Abstract
Some antimicrobial peptides (AMPs) are produced in the vaginal innate immune system and play an important role in protecting this organ against pathogenic agents. Moreover, sexually transmitted diseases have become a major problem in human societies and are rapidly spreading. The emergence of antibiotic-resistant microbes (superbugs) can pose a major threat to human societies and cause rapid spread of these diseases. Finding new antimicrobial compounds to fight superbugs is therefore essential. It has been shown that AMPs have good potential to become new antibiotics. The most important AMPs in the vaginal innate immune system are defensins, secretory leucocyte protease inhibitors, calprotectin, lysozyme, lactoferrin and elafin, which play an important role in host defence against sexually transmitted infections, modulation of immune responses and anticancer activities. Some AMPs, such as LL-37, magainin 2 and nisin, show both spermicidal and antimicrobial effects in the vagina. In this summary, we will discuss vaginal AMPs and continue to address some of the challenges of using peptides to control pathogens that are effective in sexually transmitted diseases.
Keywords: Antimicrobial peptide, defensin, LL-37, sexually transmitted diseases, spermicidal peptides, vaginal innate immune system
Introduction
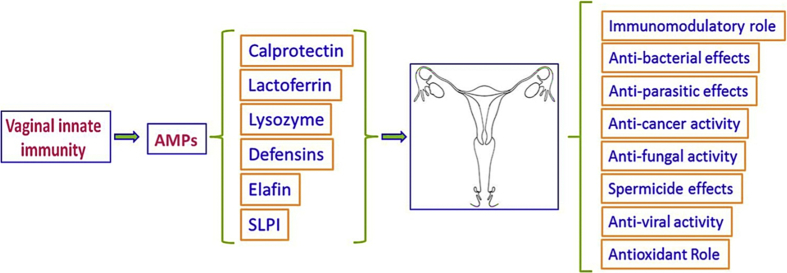
Defensins, secretory leucocyte protease inhibitors (SLPIs), calprotectin, lysozyme, lactoferrin and elafin are groups of antimicrobial peptides (AMPs) with effective roles in the innate immunity of the vagina [1]. Some of the most important functions of these peptides in the vagina are shown in Fig. 1. Sexually transmitted diseases (STDs) are defined as a group of diseases that are transmitted from person to person through sexual contact. It means that the infection has several methods of spreading, such as vaginal intercourse, or anal and oral sex [2]. Chlamydia trachomatis infection, chancroid (caused by Treponema pallidum), gonorrhoea (Neisseria gonorrhoeae), crabs (pubic lice), genital herpes (herpes simplex virus-2 (HSV-2)), hepatitis B (hepatitis B virus), human immunodeficiency virus (HIV)/AIDS, human papillomavirus (HPV), scabies, trichomoniasis (Trichomonas vaginalis), molluscum contagiosum virus and candidal vaginitis (Candida albicans) are examples of the most important STDs [2,3].
Fig. 1.
Some important functions of antimicrobial peptides in the vagina.
