Graphical abstract
Keywords: Combretum micranthum, Toxicity, Acute toxicity, Sub-chronic toxicity
Highlights
-
•
Acute and subchronic oral toxicity assessments of Combretum micranthum leaves extract were evaluated in Wistar rats of both sexes.
-
•
In acute oral toxicity assessment, LD50 of Combretum micranthum leaves extract is greater than 5000 mg/kg.
-
•
In subchronic oral toxicity assessment at doses of 500 and 1000 mg/kg/day for 28 days, No significant changes in food consumption, body weight gain, organ weights and in biochemical parameters.
-
•
The level of PLT increased in female rats in the sub-chronic study but the immune system was not affected.
-
•
No treatment related pathology was identified during histopathology.
Abstract
Background
Combretum micranthum (CM) (Combretaceae) is widely used in traditional medicine throughout West Africa for the treatment of diabetes, hypertension, inflammation, malaria and liver ailments. In our recent research we demonstrated that CM has nephroprotective potentials in diabetes mellitus, hypertension and renal disorders. However, to the best of our knowledge, no systematic study concerning its toxicity profile has been reported.
Aim of the study
The study carried out to evaluates the potential toxicity of the hydroalcoholic extract from leaves of the CM, through the method of acute and sub-chronic oral administration in rats.
Materials and methods
During the acute toxicity study, male and female rats were orally administrated with CM extract at single doses of 5000 mg/kg (n = 5/group/sex). Abnormal behaviour, toxic symptoms, weight, and death were observed for 14 consecutive days to assess the acute toxicity. For sub-chronic toxicity study, the extract was administered orally at doses of 500 and 1000 mg/kg (n = 5/group/sex) daily to Wistar rats for 28 days. The general behaviour and body weight of the rats was observed daily. A biochemical, haematological, macroscopical and histopathological examinations of several organs were conducted at the end of the treatment period. The CM extract was subjected to Fourier transform infrared spectrophotometric examination in order to detect the presence or absence of cyanide toxic compounds.
Results
The absence of absorbance peaks between the 2220−2260 cm−1 region of FT-IR spectrum of CM, indicating the absence of cyanide groups. This suggested that the CM extract may not contain toxic substances. During the acute toxicity test, no mortality or adverse effects were noted at the dose of 5000 mg/kg. In the subchronic study, the CM extract induced no mortality or treatment-related adverse effects with regard to body weight, general behaviour, relative organ weights, hematological, and biochemical parameters. Histopathological examination of vital organs showed normal architecture suggesting no morphological alterations.
Conclusion
The present study revealed that oral administration of CM extract for 28 days, at dosage up to 1000 mg/kg did not induce toxicological damage in rats. From acute toxicity study, the median lethal dose (LD50) of the extract was estimated to be more than 5000 mg/kg.
1. Introduction
The use of medicinal plants to treat various pathologies dates back to the time [[1], [2], [3]]. They are an important part of our daily lives [1]. The reasons for the usage of plants are natural, traditional, cultural, economic and other reasons [[4], [5], [6], [7]]. According to the World Health Organization (WHO), up to 80 % of the world's population uses medicinal plants for their health care [1,6,8]. The effectiveness, availability, cost and minimization of side effects would contribute to this situation [4,7,9]. Medicinal plants can play an important role in drug discovery and their studies are logical search strategies in the discovery of new drugs [2,10]. Several studies have reported the efficacy of medicinal plants in treating various diseases [4,8,10,11]. Toxicological study strategy prevailed in the 19th and 20th centuries but it is thought that toxicity is no longer a criterion of choice in ethnopharmalogical approach because the orientation is brought to consumers by traditional use based on secular knowledge in selecting interesting species [2]. In this perspectives, the search for toxicity is considered not essential in the first intention because the great majority of the experienced plant extracts are not toxic [2]. Lack of evidence-based approaches such as the legal and regulatory framework, pharmacovigilance, non-standardization and lack of toxicological profiling of herbal preparations form the biggest concern of medicinal plants use [1,2]. Plants synthesize a variety of metabolites that form complex compounds that may be beneficial or harmful to humans [10,11]. However, despite the therapeutic benefits of plants, some constituents of a few medicinal plants have been shown to be potentially toxic, carcinogenic and teratogenic [1,8,10]. The general perception is that natural products of plant origin are non-toxic and without side effects is proven false in some cases [2,9,10]. Investigations carried out on more than 1,500,000 plants, show that most of these plants contain toxic substances [5]. In addition, it is known that the consumption of medicinal plants without evaluating their efficacy and safety may lead to unexpected or toxic effects that may affect the physiology of different organs [10,12]. In our recent research, we demonstrated that hydroalcoholic extract of Combretum micranthum [CM] exhibited good antioxidant and nephroprotective potentials in in-vivo, ex-vivo and in-vitro HEK-293 (Human embryonic kidney) cell line experiments [13] and nephroprotective activity in cisplatin induced nephrotoxicity in rats [14]. To validate the nephroprotective phytomedicine, toxicological studies are necessary. Conventionally, in the presence of an unknown substance the first step in the search for a pharmacological activity begins with the study of toxicity and in particular by the evaluation of the lethal dose 50 (LD50) [15]. Toxicity study is, moreover, an excellent criterion for guiding the search for pharmacological activity [8,15]. "Everything is poison, nothing is poison, only the dose counts". This famous idea of Paracelsus leads to be careful with the drug. The border between drug and toxic is vague, it is often a question of dose [16]. Despite the widespread use of Combretum micranthum in traditional medicine, there is a lack of experimental data on its possible toxicity. In addition, nephrology enthusiasts criticize several medical specialties for causing kidney damage. To avoid this situation and to ensure the safety of Combretum micranthum, the present study aims to search for Combretum micranthum safety data focusing on acute toxicity and 28-day subchronic toxicity of hydroethanolic extract of Combretum micranthum leaves administered orally in male and female Wistar rats.
