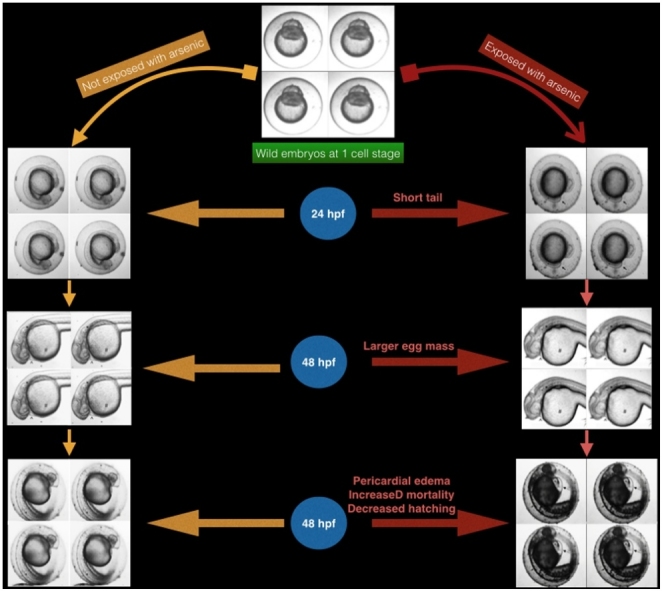
Graphical abstract
Abbreviations: mpf, minutes post-fertilization; hpf, hours post-fertilization; dpf, days post-fertilization
Keywords: Zebrafish model, Arsenic, Embryonic development, Heavy metals, Toxicology
Highlights
-
•
Exposure to arsenic results delayed and deformed embryonic development.
-
•
Arsenic exposure increased the mortality rate of embryos.
-
•
Arsenic exposure may increase miscarriage or abortion rate in the pregnant mother.
Abstract
Zebrafish (Danio rerio) has appeared as a valuable and popular model species to study the developmental and toxicological impact of environmental pollutants. To get insights on the toxicological effect of arsenic on early embryonic development, a controlled breeding of local Bangladeshi zebrafish followed by comprehensive microscopic analysis was conducted to study the embryonic development after exposure to different concentrations of arsenic ranges from 4−120 h post-fertilization. Zebrafish embryos exposed to 2 mM of arsenic displayed distinguishable developmental delay compared to control. At three days post-fertilization, a distinct phenotype appears in arsenic-treated embryos, which can be characterized by dechorionated embryos, larger egg mass, pericardial edema, abnormal heart rate, and abnormal head development. Remarkably, the death rate of the arsenic-treated embryos was significantly higher compared to control. Collectively, these findings indicate that exposure to arsenic may result in abnormal embryonic development. These results suggest for proper management of the pregnant mother in the arsenic-exposed area, and may also explain the incidence of increased miscarriage/abortion rate in arsenic water drinking pregnant mother.
1. Introduction
Heavy metals are the metal or metalloid compound that has a higher density than water, usually having density more than 5 g/cm3 [1]. Naturally, these heavy metals are essential at minute concentrations to keep different biochemical as well as physiological homeostasis in the living organisms; however, become poisonous when they surpassed particular threshold concentration [2,3]. Arsenic, the 20th most plentiful element of the world, marked as most toxicant among the heavy metals and emerged as a significant menace for the environment as well as human health. Primarily arsenic is present in the air, soils, water and food. Arsenic present in the ground-water enters in the different food chain and obsessed with grain, vegetables, and aquatic organisms [[4], [5], [6], [7], [8], [9], [10], [11]]. The maximum allowed concentration of arsenic in the drinking water is ten μg/L, which is recommended by the Environmental Protection Agency (EPA) and the World Health Organization (WHO) [12]. Chronic excessive arsenic contamination through the drinking water found to be associated with several human health issues.
Globally, around 20 crore people are directly or indirectly incorporated with excessive levels of arsenic. The scenario is even worsened in Bangladesh; with about two crore persons are regularly using arsenic-contaminated groundwater, and approximately 43,000 persons are dying every year due to arsenic poisoning [[13], [14], [15]]. In Bangladesh, hand-pumped tube well is used as the key source for drinking and household working water. Unfortunately, more than half of them (∼10 million around the country) were found to supply groundwater with arsenic load over 50 μg/L (5x higher than EPA and WHO recommendation) [[16], [17], [18]]. Furthermore, rice, which is the main food of about 16 crore persons of the country, is grown on over 70 thousand hectares of land (which constitutes ∼7 % of country’s total rice cultivated area) where the average groundwater arsenic load was reported to over 50 μg/L [19,20]. Subsequently, arsenic entered and accumulated in the food chain, and a considerable proportion of the Bangladeshi people are affected directly or indirectly by consuming those foods.
Epidemiological investigations have affirmed that both the intense and contineous exposure to arsenic are harmful to human health; can cause many diseases including cancer (in lung, bladder, and skin), cardiovascular diseases, diabetes, developmental, reproductive and neurological disorders [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]]. Most importantly, arsenic is well known for its teratogenic effect, can cross the mammalian placenta during pregnancy period and cause an increased rate of abortion or miscarriage, lower intelligence quotient (IQ) and developmental retardation in humans [[32], [33], [34]]. Previous investigation reported that arsenic exposure causes inhibition of stem cell differentiation by altering multiple signaling pathways including Notch and Wnt [35]. Vitamin supplimenation during gestation are common practice in many countries for the better outcomes, but many of the prenatal vitamins were found to contaminated with heavy metals including arsenic [36]. Thus, it is urgent to assess the toxic effect of arsenic on human health, particularly during the early embryonic development.