The rapid growth and spread of these diseases is a major threat to human health so research into the diagnosis and treatment of these diseases should be taken seriously. Despite all advances in the field of antibiotics, microbial resistance is a significant issue that can eliminate the effects of most antimicrobial drugs a few years after their introduction as therapeutic compounds. Recently, AMPs have been considered by many scientists as suitable therapeutic compounds [4,5]. AMPs are antimicrobial compounds produced by the innate immune system of all organisms [6]. Much research has shown that antimicrobial peptides and proteins can exert their antimicrobial effects on the pathogens that are effective in STDs [[7], [8], [9], [10]]. Many of these peptides, including natural peptides derived from the vaginal innate immune system and synthetic AMPs, have been tested as antimicrobial compounds against the bacteria and viruses that cause STDs. The results of all these tests indicate that AMPs are suitable candidates for the fight against STDs. For example, anti-HIV activity is one of the important functions for both α- and β-defensins. α-Defensins have direct inhibitory effects on viral replication [1,11] whereas β-defensins inhibit viral entry into cells by reducing expression of its related co-receptors [1]. Regulation of sex hormones is another characteristic of some AMPs. Levels of α- and β-defensins in cervico-vaginal lavage liquid vary during the menstrual cycle, with differences up to 50-fold [1,12]. Such differences in concentration indicate that levels of estrogen can affect the operational vaginal innate immunity status [1,13]. SLPIs have inhibitory actions on HIV-1 [14], and women with lower genital tract infections by T. vaginalis, C. trachomatis, N. gonorrhoeae and Candida express lower SLPI levels [13]. Calprotectin concentrations have a direct relationship with inflammatory cytokines in cervical mucus, proving that it has a key role in local inflammation. Calprotectin also has anti-candida properties [1,15]. Elafin, like defensins and SLPIs, has anti-HIV activity, and there is evidence that the presence of elafin may lead to HIV infection resistance [16].
AMPs that act against effective viruses in STDs
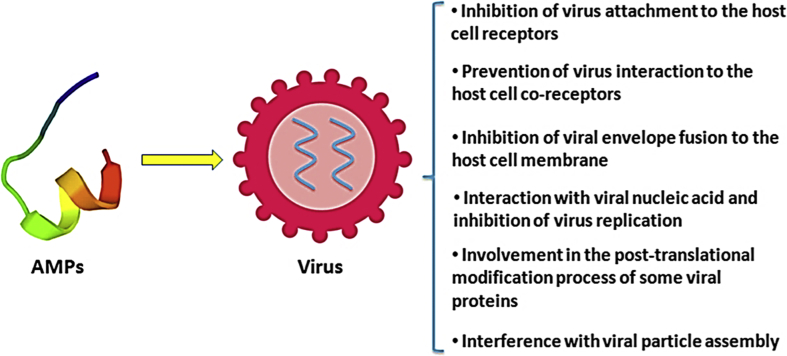
One of the AMP classes comprises anti-viral peptides, which can inhibit the replication of viruses or prevent their pathogenesis by various mechanisms (Fig. 2). For a long time HIV-1 infection as an STD was an insoluble global health problem. New therapeutic compounds and methods to fight against this infection are needed [17]. A total of 109 anti-HIV peptides have been registered in the AMP database or APD3 (http://aps.unmc.edu/AP/database/antiH.php/2019) up to now. With advances in peptide stability, production, formulation and delivery methods, it is possible that some of these complexes may ultimately become the source of novel anti-HIV agents and medications [17]. Some of the most important of these peptides include defensins, LL-37 [18], gramicidin D, caerin1 [19], maximin 3, magainin 2, dermaseptin-S1, dermaseptin-S4, siamycin I, siamycin II and RP 71955 (http://aps.unmc.edu/AP/database/antiH.php/2019). Infection with HSV-2 elevates the risk ratio of catching HIV from 2 to 4 [20,21]. Keratinocytes infected by HSV-2 release several AMPs, including LL-37, which is the most notable peptide. LL-37 increases the expression of the HIV-1 receptors CD4 and CCR5 on T cells, which leads to elevation of the susceptibility and possibility of catching HIV-1. Hence, cleaning LL-37 from HSV-2-infected cells decreases the cells' HIV-1 susceptibility [18,20]. Innate immunity has a key role against HSV [22]. In an in vitro study a complex of native cytokines and AMPs (CCAP or Super lymph) had an inhibitory effect on virus reproduction. Protegrines, as a CCAP element, have an active role against the virus. The mechanism of this mentioned CCAP is to produce both immune modulating and antiviral effects [22]. In another study, a microbicide compound combining the lipid-ether 1-0-octyl-sn-glycerol (OG; 3 mm) and peptide D2A21 (9 μm) decreased the titers of herpes simplex virus type 1 (HSV-1) and HSV-2 by at least 1000-fold, more than the sum of the decreased titers under the induction of OG and D2A21 alone [23]. There are other compounds, like penaeidin-3, that have inhibited over 85% of the HSV replication at 100 μm [24]. HPV is the cause of common warts such as laryngeal papilloma and genital condylomata and it can lead to cervical cancer [25]. Lactoferrin acts as an antiviral compound against HPV-16 and other types are under the influence of lactoferricin [25]. Successful E6-binding peptides against HPV-16 have been identified [26]. Another study reported that β-defensin-1 peptide is a natural shield against HPV in the mucosa of the genital tract [27]. Human α-defensins 1–3 (also called human neutrophil peptides 1–3) and human α-defensin 5 have antiviral activities against both cutaneous and mucosal papillomavirus types [28]. The changes in AMP expression in vulvo-vaginal biopsies from HPV-infected individuals compare with those from non-infected people indicate that these peptides have key roles in local immune responses. It is strange that human β-defenisin-1 has a notable induction whereas RNase7 has not, so there is different regulation of AMPs between bacterial and viral infections [29]. Given this, can these peptides be used in the preparation of pre-sex lotions?
