Abstract
A 51 years old female patient with granulomatous mastitis diagnosis, was referred to the Medical Mycology Department “Dr. Dante Borelli” at the Instituto de Medicina Tropical, Universidad Central de Venezuela (IMT-UCV). Mycological diagnosis demonstrated the presence of intracellular yeast structures suggesting Histoplasma sp. and also multi-budding structures compatible with Paracoccidioides sp. To the best of our knowledge, this is the first case report of a granulomatous mastitits due to coinfection of both fungi. 2012 Elsevier Ltd. All rights reserved.
Keywords: Histoplasmosis, Paracoccidioidomycoses, Granulomatous, Mycoses, Mastitits
1. Introduction
Deep mycoses such as Histoplasmosis and Paracoccidioidomycoses (PCM) may have varied clinical presentations when disseminated. Patients with HIV/AIDS or any other form of immunosuppression, frequently develop disseminated forms with muco-cutaneous involvement. PCM occurs in adults between the ages of 30- and 60-years-old, mainly in male patients as result of endogenous latent foci reactivation. Most infected individuals living in endemic areas from southern Mexico to Argentina, will not develop any illness. PCM has a range of clinical presentations along a spectrum, associated with a specific T cell immunity pattern. These individuals exhibit a T-helper [Th-1] immune response characterized by the release of cytokines that activate macrophages, TCD4+, and TCD8+ cells, resulting in the formation of compact granulomas and control of fungal replication. In patients with faulty T-cell mediated immunity, dissemination may occur long after first exposure, up to 30 years. Symptomatic disease can be unifocal or disseminated, involving any organ. Most patient have pulmonary involvement; the most common extrapulmonary sites are skin and mucous membranes. Several studies have shown that estrogens as 17β-estradiol (E2), impair Paracoccidioides spp. morphological transformation of the mycelial to the yeast form, which may explain the strong gender differences among adult population. The exact mechanism involved in cell wall remodeling, energy metabolism, and cell signaling during the mycelium-to-yeast transition remains unclear. For both entities, the clinical presentation can mimic other conditions, including malignancy. A case of granulomatous mastitis associated with infection to Histoplasma sp. and Paracoccidioides sp. is described.
2. Case
A 51-year-old female referred to the Medical Mycology Department “Dr. Dante Borelli” (IMT-UCV) with diagnosis of granulomatous mastitis from an oncologist, presenting with a 4-month history of cutaneous ulcers. The initial lesion (day 0) started as a 4 centimeter deep nodule that eventually fistulized draining a serous discharge. During 3 months, she received several antibiotic treatments including oral Ampicilline/Sulbactam (day +15) for a month, intravenous Meropenem (day +45) for ten days and Amikacin (day +60) for ten days, without improvement. Tuberculine test, baciloscopy and culture done on day 80th were all negative.
A biopsy of the breast tissue performed on day 90th revealed pseudoepitheliomatous hyperplasia and non-caseating chronic granulomatous inflammation. No foreign material was identified and malignancy was ruled out.
On day 100, the patient's breast showed asymmetric gynecomastia with abnormal growth of the left breast and multiple painful, non-healing ulcers with indurated edges and granulomatous aspect affecting the total surface of the breast (Fig. 1). There were no palpable lymphadenopathies. As epidemiologic background, the patient referred contact with barnyard birds and farming as a hobby for the previous 10 years using recently for this activity, soil recovered from a racecourse near of her house. Two months before to the appearance of the cutaneous lesions, she experienced productive cough and night fever that resolved spontaneously, being diagnosed as pneumonia. The patient referred weight loss of approximately 20 kg in the last 6 months attributed to economic shortage. A chest X-ray, showed patchy opacities involving both lungs with interstitial pneumonitis pattern (Fig. 2). Laboratory exams revealed anemia, leukopenia and thrombocytopenia; the other values were not available. During clinical evaluation, a sample of a breast ulcer was taken (day +100) and stained with Giemsa, revealing in the microscope exam 10-μm multi budding anisometric blastoconidia that suggested infection with Paracoccidioides sp (Fig. 3). Serological screening for HIV infection was negative and subsequent serological tests done with Histoplasma sp. and Paracoccidioides sp. antigens performed on day +110 showed negative results.
Fig 1.

Asymmetric and abnormal growth of the left breast and multiple, non-healing ulcers with indurated edges and granular aspect affecting the total surface of the breast, additionally nipple inversion.
Fig. 2.
Chest X-ray showing patchy opacity involving both lungs with interstitial pattern.