2. Material and methods
2.1. Material
2.1.1. Chemicals and reagents
Commercial reagent kits for determination of creatinine (CRE), urea (UR), glucose (Glu), total protein (TP), albumin (Alb), calcium (Ca2+), phosphorus (P), triglycerides (TG), total cholesterol (TC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and gamma glutamyl transferase (γGT) activities were purchased from Biolabo S.A. (Paris, France). All other chemicals and reagents used were of the highest quality analytical obtained from commercial sources.
2.1.2. Plant material
Fresh leaves of Combretum micranthum (CM) were collected in December 2016 from Alibi I, a locality at North West of Tchamba (Togo). Botanical authentication was confirmed at the Laboratory of Botany and Vegetable Ecology, Faculty of Science, University of Lomé, Togo and the voucher specimen was deposited at the herbarium (N° TOGO151085). The leaves were washed, dried under shade and were coarsely powdered.
2.1.3. Animals
Albino Wistar rats of either sex of 6-8 weeks weighing 200-–8 weeks weighing 200−250 g were procured from Nigerian Institute of Medical Research, Lagos, Nigeria. Animals were housed in standard cages and maintained under standard laboratory conditions. Experimental protocols adopted were based on World Health Organization Guidelines for care and use of laboratory animals. The experimental usage of the animals was approved by the Ethics Committee of the University of Lomé, a branch of the National Ethics Committee for control and supervision of experiments on animals (N° SBM/UL/14/NS0004). They were acclimatized for two weeks before the experiments and fed with normal pellet diet and water ad libitum.
2.2. Methods
2.2.1. Extraction
The powder (830 g) was macerated at room temperature with 5 L of ethanol-water (8:2 v/v) for 72 h. The filtrate was evaporated under vacuum at 45 °C by a rotary evaporator (Rotavapor Buchi R100) [13,14]. The yield of hydroalcoholic extract of Combretum micranthum (CM) was 12.15 %.
2.2.2. Acute toxicity
Healthy male and female Wistar rats were used in this study according to the instructions of the Organization for Economic Cooperation and Development (OECD) for acute oral toxicity tests [17,18]. All animals were fasted overnight, but with free access to water and weighed before administration of the extract. The animals were randomly divided into two groups according to their sex (n = 10; 5 males and 5 females per group). Group 1 (Control) received distilled water orally; Group 2 (Acute toxicity) received a limit dose of the CM extract of 5 g/kg.
The animals are then observed for mortality, signs of acute toxicity and behavioural changes (aggression, unusual vocalisation, agitation, sedation and somnolence, convulsions, tremors, ataxia, catatonia, paralysis, fasciculation, prostration and unusual locomotion and asphyxia) for the first thirty minutes and the first hour, then every hour for 5 h and finally periodically up to 48 h. All experimental animals were individually observed daily for general behaviour and body weight changes, dangerous symptoms and mortality for 14 days after treatment. The LD50 should be greater than 5 g/kg if three or more rats survived. At the end of the experimental period, all animals were weighed and sacrificed by cervical dislocation, and the organs were removed for necropsy.
2.2.3. 28-day subchronic toxicity
The experiment was conducted according to the protocols described by OECD Guideline 407 [19] and Agyigra, Ejiofor and Magaji [20], Lee, Kim, Kim, Shin, Lee, Cho, Lee and Lee [21], Atsamo, Nguelefack, Datte and Kamanyi [22] and Tchoumtchoua, Mouchili, Ateba, Zingue, Halabalaki, Mbanya, Skaltsounis and Njamen [18] previous studies with minor modifications. A total of 30 rats of both sexes were used in this study. The rats were divided into three groups of 10 (n = 10; 5 males and 5 females per group) and their weights were recorded. Prior to treatment, rats were handled individually and carefully examined for abnormal behaviour and appearance. The CM extract dissolved in distilled water, was administered orally once a day for 28 consecutive days. Group1 (Control rats) received distilled water; Group 2 (CM 500 mg/kg) received extract at a dose of 500 mg/kg; Group 3 (CM1000 mg/kg) received the extract at a dose of 1000 mg/kg.
The animals were observed daily during the experimental period for mortality or morbidity, changes in posture, changes in the fur of the skin, eyes, mucous membranes and behaviours. At the end of the 28 days of administration, the animals were fasted overnight, but had free access to the water. On 29th day, they were anesthetized with ether and blood samples were taken by retro-orbital puncture using capillary tubes for hematological and biochemical studies. After blood collection, the rats were sacrificed by cervical dislocation. Organs such as liver, kidneys, sex organs (testes, seminal vesicles and epididymis in male rats, ovaries and uterus for female rats), brain, spleen, lungs and heart were collected, washed immediately in NaCl (0.9 %), weighed individually and examined macroscopically. The organs removed were weighed and the relative weight of the organs was calculated.