Fig. 2.
This figure represents the main mechanisms of action of antiviral peptides.
Antibacterial peptides that combat STDs
Data show that there are several antibacterial compounds that combat STDs. For instance, in gonorrhoea, decreasing LL-37 expression gives a survival advantage in the female genital tract to pathogenic Neisseria [30]. Another study introduced a cell-penetrating peptide with 12 residues that was able to kill 100% of N. gonorrhoeae strains at a concentration of 100 mm [31]. Another study on a new AMP revealed its ability to combat all strains of N. gonorrhoeae, especially some reference and clinical N. gonorrhoeae strains, such as penicillin-resistant strains. Dermaseptin is a new AMP obtained from frog skin [32].
Other diseases can be treated by AMPs. Studies have indicated the antibacterial effects of cathelicidin peptides against C. trachomatis, Chlamydia pneumoniae and Chlamydia psittaci [33] but Chlamydial Protease-Like Activity Factor (CPAF), a serine protease that releases from C. trachomatis, can stop the LL-37 anti-chlamydial activity due to CPAF's proteolytic activity [34]. Some studies revealed defensin and cathelicidin-derived peptide activities against Leptospira, Borrelia and Treponema pallidum, and an in vitro rabbit model showed that synthetic truncated LL-37-derived peptide WS22–N-amide prevented T. pallidum infection [[35], [36], [37]].
Another example of an AMP compound is cell-penetrating peptide, Pep-1, which prevents the growth of intracellular, but not extracellular, forms of C. trachomatis [38]. This bacteria is a human obligate intracellular pathogen and causes STDs in both men and women [39]. In 2013, researchers studied the anti-chlamydial activity of cyto-insectotoxin 1a (CIT 1a), a unique class of AMP that was collected from some of a central Asian spider's venom – Lachesanatarabaevi [40].
Anti-parasitic and antifungal peptides against sexually transmitted infections
Trichomoniasis
We know that AMP expression rises during T. vaginalis infection in the cervix [41]. Trichomonas vaginalis is a pathogen that causes trichomoniasis. There are two serious problems with trichomoniasis. On the one hand, if it becomes chronic the it can lead to more serious effects in patients, and on the other, there are drug-resistant strains of T. vaginalis that require novel clinical therapeutic compounds like AMPs to be designed [42]. Metronidazole resistance in T. vaginalis can be compensated for by epinecidin-1, which is a synthetic marine AMP with anti-T. vaginalis activity. Epinecidin-1 in in vitro conditions, destroys the membrane of T. vaginalis, and at a concentration of 12.5 mg/L kills it [42,43]. C-amidated tritrpticin is an antimicrobial tryptophan-rich peptide derived from a porcine cathelicidin. It can disrupt T. vaginalis survival and growth [44]
Antifungal peptides
Antifungal peptides reported in AMP database or APD3 (http://aps.unmc.edu/AP/database/antiH.php/2019) such as aureins, maximins, magainin, brevinin, ranatuerin and cecropins and also many synthetic peptides such as AurH1, which derives from Aurein1.2 and 1127 other antifungal peptides can effectively treat Candida albicans infection [45]. Candidal vaginitis is the second most common vaginal infection in the USA, that usually detect simultaneously by physician in gynecologic problems [46].