Danio rerio, commonly known as zebrafish, has arisen as a vital model animal for toxicology related research in early developmental stages [[37], [38], [39]]. Besides high sequence homology with human genes, other advantages include rapid external development, optical transparency during early embryonic development, permeability to low molecular weight molecules which make them ideal for high-throughput screening against teratogenic, cardiotoxic and neurotoxic drugs [[37], [38], [39], [40], [41], [42], [43], [44], [45]]. Their small size, easy maintenance, and high fecundity make them highly favorable for the experiments demanding a large number of replicates [46]. The current study utilized wild-caught zebrafish embryos from Bangladesh to gain insights into the impact of arsenic during the initial embryonic growth.
2. Methodology
2.1. Collection of zebrafish and optimization of culture condition
Adult zebrafish were collected after being adopted from Mymensingh freshwater. Fishes were housed and cultured at the zebrafish facility of Shahjalal University of Science and Technology (SUST) under standard laboratory environments. A strict light cycle of 14 h light following a dark period of 10 h, was maintained. The water quality parameters temperature and pH were recorded once daily. Tanks were cleaned every three days and refreshed with clean water and sterilized every two weeks. Embryos are reared in an E3 medium (containing methylthioninium chloride) incubated at 28 °C [46] up to 9dpf for normal growth. Dead embryos were removed, and the E3 medium is refreshed every day by replacing half of the fresh E3 medium. All procedure for animal use were reviewed and approved by the SUST Research Center (authorized institutional body for research care).
2.2. Feeding
Adult fish were fed with pellet food (TetraBits® Complete, Tetra GmbH, Germany) twice a day. On the contrary, embryos from the beginning of the 6th day were fed with 50 u M solid dry granules once a day and Artemia spore once a day. Artemia decapsulation was done by the removal of the hard shell by exposure of Artemia cyst to a sodium chloride solution with aeration for 24 h in light.
2.3. Zebrafish breeding
Breeding tanks of 1.5-liter capacity (locally made) were used for breeding. On the day before breeding, during the late evening, two females and two males were kept separated by a transparent separation sheet. Breeding tanks were then filled with about 2/3 of tap water. In the next morning, the separator was removed, refreshed with clean water and checked time to time for successful breeding. Fertilized eggs were deposited through a perforated net on the floor of the breeding tank and were then collected in E3 medium. The breeding technique was repeated for fishes at 8–10 days intervals.
2.4. Mathematical analysis
The breeding performance indicators including the spawning success, fecundity, fertilization rate, hatching rate, and larvae’s survival rate up to 20 days post-fertilization (dpf) were calculated by using standard formula.
2.5. Preparation of arsenic solution
Serial dilution of sodium arsenate (CAS NO-10048-95-0, Loba Chemie, Mumbai, India; MW 312.01 g/l) ranges from 0.2 to 1.5 mM was tested to get the minimum morphological effect. Further, sodium arsenate concentration of 1.0 mM, 1.5 mM, and 2.0 mM was tested to observe their effect on embryonic development.
2.6. Evaluation of arsenic concentration
The effective level of arsenic was evaluated as described by Haley and Turner (2016), and Chakraborty et al. (2012) with minor modification [48,49]. Sodium arsenate solution (Cat: A6756; Merck KGaA, Darmstadt, Germany; MW 312.01 g/l) was used as a standard for evaluation of arsenic solutions.
2.7. Experimental setup
Three replicates, each constituting of 30 embryos, were used for every treatment. For arsenic exposure respective concentration of sodium arsenate + E3 medium was treated, while control remained arsenic-free. Also, to observe the internal organ formation clearly under a microscope, 0.003 % 1-phenyl-2-thiourea (PTU; CAS NO- 103-85-5, Merck KGaA, Darmstadt, Germany) was supplemented to each petri dish to arrest pigmentation.
2.8. Growth measuring and survival rate counting of zebrafish embryos
Rate (%) of embryos come out from the chorion at a given time point was enumerated to analyze the hatching rate. The embryos were analyzed under an inverted and dissecting microscope at different time points to observe any phenotypic deformities as well as the rate of survivability.
2.9. Irregular heart pump measuring
To estimate the effect of arsenic in cardiovascular activity, heart pump was counted per minute in a cohort of arsenic-treated embryos and compared with control at 48 hpf. Also, the heart shape was observed under a light microscope.
