Abstract
Parenteral nutrition-associated liver disease (PNALD) is a liver dysfunction caused by various risk factors presented in patients receiving total parenteral nutrition (TPN). Omega-6 rich Intralipid® and omega-3 rich Omegaven® are two intravenous lipid emulsions used in TPN. TPN could affect the hepatic expression of genes in anti-oxidative stress, but it's unknown whether TPN affects genes in drug metabolism. In this study, either Intralipid®- or Omegaven®-based TPN was administered to mice and the expression of a cohort of genes involved in anti-oxidative stress or drug metabolism was analyzed, glutathione (GSH) levels were measured, and protein levels for two key drug metabolism genes were determined. Overall, the expression of most genes was downregulated by Intralipid®-based TPN (Gstp1, Gstm1, 3, 6, Nqo1, Ho-1, Mt-1, Gclc, Gclm, Cyp2d9, 2f2, 2b10, and 3a11). Omegaven® showed similar results as Intralipid® except for preserving the expression of Gstm1 and Cyp3a11, and increasing Ho-1. Total GSH levels were decreased by Intralipid®, but increased by Omegaven®. CYP3A11 protein levels were increased by Omegaven®. In conclusion, TPN reduced the expression of many genes involved in anti-oxidative stress and drug metabolism in mice. However, Omegaven® preserved expression of Cyp3a11, suggesting another beneficial effect of Omegaven® in protecting liver functions.
Key words: Total parenteral nutrition, Glutathione, Drug metabolism, Liver, Parenteral nutrition-associated liver disease
Abbreviations: CYP450, cytochrome p450; FAs, fatty acids; GADPH, glyceraldehyde 3-phosphate dehydrogenase; Gclc: glutamate-cysteine ligase catalytic subunit, Gclm: glutamate-cysteine ligase modifier subunit; Gpx3, glutathione peroxidase 3; GSH, glutathione; GSSG, GSH/glutathione disulfide; Gstm1, glutathione S-transferase, mu 1; Gstm3, glutathione S-transferase, mu 3; Gstm6, glutathione S-transferase, mu 6; Gstp1, glutathione S-transferase, pi 1; Ho-1, heme oxygenase 1; Mt-1, metallothionein 1; NQO1, NAD(P)H:quinone acceptor oxidoreductase 1; PNALD, parenteral nutrition-associated liver disease; ROS, reactive oxygen species; TPN, total parenteral nutrition
Graphical abstract
Total parenteral nutrition reduces the expression of genes involved in drug metabolism in mice, including Cyp3a11, with the degree more severe by a plant lipid-based formulation than fish-oil-based formulation. Therefore, patients on total parenteral nutrition should be monitored closely for drug–drug interactions.
1. Introduction
Total parenteral nutrition (TPN) has significantly improved the quality and survival of patients who cannot tolerate enteral nutrition. Despite its numerous medical benefits, TPN is associated with several adverse outcomes, including liver steatosis, cholestasis and fibrosis. There are several different types of lipid emulsions used in TPN; these emulsions vary in their composition of omega-3 and omega-6 fatty acids (FAs), phytosterol concentrations, and vitamin E composition and concentration. The commonly used lipid emulsions are Intralipid®, Omegaven® and Smoflipid®, with Intralipid® composed of lipids rich in omega-6 FAs, Omegaven® of omega-3 FAs and Smoflipid® of both types of lipids. Use of Omegaven® has been associated with decreased incidence of parenteral nutrition-associated liver disease (PNALD)1.
Lipids administered enterally are absorbed by the enterocyte in the form of a micelle and packaged into chylomicrons for liver metabolism. The lipid particles in parenteral soybean oil are similar in size and structure to the chylomicrons, but primarily contain omega-6 FAs and triglycerides. These lipid particles lack cholesterol or protein. Reduced cholesterol leads to limited lipolysis, resulting in an accumulation of lipid particles in the liver2.
Omega-3 FAs are thought to exhibit anti-inflammatory effects by causing a shift from omega-6 FA-derived proinflammatory eicosanoids to the anti-inflammatory variety derived from omega-3 FAs3. Absorption of phytosterols is limited to 5%–10% when plant products are consumed enterally. However, when given parenterally, phytosterols are fully bioavailable, allowing high, nonphysiologic serum concentrations. It has been shown that high concentrations of serum phytosterols in TPN-dependent children are correlated with PNALD and the severity of cholestasis2.