Spermicide and microbicide peptides
Although there is only three AMPs – LL-37, magainin 2 and nisin – of which the contraceptive effects have been proved in animal models, there are many AMPs, including LL-37, maximin 1, maximin3, magainin 2, dermaseptin-S1, dermaseptin-S4, subtilosin A, pediocin PA-1/AcH, nisin A, lacticin 3147, sarcotoxinPd and gramicidin A, that may have ambivalent spermicidal/microbicidal activity (Fig. 1) [47,48]. However, there are two serious problem: infertility and disruption of the normal flora. Some AMPs, like nisin A, have a dual killing effect on both microbes and beneficial commensal lactobacilli that exist in the vagina. Lactobacilli are responsible for the acidic pH in the healthy human vagina, so nisin A is not a good choice as an antimicrobial drug [48]. On the one hand we have cervicovaginal epithelial cells that naturally release LL-37 and on the other it has spermicideal effects. Therefore, high levels of natural LL-37 can cause infertility. Now there is a new question: ‘What factors can elevate the natural LL-37 production or release from cervico-vaginal epithelial cells?’
Discussion
New clinical procedures have some problems, for example, antimicrobial peptide synthesis is a costly and time-consuming procedure. To overcome such a problem, in silico analysis is suggested before clinical experiments, which can decrease production costs and activity time, and even down-regulate the toxicity [4]. However, nowadays natural AMPs have clinical and commercial functions so that we have many AMPs for every pathogen responsible for sexually transmitted disease whether bacteria and viruses or fungi, yeasts and protozoa. Some of them have direct antimicrobial effects and can defend the host against pathogens, and some are chemotactic agents that direct the attention of inflammatory cells to the infection location [1,4,49]. In future we have to improve their antimicrobial activity and stability, and also minimize the cytotoxicity and immunogenicity, and decrease the proteolytic self-destruction or the biological fluid inhibition [4,50].
In conclusion we find LL-37 as a microbicidal AMP against Chlamydia trachomatis, N. gonorrhoeae, Trpeonema pallidum, Candida albicans, Staphylococcus aureus, Pseudomonas aeruginosa, Prevotella intermedia, Porphyromonas gingivalis, Klebsiella pneumoniae, Escherichia coli, Enterococcus faecalis and HIV and HSV [48]. LL-37 has several notable properties such as dual spermicidal/microbicidal activity. It destroys the sperm surface membrane and stops sperm motility in humans and mice. This takes about 5 min with a dosage of 10.8 μm and then 3.7 μm [48]. The LL-37 microbicidal activity affects STDs, vaginitis and urinary tract infection by controlling involved microorganisms [48]. LL-37 used as a pharmaceutical gel and administered into the vagina induces microbicidal effects on uropathogenic opportunistic microbes. The vaginal secretions, which contain both naturally secreted LL-37 and the LL-37 released from the gel can move from the vagina to the urinary tract, where it can also exert its microbicidal effects [48].
Conclusion
As mentioned above, AMPs, especially LL-37, can inhibit STDs. Any new medical compounds containing LL-37 have problems in several stages of their production, but changes in AMPs can be made, such as structural changes or changes in method of consumption, then LL-37 could have more benefits and fewer limitations. First, there are economic problems in the production stage; because LL-37 is a long peptide, it is expensive to synthesize. Hence it is essential to discover a mimetic of the shortest possible truncated LL-37 peptide with no decrease in spermicidal and microbicidal activities, and to prepare recombinant LL-37. Novel methods like in silico analysis can have key roles in solving this problem. Second, there are problems in the delivery stage. It is necessary to find a method to deliver LL-37 into the human vagina [48]. Also in the consumption stage, modified AMPs can be designed as ointments or topical lotions to have better efficiency in women's health.