2.2.3.1. Hematological and biochemical analyzes
Blood samples taken in EDTA tubes were used for hematological treatment using an automated hematology analyzer (ABX Pentra XL 80, France). The differential count of leukocytes was performed with light microscopy (Optika, Italy) after haematological staining (fixation with May Grunewald and staining with Giemsa stain (Atom Scientific, UK). In each case, 100 cells were counted. Blood samples taken in anticoagulant-free tubes were used for biochemical analysis and were centrifuged at 3000 rpm for 10 min. The sera were separated, stored at -20 °C and used for evaluation. Biochemical parameters were estimated using URIT 8021A automated analyzer (URIT Medical Electronic Group Co., Ltd.).
2.2.3.2. Histological examination
Tissue samples from kidney, liver, lung, brain, heart, spleen, gastrointestinal tract, pancreas, ovaries and testes were collected and fixed in 10 % phosphate-buffered formalin (pH 7.0) for 24 h, routinely processed, embedded in paraffin wax, cut into 2–3 μm (μm) sections and stained with hematoxylin and eosin (H&E). The photomicrographs were taken using an Olympus SP 350 digital camera and Stream Basic imaging software (Olympus Corporation, Tokyo, Japan) [14].
2.2.4. Statistical analyses
All the values are expressed as mean ± SEM (n = 5). Statistical analysis was performed in Graph Pad Prism 7 software (San Diego, CA, USA) using One-way analysis of variance (ANOVA) followed by Tukey’s test as a post hoc analysis. The value of p < 0.05 was considered to be statistically significant.
3. Results
3.1. Acute toxicity
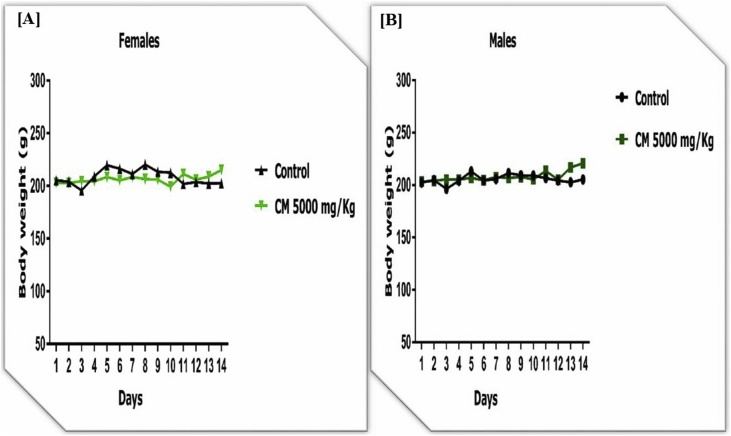
Oral administration of CM extract at a dose of 5000 mg/kg resulted in no mortality or clinical signs of acute toxicity in rats as observed for a short period of 48 h and a prolonged period of 14 days. The 10 rats (5 males and 5 females) survived until the end of the observation period. No abnormalities were found in the organs at autopsy. The body weight did not change during the 14 days period (Fig. 1).
Fig. 1.
Effect of Combretum micranthum (CM) extract on body weight changes in females [A] and males [B] in acute toxicity in rats. Data are expressed as Mean ± SEM (n = 5). One-way ANOVA followed by Tukey's multiple comparisons test.
3.2. Subchronic (28 days) toxicity
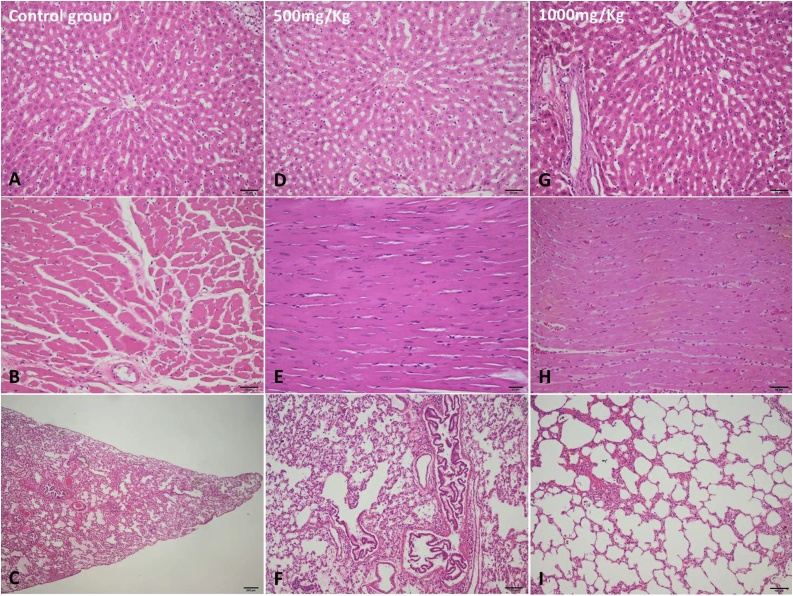
Daily oral administration of the CM extract at doses of 500 mg/kg and 1000 mg/kg for 28 consecutive days did not result in any significant change in the overall behaviour of treated rats compared to controls. Control rats and treated rats appeared uniformly healthy and no lethality was observed throughout the 28-day treatment period. No differences in food and water consumption were observed between groups of rats. Similarly, no significant differences in body weight gain or relative organ weight were observed between control and treated groups (Fig. 2). Macroscopic observations of treated animal organs showed no significant changes in colour and texture between the control and treated groups. Similarly, subchronic oral administration of the extract did not result in significant changes in the hematological parameters of male and female rats (Table 1). However, platelet count increased (P < 0.001) in the female treated rats. The extract did not cause significant changes in hepatic enzymes (AST, ALT, PAL and γGT), serum glucose (Glu), calcium (Ca2+), phosphorus (P), triglycerides (TG), total cholesterol (TC), total protein (TP), albumin (Alb), creatinine (CRE) and urea (UR) in male and female rats (Table 2). Microscopically, no histological changes were identified in the evaluated organs (Liver, Heart and Lungs) from all the groups (Fig. 3).