Fig. 3.
Large spherical yeast with buds attached by narrow necks and large blastoconidia with multiple buds suggestive of Paracoccidioides sp. Giemsa stain (400X).
A histological review of the original tissue sampled (day +115) was performed, and non-caseating granulomatous inflammation as well as numerous intracellular round shaped microorganisms surrounded by a clear halo were evidenced (Fig. 4). Special stains (Periodic acid-Schiff, Gomori's methanamine silver and Acid Fast Bacilli) were performed confirming the presence of intracellular small yeasts compatible with Histoplasma sp. and multi-budding anisometric rounded yeasts consistent with Paracoccidioides sp (Fig. 5).
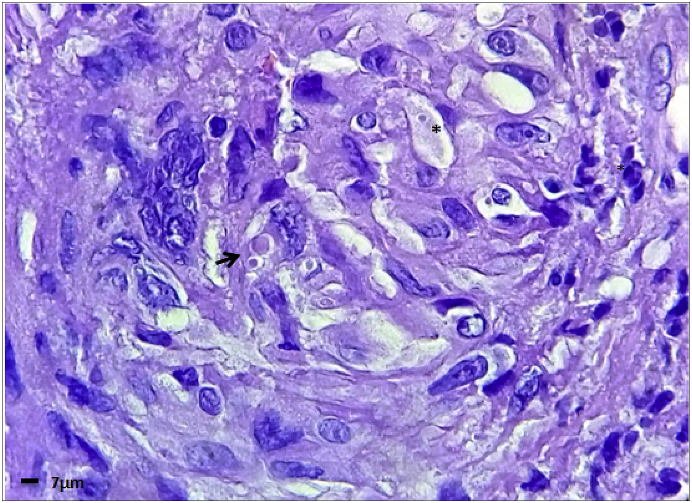
Fig. 4.
Non-necrotizing granuloma, histologically formed by epithelioid histiocytes with small intracellular yeast (asterisk) suggestive of Histoplasma sp. Large spherical blastoconidia with buds attached by narrow necks (arrow) and large yeast with multiple buds remindful of Paracoccidioides sp H&E (1000x).
Fig. 5.
Microphotography showing suggestive blastoconidia of Histoplasma sp (red asterisk) and Paracoccidioides sp (red arrow). Grocott methenamine silver stain (400x). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The patient was treated with 400 mgs of Itraconazole for 1 year, with complete resolution of the cutaneous ulcers and disappearance of the patchy opacities on the chest X-rays.
3. Discussion
Granulomatous mastitis (GM) is a rare chronic benign inflammatory pathology of the breast, with remarkable variable etiology, including infectious and non-infectious causes. Recognizing this entity is important because it can mimic breast carcinoma, both clinically and radiographically. Infectious causes of GM include tuberculosis, actinomycosis, syphilis and fungal infections among others. It is thought that those agents trigger the immune system, leading to granuloma formation. Non-infectious causes include autoimmune diseases such as Granulomatosis with polyangiiitis, giant cell arteritis, sarcoidosis and others. All these entities are difficult to distinguish clinically and radiographically.
In our case, GM was a muco-cutaneous manifestation of the co-infection with Histoplasma sp., and Paracoccidioides sp.
Paracoccidioidomycosis and Histoplasmosis are the most relevant endemic mycoses in Venezuela [1,2]. In both cases, infection occurs following inhalation of spores from the mold of the fungus and is usually asymptomatic and self-limited [[3], [4], [5], [6]]. Sub-clinical hematogenous dissemination into other organs after a benign primary lung infection is well documented [1]. The occurrence of mammary gland infection in a highly endemic region such as Venezuela, does not surprise [7].
Paracoccidioidomycosis and Histoplasmosis share pathogenesis; soil-borne spore-containing dust is inhaled by patients and a pulmonary complex is formed. It is widely documented that Histoplasmosis is a cosmopolitan disease. Emmons's discovery of Histoplasma sp. in soil, revealed the basic source of all human and animal infection, and the recovery of this fungus from the air, substantiate the hypothesis that air currents spread the spores in the soil reservoirs. The excreta of various bird species and mammals, when incorporated into the soil, provide conditions that permits Histoplasma sp. survive the competition of other soil microorganisms [8].
Paracoccidioidomycosis is restricted to Latin America. The mechanism of how Paracoccidioides sp. reaches the soil is unknown. People at major risk for acquiring the infection are those having a profession or activity related to the management of soil that could be contaminated with the fungus, such as agriculture, earthwork, transportation of vegetable products and soil preparation for gardening, as in our case [2,5].