The etiology of PNALD is multifactorial3, and limited clinical studies have shown that oxidative stress is associated with PNALD4. Glutathione (GSH), an intracellular peptide present in all tissues with a particularly high concentration in the liver, has diverse functions that include detoxification and antioxidant defense5. GSH deficiency contributes to oxidative stress in the liver6. The genes involved in the GSH anti-oxidative stress pathway in the liver include, but are not limited to, glutathione S-transferase, pi 1 (Gstp1), glutathione S-transferase, mu 1 (Gstm1), glutathione S-transferase, mu 3 (Gstm3), glutathione S-transferase, mu 6 (Gstm6), and glutathione peroxidase 3 (Gpx3)7, 8, 9, 10. Additional genes involved in anti-oxidative stress are NAD(P)H quinone dehydrogenase 1 (Nqo1), heme oxygenase 1 (Ho-1), metallothionein 1 (Mt-1), glutamate-cysteine ligase catalytic subunit (Gclc), and glutamate-cysteine ligase modifier subunit (Gclm)11, 12, 13, 14, 15, 16. Studies on effects of TPN on the expression of these various genes are currently lacking.
Additionally, there are no reports on the effects of TPN on the expression of genes responsible for drug metabolism. Phase I cytochrome P450 (CYP) enzymes are necessary for detoxification of chemicals including metabolism of drugs17. The cytochrome P450 genes involved in drug and xenobiotic metabolism pathways in the liver of mice include, but are not limited to, Cyp3a11 (human: CYP3A4), Cyp2b10 (human: CYP2B6), Cyp2d9 (human: CYP2D9), and Cyp2f2 (human: CYP2F1).
Previously, we performed a microarray to evaluate the changes of expression of genes in the livers of mice receiving Intralipid®18. There was downregulation of the expression of several genes involved in GSH metabolism. We also discovered that the expression of genes involved in the drug metabolism pathway were downregulated in mice receiving Intralipid®. The objective of the current study is to determine the mRNA expression and protein levels of genes involved in oxidative stress and drug metabolism in a mouse model of TPN with Intralipid® or Omegaven®. We hypothesize that Intralipid® reduces the expression of genes involved in oxidative stress and drug metabolism. We, additionally, hypothesize that Omegaven® compared to Intralipid, results in less oxidative injury due to higher concentrations of antioxidative omega-3 FAs and lack of phytosterols.
2. Material and methods
2.1. Animals and treatments
Saline, Intralipid® or Omegaven® was administered to 20–25 g male C57BL/6J mice at 6–10 weeks of age. Saline, the components of Intralipid® or Omegaven® were obtained from CAPS pharmacy (Allentown, PA, USA). Mice receiving saline were allowed to feed ad libitum. Mice receiving Intralipid® or Omegaven® were kept nil per os. In order to administer TPN, mouse jugular veins were cannulated and TPN was infused continuously at 8–10 mL/day. Dosing was determined from previously published works18. After 14 days of Intralipid® (n = 5), Omegaven® (n = 5), or saline (n = 6) infusion, mice were euthanized and tissues were harvested for analysis. There was no period of fasting between time of last TPN infusion and sacrifice. All animal experiments were conducted with the approval of the Rutgers University Institutional Animal Care and Use Committee.
2.2. RNA isolation and gene expression measurement
Total RNA was isolated using TRI reagent (Sigma, St. Louis, MO, USA) according to the manufacturer's instructions. Reverse transcription was performed on mRNA to make cDNA. Liver mRNA expression of genes was analyzed by RT-qPCR with the details described in our previous publication19. The mRNA expression levels of the following genes were measured, including Gpx3, Gstp1, Gstm1, Gstm3, Gstm6, Nqo1, Ho-1, Mt-1, Gclc, Gclm, Cyp2d9, Cyp2f2, Cyp2b10, and Cyp3a11. The sequences of the primers will be provided upon request.
2.3. GSH level measurement
Total, oxidized (glutathione disulfide, GSSG) and reduced (GSH) glutathione in mouse liver lysates were measured using a glutathione kit assay (Cayman Chemical, Ann Arbor, MI, USA), according to supplier's instruction. Briefly, 0.1 g mouse liver tissue was homogenated in 1 mL cold GSH assay buffer homogenates, and supernatant after centrifugation at 100,000×g for 10 min was used to perform the assay. GSH and GSSH levels were normalized to liver weight.
2.4. Western blot to determining protein levels
Western blot was performed to measure the protein levels of CYP2B10 and CYP3A11 with glyceraldehyde 3-phosphate dehydrogenase (GADPH) as a loading control. Antibodies against mouse CYP2B10 (AB9916) and CYP3A11 (MAB10041) were from EMD Millipore (Burlington, MA, USA).