Conflict of interest
The authors declared that they have no competing interests.
Funding
There was no financial support for this research.
Authors' contributions
HM, MSH and HHK provided direction and guidance throughout the preparation of this manuscript. SS and HHK conducted the literature search and drafted the manuscript. HM and MSH reviewed the manuscript and made significant revisions to the drafts. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Acknowledgements
The authors wish to express their appreciation for the full cooperation of the personnel who participated in this review study.
Contributor Information
M. Shoushtari, Email: mb.shoushtari@gmail.com.
H.H. Kashani, Email: hamedir2010@gmail.com.
S. Sardari, Email: ssardari@hotmail.com.
References
- 1.Mendez-Figueroa H., Anderson B. Vaginal innate immunity: alteration during pregnancy and its impact on pregnancy outcomes. Exp Rev Obstet Gynecol. 2011;6(6):629–641. [Google Scholar]
- 2.Da Ros C.T., da Silva Schmitt C. Global epidemiology of sexually transmitted diseases. Asian J Androl. 2008;10(1):110–114. doi: 10.1111/j.1745-7262.2008.00367.x. [DOI] [PubMed] [Google Scholar]
- 3.Fahimi H., Mohammadipour M., Kashani H.H., Parvini F., Sadeghizadeh M. Dengue viruses and promising envelope protein domain III-based vaccines. Appl Microbiol Biotechnol. 2018;102(7):2977–2996. doi: 10.1007/s00253-018-8822-y. [DOI] [PubMed] [Google Scholar]
- 4.Maccari G., Nifosi R., Di Luca M. Rational development of antimicrobial peptides for therapeutic use: design and production of highly active compounds. Microb Pathog Strat Combat: Sci Technol Educ. 2013:1265–1277. [Google Scholar]
- 5.Kashani H.H., Nikzad H., Mobaseri S., Hoseini E.S. Synergism effect of nisin peptide in reducing chemical preservatives in food industry. Life Sci J. 2012;9(1) [Google Scholar]
- 6.Wang G., Mishra B., Lau K., Lushnikova T., Golla R., Wang X. Antimicrobial peptides in 2014. Pharmaceuticals. 2015;8(1):123–150. doi: 10.3390/ph8010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters B.M., Shirtliff M.E., Jabra-Rizk M.A. Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathogens. 2010;6(10) doi: 10.1371/journal.ppat.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haddad Kashani H., Fahimi H., Dasteh Goli Y., Moniri R. A novel chimeric endolysin with antibacterial activity against methicillin-resistant Staphylococcus aureus. Front Cell Infect Microbiol. 2017;7:290. doi: 10.3389/fcimb.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashani H.H., Moniri R. Expression of recombinant pET22b-LysK-cysteine/histidine-dependent amidohydrolase/peptidase bacteriophage therapeutic protein in Escherichia coli BL21 (DE3) Osong Public Health Res Perspect. 2015;6(4):256–260. doi: 10.1016/j.phrp.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosseini E.S., Moniri R., Goli Y.D., Kashani H.H. Purification of antibacterial CHAP K protein using a self-cleaving fusion tag and its activity against methicillin-resistant Staphylococcus aureus. Probiot Antimicrob Proteins. 2016;8(4):202–210. doi: 10.1007/s12602-016-9236-8. [DOI] [PubMed] [Google Scholar]