3. Results
3.1. Embryogenesis of local Bangladeshi zebrafish
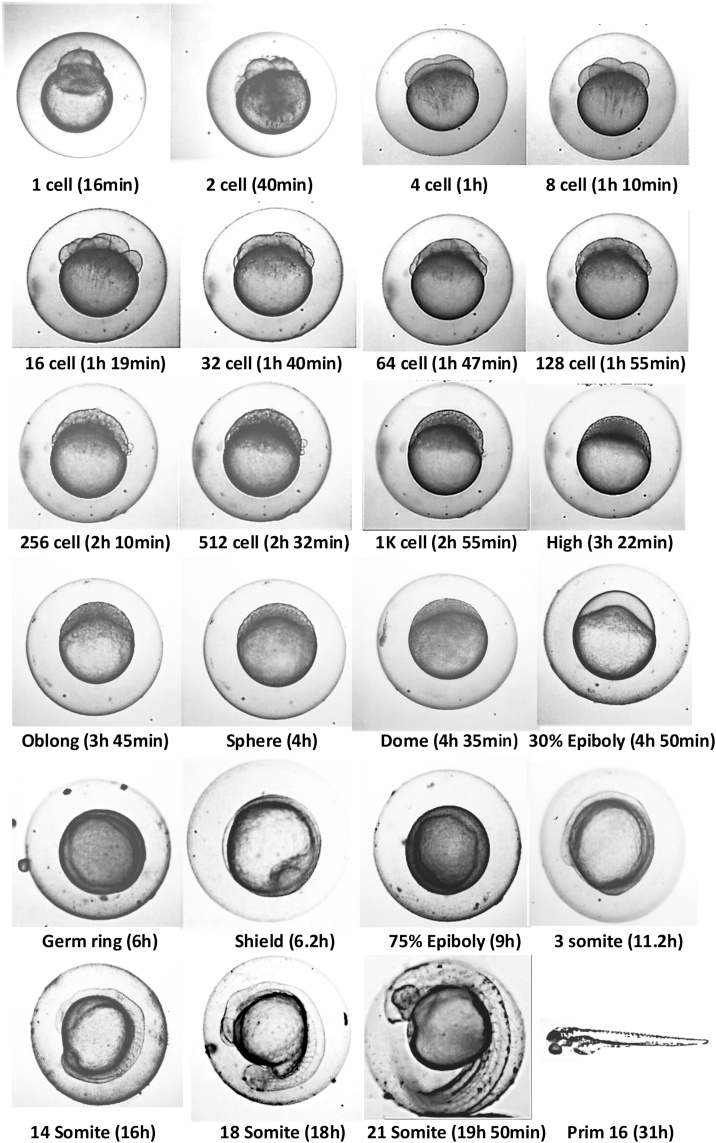
Zebrafish embryogenesis was observed under a microscope (40X) from fertilization to adult stage (Fig. 1). Different time points were documented for some specific developmental stages and found that zygote (4 cells) forms 40 min post-fertilization (pf), and it takes 48 hpf for hatching (Table 1).
Fig. 1.
Different stages of embryogenesis of local Bangladeshi zebrafish under a microscope (40X). Some key diagnostic features at the following stages are indicated with arrows; 1 K cell: yolk syncytial layer (YSL) nuclei. Germ ring: germ ring. Shield: embryonic shield. 75 % epiboly: Brachet’s cleft. Three somites: third somite. 21 somite: lens primordium.
Table 1.
Time point of the different developmental stage of Bangladeshi local zebrafish embryogenesis.
| Developmental stage | Time (hpf) |
|---|---|
| Zygote | 0-0.75 |
| Cleavage | 0.75-2.42 |
| Blastula | 2.42-5.42 |
| Gastrula | 5.42-10 |
| Segmentation | 10-24 |
| Pharyngula | 24-48 |
| Hatching | 48-72 |
| Early Larva | 72 hpf-29dpf |
3.2. Breeding performance
To get insights into the breeding performance, the key indicators, including spawning, fecundity, fertilization rate, and hatching rate, were checked in a cohort of 30 embryos (Table 2). Spawning success rate and fecundity rate were calculated as 80 % and 81.67 % respectively. Also, the fertilization rate and hatching rate of embryos were found to be 75 % and 95 %, respectively.
Table 2.
Key indicators of the breeding performance of local Bangladeshi zebrafish.
| Breeding performance indicator | Mean ± S.D | Sample size |
|---|---|---|
| Spawning Success (%) | 80 % ±16.33 | 20 |
| Fertilization rate (%) | 75 % ± 8.164 | 20 |
| Hatching rate (%) | 95 % ± 4.08 | 20 |
| Survival rate (%) | 81.67 % ± 6.236 | 20 |
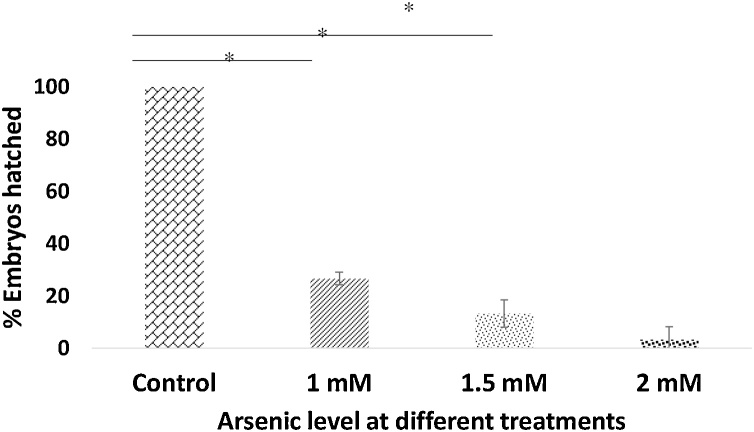
3.3. Arsenic exposure decreases hatching rate
Arsenic-treated (1 mM, 1.5 mM, 2 mM) embryos showed an extended time (approximately 96–120 hpf) to hatch from the chorion compared to control. Arsenic exposure revealed that hatching rate decreased with increased arsenic concentration; at 72 hpf hatching rate of 27 %, 13 %, and 3 % embryos were found in 1 mM, 1.5 mM, 2 mM arsenic concentration respectively (Fig. 2). Untreated control embryos showed 100 % hatching rate.