2.5. Statistical analysis
Data are expressed as mean ± SD. A Student's t-test was performed for statistical significance to show the difference between Intralipid® and saline. One-way ANOVA testing was performed to determine the difference between Intralipid®, saline, and Omegaven® and Tukey testing was performed to determine the difference between the individual groups. A P value less than 0.05 was considered statistically significant.
3. Results
3.1. Expression of genes in oxidative stress
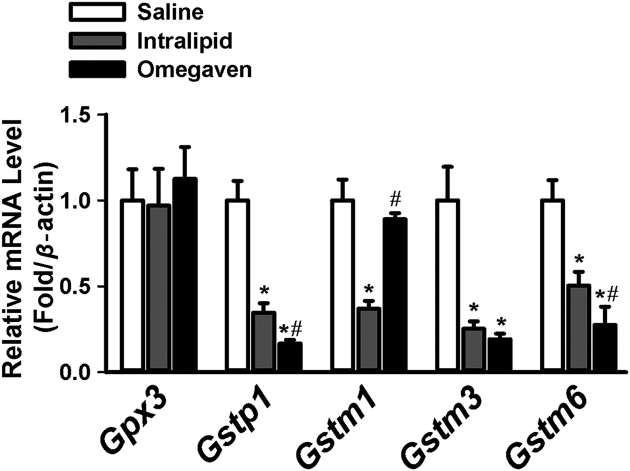
The mRNA levels of several genes involved in GSH metabolism were significantly down-regulated in mice receiving Intralipid® when compared to saline (Fig. 1). Overall, both Intralipid® and Omegaven® downregulated the expression of these genes consistently: Gstp1, Gstm1, Gstm3, and Gstm6. However, compared to mice receiving Intralipid®, there was preservation of Gstm1 with Omegaven® (Fig. 1).
Figure 1.
RT-PCR results of liver gene expression in mice with Saline (n = 6), Intralipid® (n = 5) and Omegaven® (n = 5). RT-PCR results comparing mRNA levels of Gpx3, Gstp1, Gstm1, Gstm3, and Gstm6. ∗indicates statistical significance (P < 0.05) between TPN and saline group. #indicates statistical significance (P < 0.05) between Intralipid® and Omegaven® treatment.
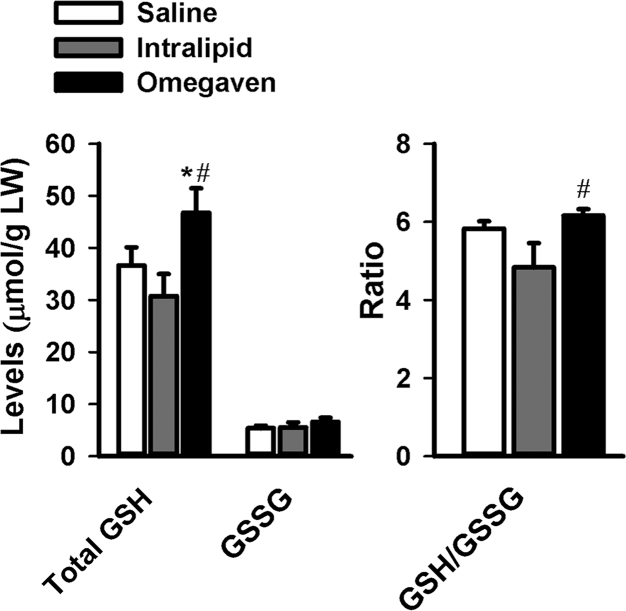
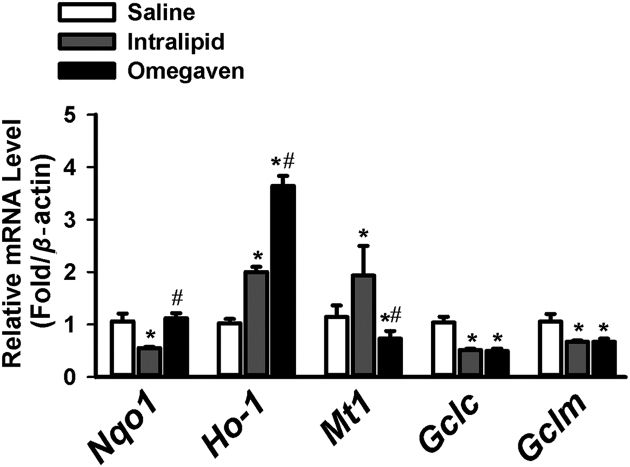
A low GSH/GSSG ratio occurs during oxidative stress. Whereas the GSH/GSSG ratio was decreased with Intralipid®, this ratio was preserved with Omegaven® (Fig. 2). TPN, regardless of lipid solution, significantly downregulated the mRNA levels of Gclc and Gclm in comparison to saline (Fig. 3). We measured the mRNA expression of several genes important in anti-oxidative stress. The mRNA levels of Nqo1 were downregulated with the use of Intralipid®, and there was a statistically significant difference noted for Nqo1 mRNA levels when comparing Intralipid® to Omegaven®. Ho-1 expression was increased with the use of both lipid solutions in comparison to saline, as well as when comparing intralipid® to Omegaven®. Mt-1 expression followed the same trend as other genes.