- 11.Chang T.L., Vargas J., DelPortillo A., Klotman M.E. Dual role of α-defensin-1 in anti–HIV-1 innate immunity. J Clin Invest. 2005;115(3):765–773. doi: 10.1172/JCI200521948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole A.M., Cole A.L. Antimicrobial polypeptides are key anti-HIV-1 effector molecules of cervicovaginal host defense. Am J Reprod Immunol. 2008;59(1):27–34. doi: 10.1111/j.1600-0897.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 13.Novak R.M., Donoval B.A., Graham P.J., Boksa L.A., Spear G., Hershow R.C. Cervicovaginal levels of lactoferrin, secretory leukocyte protease inhibitor, and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus (HIV)-seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin Vaccine Immunol. 2007;14(9):1102–1107. doi: 10.1128/CVI.00386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quiñones-Mateu M.E., Lederman M.M., Feng Z., Chakraborty B., Weber J., Rangel H.R. Human epithelial β-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17(16):F39–F48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 15.Que M.L., Andersen E., Mombelli A. Myeloid-related protein (MRP) 8/14 (calprotectin) and its subunits MRP8 and MRP14 in plaque-induced early gingival inflammation. J Clin Periodontol. 2004;31(11):978–984. doi: 10.1111/j.1600-051X.2004.00594.x. [DOI] [PubMed] [Google Scholar]
- 16.Iqbal S.M., Ball T.B., Levinson P., Maranan L., Jaoko W., Wachihi C. Elevated elafin/trappin-2 in the female genital tract is associated with protection against HIV acquisition. AIDS. 2009;23(13):1669–1677. doi: 10.1097/QAD.0b013e32832ea643. [DOI] [PubMed] [Google Scholar]
- 17.Wang G. Natural antimicrobial peptides as promising anti-HIV candidates. Curr Top Pept Protein Res. 2012;13:93. [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa Y., Kawamura T., Matsuzawa T., Aoki R., Gee P., Yamashita A. Antimicrobial peptide LL-37 produced by HSV-2-infected keratinocytes enhances HIV infection of Langerhans cells. Cell Host Microbe. 2013;13(1):77–86. doi: 10.1016/j.chom.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 19.VanCompernolle S., Smith P.B., Bowie J.H., Tyler M.J., Unutmaz D., Rollins-Smith L.A. Inhibition of HIV infection by caerin 1 antimicrobial peptides. Peptides. 2015;71:296–303. doi: 10.1016/j.peptides.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Guaní-Guerra E., Santos-Mendoza T., Lugo-Reyes S.O., Terán L.M. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010;135(1):1–11. doi: 10.1016/j.clim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Piroozmand A., Kashani H.H., Zamani B. Correlation between Epstein-Barr virus infection and disease activity of systemic lupus erythematosus: a cross-sectional study. Asian Pac J Cancer Prevent: APJCP. 2017;18(2):523. doi: 10.22034/APJCP.2017.18.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovalchuk L.V., Gankovskaya L.V., Gankovskaya O.A., Lavrov V.F. Immune-Mediated Diseases. Springer; 2007. Herpes simplex virus: treatment with antimicrobial peptides; pp. 369–376. [DOI] [PubMed] [Google Scholar]
- 23.Isaacs C.E., Jia J.H., Xu W. A lipid-peptide microbicide inactivates herpes simplex virus. Antimicrob Agents Chemother. 2004;48(8):3182–3184. doi: 10.1128/AAC.48.8.3182-3184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carriel-Gomes M.C., Kratz J.M., Barracco M.A., Bachére E., Barardi C.R.M., Simões C.M.O. In vitro antiviral activity of antimicrobial peptides against herpes simplex virus 1, adenovirus, and rotavirus. Mem Inst Oswaldo Cruz. 2007;102(4):469–472. doi: 10.1590/s0074-02762007005000028. [DOI] [PubMed] [Google Scholar]