Fig. 2.
Effect of Combretum micranthum (CM) extract on body weight of females [A] & males [A] and relative weight of organ of on females [C] & males [D] in subchronic toxicity in rats. Data are expressed as Mean ± SEM (n = 5). One-way ANOVA followed by Tukey's multiple comparisons test.
Table 1.
Effect of CM on hematological parameters.
| Female rats | |||
|---|---|---|---|
| Parameters | Control | CM 500 mg/Kg | CM 1000 mg/Kg |
| WBC (103 cells/μL) | 13.93 ± 3.20 | 10.23 ± 1.03 | 13.96 ± 3.27 |
| RBC (106 cells/μL) | 7.14 ± 0.23 | 6.56 ± 0.35 | 6.61 ± 0.12 |
| HGB (g/dL) | 15.06 ± 0.49 | 12.8 ± 0.35 | 12.6 ± 0.47 |
| HCT (%) | 44.5 ± 1.98 | 41.66 ± 0.81 | 37.93 ± 1.37 |
| MCV (fL) | 62.33 ± 1.98 | 63.96 ± 4.75 | 57.33 ± 1.27 |
| MCH (pg) | 21.13 ± 0.75 | 19.43 ± 0.51 | 19.03 ± 0.48 |
| MCHC (g/dL) | 33.9 ± 0.66 | 30.46 ± 1.73 | 33.23 ± 0.06 |
| PLT (103 cells/μL) | 814.66 ± 31.85 | 877 ± 97.07 | 1164.66 ± 175.23*** |
| RDW-CV (fL) | 31.76 ± 1.30 | 42.9 ± 10.58 | 33.4 ± 2.23 |
| PDW (fL) | 12.26 ± 0.43 | 17.7 ± 3.85 | 15.86 ± 2.22 |
| MPV (fL) | 10.26 ± 0.08 | 10.26 ± 0.60 | 10.53 ± 0.26 |
| P-LCR (%) | 8.36 ± 0.06 | 8.03 ± 0.29 | 8.2 ± 0.2 |
| PNN (%) | 13.83 ± 0.58 | 12.73 ± 1.66 | 13.53 ± 1.23 |
| PNE (%) | 17.66 ± 7.17 | 25.66 ± 6.17 | 32 ± 10.44 |
| PNB (%) | 3.66 ± 1.76 | 3.66 ± 1.45 | 2 ± 1.52 |
| LYM (%) | 0.33 ± 0.33 | 0.6 ± 0.66 | 1 ± 0.57 |
| MON (%) | 77 ± 8.50 | 67 ± 6 | 61 ± 8.71 |
| Male rats | |||
|---|---|---|---|
| Parameters | Control | CM 500 mg/Kg | CM 1000 mg/Kg |
| WBC (103 cells/μL) | 13.13 ± 1.61 | 10.76 ± 0.31 | 12.3 ± 2.67 |
| RBC (106 cells/μL) | 8.39 ± 0.23 | 8.05 ± 0.40 | 8.59 ± 0.13 |
| HGB (g/dL) | 14.93 ± 0.32 | 14.16 ± 0.24 | 15.03 ± 0.27 |
| HCT (%) | 47.73 ± 0.77 | 45.6 ± 0.65 | 48.4 ± 0.75 |
| MCV (fL) | 56.96 ± 1.15 | 56.8 ± 2.00 | 56.2 ± 0.91 |
| MCH (pg) | 17.8 ± 0.30 | 17.66 ± 0.75 | 17.5 ± 0.40 |
| MCHC (g/dL) | 31.3 ± 0.17 | 31.06 ± 0.38 | 31.03 ± 0.18 |
| PLT (103 cells/μL) | 754.33 ± 75.64 | 794 ± 45.40 | 801.66 ± 89.27 |
| RDW-CV (fL) | 34.16 ± 0.66 | 36.53 ± 0.23 | 35.23 ± 0.98 |
| PDW (fL) | 16.86 ± 0.77 | 18.46 ± 1.58 | 18.3 ± 0.17 |
| MPV (fL) | 11.9 ± 0.15 | 10.56 ± 0.23 | 11.2 ± 0.62 |
| P-LCR (%) | 8.86 ± 0.18 | 8.36 ± 0.14 | 8.46 ± 0.21 |
| PNN (%) | 18.43 ± 1.41 | 14.03 ± 0.95 | 15.23 ± 1.81 |
| PNE (%) | 36.66 ± 4.05 | 24 ± 3.51 | 18.66 ± 0.88 |
| PNB (%) | 3.66 ± 0.88 | 0.33 ± 0.33 | 3.66 ± 0.88 |
| LYM (%) | 0.66 ± 0.33 | 1 ± 0.57 | 2 ± 1.15 |
| MON (%) | 58 ± 2.88 | 72 ± 3.60 | 69.66 ± 1.45 |
Effect of CM extract on serum biochemical parameters in subchronic toxicity in rats. Data are expressed as Mean ± SEM (n = 5). One-way ANOVA followed by Tukey's multiple comparisons test. Compared to control group: *** P < 0.001.