Fig. 2.
Hatching rate (%) of As treated embryos compared to control after 48hpf. As exposed embryos exhibit decreased hatched rate proportion to increase As concentration. Here, the sample size for each treatment is 30, and they were replicated three times (at 28.5 °C). *p ≤ 0.001.
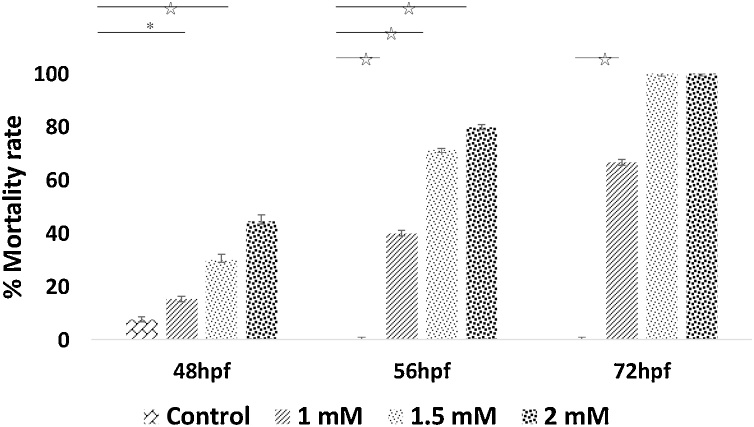
3.4. Arsenic exposure causes increased mortality rate
The rate of mortality was found significantly and proportionally higher with concentraion in the arsenic-exposed embryos compared to control until 56 hpf (Fig. 3). All of the embryos (100 %) treated at 1.5 mM or 2.0 mM of arsenic died at 72 hpf (Fig. 3).
Fig. 3.
Mortality rate (% of embryos died) of arsenic-exposed embryos at different time point. Arsenic-treated embryos showed, higher mortality compared to control. Mortality rate increased with increasing As concentration and time point. Sample size = 30, for each treatment, and they were replicated three times. *p ≤ 0.05; ⋆p ≤ 0.001.
3.5. Arsenic exposure persuades irregular morphology and impaired development
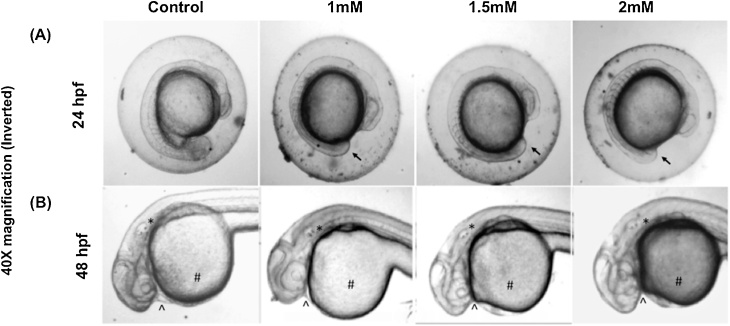
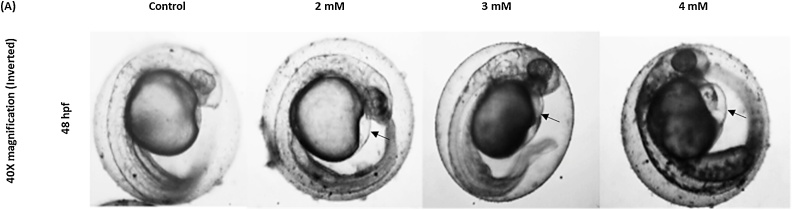
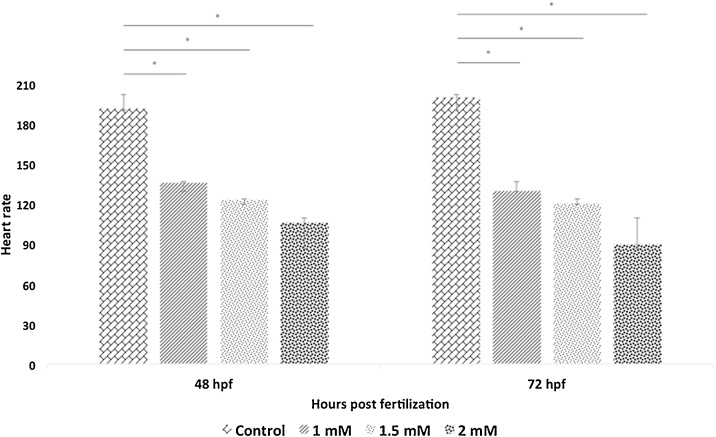
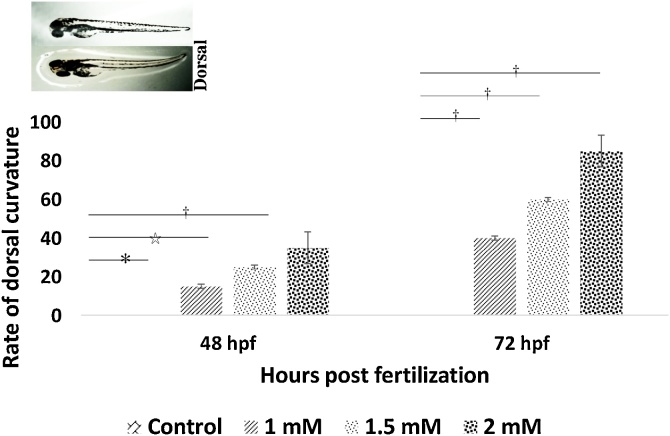
Embryos exposed to 1.0 mM and higher concentrations of arsenic results morphological and developmental abnormalities, which can be characterized as deformation in the midbrain, tail bud, egg mass, otic vesicle, and hatching gland cell (Fig. 4A, B). These anomalies were found most distinguished within the 1.5 mM and 2.0 mM of arsenic-treated embryos. One of the most remarkable features found in the arsenic-treated embryos was pericardial edema (an abnormal accumulation of fluid between the heart and sac surrounding the heart) of the heart at 48 hpf (Fig. 5). Remarkably, the decreased rate of heart pump also exhibited in the embryos proportionally to increase arsenic concentration (Fig. 6). Another distinct feature named “dorsal curvature” was found prominent in the arsenic-treated embryos (Fig. 7).
