions of active efflux pump in the experimental environment.
Fig. 3.
The relationship between logPapp values of the secondary set of 34 compounds calculated using multivariate linear regression analysis and those of experimentally observed in the Caco-2 cell system. The multivariate prediction equation set up using the dataset shown in Fig. 2H was applied to the secondary set of 34 compounds in this Figure: Predicted logPapp = 2.9 − 0.0032 × (MW) + 0.49 × (logDapical) − 0.38 × (logDbasal). Each observed logPapp value represents the mean of triplicate determinations with standard deviation as shown in Table 2. Solid and dashed/dotted lines indicate linear regression and twofold/threefold ranges, respectively.
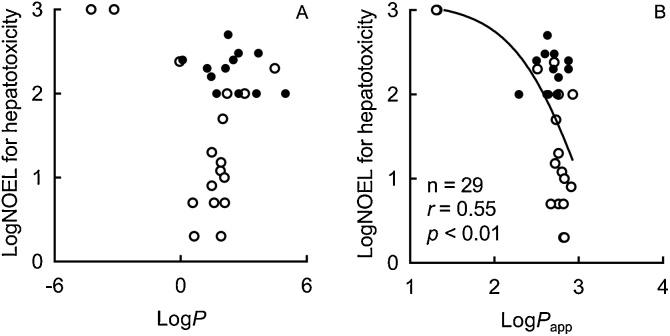
To investigate the relevance of in vitro pH-dependent Caco-2 monolayer systems to in vivo absorption rates, the relationship was examined between the measured logPapp values for pharmaceutical drugs and their reported absorption (fraction absorbed, Fa) in humans (Fig. 4). A significant sigmoidal correlation was observed between the experimental logPapp and reported Fa values (r = 0.61, p < 0.01, n = 28); a similar nonlinear shape has been previously reported for this relationship [5,13]. Furthermore, under the present conditions, a significant inversely non-linear relationship was found between the logarithmic transformed values of observed Papp and reported hepatic NOELs of industrial chemicals and acetaminophen (r = –0.55, p < 0.01, n = 29; Fig. 5B), but not with the calculated logP (r = –0.27, p = 0.2, n = 29; Fig. 5A).
Fig. 4.
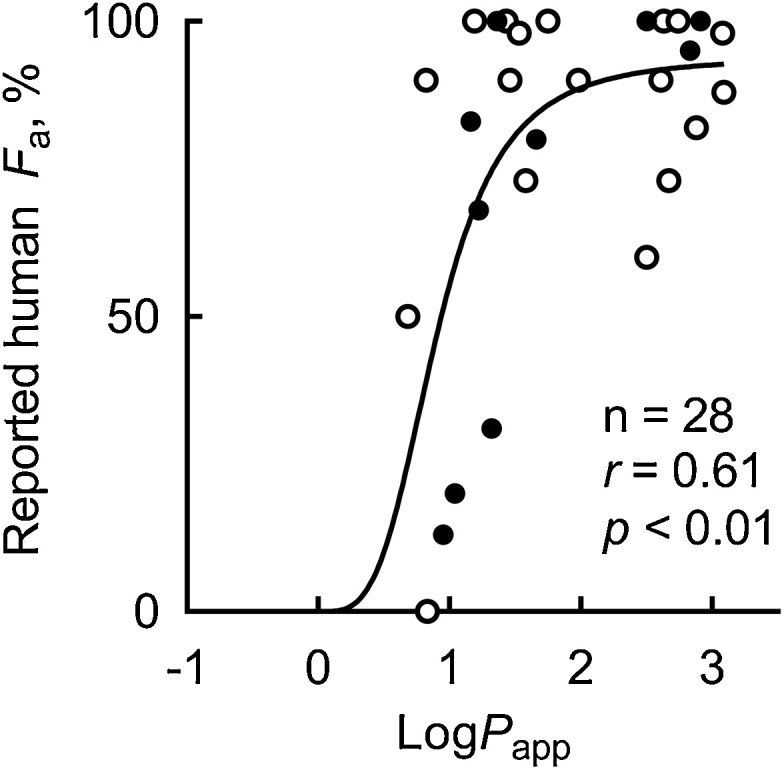
The relationship between observed logPapp values and fraction of oral absorption (Fa) values of medicines reported in humans among the primary set of 56 compounds (open circles) and the secondary set of 34 (solid circles) compounds. Solid line indicates non-linear regression curve: Reported human Fa = 94 × (logPapp)3.6 / (0.69 + (logPapp)3.6).