Table 2.
Effect of CM on serum biochemical parameters.
| Females rats | |||
|---|---|---|---|
| Parameters | Control | CM 500 mg/Kg | CM 1000 mg/Kg |
| ALT (U/l) | 42.50 ± 3.52 | 34.07 ± 2.98 | 39.86 ± 6.03 |
| AST (U/l) | 181.53 ± 15.09 | 183.73 ± 14.58 | 175.70 ± 25.76 |
| ALP (U/l) | 130.23 ± 20.66 | 137.30 ± 15.49 | 138.43 ± 17.73 |
| γGT (UI/L) | 2.98 ± 0.06 | 2.80 ± 0.28 | 3.48 ± 0.16 |
| Glu (mg/dL) | 85.33 ± 4.18 | 80.67 ± 2.33 | 88.33 ± 5.78 |
| TP (g/L) | 71.83 ± 3.43 | 66.40 ± 2.15 | 69.87 ± 3.49 |
| Alb (g/L) | 44.37 ± 7.33 | 44.97 ± 1.43 | 31.78 ± 2.33 |
| TC (g/L) | 1.70 ± 0.06 | 1.32 ± 0.14 | 1.42 ± 0.18 |
| TG (g/L) | 1.83 ± 0.18 | 1.50 ± 0.18 | 1.76 ± 0.11 |
| Ca2+(mg/L) | 102.00 ± 5.20 | 104.20 ± 1.20 | 113.70 ± 5.41 |
| P (mg/L) | 47.20 ± 2.37 | 36.83 ± 4.20 | 46.60 ± 1.50 |
| CRE (mg/dL) | 0.93 ± 0.02 | 0.90 ± 0.05 | 1.00 ± 0.01 |
| UR (mg/dL) | 53.69 ± 2.22 | 52.52 ± 3.21 | 49.40 ± 5.21 |
| Males rats | |||
|---|---|---|---|
| Parameters | Control | CM 500 mg/Kg | CM 1000 mg/Kg |
| ALT (U/l) | 73.42 ± 5.20 | 51.61 ± 2.75 | 49.92 ± 2.97 |
| AST (U/l) | 277.60 ± 14.35 | 181.80 ± 4.99 | 224.63 ± 28.88 |
| ALP (U/l) | 350.03 ± 84.75 | 451.83 ± 75.41 | 330.97 ± 66.89 |
| γGT (UI/L) | 1.62 ± 0.23 | 1.67 ± 0.06 | 1.59 ± 0.25 |
| Glu (mg/dL) | 72.33 ± 0.67 | 81.00 ± 5.57 | 83.67 ± 1.33 |
| TP (g/L) | 64.93 ± 3.55 | 61.30 ± 4.10 | 64.37 ± 0.85 |
| Alb (g/L) | 42.81 ± 1.30 | 37.79 ± 2.41 | 40.70 ± 1.94 |
| TC (g/L) | 1.88 ± 0.17 | 1.45 ± 0.10 | 1.74 ± 0.06 |
| TG (g/L) | 1.93 ± 0.09 | 1.64 ± 0.07 | 1.66 ± 0.22 |
| Ca2+(mg/L) | 96.00 ± 13.30 | 92.20 ± 4.92 | 118.73 ± 8.72 |
| P (mg/L) | 40.37 ± 1.82 | 45.90 ± 3.35 | 48.00 ± 4.70 |
| CRE (mg/dL) | 0.83 ± 0.12 | 0.91 ± 0.11 | 0.86 ± 0.04 |
| UR (mg/dL) | 58.06 ± 7.15 | 50.03 ± 4.06 | 55.10 ± 6.13 |
Effect of CM extract on serum biochemical parameters in subchronic toxicity in rats. Data are expressed as Mean ± SEM (n = 5). One-way ANOVA followed by Tukey's multiple comparisons test.
Fig. 3.
Histological findings in normal control (A-liver, B-heart, C-lung), CM (500 mg/kg) [D -liver, E-heart, F-lung] and CM (1000 mg/kg) [G -liver, H-heart, I-lung] groups. Hematoxylin and eosin (H&E) stain.