Fig. 4.
Embryos exposed to As exhibited morphological anomalies. Showed tail bud deformation of embryos at 24 hpf. (B) * showed otic vesicle distortion, # showed egg mass deformation, and ^ showed hatching gland cell abnormalities at 48 hpf.
Fig. 5.
Embryos exposed to As exhibited anomalies in the cardiovascular system. At 48hpf As exposed Embryos showed pericardial edema (black arrows).
Fig. 6.
Embryos exposed to arsenic exhibited a dropped heart rate. Heart pump decreased as the arsenic concentration and time point extended. *p ≤ 0.05.
Fig. 7.
Embryos exposed to arsenic causes an increased rate of dorsal curvature (lordosis). The percentage of embryos affected by dorsal curvature plotted against the hpf. No signs of dorsal curvature were seen in the control at any time point. Statistical significance was calculated comparing against the control. *p ≤ 0.05; ⋆p ≤ 0.005; †p ≤ 0.001.
4. Discussion
This study has determined the impacts of arsenic on the early embryonic development of local zebrafish. Embryonic exposure to higher levels of arsenic increased mortality and induced physiological abnormalities in early development, including prolonged hatching time, abnormalities in cardiovascular systems, and lowered growth. Previously reported findings in rodents also showed that arsenic treatment results decreased reproduction rate and development. Furthermore, it was also reported that oral treatment of arsenic during the pregnancy period contributes to the fetus neural tube defects (NTDs) [50,51]. In zebrafish, arsenic was also reported to cause abnormal neural development, including impairment of patterning in the central nervous system (CNS) [52]. Toxicology experiments of some chemicals revealed that neural deficiencies have crosstalk with cardiac dysfunction [53]. Besides cardiac dysfunction, arsenic exposure could induce neural defects during embryogenesis [54]. Our result certainly showed that arsenic exposure led to cardiac dysfunction, which may also have contributed to the abnormal heart rate [55,56]. Another study suggested that cleft spine (spina bifida), sticking-ribs, skeletal malformation and a stress fracture in one of the vertebrae (Spondylolysis) are among the noteworthy abnormalities caused by sodium arsenate in human [50,57,58]. The present study has also shown that arsenic-treated embryos are affected by the development of lordosis (dorsal curvature). Ahmad et al. (2001) showed that the rates of miscarriages, stillbirth and preterm delivery in pregnant women, who were exposed to arsenic, were remarkably higher than the pregnant women who were unexposed [59]. Overall, these results indicate the impacts of preeminent levels of arsenic in drinking water and its relationship with pregnancy and fetal complications in human. Arsenic has been reported to associated with hatching delay, increase mortality and fish demormities including anomaly in spine in both natural and loboratory population [60]. Comparing to our findings on impacts of arsenic on loacal Bangladesih/wild zebrafish with normal/AB line revealed that minimum concentration to recapitulate phenotype in both group is similar, no hornesis phenotype found in wild embryos, hatching rate and mortality rate are much higher compare to the normal embryos (Table 3). Interestingly, embryogenesis process was found slower in the loacal embryos compare to the normal embryos (Table 3).
Table 3.