Figure 2.
Total levels of GSH and GSSG, and GSH/GSSG ratio in livers of mice treated with Saline (n = 6), Omegaven® (n = 5) and Intralipid® (n = 5). ∗indicates statistical significance (P < 0.05) between TPN and saline group. #indicates statistical significance (P < 0.05) between Intralipid® and Omegaven® treatment.
Figure 3.
Expression level of liver genes involved in anti-oxidative stress in mice with Saline (n = 6), Intralipid® (n = 5) and Omegaven® (n = 5). RT-PCR results comparing mRNA levels of Nqo1, Ho-1, Mt-1, Gclc, and Gclm with saline vs. Intralipid® vs. Omegaven®. ∗indicates statistical significance (P < 0.05) between TPN and saline group. #indicates statistical significance (P < 0.05) between Intralipid® and Omegaven® treatment.
3.2. Expression of genes in drug metabolism
Overall, the results comparing the mRNA levels of Cyp2d9, Cyp2f2, Cyp2b10, and Cyp3a11 revealed a trend of reduction by TPN regardless of lipid composition. There was statistical significance observed for each gene (Cyp2d9, P < 0.01; Cyp2f2, P < 0.0001; Cyp2b10, P < 0.01; and Cyp3a11, P < 0.01). Statistically significant downregulation of all four genes was observed on Tukey test comparing saline to Intralipid® (P < 0.05, 0.01, 0.05, 0.01 for Cyp2d9, Cyp2f2, Cyp2b10, Cyp3a11, respectively). Statistically significant downregulation of three of the genes was observed on Tukey test comparing saline to Omegaven® (Cyp2d9, P < 0.05, Cyp2f2, P < 0.001, Cyp2b10, P < 0.05). Interestingly, the mRNA levels of Cyp3a11 (equivalent to CYP3A4 in humans and responsible for the metabolism of majority of medications) were not statistically different from saline when compared to Omegaven® (Fig. 4).
Figure 4.
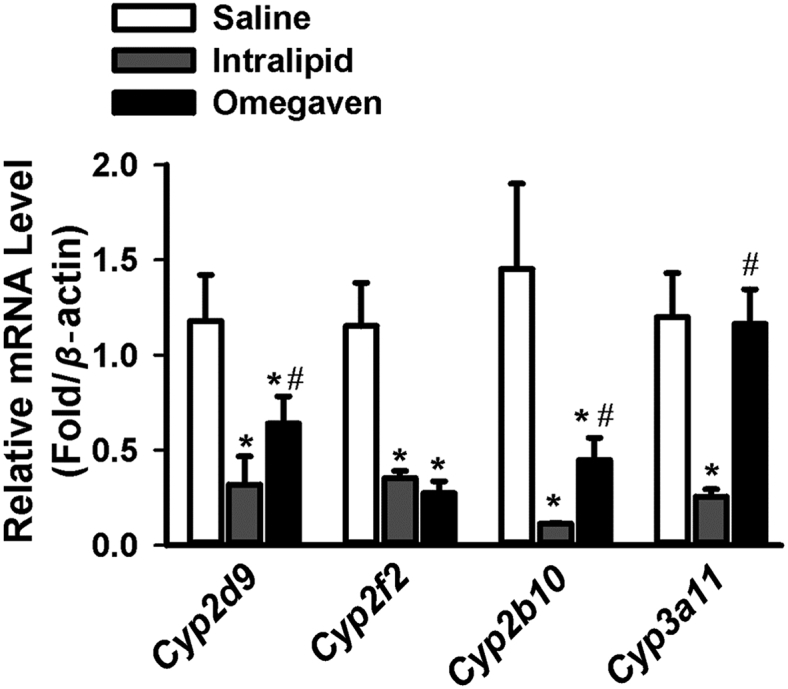
Changes in liver drug metabolism gene expression at mRNA levels with Saline (n = 6), Intralipid® (n = 5) and Omegaven® (n = 5). RT-PCR results were shown comparing the mRNA levels of Cyp2d9, Cyp2f2, Cyp2b10, and Cyp3a11. ∗indicates statistical significance (P < 0.05) between TPN and saline group. #indicates statistical significance (P < 0.05) between Intralipid® and Omegaven® treatment.