4. Discussion
Traditional and complementary medicine has been used for centuries. In addition, its popularity and widespread use make it an evidence-based medicine [1,23]. Lack of regulation and factual approach are common and negatively impact. This may be due to the lack of scientific research data and adequate research methodology to evaluate drugs [1,24]. Thus, several herbs and their preparations have been identified for toxicity worldwide [[25], [26], [27]]. The Food and Drug Administration has issued warnings regarding the potential toxic effects of many herbal remedies and/or herbal preparations commonly consumed [1,26]. The toxicological risks of medicinal plants used in different parts of the world have been documented [15]. Safety tests are needed to popularize the acceptance, standardization and or regulation of the market for herbal medicines currently on sale [1,6]. Prior to any pharmacological validation and the development of a phytomedicine of any medicinal plant, acute and subacute toxicity is mandatory according to standard guidelines [15,28]. It is also a necessary process for preclinical dose determination in drug discovery and development [15,29]. Thus, toxicity provides accurate information on potentially relevant adverse effects for the substance being evaluated [30]. Thus, in recent times, scientists have examined the safety and efficacy potential in traditional medicine in order to provide data that meet the criteria required to support its worldwide use [1,10,31]. CM is a medicinal plant traditionally used in the treatment and management of several diseases, but no scientific report is available to date on its safety. From a pharmacological point of view, this plant belongs to the group of those used as antioxidant, cicatrizing, antimicrobial, anti-inflammatory, antidiabetic, hypotensive and other treatments [13]. In our previous work, we proved nephroprotective potential of CM in three models of nephropathies such as diabetes, hypertension and cisplatin induced in-vitro and in-vivo experiments. The medicinal properties of the plant are due to the presence of alkaloids, flavonoids, phenolics, tannins and other phytoconstituents [13]. Several reports have shown that these secondary plant metabolites exert a variety of biological effects [15,32] and are not as free of toxic effects as any substances [33]. In the present study, Wistar rats were used to evaluate the safety of the CM extract with estimates of physical signs, biochemical, hematological and histopathological vital organs in both acute and subchronic toxicity studies. In the acute toxicological study, a single dose treatment of 5000 mg/kg of CM showed no mortality, no clinical signs indicating elevation or decrease in temperature, change of skin colour, change in eye colour, general appearance, diarrhea or sedation. The LD50 of the extract is therefore greater than 5000 mg/kg. The subchronic toxicity study evaluated for 28 days with two doses of 500 and 1000 mg/kg/day was conducted. No toxicity or mortality was observed in the treated groups. After 28 days of oral administration of CM, food and water consumption was not affected. It indicates that the extract had no effect on appetite or adverse effects on the growth of the animals. The results revealed a significant increase in platelet count in female rats at a dose of 1000 mg/kg of CM as compared to normal control group. These results suggest that CM at the highest tested dose can induced thrombocytopenia in female rats by increasing platelets production [1,18]. Hence, this significant change in platelets noted in the female rats was considered as physiological variation, as they were within the normal range and were considered to be incidental due to the lack of dose-dependency, they are generally slightly more sensitive [34]. Our therapeutic doses used were 200 mg/kg and 400 mg/kg [14] which are less than 500 mg/kg and 1000 mg/kg. Hepatic and renal function are crucial, with one being used for the metabolism of ingestion and the other for excretion of the waste product respectively [3,15]. To evaluate the toxicity of any new compound, it is essential to know the state of these two vital organs, which can be verified by biochemical estimation [3]. Renal and hepatic biomarkers showed that the extract is not toxic at the doses studied. In male and female rats, CM at all the doses administered did not alter the levels of total protein and albumin. Similarly, serum lipid parameters have not changed in animals. The CM extract was subjected to Infrared analysis in order to detect within it the presence or absence of toxic compounds [13]. The FT-IR spectrum showed that there is no absorbance in the 2220−2260 cm−1 band, indicating the absence of cyanide groups in the extract. This result suggests that the CM extract may not contain toxic substances [35], which would partly explain its safety.
5. Conclusion
The oral dose up to 5000 mg/kg hydroalcoholic extract of CM leaves showed no evidence of toxicity or treatment-related mortality in animals. Repeated doses of CM up to 1000 mg/kg for 28 days showed no significant change in food and water intake. Hematology, serum biochemical analysis and histopathology at the cellular level also showed no marked effects. The risk /benefit ratio evaluated in this study is in favour of usage of this traditional medicinal plant. Thus, CM extract can be used for its proven nephroprotective effects because the therapeutic doses used are far from the LD50 and toxic doses. The toxic effect on fertility, mutagenicity, teratogenicity and carcinogenic potential may be realized in the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Government of India through CV Raman International Fellowship for African Researchers (DST/INT/CVRF/2016 dated 01/08/2017) and the Government of Romania through Eugen Ionescu Fellowship for African Researchers (CE/DG/28/2017). The authors also appreciate University of Lomé, Togo, Sree Siddaganga College of Pharmacy, Government of Karnataka, India and University of Agricultural Science and Veterinary Medicine, Cluj-Napoca, Romania for their support in providing with the required resources for carrying out this project.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.01.007.
Contributor Information
Mabozou Kpemissi, Email: mabozou@gmail.com.