Comparative features of arsenic treated embryoes between AB line and local zebrafish.
| Features | Normal/AB line | Local BD/wild | |
|---|---|---|---|
| Embryogenesis | Cleavage | 45 min to 2 h [47] | 40 min to 1 h 47 min |
| Blastula | 2 h 15 min to 4 h 40 min [47] | 1 h 55 min to 4 h 50 min | |
| Gastrula | 5h 40 min [47] | 6h | |
| Germ-ring | 2 h [47] | 1 h 47 min | |
| Shield | 6h [47] | 6h 20 min | |
| 75 %-epiboly | 8h [47] | 9h | |
| Concentration of arsenic used in the experiment | 0.5−10 mM [52] | 0.2−2 mM | |
| Minimum concentration of phenotype/effect | 1.mM [52] | 1 mM | |
| Hormesis phenotype | Found at 2 mM after 48hpf [52] | Not found | |
| Hatching rate (1 mM arsenic at 48hpf) | 1.10 ± 0.03 % [52] | 26.67 % | |
| Mortality rate (1 mM arsenic at 48hpf) | ∼9 % [52] | ∼15 % | |
| Mortality rate (1 mM arsenic at 48hpf) | ∼22 % [52] | ∼44 % | |
5. Conclusion
The overall results suggest that chronic higher level of arsenic exposure causes abnormal embryonic development, including severe heart defects in embryos. These results essence the proper management of pregnant mothers in the arsenic-exposed area. This study stressed the essense of molecular analysis to obtain further insights into the effects of arsenic on embryonic growth.
Funding
This study was funded by the Bangladesh University Grant Commission (UGC); Grant ID: Medical-17/2016/6467 of Bangladesh and SUST-Research center to Prof. Dr. Mohammad Jakir Hosen.
CRediT authorship contribution statement
Tamanna Kabir: Investigation, Writing - original draft. Saeed Anwar: Investigation, Validation, Writing - original draft. Jarin Taslem Mourosi: Validation, Writing - original draft. Jakir Hossain: Resources. Md. Golam Rabbane: Resources. Md. Masuder Rahman: Formal analysis. Tohura Tahsin: Writing - review & editing. Md. Nazmul Hasan: Formal analysis. Manik Chandra Shill: Validation. Mohammad Jakir Hosen: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing - review & editing.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgment
The authors are grateful to Prof. Dr. Golam Rabbane for supplying zebrafish.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2019.12.009.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Järup L. Hazards of heavy metal contamination. Br. Med. Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 2.Jaishankar M., Mathew B.B., Shah M.S., Gowda K.R.S. Biosorption of few heavy metal ions using agricultural wastes. J. Environ. Pollut. Hum. Health. 2014;2:1–6. [Google Scholar]
- 3.Nagajyoti P.C., Lee K.D., Sreekanth T.V.M. Heavy metals, occurrence and toxicity for plants: a review. Environ. Chem. Lett. 2010;8:199–216. [Google Scholar]
- 4.Yedjou C., Moore P., Tchounwou P. Dose- and time-dependent response of human leukemia (HL-60) cells to arsenic trioxide treatment. Int. J. Environ. Res. Public Health. 2006;3:136–140. doi: 10.3390/ijerph2006030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchounwou P.B., Centeno J.A., Patlolla A.K. Arsenic toxicity, mutagenesis, and carcinogenesis – a health risk assessment and management approach. Mol. Cell. Biochem. 2004;255:47–55. doi: 10.1023/b:mcbi.0000007260.32981.b9. [DOI] [PubMed] [Google Scholar]
- 6.Yedjou C.G., Tchounwou P.B. In-vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia (HL-60) cells using the MTT and alkaline single cell gel electrophoresis (Comet) assays. Mol. Cell. Biochem. 2007;301:123–130. doi: 10.1007/s11010-006-9403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes M.F., Beck B.D., Chen Y., Lewis A.S., Thomas D.J. Arsenic exposure and toxicology: a historical perspective. Toxicol. Sci. 2011;123:305–332. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng W.P., Chu H., How S.W., Fong J.M., Lin C.S., Yeh S.H.U. Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J. Natl. Cancer Inst. 1968;40:453–463. [PubMed] [Google Scholar]
- 9.Mazumder D.G. Chronic arsenic toxicity & human health. Indian J. Med. Res. 2008;128:436–447. [PubMed] [Google Scholar]
- 10.Kurosawa K., Egashira K., Tani M., Jahiruddin M., Moslehuddin A.Z.M., Rahman Z.M. Groundwater–soil–crop relationship with respect to arsenic contamination in farming villages of Bangladesh – a preliminary study. Environ. Pollut. 2008;156:563–565. doi: 10.1016/j.envpol.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Smith N.M., Lee R., Heitkemper D.T., Cafferky K.D., Haque A., Henderson A.K. Inorganic arsenic in cooked rice and vegetables from Bangladeshi households. Sci. Total Environ. 2006;370:294–301. doi: 10.1016/j.scitotenv.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Smith A.H., Lingas E.O., Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull. World Health Organ. 2000;78:1093–1103. [PMC free article] [PubMed] [Google Scholar]
- 13.Roy P. The Daily Star; 2016. Arsenic Danger Still Not Over. [Google Scholar]
- 14.Jahan H. Arsenic in Bangladesh: how to protect 20 million from the world’s largest poisoning. The Gurdian. 2016 [Google Scholar]