Fig. 5.
Relationships between hepatic NOEL values of industrial chemicals and acetaminophen reported in rats and their chemical lipophilicity (logP, A) and apparent permeability data (Papp, B) among the primary set of 56 compounds (open circles) and the secondary set of 34 (solid circles) compounds.
4. Discussion
Conditions that mimic the in vitro pH gradient found between the gastrointestinal lumen and plasma have been shown to well reflect human oral absorption of drugs in the gut [6,14]. Furthermore, simple pH-dependent Caco-2 monolayer systems have proven advantageous in predicting in vivo drug absorption as a part of pharmaceutical research [2,5,13,[15], [16], [17], [18], [19]]. It has been reported recently that Caco-2 permeability coefficients for 768 diverse drugs and druglike compounds could account for passive diffusion across the mucosal epithelium [9] using a minimal set of physicochemical descriptors (octanol–water logD, pKa, hydrogen bonding potential, and molecular size), a model has been successfully set up to predict Caco-2 permeability coefficients [9]. However, the pharmacokinetics and/or toxicokinetics of industrial chemicals are not usually investigated as part of their extensive acute toxicity studies [1]. Therefore, the relationship between Papp and the hepatic NOEL values of chemical substances were examined in the present study.
In our previous report, suitable concentrations of albumin for in vitro assays of drug oxidations by human liver microsomal cytochrome P450 2C enzymes could be multivariately estimated using the drugs’ physicochemical properties in combination [20]. In the current study, multivariate regression analysis with three physicochemical properties in combination (reflecting the experimental apical and basal pH conditions in the current monolayer cell assays) showed that the in silico predicted and in vitro measured Papp values of a total of 90 chemicals were well correlated (Fig. 2). These results suggest that our proposed multivariate regression equation using the physicochemical properties of compounds in combination could predict the permeability coefficients across the Caco-2 cell sheets of a wide variety of chemicals. Analysis of the combined 90 tested chemical substances allowed us to update the multivariate equation as follows: Predicted logPapp value = 3.0 − 0.0038 × (MW) + 0.41 × (logDapical) − 0.30 × (logDbasal). The reason why some predicted logPapp of drugs were out of threefold areas are not known under the present conditions, presumably because of some contributions of active efflux/influx pump in the actual experimental Caco-2 environment. Predictions for any uptake/efflux transport potential of substances in the current models using simple physiological parameters may have some limitation at present and would be another big project expected in this research area. In another viewpoint, a multivalent equation fortified with more chemical descriptors might be solved for good prediction in future.
The Papp values obtained from experiments in this study could reflect the in vivo intestinal absorption of known medicines (Fig. 4), although some absolute values were different in in vitro systems (Table 1, Table 2). Our current inverse correlation between logarithmic transformed values of reported NOEL and measured Papp values of general chemicals was able to apply for a drug, acetaminophen (Fig. 5). The in vivo oral absorption, rather than partition coefficients, is considered to be one of the many determinant factors predicting the pharmacokinetics and/or potential hepatotoxicity (Fig. 5) of intentionally or unintentionally orally ingested chemical substances. In the present study, if one (5-amino-2-chlorotoluene-4-sulfonic acid) and two points, respectively, from two compounds implying moderate absorption would be omitted in correlation assays shown in Fig. 5B, both the non-linear correlation coefficients were still significant (r = –0.49, p < 0.01, n = 28; and r = –0.39, p < 0.05, n = 27). Under the present relationship analyses, although NOEL values of chemicals are generally determined in discreet numbers dependent on animal dosing levels, continuous variable in vitro apparent permeability data (Papp) of industrial chemicals would be one of the diverse determinant factors predicting in vivo potential hepatotoxicity, in comparison with chemical lipophilicity (logP). It could be of use to have more NOEL values of chemicals from any toxicity/regulatory databases with similar evaluation criteria to help correlations between the Papp and NOEL values to the toxicity conclusions. Anyway, it should be noted that chemical exposure levels via intestinal absorption after oral doses should be one of the primary key steps and following species-specific metabolic activations in livers and their mechanistically modifications would be the secondary critical points to understand potential hepatic risk from multiple exposures in chemical toxicology. Gastrointestinal epithelial Caco-2 cells have been also reportedly used in the other toxicological research such as cytotoxic effects of pesticides in combination [21] or gene expression profiles by nanosiliver [22].