Veeresh P. Veerapur, Email: veeresh36@gmail.com.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Kale O.E., Awodele O., Akindele A.J. Subacute and subchronic oral toxicity assessments of Acridocarpus smeathmannii (DC.) Guill. & Perr. root in Wistar rats. Toxicol. Rep. 2019;6:161–175. doi: 10.1016/j.toxrep.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad L., He Y., Hao J.C., Semotiuk A., Liu Q.R., Mazari P. Toxic pyrrolizidine alkaloids provide a warning sign to overuse of the ethnomedicine Arnebia benthamii. J. Ethnopharmacol. 2018;210:88–94. doi: 10.1016/j.jep.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Sureshkumar D., Begum S., Johannah N.M., Maliakel B., Krishnakumar I.M. Toxicological evaluation of a saponin-rich standardized extract of fenugreek seeds (FenuSMART®): acute, sub-chronic and genotoxicity studies. Toxicol. Rep. 2018;5:1060–1068. doi: 10.1016/j.toxrep.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upadhyay P., Purohit S., Mishra S.K., Tiwari K.N., Dubey G.P., FARAG M.R., Shah H. Pharmacognostic standardization of Asian folk medicinal plant Reinwardtia Indica Dumort. Int. J. Green Pharm. 2018;12(2):s385. [Google Scholar]
- 5.Boukandou Mounanga M., Mewono L., Aboughe Angone S. Toxicity studies of medicinal plants used in sub-Saharan Africa. J. Ethnopharmacol. 2015;174:618–627. doi: 10.1016/j.jep.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Habbu P., Madagundi S., Kulkarni R., Jadav S., Vanakudri R., Kulkarni V. Preparation and evaluation of Bacopa–phospholipid complex for antiamnesic activity in rodents. Drug Invent. Today. 2013;5(1):13–21. [Google Scholar]
- 7.Njan A.A., Olaoye S.O., Afolabi S.O., Ejimkonye B.C., Soje A., Olorundare O.E., Iwalewa E.O. Safety effect of fractions from methanolic leaf extract of Ocimum gratissimum on reproduction in male wistar rats. Toxicol. Rep. 2019;6:496–504. doi: 10.1016/j.toxrep.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ugwah-Oguejiofor C.J., Okoli C.O., Ugwah M.O., Umaru M.L., Ogbulie C.S., Mshelia H.E., Umar M., Njan A.A. Acute and sub-acute toxicity of aqueous extract of aerial parts of Caralluma dalzielii N. E. Brown in mice and rats. Heliyon. 2019;5(1) doi: 10.1016/j.heliyon.2019.e01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takke A., Shende P. Nanotherapeutic silibinin: an insight of phytomedicine in healthcare reformation. Nanomedicine. 2019;21 doi: 10.1016/j.nano.2019.102057. [DOI] [PubMed] [Google Scholar]
- 10.Singh R.P., Gangadharappa H.V., Mruthunjaya K. Phospholipids: Unique carriers for drug delivery systems. J. Drug Deliv. Sci. Technol. 2017;39:166–179. [Google Scholar]
- 11.Sani T.A., Mohammadpour E., Mohammadi A., Memariani T., Yazdi M.V., Rezaee R., Calina D., Docea A.O., Goumenou M., Etemad L. Cytotoxic and apoptogenic properties of Dracocephalum kotschyi aerial part different fractions on calu-6 and mehr-80 lung cancer cell lines. Farmacia. 2017;65(2):189–199. [Google Scholar]
- 12.Shaw D. Toxicological risks of Chinese herbs. Planta Med. 2010;76(17):2012–2018. doi: 10.1055/s-0030-1250533. [DOI] [PubMed] [Google Scholar]
- 13.Kpemissi M., Eklu-Gadegbeku K., Veerapur V.P., Potârniche A.-V., Adi K., Vijayakumar S., Banakar S.M., Thimmaiah N., Metowogo K., Aklikokou K. Antioxidant and nephroprotection activities of Combretum micranthum: a phytochemical, in-vitro and ex-vivo studies. Heliyon. 2019;5(3) doi: 10.1016/j.heliyon.2019.e01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romanucci V., Agarwal C., Agarwal R., Pannecouque C., Iuliano M., De Tommaso G., Caruso T., Di Fabio G., Zarrelli A. Silibinin phosphodiester glyco-conjugates: synthesis, redox behaviour and biological investigations. Bioorg. Chem. 2018;77:349–359. doi: 10.1016/j.bioorg.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 15.Paré D., Hilou A., N’do J., Guenne S., Ernest S.N., Kpemissi M. Protective effect of bioactive fractions of C. Dalzielii against weight gain in mice feed with high fat-diet. Int. J. Recent Sci. Res. 2019;10(8):34144–34153. [Google Scholar]
- 16.Fleurentin J., Cabalion P., Mazars G., Santos J., Younos C. 1991. Ethnopharmacologie: Sources, Méthodes, Objectifs Ethnopharmacology: Sources, Methods, Objectives; p. 495. documentation.ird.fr. [Google Scholar]
- 17.Prochazkova D., Bousova I., Wilhelmova N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82(4):513–523. doi: 10.1016/j.fitote.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Tchoumtchoua J., Mouchili O.R., Ateba S.B., Zingue S., Halabalaki M., Mbanya J.C., Skaltsounis A.L., Njamen D. Safety assessment of the methanol extract of the stem bark of Amphimas pterocarpoides harms: acute and subchronic oral toxicity studies in Wistar rats. Toxicol. Rep. 2014;1:877–884. doi: 10.1016/j.toxrep.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.OECD . OECD Guidelines for the Testing of Chemicals. Organization for Economic Cooperation and Development; Paris, France: 2008. Test No. 407: repeated dose 28-day oral toxicity study in rodents. [Google Scholar]