The ecological and weather changes, possibly leads to the hypothesis that both fungi inhabit in the same ecological niche. In Venezuela, PCM reservareas and endemic areas of histoplasmosis geographically overlaps [1,2]. The primary infection may precede by many years the clinical manifestations in the chronic forms, appearing when the individual no longer lives in an endemic area [2].
There are several reports that associates GM with bacteria such as Corynebacterium sp. and Mycobacterium tuberculosis [9,10]. However we found few reports of granulomatous mastitis associated to infection with Histoplasma sp. or Paracoccidioides sp. in the literature [7,[11], [12], [13], [14], [15]]. To the best of our knowledge, there have been few cases describing coinfection of both mycoses in a single human host [2,16]. This may be due to either lack of suspicion or the failure to recognize the causative agents in tissue sections [[17], [18], [19], [20]]. Nevertheless, it is fair to point out, that both fungi are difficult to grow and slow-growing in culture, resulting frequently in false-negative cultures [1,7,20].
It is highly recommended to use several diagnostic methods, to improve the possibility of achieving the diagnosis. However, the direct microscopic examination of fresh clinical specimens, as well as biopsy staining with Giemsa or Wright-Giemsa, Gomori-Grocott and PAS, may facilitate the rapid diagnosis of patients with both mycoses; the thorough search and review of the slides stained especially for fungus by well-trained personnel, demonstrated the presence of Histoplasma sp. and Paracoccidioides sp yeast in this case [1,7,11,14]. On the other hand, delay in the diagnosis while awaiting the results of fungal cultures may lead to a fatal outcome in more severe cases [1].
Fungal agents should be considered always in cases of GM, especially if the patient has any form of immunosuppression (e.g: post-transplant, immunotherapy, chemotherapy, malnutrition, stress, etc) [[10], [11], [12]]. Clinical manifestations in addition to epidemiological data, must be thoroughly gathered as they may aid in the diagnosis of the disease [1]. In our case, the patient referred gardening as hobby and also was malnourished. Use of fungal-specific stains, culture, histopathological exam of clinical samples obtained from suspected lesions and serology should be done to confirm the clinical diagnosis [[17], [18], [19]].
We recommend treatment of the co-infection with these fungi using 6–11mg/kg of oral itraconazole for a 1-year period.
In Venezuela, as well as in other countries where PCM and Histoplasmosis are endemic, clinicians must bear these mycoses in mind, specially when dealing with patients having non-healing granulomatous cutaneous lesions. Seeking epidemiological clues and a throughful evaluation of general health conditions and backgrounds during examination is very important. Proper mycological and histopathological exam of the lesions is also a key tool to achieve an early diagnosis of both mycoses and avoid evolution of the disease [1,2,15]. As more cases of GM clinically simulating carcinoma are detected, mycotic mastitis should become more frequently recognized [7].
Declaration of competing interest
There are no conflict of interest.
Acknowledgements
We would like to acknowledge Dr. Carolina Olaizola for reviewing this manuscript and helping us with the final edition.