Consequently, being able to predict the permeability of a diverse range of industrial chemicals across the intestinal epithelial cell monolayer using their physicochemical properties in combination could be of use for estimating systemic exposure via oral absorption as one of putative toxicokinetic markers of hepatotoxicity. With a view to predicting hepatic toxicity after oral absorption of chemicals as a part of risk assessment, simple physiologically based pharmacokinetic models (consisting of gut, liver, and central compartments) were recently used to estimate the plasma/hepatic concentrations of chemicals after virtual oral doses [10]. In conclusion, the in vitro determination and/or in silico prediction of permeability coefficients across the intestinal cell monolayer of a diverse range of industrial chemicals/drugs demonstrated in the current study represent useful tools for estimating oral absorption as a possible indicator of hepatotoxicity in vivo.
CRediT authorship contribution statement
Yusuke Kamiya: Data curation, Formal analysis, Methodology. Hiroka Takaku: Investigation. Rio Yamada: Investigation. Chisato Akase: Investigation. Yuto Abe: Investigation. Yuko Sekiguchi: Investigation. Norie Murayama: Validation. Makiko Shimizu: Validation. Masato Kitajima: Software. Fumiaki Shono: Funding acquisition. Kimito Funatsu: Funding acquisition. Hiroshi Yamazaki: Project administration, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
We are grateful to Tomonori Miura, Miyu Iwasaki, Yui Kobayashi, Ayane Nakano, Ushio Onishi, Tatsurou Sasaki, and Manae Yoshizawa for their assistance. We also thank David Smallbones for copyediting a draft of this article. This work was supported in part by the METI Artificial Intelligence-based Substance Hazard Integrated Prediction System Project, Japan and by the JSPS KAKENHI Grant Number JP 19K16422 for YK.
References
- 1.Bell S.M., Chang X., Wambaugh J.F., Allen D.G., Bartels M., Brouwer K.L.R., Casey W.M., Choksi N., Ferguson S.S., Fraczkiewicz G., Jarabek A.M., Ke A., Lumen A., Lynn S.G., Paini A., Price P.S., Ring C., Simon T.W., Sipes N.S., Sprankle C.S., Strickland J., Troutman J., Wetmore B.A., Kleinstreuer N.C. In vitro to in vivo extrapolation for high throughput prioritization and decision making. Toxicology In Vitro. 2018;47:213–227. doi: 10.1016/j.tiv.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamashita S., Tanaka Y., Endoh Y., Taki Y., Sakane T., Nadai T., Sezaki H. Analysis of drug permeation across Caco-2 monolayer: implication for predicting in vivo drug absorption. Pharmaceut. Res. 1997;14(4):486–491. doi: 10.1023/a:1012103700981. [DOI] [PubMed] [Google Scholar]
- 3.Hilgers A.R., Conradi R.A., Burton P.S. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharmaceut. Res. 1990;7(9):902–910. doi: 10.1023/a:1015937605100. [DOI] [PubMed] [Google Scholar]
- 4.Leonard M., Creed E., Brayden D., Baird A.W. Iontophoresis-enhanced absorptive flux of polar molecules across intestinal tissue in vitro. Pharmaceut. Res. 2000;17(4):476–478. doi: 10.1023/a:1007541423500. [DOI] [PubMed] [Google Scholar]
- 5.Artursson P., Palm K., Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2001;46(1–3):27–43. doi: 10.1016/s0169-409x(00)00128-9. [DOI] [PubMed] [Google Scholar]