- 25.Mistry N., Drobni P., Näslund J., Sunkari V.G., Jenssen H., Evander M. The anti-papillomavirus activity of human and bovine lactoferricin. Antiviral Res. 2007;75(3):258–265. doi: 10.1016/j.antiviral.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y., Liu Z., Androphy E., Chen J., Baleja J.D. Design and characterization of helical peptides that inhibit the E6 protein of papillomavirus. Biochemistry. 2004;43(23):7421–7431. doi: 10.1021/bi049552a. [DOI] [PubMed] [Google Scholar]
- 27.Segat L., Zupin L., Moura R.R., Coelho A.V.C., Chagas B.S., Freitas ACd. DEFB1 polymorphisms are involved in susceptibility to human papillomavirus infection in Brazilian gynaecological patients. Mem Inst Oswaldo Cruz. 2014;109(7):918–922. doi: 10.1590/0074-0276140220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buck C.B., Day P.M., Thompson C.D., Lubkowski J., Lu W., Lowy D.R. Human α-defensins block papillomavirus infection. Proc Natl Acad Sci. 2006;103(5):1516–1521. doi: 10.1073/pnas.0508033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erhart W., Alkasi Ö., Brunke G., Wegener F., Maass N., Arnold N. Induction of human β-defensins and psoriasin in vulvovaginal human papillomavirus–associated lesions. J Infect Dis. 2011;204(3):391–399. doi: 10.1093/infdis/jir079. [DOI] [PubMed] [Google Scholar]
- 30.Bergman P., Johansson L., Asp V., Plant L., Gudmundsson G.H., Jonsson A.B. Neisseria gonorrhoeae downregulates expression of the human antimicrobial peptide LL-37. Cell Microbiol. 2005;7(7):1009–1017. doi: 10.1111/j.1462-5822.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 31.Handing J.W., Ragland S.A., Bharathan U.V., Criss A.K. The MtrCDE efflux pump contributes to survival of Neisseria gonorrhoeae from human neutrophils and their antimicrobial components. Front Microbiol. 2018;9:2688. doi: 10.3389/fmicb.2018.02688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zairi A., Tangy F., Ducos-Galand M., Alonso J.-M., Hani K. Susceptibility of Neisseria gonorrhoeae to antimicrobial peptides from amphibian skin, dermaseptin, and derivatives. Diagn Microbiol Infect Dis. 2007;57(3):319–324. doi: 10.1016/j.diagmicrobio.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Donati M., Di Leo K., Benincasa M., Cavrini F., Accardo S., Moroni A. Activity of cathelicidin peptides against Chlamydia spp. Antimicrob Agents Chemother. 2005;49(3):1201–1202. doi: 10.1128/AAC.49.3.1201-1202.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang L., Chen J., Zhou Z., Yu P., Yang Z., Zhong G. Chlamydia-secreted protease CPAF degrades host antimicrobial peptides. Microb Infect. 2015;17(6):402–408. doi: 10.1016/j.micinf.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Sambri V., Marangoni A., Giacani L., Gennaro R., Murgia R., Cevenini R. Comparative in vitro activity of five cathelicidin-derived synthetic peptides against Leptospira, Borrelia and Treponema pallidum. J Antimicrob Chemother. 2002;50(6):895–902. doi: 10.1093/jac/dkf220. [DOI] [PubMed] [Google Scholar]
- 36.Borenstein L., Selsted M., Lehrer R., Miller J. Antimicrobial activity of rabbit leukocyte defensins against Treponema pallidum subsp. pallidum. Infect Immun. 1991;59(4):1359–1367. doi: 10.1128/iai.59.4.1359-1367.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox D.L., Sun Y., Liu H., Lehrer R.I., Shafer W.M. Susceptibility of Treponema pallidum to host-derived antimicrobial peptides. Peptides. 2003;24(11):1741–1746. doi: 10.1016/j.peptides.2003.07.026. [DOI] [PubMed] [Google Scholar]