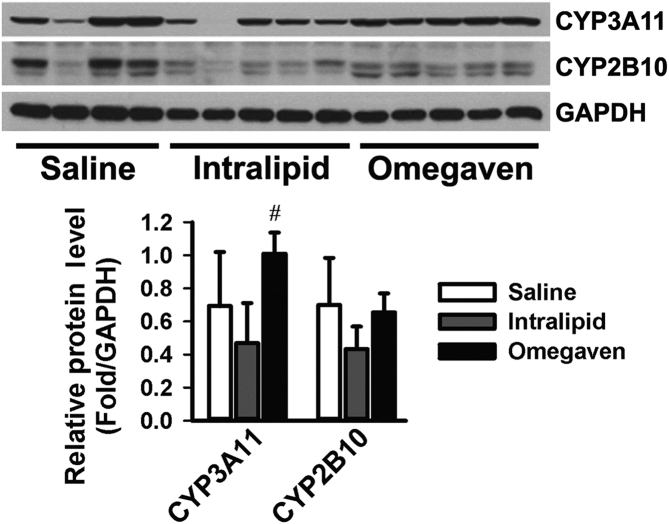
From Western blot analysis of the protein expression levels of CYP3A11 and CYP2B10, there was a statistically significant difference for CYP3A11 levels (P < 0.05), with Omegaven® upregulating the levels of CYP3A11. Furthermore, there was a statistically significant difference in the levels of CYP3A11 when comparing mice receiving Intralipid® to mice receiving Omegaven® (Fig. 5, P < 0.05).
Figure 5.
Protein levels of drug metabolism enzyme CYP2B10 and CYP3A11 in livers of mice treated with Saline (n = 6), Intralipid® (n = 5) or Omegaven® (n = 5). CYP2B10 and CYP3A11 protein levels were measured by Western blot, and GAPDH was used as the loading control. The levels of protein were quantified with #indicating statistical significance (P < 0.05) between Intralipid® and Omegaven® treatment.
4. Discussion
Reactive oxygen species (ROS) cause cellular injury and have been shown to play an important role in the pathogenesis of PNALD3. ROS species are generated continuously in every cell that depends on aerobic metabolism and can be detrimental to cellular function. During cholestasis, bile acids stimulate hepatocytes to produce proinflammatory mediators that attract neutrophils. Neutrophils release ROS that injure hepatocytes, stimulating release of cytokines from Kupffer cells or even directly activating neutrophils20. Healthy cells contain several intracellular enzyme systems that limit the production of ROS and maintain extremely low cellular levels of these oxidants21. Additionally, cells contain several low-molecular-weight antioxidants, such as GSH, which effectively eliminate ROS20.
GSH is the most abundant low-molecular-weight thiol, and GSH/glutathione disulfide is the major redox couple in animal cells. GSH exists in all mammalian tissues and is highly concentrated in the liver. Glutathione exists in the thiol-reduced (GSH) and disulfide-oxidized (GSSG) forms, with GSH as the predominant form5. We found that the mRNA expression of genes involved in GSH metabolism, including Gstp1, Gstm1, Gstm3, and Gstm6, was down-regulated in mice receiving Intralipid®. We speculate that the dysregulation of GSH homeostasis may contribute to PNALD as a result of diminished antioxidative activity in the liver. Compared with mice receiving Intralipid®, there is preservation of one of the GSH pathway genes, Gstm1, with use of Omegaven®. We also found that GSH levels were decreased in mice receiving Intralipid®, but increased in those receiving Omegaven®. We speculate that the increased omega-3/omega-6 ratio, decreased phytosterol concentrations, and/or increased vitamin E concentrations in Omegaven are protective against oxidative stress by preserving glutathione and GSH/GSSG ratios. This information may be helpful in identifying future strategies for PNALD intervention.