- 6.Neuhoff S., Ungell A.L., Zamora I., Artursson P. pH-Dependent passive and active transport of acidic drugs across Caco-2 cell monolayers. Eur. J. Pharm. Sci. 2005;25(2–3):211–220. doi: 10.1016/j.ejps.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Varma M.V., Obach R.S., Rotter C., Miller H.R., Chang G., Steyn S.J., El-Kattan A., Troutman M.D. Physicochemical space for optimum oral bioavailability: contribution of human intestinal absorption and first-pass elimination. J. Med. Chem. 2010;53(3):1098–1108. doi: 10.1021/jm901371v. [DOI] [PubMed] [Google Scholar]
- 8.Broccatelli F., Salphati L., Plise E., Cheong J., Gobbi A., Lee M.L., Aliagas I. Predicting passive permeability of drug-like molecules from chemical structure: where are we? Mol. Pharm. 2016;13(12):4199–4208. doi: 10.1021/acs.molpharmaceut.6b00836. [DOI] [PubMed] [Google Scholar]
- 9.Lanevskij K., Didziapetris R. Physicochemical QSAR analysis of passive permeability across Caco-2 monolayers. J. Pharm. Sci. 2019;108(1):78–86. doi: 10.1016/j.xphs.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Kamiya Y., Otsuka S., Miura T., Takaku H., Yamada R., Nakazato M., Nakamura H., Mizuno S., Shono F., Funatsu K., Yamazaki H. Plasma and hepatic concentrations of chemicals after virtual oral administrations extrapolated using rat plasma data and simple physiologically based pharmacokinetic models. Chem. Res. Toxicol. 2019;32:211–218. doi: 10.1021/acs.chemrestox.8b00307. [DOI] [PubMed] [Google Scholar]
- 11.Venkatesan P.S., Deecaraman M., Vijayalakshmi M., Sakthivelan S.M. Sub-acute toxicity studies of acetaminophen in Sprague Dawley rats. Biol. Pharm. Bull. 2014;37(7):1184–1190. doi: 10.1248/bpb.b14-00066. [DOI] [PubMed] [Google Scholar]
- 12.Sakuratani Y., Zhang H.Q., Nishikawa S., Yamazaki K., Yamada T., Yamada J., Gerova K., Chankov G., Mekenyan O., Hayashi M. Hazard Evaluation Support System (HESS) for predicting repeated dose toxicity using toxicological categories. SAR QSAR. Environ. Res. 2013;24(5):351–363. doi: 10.1080/1062936X.2013.773375. [DOI] [PubMed] [Google Scholar]
- 13.Cheng K.C., Li C., Uss A.S. Prediction of oral drug absorption in humans--from cultured cell lines and experimental animals. Expert Opin. Drug Metab. Toxicol. 2008;4(5):581–590. doi: 10.1517/17425255.4.5.581. [DOI] [PubMed] [Google Scholar]
- 14.Neuhoff S., Ungell A.L., Zamora I., Artursson P. pH-dependent bidirectional transport of weakly basic drugs across Caco-2 monolayers: implications for drug-drug interactions. Pharmaceut. Res. 2003;20(8):1141–1148. doi: 10.1023/a:1025032511040. [DOI] [PubMed] [Google Scholar]
- 15.Avdeef A., Artursson P., Neuhoff S., Lazorova L., Grasjo J., Tavelin S. Caco-2 permeability of weakly basic drugs predicted with the double-sink PAMPA pKa(flux) method. Eur. J. Pharm. Sci. 2005;24(4):333–349. doi: 10.1016/j.ejps.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Furubayashi T., Kamaguchi A., Kawaharada K., Masaoka Y., Kataoka M., Yamashita S., Higashi Y., Sakane T. Kinetic model to predict the absorption of nasally applied drugs from in vitro transcellular permeability of drugs. Biol. Pharm. Bull. 2007;30(5):1007–1010. doi: 10.1248/bpb.30.1007. [DOI] [PubMed] [Google Scholar]
- 17.Kamiya Y., Yamaki T., Uchida M., Hatanaka T., Kimura M., Ogihara M., Morimoto Y., Natsume H. Preparation and evaluation of PEGylated Poly-L-ornithine complex as a novel absorption enhancer. Biol. Pharm. Bull. 2017;40(2):205–211. doi: 10.1248/bpb.b16-00781. [DOI] [PubMed] [Google Scholar]