- 15.Smedley P.L., Kinniburgh D.G. A review of the source, behavior and distribution of arsenic in natural waters. Appl. Geochem. 2002;17:517–568. [Google Scholar]
- 16.Center for Disease Control, Atlanta; Georgia: 2000. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Arsenic. [PubMed] [Google Scholar]
- 17.Argos M., Kalra T., Rathouz P.J., Chen Y., Pierce B.B., Parvez F. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a perspective cohort study. Lancet. 2010;376:252–258. doi: 10.1016/S0140-6736(10)60481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tchounwou P. Development of public health advisories for arsenic in drinking water. Rev. Environ. Health. 1999;14:211–230. doi: 10.1515/reveh.1999.14.4.211. [DOI] [PubMed] [Google Scholar]
- 19.Ross Z., Duxbury J.M., Degloria S.D., Paul D.N.R. Potential for arsenic contamination of rice in Bangladesh: spatial analysis and mapping of high-risk areas. Int. J. Risk Assess. Manag. 2006;6:298–315. [Google Scholar]
- 20.Bangladesh Rice Knowledge Bank; 2018. Bangladesh Rice Research Institute (BRRI), Rice in Bangladesh. [Google Scholar]
- 21.Nuckols J.R., Beane-Freeman L., Baris D., Lubin J.H., Ayotte J.D., Schwenn M. Arsenic exposure assessment in the new england bladder Cancer study. Epidemiology. 2008;19:23–24. [Google Scholar]
- 22.Mandal B.K., Suzuki K.T. Arsenic round the B. Mandal, Arsenic round the world: a review. Talanta. 2002;58:201–235. [PubMed] [Google Scholar]
- 23.Mink P.J., Alexander D.D., Barraj L.M., Kelsh M.A., Tsuji J.S. Low-level arsenic exposure in drinking water and bladder cancer: a review and meta-analysis. Regul. Toxicol. Pharmacol. 2008;52:299–310. doi: 10.1016/j.yrtph.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Platanias L.C. Biological responses to arsenic compounds. J. Biol. Chem. 2009;284:18583–18587. doi: 10.1074/jbc.R900003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuhmacher–Wolz U., Dieter H.H., Klein D., Schneider K. Oral exposure to inorganic arsenic: evaluation of its carcinogenic and non-carcinogenic effects. Crit. Rev. Toxicol. 2009;39:271–298. doi: 10.1080/10408440802291505. [DOI] [PubMed] [Google Scholar]
- 26.Gebel T.W. Genotoxicity of arsenical compounds. Int. J. Hyg. Environ. Health. 2001;203:249–262. doi: 10.1078/S1438-4639(04)70036-X. [DOI] [PubMed] [Google Scholar]
- 27.Hughes M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002;133:1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 28.Nickson R., McArthur J., Burgess W., Ahmed K.M., Ravenscroft P., Rahman M. Arsenic poisoning of Bangladesh groundwater. Nature. 1998;395:338. doi: 10.1038/26387. [DOI] [PubMed] [Google Scholar]
- 29.Rahman A., Vahter M., Smith A.H., Nermell B., Yunus M., Arifeen S.E. Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangladesh. Am. J. Epidemiol. 2008;169:304–312. doi: 10.1093/aje/kwn332. [DOI] [PubMed] [Google Scholar]
- 30.Tsai S.Y., Chou H.Y. The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. NeuroToxicology. 2003;24:747–753. doi: 10.1016/S0161-813X(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 31.Tseng C.H., Tseng C.P., Chiou H.Y., Hsueh Y.M., Chong C.K., Chen C.J. Epidemiologic evidence of diabetogenic effect of arsenic. Toxicol. Lett. 2002;133:69–76. doi: 10.1016/s0378-4274(02)00085-1. [DOI] [PubMed] [Google Scholar]
- 32.Wlodarczyk B.J., Bennett G.D., Calvin J.A., Finnell R.H. Arsenic-induced neural tube defects in mice: alterations in cell cycle gene expression. Reprod. Toxicol. 1996;10:447–454. doi: 10.1016/s0890-6238(96)00131-1. [DOI] [PubMed] [Google Scholar]
- 33.Shalat S.L., Walker D.B., Finnell R.H. Role of arsenic as a reproductive toxin with particular attention to neural tube defects. J. Toxicol. Environ. Health. 1996;48:253–272. doi: 10.1080/009841096161320. [DOI] [PubMed] [Google Scholar]
- 34.ATSDR . U.S. Department of Health and Human Services. Public Health Service; 2007. Agency for Toxic Substances and Disease Registry. [Google Scholar]
- 35.Bain L.J., Liu J., League R.E. Arsenic inhibits stem cell differentiation by altering the interplay between the Wnt3a and Notch signaling pathways. Toxicol. Rep. 2016;3:405–413. doi: 10.1016/j.toxrep.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwalfenberg G., Rodushkin L., Genuis S.J. Heavy metal contamination of prenatal vitamins. Toxocology Reports. 2018;5:390–395. doi: 10.1016/j.toxrep.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lieschke G.J., Currie P.D. Animal models of human disease: zebrafish swim into view. Nat. Rev. Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- 38.Kari G., Rodeck U., Dicker A.P. Zebrafish: an emerging model system for human disease and drug discovery. Clin. Pharmacol. Ther. 2007;82:70–80. doi: 10.1038/sj.clpt.6100223. [DOI] [PubMed] [Google Scholar]