The difference in lipid compositions of soybean oil parenteral nutrition, Intralipid®, and Omegaven® may also play a role in the development of cholestasis22. There is a much higher concentration of vitamin E in Omegaven® in comparison to Intralipid®. Omegaven® also has a lack of both phytosterols and soybean oil, but with a high concentration of fish oil22,23. Of note, the fat compositions of the two lipids are different as well22. Omegaven® may be protective against oxidative stress by preserving glutathione and GSH/GSSG ratios. We speculate that the dysregulation of GSH homeostasis may contribute to PNALD as a result of diminished antioxidative activity in the liver. This information may be helpful in identifying future strategies for PNALD intervention.
One of the mechanisms implicated in PNALD is down-regulation of hepatic anti-oxidative pathways and therefore an increase in oxidative injury to the liver. GSH depletion and oxidative stress are important factors in liver injury as well as the pathogenesis of TPN-induced liver abnormalities in weanling rats24. After 5 days of TPN, Sokol et al.25 demonstrated that hepatic GSH levels decreased to 16% of control values. After 8 days of TPN, serum alanine aminotransferase activities were elevated, hepatocellular steatosis was present, and lipid peroxidation of mitochondria was increased compared with control groups. Oxidative stress has been shown to be associated with hepatocellular injury in preterm infants. Weinberger et al.4 demonstrated that urinary levels of thiobarbituric-acid-reacting substances, indicators of lipid peroxidation and oxidative stress, are correlated with elevated serum transaminase levels.
This information led us to investigate other genes involved in antioxidative stress, such as Nqo1, Gclc, Gclm, Ho-1, and Mt-111,13,14,16. NQO1 is a flavoprotein with superoxide scavenging activity and it minimizes opportunities for generation of ROS by redox cycling. Induction of NQO1 levels is associated with decreased susceptibilities to oxidative stress. When NQO1 levels are depleted, an increase in oxidative stress is observed16. GCL is the rate-limiting step in GSH synthesis and represents a heterodimeric enzyme comprised of a two subunits: GCLC and GCLM11. In mammals, HO is the rate-limiting enzyme for the degradation of heme13.
HO is a catalyst for the oxidative degradation of heme into iron, biliverdin/bilirubin, and carbon monoxide12,13. As an adaptive cellular response to environmental stresses, HO-1 activity is induced. Though the functions of this enzyme are not entirely understood, evidence suggests a role in suppression of inflammation and oxidative stress. The protective effects of HO-1 may be due to its antioxidative product biliverdin/bilirubin, which can scavenge peroxide radicals and inhibit lipid peroxidation13. Ho-1 induction was increased with both Intralipid and Omegaven.
MT-1 is a small cysteine-rich protein that tightly binds and exchanges specific metal ions, particularly zinc14. This protein detoxifies heavy metals like mercury and cadmium, while promoting homeostasis of essential metals including copper and zinc. MT-1 also provides antioxidation against ROS, protection against DNA damage and oxidative stress15. The antioxidant property of MT-1 enhances in the presence of zinc14. Although not statistically significant, mRNA levels of Mt-1 were increased in the presence of Intralipid®.
Therefore, we speculate that reduced expression of Gclc and Gclm may lead to a decrease in GSH. Preservation of Nqo1 expression with the use of Omegaven® may lead to decreased susceptibility to oxidative stress. Increased Ho-1 expression was likely a protective mechanism to suppress oxidative stress. The increase in Mt-1 expression may be due to a difference in the concentration of copper and zinc in Intralipid® vs. Omegaven®.
CYP450 enzymes are important in the detoxification of chemicals and the metabolism of drugs and are subject to regulation in ontogeny26. CYP450 enzymes are also essential for the production of steroids, cholesterol, thromboxane A2, and prostacyclins27. In humans, there are more than 50 CYP450 enzymes identified. Six of these enzymes metabolize 90 percent of drugs. The two most important enzymes in humans are CYP3A4 and CYP2D6. CYP3A11 is the murine homolog to human CYP3A427,28. A high dosage of α-tocopherol leads to increased levels of CYP3A11 in mice. This may induce the drug-metabolizing system to an excessive level, which may lead to an interference with drug metabolism28.
We found that Intralipid® and Omegaven®, in spite of different lipid composition, reduced the expression of most drug metabolism genes in mice, including Cyp2d9, Cyp2f2, and Cyp2b10. Although Cyp3a11 was downregulated by Intralipid®, but not by Omegaven®. This suggests another beneficial effect of Omegaven® in protecting liver functions. Due to the alteration of the expression of CYP450 enzymes, drug metabolism maybe likely affected by the administration of intravenous lipid infusions. Preservation of Cyp3a11 expression by Omegaven® may be one possible mechanism of its role in preventing the PNALD development.