- 18.Konishi Y., Hagiwara K., Shimizu M. Transepithelial transport of fluorescein in Caco-2 cell monolayers and use of such transport in in vitro evaluation of phenolic acid availability. Biosci. Biotechnol. Biochem. 2002;66:2449–2457. doi: 10.1271/bbb.66.2449. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita S., Furubayashi T., Kataoka M., Sakane T., Sezaki H., Tokuda H. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur. J. Pharm. Sci. 2000;10(3):195–204. doi: 10.1016/s0928-0987(00)00076-2. [DOI] [PubMed] [Google Scholar]
- 20.Shimura K., Murayama N., Tanaka S., Onozeki S., Yamazaki H. Suitable albumin concentrations for enhanced drug oxidation activities mediated by human liver microsomal cytochrome P450 2C9 and other forms predicted with unbound fractions and partition/distribution coefficients of model substrates. Xenobiotica. 2019;49:557–562. doi: 10.1080/00498254.2018.1482576. [DOI] [PubMed] [Google Scholar]
- 21.Ilboudo S., Fouche E., Rizzati V., Toe A.M., Gamet-Payrastre L., Guissou P.I. In vitro impact of five pesticides alone or in combination on human intestinal cell line Caco-2. Toxicol. Rep. 2014;1:474–489. doi: 10.1016/j.toxrep.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahu S.C. Altered global gene expression profiles in human gastrointestinal epithelial Caco2 cells exposed to nanosilver. Toxicol. Rep. 2016;3:262–268. doi: 10.1016/j.toxrep.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levitt D.G. Quantitation of small intestinal permeability during normal human drug absorption. BMC Pharmacol. Toxicol. 2013;14:34. doi: 10.1186/2050-6511-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yee S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man--fact or myth. Pharmaceut. Res. 1997;14(6):763–766. doi: 10.1023/a:1012102522787. [DOI] [PubMed] [Google Scholar]
- 25.Velicky M., Tam K.Y., Dryfe R.A. In situ artificial membrane permeation assay under hydrodynamic control: correlation between drug in vitro permeability and fraction absorbed in humans. Eur. J. Pharm. Sci. 2011;44(3):299–309. doi: 10.1016/j.ejps.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Chen N., Wen L., Lau H., Surapaneni S., Kumar G. Pharmacokinetics, metabolism and excretion of [(14)C]-lenalidomide following oral administration in healthy male subjects. Cancer Chemother. Pharmacol. 2012;69(3):789–797. doi: 10.1007/s00280-011-1760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu C., Jiang L., Chen T.M., Hwang K.K. A comparative study of artificial membrane permeability assay for high throughput profiling of drug absorption potential. Eur. J. Med. Chem. 2002;37(5):399–407. doi: 10.1016/s0223-5234(02)01360-0. [DOI] [PubMed] [Google Scholar]
- 28.Thummel K.E., O’Shea D., Paine M.F., Shen D.D., Kunze K.L., Perkins J.D., Wilkinson G.R. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. CPT. 1996;59:491–502. doi: 10.1016/S0009-9236(96)90177-0. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann M., Kasserra C., Reyes J., Schafer P., Kosek J., Capone L., Parton A., Kim-Kang H., Surapaneni S., Kumar G. Absorption, metabolism and excretion of [14 C]pomalidomide in humans following oral administration. Cancer Chemother. Pharmacol. 2013;71(2):489–501. doi: 10.1007/s00280-012-2040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeVane C.L., Nemeroff C.B. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin. Pharmacokinet. 2001;40(7):509–522. doi: 10.2165/00003088-200140070-00003. [DOI] [PubMed] [Google Scholar]
- 31.Skolnik S., Lin X., Wang J., Chen X.H., He T., Zhang B. Towards prediction of in vivo intestinal absorption using a 96-well Caco-2 assay. J. Pharm. Sci. 2010;99(7):3246–3265. doi: 10.1002/jps.22080. [DOI] [PubMed] [Google Scholar]