- 39.Dai Y.J., Jia Y.F., Chen N., Bian W.P., Li Q.K., Ma Y.B. Zebrafish as a model system to study toxicology. Environ. Toxicol. Chem. 2014;33:11–17. doi: 10.1002/etc.2406. [DOI] [PubMed] [Google Scholar]
- 40.Westerfield M. University of Oregon Press; 1995. The Zebrafish Book: a Guide for the Laboratory Use of Zebrafish (Brachydanio Rerio) [Google Scholar]
- 41.Weigt S., Huebler N., Braunbeck T., Landenberg F.V., Broschard T.H. Zebrafish teratogenicity test with metabolic activation (mDarT): effects of phase I activation of acetaminophen on zebrafish Danio rerio embryos. Toxicology. 2010;275:36–49. doi: 10.1016/j.tox.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Ding Y.J., Chen Y.H. Developmental nephrotoxicity of aristolochic acid in a zebrafish model. Toxicol. Appl. Pharmacol. 2012;261:59–65. doi: 10.1016/j.taap.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 43.He J.H., Guo S.Y., Zhu F., Zhu J.J., Chen Y.X., Huang C.J. A zebrafish phenotypic assay for assessing drug-induced hepatotoxicity. J. Pharmacol. Toxicol. Methods. 2013;67:25–32. doi: 10.1016/j.vascn.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Nishimura Y., Murakami S., Ashikawa Y., Sasagawa S., Umemoto N., Shimada Y. Zebrafish as a systems toxicology model for developmental neurotoxicity testing. Congenit. Anom. 2015;55:1–16. doi: 10.1111/cga.12079. [DOI] [PubMed] [Google Scholar]
- 45.Liang J., Jin W., Li H., Liu H., Huang Y., Shan X. In vivo cardiotoxicity induced by sodium arscinate in zebrafish Larvae. Molecules. 2016;21:190. doi: 10.3390/molecules21030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lele Z., Krone P.H. The zebrafish as a model system in developmental, toxicological and transgenic research. Biotechnol. Adv. 1996;14:57–72. doi: 10.1016/0734-9750(96)00004-3. [DOI] [PubMed] [Google Scholar]
- 47.Kimmel C.B., Ballard W.W., Kimmel S.R., Ullmann B., Schilling T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 48.Chakraborty P., Babu P.R., Sarma V. A new spectrofluorometric method for the determination of total arsenic in sediments and its application to kinetic speciation. Int. J. Environ. Anal. Chem. 2012;92:133–147. [Google Scholar]
- 49.Haley R., Turner M. 2016. Evaluation of a Colorimetric Assay for the Detection of Arsenic in Water: a Report of a Senior Study, Thesis. [Google Scholar]
- 50.Hill D.S., Wlodarczyk B.J., Finnell R.H. Reproductive consequences of oral arsenate exposure during pregnancy in a mouse model. Birth Defects Res. B Dev. Reprod. Toxicol. 2008;83:40–47. doi: 10.1002/bdrb.20142. [DOI] [PubMed] [Google Scholar]
- 51.Rodríguez V.M., Carrizales L., Mendoza M., Fajardo O., Giordano M. Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol. Teratol. 2002;24:743–750. doi: 10.1016/s0892-0362(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 52.Li D., Lu C., Wang J., Hu W., Cao Z., Sun D. Developmental mechanisms of arsenite toxicity in zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2009;91:229–237. doi: 10.1016/j.aquatox.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Lema S.C., Schultz I.R., Scholz N.L., Incardona J.P., Swanson P. Neural defects and cardiac arrhythmia in fish larvae following embryonic exposure to 2, 2′, 4, 4′-tetrabromodiphenyl ether (PBDE 47) Aquat. Toxicol. 2007;82:296–307. doi: 10.1016/j.aquatox.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Incardona J.P., Collier T.K., Scholz N.L. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol. Appl. Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 55.Ross M.E. Cell division and the nervous system: regulating the cycle from neural differentiation to death. Trends Neurosci. 1996;19:62–68. doi: 10.1016/0166-2236(96)89622-6. [DOI] [PubMed] [Google Scholar]
- 56.Wullimann M.F., Knipp S. Proliferation pattern changes in the zebrafish brain from embryonic through early postembryonic stages. Anat. Embryol. 2000;202:385–400. doi: 10.1007/s004290000115. [DOI] [PubMed] [Google Scholar]
- 57.Machado A.F., Hovland D.N., Jr, Pilafas S., Collins M.D. Teratogenic response to arsenite during neurulation: relative sensitivities of C57BL/6J and SWV/Fnn mice and impact of the splotch allele. Toxicol. Sci. 2020;51(99):98–107. doi: 10.1093/toxsci/51.1.98. [DOI] [PubMed] [Google Scholar]
- 58.Najafzadeh H., Mahabady M.K., Haji A. A comparative study of the effects of sodium arsenite and nanoparticles of sodium arsenite on the apparent and skeletal malformations in rat embryos. Journal of Babol University of Medical Sciences. 2015;17:60–66. [Google Scholar]
- 59.Ahmad S.A., Sayed M.H., Barua S., Khan M.H., Faruquee M.H., Jalil A. Arsenic in drinking water and pregnancy outcomes. Environ. Health Perspect. 2001;109:629–631. doi: 10.1289/ehp.01109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sfakianakis D.G., Renieri E., Kentouri M., Tsatsakis A.M. Effect of heavy metals on fish larvae deformities: a review. Environ. Res. 2015;137:246–255. doi: 10.1016/j.envres.2014.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.