5. Conclusions
We have shown that the antioxidative defense pathways in the liver are affected by different lipid emulsions in TPN. Compared to Intralipid®, Omegaven® use is associated with preservation of genes essential for detoxification systems in a murine parenteral nutrition model.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01GM104037, R21ES029258, T32ES007148, and P3-ES005022, USA) and Department of Veterans Affairs (BX002741, USA).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Author contributions
Substantial contributions to the conception or design of the work: Christina Ferrucci-Da Silva, Le Zhan, Naureen Memon, Thomas Hegyi, and Grace L. Guo. Acquisition, analysis, or interpretation of data for the work: Christina Ferrucci-Da Silva, Le Zhan, Jianliang Shen, Bo Kong, Thomas Hegyi, Lucy Lu, and Grace L. Guo. Drafting the work or revising it critically for important intellectual content: Christina Ferrucci-Da Silva, Michael J. Campbell, Bo Kong, and Grace L. Guo. Final approval of the version to be published: Christina Ferrucci-Da Silva, Le Zhan, Jianliang Shen, Bo Kong, Michael J. Campbell, Naureen Memon, Thomas Hegyi, Lucy Lu, and Grace L. Guo. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Christina Ferrucci-Da Silva, Le Zhan, Jianliang Shen, Bo Kong, Michael J. Campbell, Naureen Memon, Thomas Hegyi, Lucy Lu, and Grace L. Guo.
Conflicts of interest
There is no conflict of interest among all authors contributed to this research.
References
- 1.Nandivada P., Cowan E., Carlson S.J., Chang M., Gura K.M., Puder M. Mechanisms for the effects of fish oil lipid emulsions in the management of parenteral nutrition-associated liver disease. Prostag leukotr ess. 2013;89:153–158. doi: 10.1016/j.plefa.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Nandivada P., Carlson S.J., Chang M.I., Cowan E., Gura K.M., Puder M. Treatment of parenteral nutrition-associated liver disease: the role of lipid emulsions. Adv Nutr. 2013;4:711–717. doi: 10.3945/an.113.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tillman E.M., Helms R.A. Omega-3 long chain polyunsaturated fatty acids for treatment of parenteral nutrition-associated liver disease: a review of the literature. J Pediatr Pharmacol Ther. 2011;16:31–38. [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberger B., Watorek K., Strauss R., Witz G., Hiatt M., Hegyi T. Association of lipid peroxidation with hepatocellular injury in preterm infants. Crit Care. 2002;6:521–525. doi: 10.1186/cc1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu S.C. Regulation of glutathione synthesis. Mol Asp Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu G., Fang Y.Z., Yang S., Lupton J.R., Turner N.D. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 7.Ghobadloo S.M., Yaghmaei B., Bakayev V., Goudarzi H., Noorinayer B., Rad F.H. GSTP1, GSTM1, and GSTT1 genetic polymorphisms in patients with cryptogenic liver cirrhosis. J Gastrointest Surg. 2004;8:423–427. doi: 10.1016/j.gassur.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Maurizio P., Novo E. Nrf1 gene expression in the liver: a single gene linking oxidative stress to NAFLD, NASH and hepatic tumours. J Hepatol. 2005;43:1096–1097. doi: 10.1016/j.jhep.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Qi X., Ng K.T., Lian Q.Z., Liu X.B., Li C.X., Geng W. Clinical significance and therapeutic value of glutathione peroxidase 3 (GPx3) in hepatocellular carcinoma. Oncotarget. 2014;5:11103–11120. doi: 10.18632/oncotarget.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu K., Liu S.S., Wang Z.X., Huang Z.C., Liu S.N., Chang H.L. Polymorphisms of glutathione S-transferase genes and survival of resected hepatocellular carcinoma patients. World J Gastroenterol. 2015;21:4310–4322. doi: 10.3748/wjg.v21.i14.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalton T.P., Dieter M.Z., Yang Y., Shertzer H.G., Nebert D.W. Knockout of the mouse glutamate cysteine ligase catalytic subunit (Gclc) gene: embryonic lethal when homozygous, and proposed model for moderate glutathione deficiency when heterozygous. Biochem Biophys Res Commun. 2000;279:324–329. doi: 10.1006/bbrc.2000.3930. [DOI] [PubMed] [Google Scholar]