References
- 1.Mata-Essayag S., Colella M.T., Roselló A., Hartung de Capriles C., Landaeta M.E. Histoplasmosis. A study of 158 cases in Venezuela, 2000-2005. Medicine. 2008;87(4):193–202. doi: 10.1097/MD.0b013e31817fa2a8. [DOI] [PubMed] [Google Scholar]
- 2.Merino-Alado R., Mata-Essayag S., Pineda J., Moronta G., Briceño-Caveda E. Oral manifestations associated to paracoccidioidomicosis and histoplasmosis. Pesqui. Bras. Odontopediatria Clin. Integr. 2018;(1) [Google Scholar]
- 3.Bahr N., Antinori S., Wheat J., Sarosi G. Histoplasmosis infections worldwide: thinking outside of the Ohio River valley. Curr. Trop. Med. Rep. 2015;2(2):70–80. doi: 10.1007/s40475-015-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz J.H. Environmental and wilderness-related risk factors for Histoplasmosis: more than bats in caves. Wilderness Environ. Med. 2018:1–13. doi: 10.1016/j.wem.2018.06.008. 000. [DOI] [PubMed] [Google Scholar]
- 5.Camacho E., Niño-Vega G. Paracoccidioides spp.: virulence factors and Immune-evasion strategies. Mediat. Inflamm. 2017:19. doi: 10.1155/2017/5313691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernard G. An overview of the immunopathology of human paracoccidioidomicosis. Mycopathologia. 2008;165:209–221. doi: 10.1007/s11046-007-9065-0. [DOI] [PubMed] [Google Scholar]
- 7.Salfelder K., Schwarz J. Mycotic “Pseudotumors” of the breast: report of four cases. Arch. Surg. 1975;110:751–754. doi: 10.1001/archsurg.1975.01360120069013. [DOI] [PubMed] [Google Scholar]
- 8.Ajello L. Observations on the epidemiology of histoplasmosis. Mycopathol. Mycol. Applicatta. 1961;XV:231–237. doi: 10.1007/BF02136328. [DOI] [PubMed] [Google Scholar]
- 9.Yu H.-J., Deng H., Ma J., Huang S.-J., Yang J.-M. Clinical metagenomic analysis of bacteria communities in breast abscesses from granulomatous mastitis. Int. J. Infect. Dis. 2016 doi: 10.1016/j.ijid.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez Miño JI., Ortiz Rodriguez A.M., García Orozco L., Venegas Mera B., Yepez-Yerovi F.E. Tuberculosis de mama: reporte de un caso. Rev. Peru. Med. Exp. Salud Pública. 2018;35(2):333–337. doi: 10.17843/rpmesp.2018.352.3131. [DOI] [PubMed] [Google Scholar]
- 11.Osborne B.M. Granulomatous Mastitis caused by Histoplasma and mimicking inflammatory breast carcinoma. Hum. Pathol. 1989;20(1):47–52. doi: 10.1016/0046-8177(89)90201-3. [DOI] [PubMed] [Google Scholar]
- 12.David Houn H.-Y., Granger J.K. Granulomatous mastitis secondary to Histoplasmosis: report of a case diagnosed by fine-needle aspiration biopsy. Diagn. Cytopathol. 1990;7(3):282–285. doi: 10.1002/dc.2840070314. [DOI] [PubMed] [Google Scholar]
- 13.Leandro de Oliveira D., Yampara Guarachi G., De Souza Marinho J., Silveira Vianna J., Alves de Melo A. Systemic Paracoccidioidomycosis involving breast. Breast J. 2018:1–2. doi: 10.1111/tbj.13078. [DOI] [PubMed] [Google Scholar]
- 14.Mata-Essayag S., Landaeta M.E., Colella M.T., Pineda V., Dawaher J. Histoplasmosis Mamaria. Estudio de una Serie de Casos. Inf. Med. 2014;16(2):71–74. [Google Scholar]
- 15.Fernandes F.F., Oliveira Alves V., Gavilanes Sanchez T., Diniz De Paula W., Cruz Santana A. Chylotorax in paracoccidioidomycosis. Rev. Inst. Med. Trop. Sao Paulo. 2016;58:57. doi: 10.1590/S1678-9946201658057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres Esteche V., Arteta Z., Torres G., Vaucher A., Gezuele E. Un caso excepcional de paracoccidioidomicosis e histoplasmosis pulmonares de presentación concomitante. J. Bras. Pneumol. 2012;38(2):264–268. doi: 10.1590/s1806-37132012000200017. [DOI] [PubMed] [Google Scholar]
- 17.Rocha-Silva F., Ferreira Gumaraes C., Rocha de Oliveira E., De Figueredo S., Basques R. Disseminated paracoccidioidomycosis prediagnosticated as neoplasm: an important challenge in diagnosis using rt-PCR. Med. Mycol. Case Rep. 2018;19:1–5. doi: 10.1016/j.mmcr.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golda N., Feldman M. Histoplasmosis clinically imitating a cutaneous malignancy. J. Cutan. Pathol. 2008;35(1):26–28. doi: 10.1111/j.1600-0560.2007.00961.x. [DOI] [PubMed] [Google Scholar]
- 19.Cubas W.S., Jimenez G., Vega J. Lesiones cutáneas como manifestación de una histoplasmosis diseminada en un hospital del Peru. Rev. Chil. infectol. 2017;34(6):613–614. doi: 10.4067/S0716-10182017000600613. [DOI] [PubMed] [Google Scholar]
- 20.Wheat L., Azar M.M., Bahr N.C., Spec A., Relich R.F., Hage C. Histoplasmosis. Infect. Dis. Clin. N. Am. 2016;30:207–227. doi: 10.1016/j.idc.2015.10.009. [DOI] [PubMed] [Google Scholar]