- 38.Park N., Yamanaka K., Tran D., Chandrangsu P., Akers J.C., de Leon J.C. The cell-penetrating peptide, Pep-1, has activity against intracellular chlamydial growth but not extracellular forms of Chlamydia trachomatis. J Antimicrob Chemother. 2008;63(1):115–123. doi: 10.1093/jac/dkn436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jevtuševskaja J., Uusna J., Andresen L., Krõlov K., Laanpere M., Grellier T. Combination with antimicrobial peptide lyses improves loop-mediated isothermal amplification based method for Chlamydia trachomatis detection directly in urine sample. BMC Infect Dis. 2016;16(1):329. doi: 10.1186/s12879-016-1674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lazarev V.N., Shkarupeta M.M., Polina N.F., Kostrjukova E.S., Vassilevski A.A., Kozlov S.A. Antimicrobial peptide from spider venom inhibits Chlamydia trachomatis infection at an early stage. Arch Microbiol. 2013;195(3):173–179. doi: 10.1007/s00203-012-0863-5. [DOI] [PubMed] [Google Scholar]
- 41.Makinde H., Novak R., Landay A., Spear G. Increased expression of antimicrobial peptides in the cervix during Trichomonas vaginalis infection (MUC2P. 923) Am Assoc Immnol. 2015;194(1 Supplement):65–66. [Google Scholar]
- 42.Huang H.-N., Chuang C.-M., Chen J.-Y., Chieh-Yu P. Epinecidin-1: a marine fish antimicrobial peptide with therapeutic potential against trichomonas vaginalis infection in mice. Peptides. 2019;112:139–148. doi: 10.1016/j.peptides.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Neshani A., Zare H., Eidgahi M.R.A., Khaledi A., Ghazvini K. Epinecidin-1, a highly potent marine antimicrobial peptide with anticancer and immunomodulatory activities. BMC Pharmacol Toxicol. 2019;20(1):33. doi: 10.1186/s40360-019-0309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Infante V.V., Miranda-Olvera A.D., De Leon-Rodriguez L.M., Anaya-Velazquez F., Rodriguez M.C., Avila E.E. Effect of the antimicrobial peptide tritrpticin on the in vitro viability and growth of trichomonas vaginalis. Curr Microbiol. 2011;62(1):301–306. doi: 10.1007/s00284-010-9709-z. [DOI] [PubMed] [Google Scholar]
- 45.Madanchi H., Khalaj V., Jang S., Shabani A.A., Ebrahimi Kiasari R., Seyed Mousavi S.J. AurH1: a new heptapeptide derived from Aurein1. 2 antimicrobial peptide with specific and exclusive fungicidal activity. J Pept Sci. 2019;25(7):e3175. doi: 10.1002/psc.3175. [DOI] [PubMed] [Google Scholar]
- 46.Sobel J.D. Vulvovaginal candidiasis—what we do and do not know. Ann Intern Med. 1984;101(3):390–392. doi: 10.7326/0003-4819-101-3-390. [DOI] [PubMed] [Google Scholar]
- 47.Srakaew N., Young C.D., Sae-Wu A., Xu H., Quesnel K.L., Di Brisco R. Antimicrobial host defence peptide, LL-37, as a potential vaginal contraceptive. Hum Reprod. 2014;29(4):683–696. doi: 10.1093/humrep/deu018. [DOI] [PubMed] [Google Scholar]
- 48.Tanphaichitr N., Srakaew N., Alonzi R., Kiattiburut W., Kongmanas K., Zhi R. Potential use of antimicrobial peptides as vaginal spermicides/microbicides. Pharmaceuticals. 2016;9(1):13. doi: 10.3390/ph9010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fjell C.D., Hiss J.A., Hancock R.E., Schneider G. Designing antimicrobial peptides: form follows function. Nat Rev Drug Disc. 2012;11(1):37. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 50.Maisetta G., Di Luca M., Esin S., Florio W., Brancatisano F.L., Bottai D. Evaluation of the inhibitory effects of human serum components on bactericidal activity of human beta defensin 3. Peptides. 2008;29(1):1–6. doi: 10.1016/j.peptides.2007.10.013. [DOI] [PubMed] [Google Scholar]