- 12.Sikorski E.M., Hock T., Hill-Kapturczak N., Agarwal A. The story so far: molecular regulation of the heme oxygenase-1 gene in renal injury. Am J Physiol Renal Physiol. 2004;286:F425–F441. doi: 10.1152/ajprenal.00297.2003. [DOI] [PubMed] [Google Scholar]
- 13.Wang R.Q., Nan Y.M., Wu W.J., Kong L.B., Han F. Induction of heme oxygenase-1 protects against nutritional fibrosing steatohepatitis in mice. Lipids Health Dis. 2011;10:31. doi: 10.1186/1476-511X-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis S.R., Cousins R.J. Metallothionein expression in animals: a physiological perspective on function. J Nutr. 2000;130:1085–1088. doi: 10.1093/jn/130.5.1085. [DOI] [PubMed] [Google Scholar]
- 15.Thirumoorthy N., Shyam Sunder A., Manisenthil Kumar K., Senthil Kumar M., Ganesh G., Chatterjee M. A review of metallothionein isoforms and their role in pathophysiology. World J Surg Oncol. 2011;9:54. doi: 10.1186/1477-7819-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinkova-Kostova A.T., Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys. 2010;501:116–123. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang X.L., Gonzalez F.J., Yu A.M. Drug-metabolizing enzyme, transporter, and nuclear receptor genetically modified mouse models. Drug Metab Rev. 2011;43:27–40. doi: 10.3109/03602532.2010.512294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhan L., Yang I., Kong B., Shen J., Gorczyca L., Memon N. Dysregulation of bile acid homeostasis in parenteral nutrition mouse model. Am J Physiol Gastrointest Liver Physiol. 2016;310:G93–G102. doi: 10.1152/ajpgi.00252.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong B., Zhang M., Huang M., Rizzolo D., Armstrong L.E., Schumacher J.D. FXR deficiency alters bile acid pool composition and exacerbates chronic alcohol induced liver injury. Dig Liver Dis. 2019;51:570–576. doi: 10.1016/j.dld.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Copple B.L., Jaeschke H., Klaassen C.D. Oxidative stress and the pathogenesis of cholestasis. Semin Liver Dis. 2010;30:195–204. doi: 10.1055/s-0030-1253228. [DOI] [PubMed] [Google Scholar]
- 21.Yang C.S., Lambert J.D., Sang S. Antioxidative and anti-carcinogenic activities of tea polyphenols. Arch Toxicol. 2009;83:11–21. doi: 10.1007/s00204-008-0372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlaardingerbroek H., Ng K., Stoll B., Benight N., Chacko S., Kluijtmans L.A. New generation lipid emulsions prevent PNALD in chronic parenterally fed preterm pigs. J Lipid Res. 2014;55:466–477. doi: 10.1194/jlr.M044545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calkins K.L., Venick R.S., Devaskar S.U. Complications associated with parenteral nutrition in the neonate. Clin Perinatol. 2014;41:331–345. doi: 10.1016/j.clp.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng X., Li X., Xu C., Jiang F., Mo Y., Fan X. Schisandra sphenanthera extract (Wuzhi Tablet) protects against chronic-binge and acute alcohol-induced liver injury by regulating the NRF2-ARE pathway in mice. Acta Pharm Sin B. 2017;7:583–592. doi: 10.1016/j.apsb.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokol R.J., Taylor S.F., Devereaux M.W., Khandwala R., Sondheimer N.J., Shikes R.H. Hepatic oxidant injury and glutathione depletion during total parenteral nutrition in weanling rats. Am J Physiol. 1996;270:G691–G700. doi: 10.1152/ajpgi.1996.270.4.G691. [DOI] [PubMed] [Google Scholar]
- 26.Peng L., Piekos S., Guo G.L., Zhong X.B. Role of farnesoid X receptor in establishment of ontogeny of phase-I drug metabolizing enzyme genes in mouse liver. Acta Pharm Sin B. 2016;6:453–459. doi: 10.1016/j.apsb.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch T., Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 2007;76:391–396. [PubMed] [Google Scholar]
- 28.Kluth D., Landes N., Pfluger P., Muller-Schmehl K., Weiss K., Bumke-Vogt C. Modulation of Cyp3a11 mRNA expression by alpha-tocopherol but not gamma-tocotrienol in mice. Free Radic Biol Med. 2005;38:507–514. doi: 10.1016/j.freeradbiomed.2004.11.010. [DOI] [PubMed] [Google Scholar]