- 20.Agyigra I.A., Ejiofor J.I., Magaji M.G. Acute and subchronic toxicity evaluation of methanol stem-bark extract of Ximenia americana Linn (Olacaceae) in Wistar rats. Bull. Faculty Pharm. Cairo Univ. 2017;55(2):263–267. [Google Scholar]
- 21.Lee J.S., Kim Y.H., Kim D.B., Shin G.H., Lee J.H., Cho J.H., Lee B.Y., Lee O.H. Acute and subchronic (28 days) oral toxicity studies of Codonopsis lanceolata extract in Sprague-Dawley rats. Regul. Toxicol. Pharmacol. 2015;71(3):491–497. doi: 10.1016/j.yrtph.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Atsamo A.D., Nguelefack T.B., Datte J.Y., Kamanyi A. Acute and subchronic oral toxicity assessment of the aqueous extract from the stem bark of Erythrina senegalensis DC (Fabaceae) in rodents. J. Ethnopharmacol. 2011;134(3):697–702. doi: 10.1016/j.jep.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Catarino L., Havik P.J., Romeiras M.M. Medicinal plants of Guinea-Bissau: therapeutic applications, ethnic diversity and knowledge transfer. J. Ethnopharmacol. 2016;183:71–94. doi: 10.1016/j.jep.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 24.Mazumder A., Dwivedi A., du Preez J.L., du Plessis J. In vitro wound healing and cytotoxic effects of sinigrin-phytosome complex. Int. J. Pharm. 2016;498(1-2):283–293. doi: 10.1016/j.ijpharm.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal A., Chakraborty P., Chakraborty D.D., Saharan V.A. Phytosomes: complexation, utilisation and commerical status. J. Biol. Act. Prod. From Nat. 2012;2(2):65–77. [Google Scholar]
- 26.De Smet P.A. Health risks of herbal remedies: an update. Clin. Pharmacol. Ther. 2004;76(1):1–17. doi: 10.1016/j.clpt.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Skalicka-Woźniak K., Georgiev M.I., Orhan I.E. Adulteration of herbal sexual enhancers and slimmers: the wish for better sexual well-being and perfect body can be risky. Food Chem. Toxicol. 2017;108:355–364. doi: 10.1016/j.fct.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 28.El-Gazayerly O.N., Makhlouf A.I., Soelm A.M., Mohmoud M.A. Antioxidant and hepatoprotective effects of silymarin phytosomes compared to milk thistle extract in CCl4 induced hepatotoxicity in rats. J. Microencapsul. 2014;31(1):23–30. doi: 10.3109/02652048.2013.805836. [DOI] [PubMed] [Google Scholar]
- 29.Kumar N., Rai A., Reddy N.D., Raj P.V., Jain P., Deshpande P., Mathew G., Kutty N.G., Udupa N., Rao C.M. Silymarin liposomes improves oral bioavailability of silybin besides targeting hepatocytes, and immune cells. Pharmacol. Rep. 2014;66(5):788–798. doi: 10.1016/j.pharep.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Jordan S.A., Cunningham D.G., Marles R.J. Assessment of herbal medicinal products: challenges, and opportunities to increase the knowledge base for safety assessment. Toxicol. Appl. Pharmacol. 2010;243(2):198–216. doi: 10.1016/j.taap.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Kale O.E., Oyesola T.O., Raji F.S. Celecoxib, a cyclooxygenase-2 inhibitor, offers chemoprevention against reproductive and neurobehavioural abnormalities induced by atrazine in male Wistar rats. Environ. Toxicol. Pharmacol. 2018;58:84–97. doi: 10.1016/j.etap.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 32.van Hoogevest P. Review - an update on the use of oral phospholipid excipients. Eur. J. Pharm. Sci. 2017;108:1–12. doi: 10.1016/j.ejps.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Kpemissi M., Eklu-Gadegbeku K., Veerapur V.P., Negru M., Taulescu M., Chandramohan V., Hiriyan J., Banakar S.M., Thimmaiah N., Suhas D.S., Puneeth T.A., Vijayakumar S., Metowogo K., Aklikokou k. Nephroprotective activity of Combretum micranthum G. Don in cisplatin induced nephrotoxicity in rats: in-vitro, in-vivo and in-silico experiments. Biomed. Pharmacother. 2019;116 doi: 10.1016/j.biopha.2019.108961. [DOI] [PubMed] [Google Scholar]
- 34.Ateba S.B., Simo R.V., Mbanya J.C., Krenn L., Njamen D. Safety profile and gender specific differences of a methanol extract of Eriosema laurentii (Leguminosae) in acute and subchronic (28 days) oral toxicity studies in Wistar rats. Food Chem. Toxicol. 2014;65:27–32. doi: 10.1016/j.fct.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 35.Ragavendran P., Sophia D., Arul Raj C., Gopalakrishnan V. Functional group analysis of various extracts of Aerva lanata (L.,) by ftir spectrum. Pharmacologyonline. 2011;1:358–364. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.